BACKGROUND
In addition to reducing subcutaneous fat for body contouring, some patients are interested in toning the underlying muscle layer.
OBJECTIVE
This feasibility study evaluated the safety and efficacy of electromagnetic muscle stimulation (EMMS) alone, cryolipolysis alone, and cryolipolysis with EMMS for noninvasive contouring of abdomen.
METHODS
Abdomens of 50 subjects were treated in a study with 3 cohorts: EMMS alone, Cryolipolysis alone, and Cryolipolysis + EMMS in combination. Electromagnetic muscle stimulation treatments were delivered in 4 sessions over 2 weeks. Cryolipolysis treatments were delivered in one session. Combination treatments consisted of one cryolipolysis and 4 EMMS visits. Efficacy was assessed by independent physician Global Aesthetic Improvement Scale (GAIS), circumferential measurement, Subject GAIS (SGAIS), and Body Satisfaction Questionnaire (BSQ).
RESULTS
Safety was demonstrated for all study cohorts with no device- or procedure-related adverse events. Independent photo review showed greatest mean GAIS score for the Cryolipolysis + EMMS cohort followed by Cryolipolysis only, then EMMS only cohort. BSQ showed greatest average score increase for Cryolipolysis + EMMS cohort followed by Cryolipolysis only cohort, then EMMS only cohort. Mean circumferential reduction measurements were greatest for Cryolipolysis + EMMS cohort followed by Cryolipolysis only, and then EMMS only cohort. The mean SGAIS improvement score was equal for the Cryolipolysis only and Cryolipolysis + EMMS cohorts, followed by the EMMS only cohort.
CONCLUSION
A multimodal approach using cryolipolysis and EMMS was safe and demonstrated enhanced body contouring efficacy for this feasibility study.
Noninvasive body contouring procedures continue to grow in popularity as patients seek treatments without unwanted surgical risks and down time. Cryolipolysis is a popular noninvasive fat reduction procedure that uses controlled cooling to target subcutaneous fat and induce adipocyte apoptosis. CoolSculpting is indicated for cold-assisted lipolysis of the upper arm, bra fat, back fat, banana roll, thigh, abdomen, and flank, or “love handles,” in individuals with a body mass index (BMI) of 30 or less. In addition, the device is intended for cold-assisted lipolysis of the submental and submandibular areas in individuals with a BMI up to 46.2.1 Numerous clinical studies have demonstrated the safety, efficacy, and tolerability of cryolipolysis in multiple body areas.2–26
As patients grow increasingly comfortable with aesthetic procedures and noninvasive body contouring, interest has grown to further enhance body contouring results. In addition to reducing unwanted subcutaneous fat with a modality such as cryolipolysis, some patients are interested in toning the underlying muscle layer.
Recently, electromagnetic muscle stimulation (EMMS) has been used for aesthetic body contouring. Although EMMS has been used for decades in physical therapy and urological applications, it is a new energy-based device technology in aesthetic medicine and uses different treatment parameters. Compared with neuromuscular electrical stimulation, EMMS has the advantage of not coupling through the patient's skin, thus minimizing discomfort caused by sensory nerve stimulation. Based on the inherent electrical properties of the skin, fat, and muscle, current flow can be induced directly in the muscle layer because of its greater electrical conductivity and powerful contractions can be stimulated without dermal discomfort. Clinical studies have been conducted to show safety and efficacy of EMMS for body contouring in the abdomen and buttocks.27–30 Electromagnetic muscle stimulation has also been used for toning and strengthening thighs.
Recently, CoolTone, an EMMS device, received clearance by the US FDA for improvement of abdominal tone, strengthening of the abdominal muscles, and development of firmer abdomen and for strengthening, toning, and firming of buttocks and thighs. The Emsculpt EMMS device was first FDA-cleared in 2017 and now has indications to strengthen, firm, and tone the abdomen, buttocks, thighs, arms, and calves.
To address patient concerns of excess subcutaneous fat and decreased muscle tone, a multi-modal treatment approach would be required. This feasibility study was designed to evaluate the safety and efficacy of EMMS alone, cryolipolysis alone, and the combination of cryolipolysis and EMMS for noninvasive contouring of the abdomen.
