Supplemental Digital Content is available in the text.
Keywords: Alzheimer’s disease, Alzheimer’s disease rats, Aβ, electroacupuncture, phosphorylated tau
Abstract
Acupuncture is widely used to treat various neurodegenerative diseases and can effectively improve cognitive and memory states in Alzheimer’s disease. However, its mechanism is unclear. We speculated that the effect of acupuncture on cognitive function may be associated with reductions in the levels of Aβ and phosphorylated tau in the brain. In this experiment, 60 male Sprague–Dawley rats were randomly divided into control, model, electroacupuncture and nonacupoint groups. We perform electroacupuncture at Shenting (GV24) and bilateral Benshen (GB13) acupoints once a day for 4 weeks in electroacupuncture group (with 1 day of rest after every 6 days of treatment). The electroacupuncture group showed a better performance in cognitive-related behavior tests and significantly lowers the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus. These results may suggest that electroacupuncture at the GV24 and bilateral GB13 acupoints might improve cognitive functions in Alzheimer’s disease by decreasing the levels of Aβ, p-tau (s396) and p-tau (s404) in the brain as these proteins are the main causes of neurological damage and cognitive dysfunction during the pathogenesis underlying Alzheimer’s disease.
Introduction
Alzheimer’s disease is an age-related neurodegenerative disease characterized by progressive cognitive dysfunction, memory loss, disorientation and thinking disorders accompanied by a decreased social life and reduced mobility [1]. According to data released by Alzheimer’s disease International in 2018, there were approximately 50 million Alzheimer’s disease patients worldwide as of 2015, and that number is predicted to reach approximately 152 million in 2050. The total estimated worldwide cost of dementia in 2018 was 1 trillion US dollars, and this figure is expected to double by 2030 [2]. A great deal of effort has been made to develop drugs to treat Alzheimer’s disease. However, these drugs alleviate only the learning and memory symptoms with many side effects, and do not provide a complete cure [3]. Due to the limitations of existing drugs, researchers are looking for new breakthrough therapies. Therefore, the need for clinical interventions for Alzheimer’s disease patients is particularly important and urgent. Currently, neurofibrillary tangles (NFTs) and soluble Aβ are thought to be the main causes of neurological damage and cognitive dysfunction in the pathogenesis of Alzheimer’s disease [4]. Senile plaques (SPs) are formed by aggregates of Aβ that form between neurons, destroy cell functions [5], and mainly occur in the hippocampus and important cerebral cortical and subcortical regions [6]. And tau is the microtubule-associated protein present at the highest levels in the brain, which provides cytoskeletal microtubules with stability [7]. Importantly, the aggregation of phosphorylated tau in neuronal cells blocks the neuronal transport system and plays a key role in the formation of NFTs [8]. Moreover, the levels of Aβ and phosphorylated tau are also closely related to behavioral abnormalities in cognitive disorders. Therefore, in this study, we examined the protein levels of p-tau (s396), p-tau (s404) and Aβ.
Acupuncture is a nonpharmacological traditional Chinese medicine (TCM) treatment with a history extending over 2000 years. Numerous clinical studies have shown that acupuncture can improve patient cognitive functions and quality of life without side effects [9,10]. In recent years, an increasing number of animal experiments have been performed to explore the mechanisms underlying the effects of acupuncture treatment on neurodegenerative diseases [11], providing an experimental and theoretical basis for future clinical applications. GV24 and bilateral GB13 acupoints are important when treating cognitive disorders according to TCM [12]. We previously found that performing acupuncture at these three acupoints may improve learning and memory abilities in Alzheimer’s disease rats by changing brain glucose metabolism in the hypothalamus, thalamus and brainstem [13]. In the present study, we aimed to determine whether electroacupuncture at GV24 and bilateral GB13 acupoints could improve the learning and memory by modulate the levels of p-tau (s396), p-tau (s404) and Aβ in the hippocampus. And further explored the neural mechanisms underlying the memory-enhancing effects of electroacupuncture at GV24 and bilateral GB13 acupoints.
Materials and methods
Animals
Male Sprague–Dawley rats weighing 300–320 g were cage-acclimated for 7 days prior to surgery in a temperature-controlled environment on a 12-h light/dark cycle with free access to food and water. All procedures performed in the present experiments were conducted in accordance with the requirements of the Provisions and General Recommendations for Chinese Experimental Animals and approved by the Committee of Ethics on Animal Experiments at Guangzhou University of Traditional Chinese Medicine.
60 rats were randomly divided into four groups (15 rats in each group): control, electroacupuncture (surgery and GV24 and GB13 electroacupuncture intervention), nonacupoint (surgery and nonacupoint electroacupuncture intervention) and model (only surgery) groups.
Animal model preparation
First, the model, electroacupuncture and nonacupoint groups received intraperitoneal D-galactose (D-gal) injections (300 mg/kg dissolved in 0.9% saline) every day for 6 weeks. At the seventh week, rats in the premodel group were given injection of Aβ1-40 peptide into the bilateral hippocampus. Before starting this experiment, we first dissolved 500 µg Aβ1-40 in 100 µL sterile saline to make a solution with a concentration of 5 µg/µL, and placed it at 37°C for 1 week for incubation. Then, store it in a refrigerator at 4°C for use. After that, the rats were fixed in a stereotactic apparatus for routine disinfection after anesthetized by an intraperitoneal injection of pentobarbital sodium anesthetic (50 mg/kg). The bilateral hippocampal CA1 region was selected as the target area (3.5 mm lateral and 2.0 mm posterior to bregma, 2.7 mm subdural) (Fig. 1a). A microinjector was then inserted vertically to the target area, and 2 μL of Aβ1-40 solution was injected into the hippocampus at a speed of 0.4 μL/min. The needle was then kept in place for 5 min after the injection was complete before it was slowly pulled out. The control group received daily intraperitoneal injections of 0.9% saline for 6 weeks and 2 μL of 0.9% saline at the same site in the hippocampus by using the same procedures as those described for the other groups. After the surgery, the brain scalp was sutured by simple suture method. And the sutures were not removed prior to acupuncture. Because usually the surgical sutures of these rats will fall off on their own by then. Meanwhile, anti-infection treatment was performed after the surgery (intramuscular injection of 40 000 U penicillin sodium once a day for 3 days).
Fig. 1.

The target area of bilateral hippocampal CA1 region (3.5 mm lateral and 2.0 mm anterior to bregma, 2.7 mm subdural) (Fig. 1a) and the GV24 and bilateral GB13 acupoints in electroacupuncture group (Fig. 1b).
Electroacupuncture treatment procedures
Seven days after surgery, electroacupuncture was performed at GV24 and bilateral GB13 acupoints (electroacupuncture group) (Fig. 1b) or at nonacupoints (nonacupoint group) by the same acupuncturist. The electroacupuncture and nonacupoint locations were the same as those reported in our previous study [13]. Sterilized stainless disposable needles (25 mm in length, 0.35 mm in diameter; Suzhou Medical Appliance Factory, China) were inserted 2 mm into the GV24 and bilateral GB13 acupoints or the nonacupoints and then connected to an electronic acupuncture treatment instrument (Hans Acupoint Nerve Stimulator 200E, Nanjing Jisheng Medical Technology Co., Ltd., China). The stimulation parameters were 30 Hz stimulation at 1.0 mA for 30 min once daily for 28 days (with 1 day of rest every 6 days). The rats in both the control and model groups were given the same immobilization stress but no acupuncture treatment.
Open field test
On the second day after the final treatment, the open field test (OFT) was conducted in a quiet, dark environment with white noise. The OFT consisted of a dark blue, opaque square box (0.80 m in length and 0.35 m deep). The behavior of the rats was recorded with a video, supplement digital content 1, http://links.lww.com/WNR/A596 camera mounted in the ceiling above the center of the field that was connected to a tracking program (DigBehv automatic animal behavior video, supplement digital content 1, http://links.lww.com/WNR/A596 analysis software system, Shanghai Jiliang Software Technology Co. Ltd., China). The system artificially divides the open field analysis grid into 16 small grids. The animals were placed in the center region to begin the test, and animal activity was recorded for 5 min.
Morris water maze test
The Morris water maze (MWM) test was performed in a circular pool (2 m in diameter and 0.35 m deep) that was filled with water to a depth of 0.3 m. The water in the pool was kept at 24.0 ± 3°C and made opaque by adding 1 kg black nontoxic paint. The pool was divided into four quadrants of equal area, and an escape platform (0.1 m in diameter) located in the middle of the southwest quadrant was hidden 5 cm below the water surface. During the acquisition trials, the rats were trained to find the hidden platform in four trials per day for 5 consecutive days. In each trial, the rat was placed in the pool facing the wall. When the rat successfully found the platform, it was kept on the platform for 10 s. If the rat failed to find the platform within 60 s, it was guided to the platform and kept there for 10 s. On day 6, the hidden platform was removed, and a probe trial lasting 60 s was performed. The behavior of the rats was recorded using the same video, supplement digital content 1, http://links.lww.com/WNR/A596 setup used for the OFT.