Materials and Methods
This was a multicenter, prospective, open label, nonrandomized feasibility study. The protocol was approved by an independent review board (Salus IRB, Austin, TX). Eligible subjects were men or women, between 22 and 65 years old, with a desire for subcutaneous fat reduction and firming and toning of the abdomen. Subjects with BMI ≤30 kg/m2 and abdominal skin fold thickness ranging from 2 to 5 cm as measured by caliper were eligible for trial inclusion.
Exclusion criteria included a previous surgical procedure, invasive fat reduction procedure, a prior noninvasive fat reduction, body contouring, and/or skin tightening procedure in or near the treatment area in the past 12 months, known history of cryoglobulinemia, cold urticaria, cold agglutinin disease, paroxysmal cold hemoglobinuria, Raynaud disease, bleeding disorders or concurrent medications that could increase the risk of bruising, dermatological conditions, such as excessive skin laxity or scars that may interfere with the treatment or evaluation, intrauterine contraceptive device inserted or removed within the past month, cardiac disorder, pulmonary insufficiency, and metal implant or active implanted devices such as a pacemaker, defibrillator, or drug delivery system. Subjects involved in any type of abdominal muscle training program within the previous 6 months were also excluded.
For the duration of the study, subjects were instructed to avoid implementing major diet or exercise changes to maintain their weight within ±5% of baseline measurement. Subjects also had to agree to refrain from any new abdominal training exercises during the course of the study. Before treatment and 12 weeks after final treatment, 3D and 2D clinical images were obtained and subject Body Satisfaction Questionnaires were administered. Subject Global Aesthetic Improvement Scale surveys were administered at the final follow-up visit.
The subjects were enrolled into 3 study cohorts. Cohort 1 subjects received EMMS only from a prototype (CoolTone, ZELTIQ Aesthetics, Pleasanton, CA) or commercial (Emsculpt, BTL Industries, Marlborough, MA) system. Cohort 2 subjects received a Cryolipolysis treatment and 4 EMMS treatments. Cohort 3 subjects received Cryolipolysis only.
For the cohorts receiving EMMS treatment, subjects had 4 EMMS treatment visits with one applicator placed on the center of the abdomen. Each EMMS treatment cycle was delivered at the highest tolerable intensity for 30 minutes. There were 4 EMMS treatment sessions, all treatments were separated by at least 48 hours, within a 2-week period.
For the cohorts receiving cryolipolysis treatment, subjects received treatment cycles to the abdomen in a single session. Noninvasive fat reduction procedures were performed with the FDA-cleared cryolipolysis device (CoolSculpting, ZELTIQ Aesthetics, Pleasanton, CA). Each cryolipolysis treatment cycle was delivered by a cooled cup vacuum applicator (−11°C for 35 minutes for CoolAdvantage and CoolAdvantage Petite applicators or −11°C for 45 minutes for CoolAdvantage Plus applicator). A protective gel pad (CoolAdhesive GelPad) was applied to the skin and suction was initiated. The vacuum adhered the applicator to the treatment area and the subject reclined throughout the cryolipolysis procedure. After each treatment cycle, vacuum was stopped, the applicator was removed, and a manual massage of the treatment area was performed for 2 minutes. Based on the investigator's assessment of fat presentation, additional cryolipolysis cycles were delivered to the upper and lower abdomen (3–6 total cycles per abdomen).
Patient discomfort was monitored throughout the study procedures, immediately following device removal, before patient discharge, and at the 1, 4, and 12-week follow-up visits. Safety was monitored by documentation of adverse events and clinical assessment of the treatment site. Subjects were assessed throughout the study for adverse events.
The primary study efficacy end point was a blinded, independent photo review comparing the pretreatment and 12-week post-treatment 2D photographs by a physician board-certified in dermatology. The independent reviewer evaluated the baseline and final visit photographs and assessed contour improvement per the Global Aesthetic Improvement Scale (GAIS), Table 1.
TABLE 1.
Global Aesthetic Improvement Scale to Assess Abdomen Contour Improvements From Baseline and Final Visit Photographs
| Score | Description |
| 3 | Very much improved |
| 2 | Much improved |
| 1 | Improved |
| 0 | No change |
| −1 | Worse |
| −2 | Much worse |
| −3 | Very much worse |
The primary safety end point was the incidence of unanticipated adverse device effects. Secondary study end points were abdominal circumference as assessed by 3D imaging and assessment of contour changes using a Subject Global Aesthetic Improvement Scale (SGAIS). A subject Body Satisfaction Questionnaire (BSQ), previously used in neuromuscular electrical stimulation body contouring studies,31,32 was administered to assess how subjects perceived the shape and appearance of their abdomens. A set of 10 dichotomous word pairs was presented with a selection of 5 checkboxes to assess how the subject described the appearance of their stomach at the present time (e.g., flat vs rounded, weak vs strong, hard vs soft). The total score was tabulated to quantify the subject's current perception of the abdomen area, with a higher score indicating greater improvement.