Western blotting
After the MWM test, the rats were sacrificed. The hippocampus was dissected and immediately placed at −80°C until the assay was performed. Samples were homogenized in radio immunoprecipitation assay buffer containing protease inhibitors and phosphatase inhibitors and then centrifuged at 13 000 rpm at 4°C for 20 min. The protein concentration was determined with a bicinchoninic acid protein assay kit. Protein samples were separated on 10% SDS-PAGE gels and electrophoretically transferred to polyvinyl difluoride membranes. The membranes were blocked in 5% defatted milk with 5% Tris buffered saline Tween for 1 h at room temperature. Primary antibodies including Aβ (1:1000, Abcam, Cambridge, UK), p-tau (s404) (1:1000, Abcam) and p-tau (s396) (1:500, Cell Signaling Technology, Danvers, Massachusetts, USA) were applied first followed by washing and incubation with secondary antibodies (1:3000, Servicebio, Wuhan, China). Then, the membranes were developed with an enhanced chemiluminescent kit. Images were analyzed using Image J software (National Institutes of Health, Bethesda, Maryland, USA).
Immunohistochemistry
For immunohistochemical analyses, brains were removed under deeply anesthetized, perfused through the ventricle, post-fixed with 4% formaldehyde, cryoprotected in 30% sucrose and cryosectioned at 4 mm in thickness. A nonspecific binding blockade was performed with normal goat serum (NGS, Thermo Scientific, Waltham, Massachusetts, USA) followed by overnight incubation at 4°C with primary antibodies for Aβ (1:1000, Abcam). Then, the sections were incubated for 1 h at room temperature with secondary antibodies. Sections were observed under a microscope and images were analyzed using Image Pro-Plus version 6.0 (Media Cybernetics, Rockville, Maryland, USA).
Immunofluorescence
Furthermore, we used immunofluorescence labeling to detect the protein expression levels of p-tau (s404). The brains were then managed and sectioned as described above. Nonspecific binding was blocked with NGS (Thermo Scientific), and the sections were then incubated for 24 h at 4°C with primary antibodies for p-tau (s404) (1:1000, Abcam). Then, the sections were incubated for 2 h at room temperature with secondary antibodies. Finally, the samples were observed under confocal microscope (Nikon eclipse Ti; Nikon, Melville, New York, USA) and analyzed by Nikon viewer software (Nikon).
Data analysis
The researchers worked independently to avoid bias. The data were expressed as mean ± SD. The significance of the differences was determined by one-way analysis or Student’s t-test of variance. P < 0.05 was considered to be statistically significant (α = 0.05). Postuse images were processed and drawn in Adobe Photoshop 6.0.
Results
Effect of electroacupuncture at GV24 and bilateral GB13 on the open field test
As seen in Fig. 2, the duration spent in the central zone and the frequency of crossing were significantly different between the control group and each of the other three groups (vs. model group, P < 0.05; vs. electroacupuncture group, P < 0.05; vs. nonacupoint group, P < 0.05; Fig. 2). Compared to the model group, the electroacupuncture group had a significant lower duration spent in the central zone (P < 0.05; Fig. 2a) and a significant higher frequency of crossing (P < 0.05; Fig. 2b). However, there was no significant difference between the model group and the nonacupoint group in any of the monitored behavioral changes (P > 0.05; Fig. 2).
Fig. 2.

Open field test. Duration spent in the central zone (a), and frequency of crossing (b). *P < 0.05 vs. the control group, #P < 0.05 vs. the model group, △P < 0.05 vs. the nonacupoint group. (c) Representative running paths during the OFT. OFT, open field test.
Effect of electroacupuncture at GV24 and bilateral GB13 on the Morris water maze
The effect of electroacupuncture at GV24 and bilateral GB13 on spatial learning and memory was evaluated on the MWM. In the acquisition trials, the escape distances and escape latencies were markedly reduced in all groups compared with the first trial. The rats in the control group were able to rapidly find the location of the hidden platform after five days of training. However, the rats in model group and nonacupoint group had showed a worse performance than did the control group, and effect that may be associated with memory deficits. However, electroacupuncture significantly reduced escape distance and escape latency compared with the results obtained in the model group (P < 0.05; Fig. 3a and b) and nonacupoint group (P < 0.01; Fig. 3a and b). Finally, we conducted a probe trial on day 6. The results showed that the latency to cross the platform for the first time was significantly longer (P < 0.05; Fig. 3c), the frequency of platform crossings was significantly smaller (P < 0.05; Fig. 3d), and the percentage of time swam in the target quadrant was significantly lower (P < 0.05; Fig. 3e) in the model group than in the control group. These results indicate that the rats in the model group exhibited severely impaired spatial learning and memory Besides, electroacupuncture group had a significantly shorter latency to cross the platform for the first time (P < 0.05; Fig. 3c), a significantly higher frequency of platform crossings (P < 0.05; Fig. 3d), and a significantly higher percentage of time spent in the target quadrant (P < 0.05; Fig. 3e) when compared to model group. In contrast, there were no significant differences between the model group and the nonacupoint group (P > 0.05; Fig. 3). According to these results, performing electroacupuncture at GV24 and bilateral GB13 but not at nonacupoints showed improved acquisition in this trial, which is suggested to restore cognition dysfunction-related behavior in MWM test.
Fig. 3.
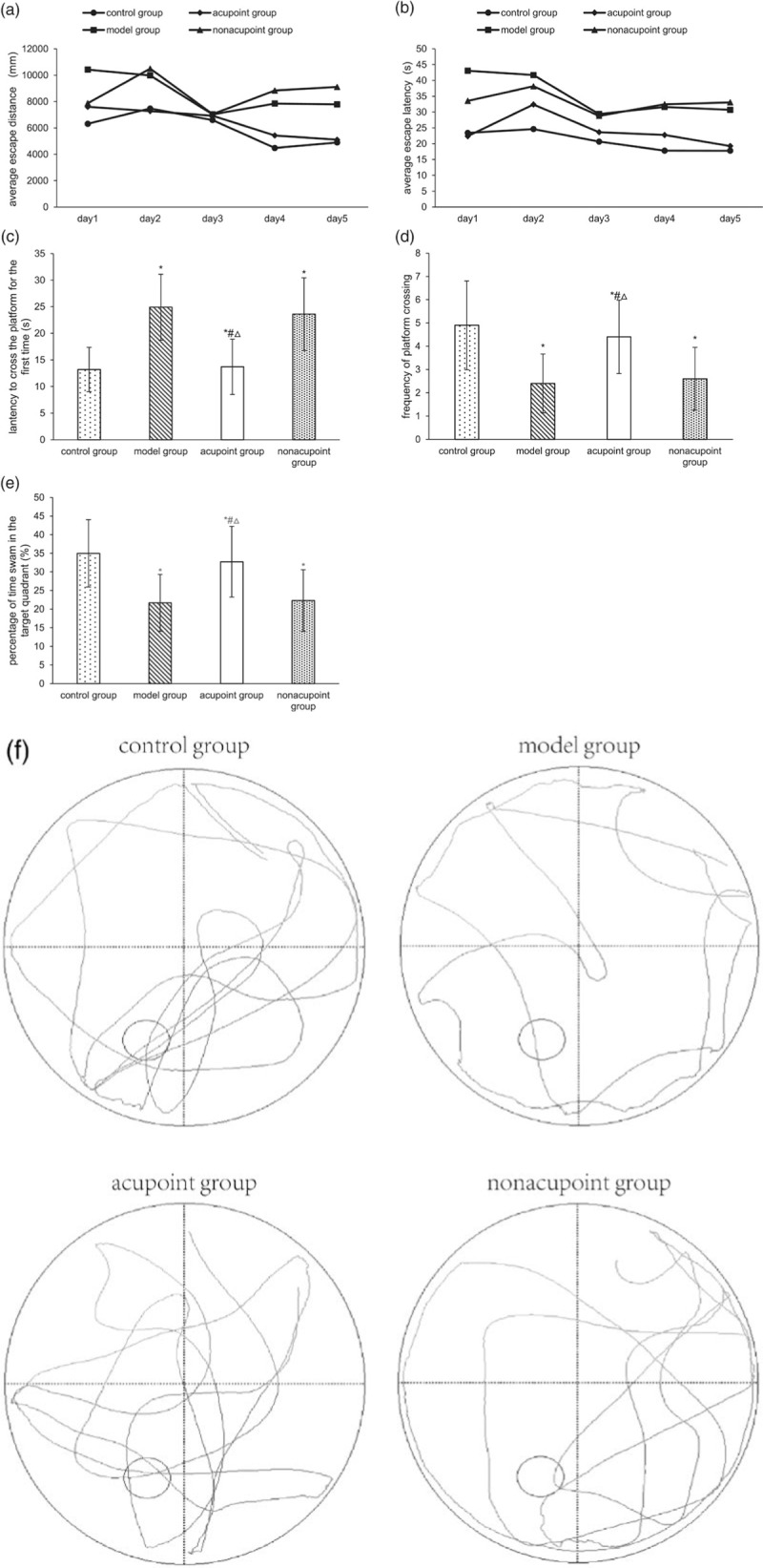
Morris water maze. The average escape distance (a) and average escape latency (b) in the acquisition trials. The latency to cross the platform for the first time (c), the frequency of platform crossing (d) and percentage of time swam in the target quadrant (e) in the probe trial. *P < 0.05 vs. the control group, #P < 0.05 vs. the model group, △P < 0.05 vs. the nonacupoint group. (f) Representative swimming paths during the probe trial of the MWM test.