Treatment efficacy was assessed by 2D clinical photographs (Nikon D800E with 85 mm lens) and circumferential measurements obtained using a 3D imaging system (LifeViz Body by QuantifiCare, San Francisco, CA). The 3D camera was mounted on a tripod to capture stereo images. The subject was positioned on a rotating stage with a fixed footprint diagram to collect images in 8 positions at 45-degree increments. Clothing was standardized and carefully adjusted to avoid affecting the appearance of fat and muscle in the abdominal area. Subjects held their arms in a fixed position and relaxed to remove muscular tension, then images were taken. Circumference measurements were taken at 3 points on the upper, middle, and lower abdomen; measurements were averaged for a mean circumference per study subject.
Statistical analysis was performed based on the nature of the data. Dichotomous (e.g., gender) and ordinal (e.g., Fitzpatrick Skin Type) data were tabulated by category. The mean, SD, maximum and minimum were tabulated for continuous data (e.g., age, circumferential reduction). The 0.05 significance level was calculated from a paired, 2-tailed test.
Results
Fifty subjects were enrolled and completed treatment. Thirty-eight of the subjects were women and 12 were men. Ethnicity of subjects comprised Caucasian (n = 43), Asian (n = 3), American Indian or Alaska Native (n = 1), or Other (n = 3) with Fitzpatrick Skin Type II (n = 16), Type III (n = 22), Type IV (n = 10), or Type V (n = 2). The subject ages ranged from 22 to 62 years (mean 42.2). The average weight was 146.4 lbs (range 110.0–201.0) with mean body mass index 23.5 kg/m2 (range 17.8–28.1).
As shown in Table 2, 20 subjects were enrolled in Cohort 1 for EMMS only, 20 subjects were enrolled in Cohort 2 for Cryolipolysis + EMMS, and 10 subjects were enrolled in Cohort 3 for Cryolipolysis only. For the EMMS treatments, subjects were evenly divided between the prototype (n = 10) and commercial (n = 10) EMMS systems for both Cohorts 1 and 2.
TABLE 2.
Study Cohort Enrollment and Treatment Cycles
| Study Cohort | Description | Subjects, n | Average Cryolipolysis Cycles | EMMS Cycles |
| 1 | EMMS only | 20 | n/a | 4 |
| 2 | Cryolipolysis + EMMS | 20 | 4.3 | 4 |
| 3 | Cryolipolysis only | 10 | 3.9 | n/a |
EMMS, electromagnetic muscle stimulation.
There were 3 cases of data exclusion. One subject had weight change greater than the ±5% weight change limit and was excluded from efficacy analysis. Another subject underwent a surgical procedure in the abdominal area and was also excluded from efficacy analysis. One subject missed the final study visit for photographs and was not included in the independent photo review GAIS or circumferential measurements.
For the independent photo review, 47 photograph pairs were available for analysis. An independent reviewer was blinded to the study cohorts but unblinded to the baseline and final photographic timepoints. The reviewer assessed each study subject's photos from baseline to final visit on a GAIS with 3 = Very much improved to 0 = No change to −3 = Very much worse. Figure 1 depicts the average GAIS scores with the EMMS only cohort scoring a mean of 1.2, Cryolipolysis only cohort scoring a mean of 1.4, and combined Cryolipolysis + EMMS cohort scoring a mean of 1.9. The Cryolipolysis + EMMS cohort improvement was statistically significant relative to the EMMS only cohort, but not relative to the Cryolipolysis only cohort. For the EMMS only cohort, 80% of subjects were scored as an improvement of 1 or greater, whereas 89% of subjects were scored as an improvement of 1 or greater in both the Cryolipolysis + EMMS and Cryolipolysis only cohorts.
Figure 1.