Effect of electroacupuncture at GV24 and bilateral GB13 on the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus
In this study, we tested whether the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus is related to the effects of electroacupuncture at GV24 and bilateral GB13 in reversing behavioral abnormalities in Alzheimer’s disease rats. Western blot analysis showed that the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus were significantly higher in the model group than in the control group (P < 0.05; Fig. 4), then they were decreased by electroacupuncture at GV24 and bilateral GB13 (P < 0. 05; Fig. 4), while performing electroacupuncture at nonacupoints resulted in no differences (P > 0.05; Fig. 4). In addition, the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus was not as low in the electroacupuncture group as in the control group (P < 0. 05; Fig. 4). The level of Aβ in the hippocampus was also detected by immunohistochemistry. The integrated optical density value of Aβ in the hippocampus was measured, with higher values indicating more Aβ content and vice versa. In Alzheimer’s disease rats, the level of Aβ was increased in the hippocampus (P < 0.05; Fig. 5). In the meantime, Aβ levels were decreased in the electroacupuncture group when compared to the model group (P < 0.05; Fig. 5). In the meantime there was no difference in the level of Aβ between the nonacupoint group and the model group (P > 0.05; Fig. 5). In agreement with the data collected by western blot, immunofluorescence results showed that compared with Alzheimer’s disease rats, performing electroacupuncture at GV24 and bilateral GB13 decreased the level of p-tau (s404) in the hippocampus (P < 0.05; Fig. 6).Additionally, performing electroacupuncture at nonacupoints did not reduce p-tau (s404) levels in Alzheimer’s disease rats (P > 0.05; Fig. 6).
Fig. 4.
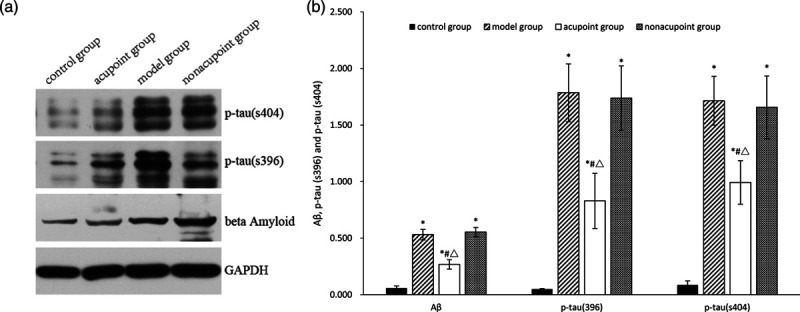
p-tau (s396), p-tau (s404) and Aβ protein levels in the hippocampus. *P < 0.05 vs. the control group, #P < 0.05 vs. the model group, △P < 0.05 vs. the nonacupoint group.
Fig. 5.

Aβ distribution (a–h) and the average IOD (i) of Aβ labeling in the hippocampus. (a–b): Aβ level in the control group, (c–d): Aβ level in the model group, (e–f): Aβ level in the electroacupuncture group, (g–h): Aβ level in the nonacupoint group. Sections were cut coronally at 4 mm. Scale bars represent 200 and 50 μm in columns 1 and 2, respectively. *P < 0.05 vs. the control group, #P < 0.05 vs. the model group, △P < 0.05 vs. the nonacupoint group. IOD, integrated optical density.
Fig. 6.
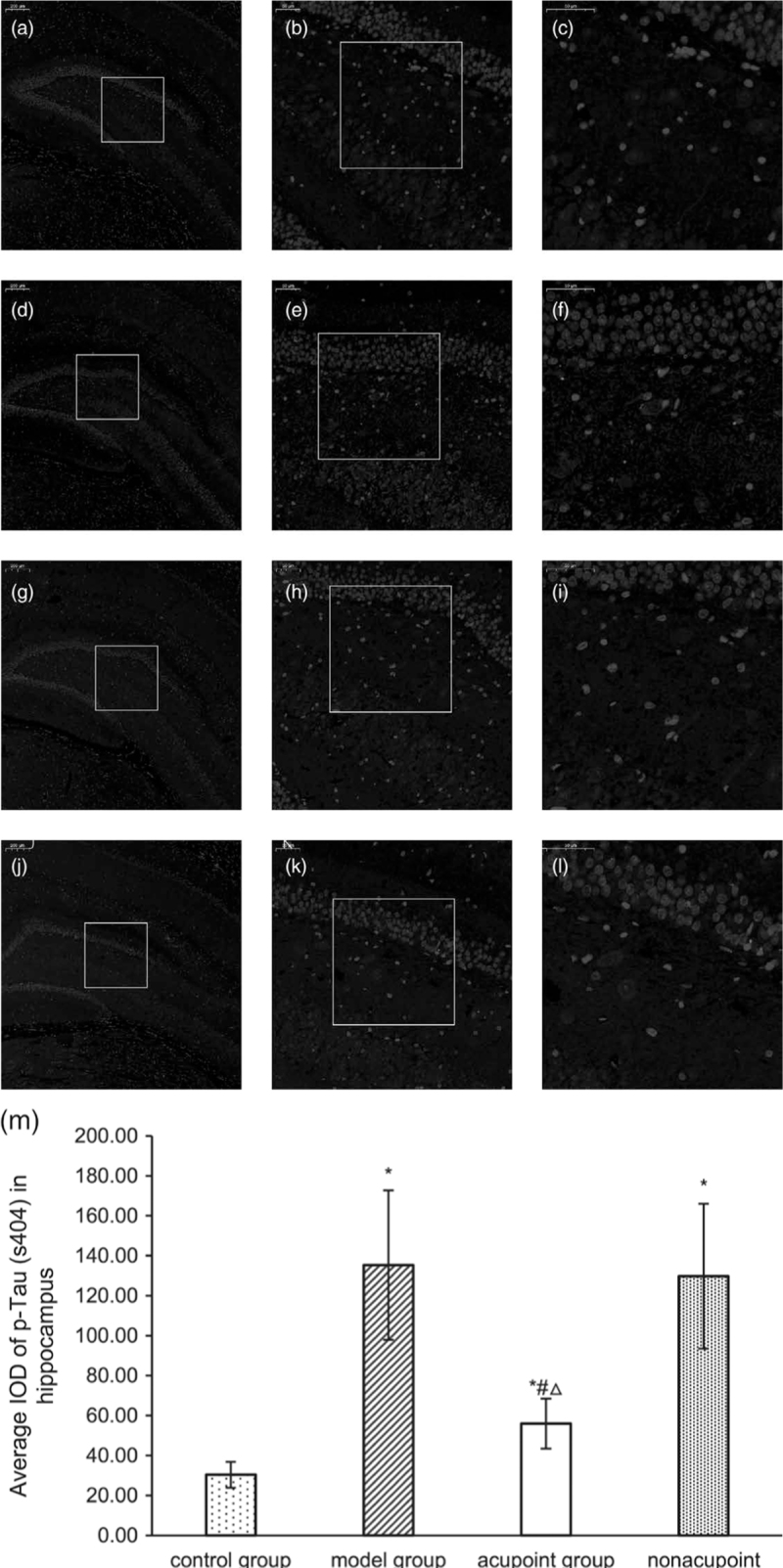
p-tau (s404) distribution (a–l) and the average IOD (m) of p-tau (s404) labeling in the hippocampus. (a–c): p-tau (s404) expression in the control group, (d–f): p-tau (s404) expression in the model group, (g–i): p-tau (s404) expression in the electroacupuncture group, (j–l): p-tau (s404) expression in the nonacupoint group. Sections were cut coronally at 4 mm. Scale bars represent 200, 50 and 50 μm in columns 1, 2 and 3, respectively. *P < 0.05 vs. the control group, #P < 0.05 vs. the model group, △P < 0.05 vs. the nonacupoint group. IOD, integrated optical density.
Discussion
The major finding of the present study is that performing electroacupuncture at GV24 and bilateral GB13 acupoints significantly reversed behavioral deficits in MWM test and OFT and reduced Aβ, p-tau (s396) and p-tau (s404) level in the hippocampus in Alzheimer’s disease rats.
An acupuncture treatment that combines GV24 and bilateral GB13 was created by Prof. Jin Rui, one of the most famous acupuncture experts in China. Shenting (GV24) belongs to the Du Meridian, which is, according to the theory of TCM, named for the place where the human spirit, consciousness, intelligence and cognition are hidden. Benshen (GB13) belongs to the Gall Bladder Channel of Foot-Shaoyang and, according to the theory of TCM, is the source and the foundation of the human spirit, consciousness, intelligence and cognition. These three acupoints are widely used in the clinical treatment and proved to have positive effects on learning and memory abilities in Alzheimer’s disease patients [14]. As a comparison, we also performed stimulation at nonacupoints which are under the bilateral costal arch regions and 10 mm above the crista iliaca, neither of which is part of any meridian in TCM theory.