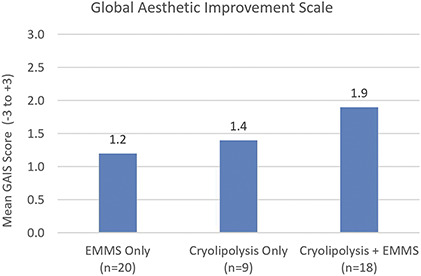
The mean Global Aesthetic Improvement Scale score assessed by independent reviewer at 12 weeks after final treatment demonstrates greatest body contour improvement for the Cryolipolysis + EMMS study cohort. EMMS, electromagnetic muscle stimulation.
Figures 2–4 show representative subjects from each study cohort at baseline and 12 weeks after final treatment and demonstrate visible changes to abdominal contour.
Figure 2.
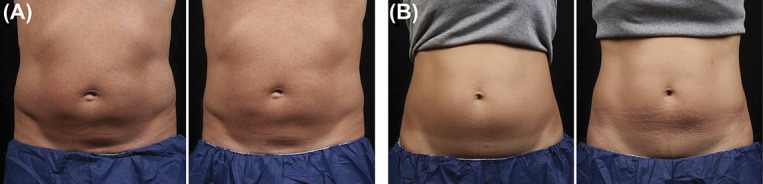
Representative baseline (left) and 12-week post-treatment (right) photographs following EMMS only treatment, Cohort 1, weight change shown from baseline. (A) 58-year-old man, +5.2 lbs. (B) 43-year-old woman, +6.0 lbs. EMMS, electromagnetic muscle stimulation.
Figure 4.
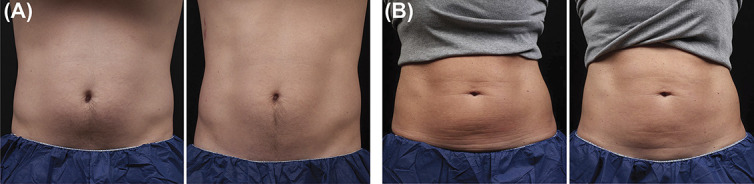
Representative baseline (left) and 12-week post-treatment (right) photographs following Cryolipolysis + EMMS treatment, Cohort 2, weight change shown from baseline. (A) 31-year-old male, −8.2 lbs. (B) 46-year-old female, 0.0 lbs. EMMS, electromagnetic muscle stimulation.
Figure 3.
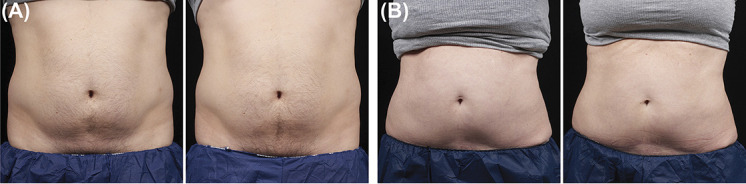
Representative baseline (left) and 12-week post-treatment (right) photographs following Cryolipolysis only treatment, Cohort 3, weight change shown from baseline. (A) 35-year-old male, +1.0 lbs. (B) 57-year-old female, 0.0 lbs.
The Subject Global Aesthetic Improvement Scale (SGAIS) data showed improvement for all study cohorts. SGAIS improvement was comparable between cohorts with a mean score of 1.2 for Cryolipolysis only and Cryolipolysis + EMMS cohorts and a mean of 1.0 for the EMMS only cohort. An assessment of subjects reporting a SGAIS improvement of 1 or greater found 100% of subjects reporting improvement for the Cryolipolysis only cohort, followed by 89% for Cryolipolysis + EMMS cohort and 70% for EMMS only cohort.
Circumference measurement was performed on the 3D images before treatment and at the 12-week post-treatment visit. The measured change in abdomen circumference ranged from a max increase of 33 mm to a max decrease of 37 mm, with a mean reduction in circumference of 4 mm for the EMMS only, 10 mm for Cryolipolysis only, and 15 mm for the Cryolipolysis + EMMS cohorts (Figure 5). Statistically significant improvement for circumferential reduction was found between EMMS only and Cryolipolysis + EMMS cohorts.
Figure 5.
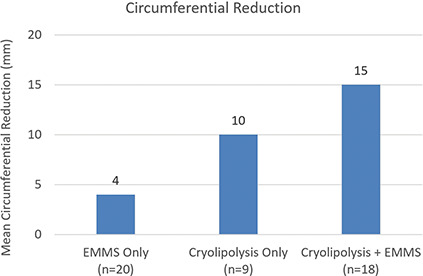
Mean change in abdominal circumference at 12 weeks post-final treatment showed greatest reduction for the Cryolipolysis + EMMS cohort. EMMS, electromagnetic muscle stimulation.