In this study, we performed bilateral intra-hippocampal injection of Aβ1-40 combining with intraperitoneal injection of D-gal to induce Alzheimer’s disease rat model. Intraperitoneal injection of D-galactose (D-gal) is widely used in the study of aging-related diseases [15]. Multiple experiments have confirmed that intraperitoneal injection of D-gal can induce oxidative damage, leading to increased levels of malondialdehyde, and reduce the activity of superoxide dismutase and glutathione peroxidase [16]. So it can cause subacute aging animal models in a short time [17]. For example, D-gal alone did damage learning and memory function in behavior tests and enhanced Aβ deposition and p-tau accumulation in mouse brains [18,19]. But these researches also proved that more serious learning and memory dysfunction and higher levels of SP-like or NFT-like structures in combined use of D-gal and aluminum compared to treating with D-gal alone [18,19]. In the meantime, there are experiments have shown that injecting Aβ1-40 directly into the hippocampus can induce the changes in the main pathological characteristics and cognitive dysfunction in Alzheimer’s disease model rats [20,21]. Despite there were studies showed that intraperitoneal injection of D-gal alone [22] or injection of Aβ1-40 alone [20] will results in changes of Aβ levels in the brain and caused cognitive dysfunction. But there is no research directly compared the combination of these two modeling methods with use of them individually. Although our previous findings demonstrated that the intrahippocampal injection of Aβ1-40 combining intraperitoneal injection of D-gal did successfully simulate Alzheimer’s disease-like behavior changes [13,23], but we cannot distinguish which modeling method contributed more to the change of the Aβ and p-tau level in the brain as well as cognitive dysfunctions in behavior test.
In the OPT, the duration spent in the central zone reflects the animal’s cognitive ability [24]. Rats are prone to anxiety and fear when in a new open space, leading to avoidance of the open environment and an increase in movement [25]. If its cognitive ability is poor, the rat will spend more time in the central grid. In the meantime, curiosity and exploratory nature of the rat will lead it to cross the center. Our study found that the Alzheimer’s disease rats spent significantly more time in the central zone but exhibited a significantly lower frequency of crossing the central zone, indicating poor cognitive ability and reduced exploratory drive. Electroacupuncture at GV24 and bilateral GB13 caused a reversal of these changes, suggesting that electroacupuncture improved cognitive function and exploratory drive in Alzheimer’s disease rats. But we cannot rule out the difference that exploratory drive attributed to behavior tests. Moreover, performing electroacupuncture at nonacupoints had no effect on the behavioral changes mentioned above. However, this results cannot distinguish which modeling method caused the change of the OPT in the model group.
The MWM test is one of the most reliable methods for studying hippocampal-dependent spatial reference learning and memory in rodents [26]. Our results showed that the memory deficits in premodel groups produced impaired behavioral performance, indicating that impaired spatial memory in the premodel group made finding the platform using the marker points more difficult and suggesting that this model was successful. However, performing electroacupuncture at GV24 and bilateral GB13 resulted in significant reductions in escape latency and escape distance, suggesting that electroacupuncture improved cognitive performance and ameliorated memory deficits in Alzheimer’s disease rats. In the probe trial, we confirmed again that both spatial learning and memory were impaired in Alzheimer’s disease rats. Meanwhile, we found electroacupuncture effectively improved MWM performance by enhancing learning and memory abilities of Alzheimer’s disease rats. Conversely, performing electroacupuncture at nonacupoints resulted in no difference in behavioral performance in the MWM. Still, these results cannot distinguish which modeling method caused the change of the MWM in the model group.
Aβ and phosphorylated tau, the characteristic pathological products and therapeutic targets of Alzheimer’s disease [27], have been studied for their roles in cognitive diseases for many years. Although, it has been proved that Aβ can form SPs and cause neuronal death, as well as cognitive and memory impairments [28,29]. Phosphorylated tau is another important factor involved in the pathogenesis of Alzheimer’s disease. Tau protein is a microtubule-associated protein and an important component of the cytoskeleton that is key to ensuring the integrity and stability of the microtubule system [30]. So the level of phosphorylated tau protein increases will affect the stability of microtubules, eventually resulting in the formation of NFTs and leading to Alzheimer’s disease [31]. At present, various phosphorylation sites have been identified on the tau protein, with s396 and s404 identified as key sites involved in the regulation of microtubule binding to tau protein and strongly associated with plaques [32,33]. So the detection of the levels of Aβ, p-tau (s404) and p-tau (s396) in the hippocampus is key to evaluating the effectiveness of any Alzheimer’s disease treatment. According to our results, performing electroacupuncture at GV24 and bilateral GB13 ameliorated learning and spatial memory impairments in behavioral tests, and this effect was related to decreases in Aβ, p-tau (s404) and p-tau (s396) in the hippocampus. But anyway, the results above both cannot distinguish which modeling method caused the change of the Aβ, p-tau (s404) and p-tau (s396) in the hippocampus in the model group.