The Body Satisfaction Questionnaire (BSQ) was administered to assess subject perception of the shape and appearance of their abdomens. Figure 6 shows the mean BSQ improvement scores between baseline and final visits. All cohorts showed statistically significant improvement from baseline with the greatest average BSQ score increase of 7.6 for the Cryolipolysis + EMMS cohort, followed by 5.7 for Cryolipolysis only cohort, and then 3.6 for EMMS only cohort. Between cohorts, statistically significant difference for BSQ score was only found between EMMS only and Cryolipolysis + EMMS treatment groups.
Figure 6.
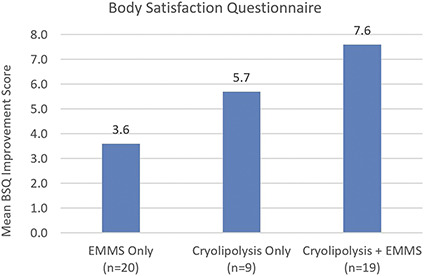
Body satisfaction questionnaire showed mean improvement for all study cohorts between baseline and 12 weeks post-final visit. Greatest BSQ score increase was found in the Cryolipolysis + EMMS cohort. EMMS, electromagnetic muscle stimulation.
Clinical assessment of the treatment areas was performed immediately post-treatment and at each follow-up visit. For the Cryolipolysis only cohort, side effects were typical, including mild-to-moderate numbness and erythema. At the 12-week final visit, all cryolipolysis side effects resolved without intervention. For the EMMS only cohort, side effects were absent in most subjects immediately after treatment with 3 instances of mild erythema that resolved without intervention by the 2-week follow-up visit. For the Cryolipolysis + EMMS cohort, immediate post-treatment side effects were consistent for each treatment modality alone and self-resolved by the 12-week final visit.
There were no reported device- and/or procedure-related adverse events for all 3 study cohorts. The EMMS only, Cryolipolysis only, and Cryolipolysis + EMMS cohorts all demonstrated procedural safety.
Discussion
This feasibility study is the first to evaluate safety and efficacy of the cryolipolysis and EMMS non-invasive body contouring procedures when used separately and in combination for body contouring. Both technologies have published clinical studies demonstrating safety and efficacy alone, whereas here, we establish safety and enhanced GAIS, BSQ scores, and circumferential reduction with cryolipolysis and EMMS used in combination for abdominal contouring.
For efficacy assessments, the Cryolipolysis + EMMS cohort showed the greatest improvement 12 weeks after treatment, except for SGAIS, in which the combination treatment and the Cryolipolysis only cohorts had the same mean SGAIS scores at week 12. For GAIS, BSQ, and circumferential reduction, all cohorts showed improvement, but the Cryolipolysis + EMMS cohort showed greatest improvement.
For logistical reasons, different clinical sites in the multicenter study received either the prototype (CoolTone) or commercially-available (Emsculpt) EMMS systems. These systems were evenly divided with n = 10 subjects each in study Cohorts 1 and 2. Although not prospectively defined, statistical analysis showed no significant difference between the systems for efficacy assessments of BSQ, SGAIS, GAIS, and circumferential reduction. Thus, the data for all subjects treated with EMMS are pooled in this feasibility study.
EMMS only treatments in Figure 2 seem to show clinical improvement in contouring and muscle definition. This presumed muscle hypertrophy may have detracted from the circumferential reduction measurements. Even for subjects that had weight increase from baseline, visible improvement in abdominal contour was shown in Figures 2–4.
Between cohorts, statistically significant difference was only found between the EMMS only and Cryolipolysis + EMMS cohorts for BSQ, GAIS, and circumferential reduction. This study is limited, however, by the small study size which is not powered sufficiently to establish significant difference between all study cohorts. Based on the trends observed in these efficacy assessments, the Cryolipolysis + EMMS cohort suggests improved abdominal contouring relative to either cryolipolysis or EMMS alone.
Electromagnetic muscle stimulation is a relatively new energy-based device treatment for noninvasive body contouring and needs further study to establish the optimal treatment protocol. Currently, we are treating patients with 4 EMMS procedures within approximately 2 weeks. In clinical practice, our patients typically choose to return in 1-to-3-month intervals for a single EMMS treatment to maintain their firmer, more toned results.
In addition to studying optimal timing for EMMS initial and maintenance treatments, combination treatment protocols should also be studied further to ensure safety and efficacy while enhancing convenience for the patient and the practice. To ensure the appropriate assessment of outcomes, the cryolipolysis and EMMS procedures were delivered on separate visits during this study. In clinical practice, however, clinicians have scheduled patients to receive both cryolipolysis and EMMS treatments on the same day, alternating which procedure takes place first based on scheduling and device availability.
In addition to the efficacy data gathered in this clinical study, future clinical studies can collect data that more clearly quantifies the patient experience following EMMS. Anecdotally, patients report not only aesthetic improvement, but functional changes that last months, as well. Patients have reported core strengthening leading to better posture and ability to accomplish physical tasks, such as holding a yoga plank position longer, achieving a stronger golf swing, and delivering a more powerful tennis serve. Substantial balance and posture benefits have also been reported among middle-aged and elderly patients that were not able to improve core strength through traditional exercise. Further study of these functional changes following EMMS treatment to quantify the changes in strength and endurance, and to objectively measure the duration of the improvement, are warranted.
Following subcutaneous fat reduction, muscle toning further refines body contouring results. This feasibility study demonstrates the safety and efficacy of Cryolipolysis and EMMS alone and in combination for abdominal contouring.
Conclusion
Standalone treatments of EMMS and cryolipolysis produced improvements in abdominal body contour. A comprehensive approach using Cryolipolysis + EMMS, however, produced the greatest changes overall as assessed by independent photo review GAIS, BSQ, and circumferential reduction. This feasibility study demonstrates the safety and efficacy of these noninvasive body contouring procedures alone and in combination with greater contour improvements found when the procedures are used together.
Footnotes
S. L. Kilmer, S. E. Cox, B. D. Zelickson, and W. G. Stevens are Advisory Board members and received research grant support from Allergan plc. E.P. Bachelor is a consulting Medical Director and received research grant support from Allergan plc. S. Gamio, R. Ostrowski, and L. D. Pham are employees of Allergan plc.
The authors have indicated no significant interest with commercial supporters.
References
- 1.CoolSculpting System User Manual, BRZ-101. ZELTIQ; 2016.
- 2.Derrick CD, Shridharani SM, Broyles JM. The safety and efficacy of cryolipolysis: a systematic review of available literature. Aesthet Surg J 2015;35:830–6. [DOI] [PubMed] [Google Scholar]
- 3.Ingargiola MJ, Motakef S, Chung MT, Vasconez HC, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg 2015;135:1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilmer SL. Prototype CoolCup cryolipolysis applicator with over 40% reduced treatment time demonstrates equivalent safety and efficacy with greater patient preference. Lasers Surg Med 2017;49:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein EF, Bloom JD, Basilavecchio LD, Plugis JM. Non-invasive fat reduction of the flanks using a new cryolipolysis applicator and overlapping, two-cycle treatments. Lasers Surg Med 2014;46:731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garibyan L, Sipprell WH, III, Jalian HR, Sakamoto FH, et al. Three-dimensional volumetric quantification of fat loss following cryolipolysis. Lasers Surg Med 2014;46:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein KB, Bachelor EP, Becker EV, Bowes LE. Multiple same day cryolipolysis treatments for the reduction of subcutaneous fat are safe and do not affect serum lipid levels or liver function tests. Lasers Surg Med 2017;49:640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boey GE, Wasilenchuk JL. Fat reduction in the inner thigh using a prototype cryolipolysis applicator. Dermatol Surg 2014;40:1004–9. [DOI] [PubMed] [Google Scholar]
- 9.Zelickson BD, Burns AJ, Kilmer SL. Cryolipolysis for safe and effective inner thigh fat reduction. Lasers Surg Med 2015;47:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens WG, Bachelor EP. Cryolipolysis conformable-surface applicator for nonsurgical fat reduction in lateral thighs. Aesthet Surg J 2015;35:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki GH, Abelev N, Tevez-Ortiz A. Noninvasive selective cryolipolysis and reperfusion recovery for localized natural fat reduction and contouring. Aesthet Surg J 2014;34:420–31. [DOI] [PubMed] [Google Scholar]
- 12.Boey GE, Wasilenchuk JL. Enhanced clinical outcome with manual massage following cryolipolysis treatment: a 4-month study of safety and efficacy. Lasers Surg Med 2014;46:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J 2013;33:835–46. [DOI] [PubMed] [Google Scholar]
- 14.Dierickx CC, Mazer JM, Sand M, Koenig S, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg 2013;39:1209–16. [DOI] [PubMed] [Google Scholar]
- 15.Suh DH, Park JH, Jung HK, Lee SJ, et al. Cryolipolysis for submental fat reduction in Asians. J Cosmet Laser Ther 2018;20:24–7. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein EF, Bloom JD. Safety and efficacy of bilateral submental cryolipolysis with quantified 3-dimensional imaging of fat reduction and skin tightening. JAMA Facial Plast Surg 2017;19:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non-invasive reduction of submental fat. Lasers Surg Med 2016;48:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington JL, Capizzi PJ. Cryolipolysis for nonsurgical reduction of fat in the lateral chest wall post-mastectomy. Aesthet Surg J 2017;37:715–22. [DOI] [PubMed] [Google Scholar]
- 19.Munavalli GS, Panchaprateep R. Cryolipolysis for targeted fat reduction and improved appearance of the enlarged male breast. Dermatol Surg 2015;41:1043–51. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Jang HW, Kim H, Suh DH, et al. Non-invasive cryolipolysis to reduce subcutaneous fat in the arms. J Cosmet Laser Ther 2016;18:126–9. [DOI] [PubMed] [Google Scholar]
- 21.Wanitphakdeedecha R, Sathaworawong A, Manuskiatti W. The efficacy of cryolipolysis treatment on arms and inner thighs. Lasers Med Sci 2015;30:2165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carruthers JD, Humphrey S, Rivers JK. Cryolipolysis for reduction of arm fat: safety and efficacy of a prototype CoolCup applicator with flat contour. Dermatol Surg 2017;43:940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivers JK, Ulmer M, Vestvik B, Santos S. A customized approach for arm fat reduction using cryolipolysis. Lasers Surg Med 2018;50:732–7. [DOI] [PubMed] [Google Scholar]
- 24.Friedmann DP. Cryolipolysis for noninvasive contouring of the periumbilical abdomen with a nonvacuum conformable-surface applicator. Dermatol Surg 2019;45:1185–90. [DOI] [PubMed] [Google Scholar]
- 25.Suh DH, Park JH, Kim BY, Lee SJ, et al. Double stacking cryolipolysis treatment of the abdominal fat with use of a novel contoured applicator. J Cosmet Laser Ther 2019;21:238–42. [DOI] [PubMed] [Google Scholar]
- 26.Jones IT, Vanaman Wilson MJ, Guiha I, et al. A split-body study evaluating the efficacy of a conformable surface cryolipolysis applicator for the treatment of male pseudogynecomastia. Lasers Surg Med 2018;50:608–12. [DOI] [PubMed] [Google Scholar]
- 27.Katz B, Bard R, Goldfarb R, Shiloh A, Wu DC, et al. Ultrasound assessment of subcutaneous abdominal fat thickness after treatments with a high-intensity focused electromagnetic field device: a multicenter study. Dermatol Surg 2019;45:1542–1548. [DOI] [PubMed] [Google Scholar]
- 28.Jacob C, Kinney B, Busso M, Chilukuri S, et al. High intensity focused electro-magnetic technology (HIFEM) for non-invasive buttock lifting and toning of gluteal muscles: a multi-center efficacy and safety study. J Drugs Dermatol 2018;17:1229–32. [PubMed] [Google Scholar]
- 29.Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg Med 2019;51:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacob CI, Paskova K. Safety and efficacy of a novel high-intensity focused electromagnetic technology device for noninvasive abdominal body shaping. J Cosmet Dermatol 2018;17:783–7. [DOI] [PubMed] [Google Scholar]
- 31.Porcari JP, Miller J, Cornwell K, Foster C, et al. The effects of neuromuscular electrical stimulation training on abdominal strength, endurance, and selected anthropometric measures. J Sports Sci Med 2005;4:66–75. [PMC free article] [PubMed] [Google Scholar]
- 32.Porcari J, Ryskey A, Foster C. The effects of high intensity neuromuscular electrical stimulation on abdominal strength and endurance, core strength, abdominal girth, and perceived body shape and satisfaction. Int J Kines Sports Sci 2018;6:19–25. [Google Scholar]