Conclusion
In conclusion, this is the first article to evaluate the therapeutic effects of electroacupuncture at GV24 and bilateral GB13 acupoints in a rat model of Alzheimer’s disease to determine its effects on the levels of Aβ, p-tau (s396) and p-tau (s404) in the brain. We found that performing electroacupuncture at GV24 and bilateral GB13 significantly improved the learning and memory abilities of Alzheimer’s disease rats and delayed the development of Alzheimer’s disease, perhaps by reducing Aβ levels and downregulating the overexpression of p-tau (s396) and p-tau (s404).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 8187151425) and High Level University Fund of China (A1-AFD018161Z0201).
The data used to support the findings presented in this study are available from the corresponding author upon request.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Yang Yang and Shaowen Hu contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.
References
- 1.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006; 314:777–781 [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer’s Disease International. World Alzheimer Report 2019 Attitudes to Dementia. 2019, London, UK: Alzheimer’s Disease International; 1–160 [Google Scholar]
- 3.Bondi MW, Edmonds EC, Salmon DP. Alzheimer’s disease: past, present, and future. J Int Neuropsychol Soc. 2017; 23:818–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox PA, Davis DA, Mash DC, Metcalf JS, Banack SA. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc Biol Sci. 2016; 283:20152397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging. 2005; 26:1235–1244 [DOI] [PubMed] [Google Scholar]
- 6.Robinson M, Lee BY, Hanes FT. Recent progress in Alzheimer’s disease research, part 2: genetics and epidemiology. J Alzheimers Dis. 2018; 61:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, et al. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci USA. 2015; 112:7501–7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986; 261:6084–6089 [PubMed] [Google Scholar]
- 9.Zhou J, Peng W, Xu M, Li W, Liu Z. The effectiveness and safety of acupuncture for patients with Alzheimer disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015; 94:e933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Y, Zhang X, Yu J, Han J, Yu T, Shi J, et al. Acupuncture for patients with mild to moderate Alzheimer’s disease: a randomized controlled trial. BMC Complement Altern Med. 2017; 17:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y, Zhang LW, Wang J, Du SQ, Xiao LY, Tu JF, Liu CZ. Mechanisms of acupuncture effect on Alzheimer’s disease in animal-based researches. Curr Top Med Chem. 2016; 16:574–578 [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Yuan Q, Luo Q, Zeng H, Zheng X, Huang X, et al. Clinical efficacy on mental retardation in the children treated with JIN’s three scalp needling therapy and the training for cognitive and perceptual disturbance. Zhongguo Zhen Jiu. 2015; 35:651–656 [PubMed] [Google Scholar]
- 13.Cui S, Xu M, Huang J, Wang QM, Lai X, Nie B, et al. Cerebral responses to acupuncture at GV24 and bilateral GB13 in rat models of Alzheimer’s disease. Behav Neurol. 2018; 2018:8740284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Guangxing TL, Han X. Clinical effects and related mechanism of Huanglian Wendan decoction combined with Zhisan acupuncture on mild cognitive impairment after stroke. World Chin Med. 2018; 13:3011–3014 [Google Scholar]
- 15.Wei H, Li L, Song Q, Ai H, Chu J, Li W. Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behav Brain Res. 2005; 157:245–251 [DOI] [PubMed] [Google Scholar]
- 16.Sadigh-Eteghad S, Majdi A, McCann SK, Mahmoudi J, Vafaee MS, Macleod MR. D-galactose-induced brain ageing model: a systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. PLoS One. 2017; 12:e0184122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu J, Wu DM, Hu B, Zheng YL, Zhang ZF, Wang YJ. NGF-dependent activation of TrkA pathway: a mechanism for the neuroprotective effect of troxerutin in D-galactose-treated mice. Brain Pathol. 2010; 20:952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y, Niu F, Sun Z, Cao W, Zhang X, Guan D, et al. Altered expression of Abeta metabolism-associated molecules from D-galactose/AlCl(3) induced mouse brain. Mech Ageing Dev. 2009; 130:248–252 [DOI] [PubMed] [Google Scholar]
- 19.Xiao F, Li XG, Zhang XY, Hou JD, Lin LF, Gao Q, Luo HM. Combined administration of D-galactose and aluminium induces Alzheimer-like lesions in brain. Neurosci Bull. 2011; 27:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda S, Sato N, Niisato K, Takeuchi D, Kurinami H, Shinohara M, et al. Validation of Abeta1-40 administration into mouse cerebroventricles as an animal model for Alzheimer disease. Brain Res. 2009; 1280:137–147 [DOI] [PubMed] [Google Scholar]
- 21.Shi Z, Sun X, Liu X, Chen S, Chang Q, Chen L, et al. Evaluation of an Aβ(1-40)-induced cognitive deficit in rat using a reward-directed instrumental learning task. Behav Brain Res. 2012; 234:323–333 [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury AA, Gawali NB, Bulani VD, Kothavade PS, Mestry SN, Deshpande PS, Juvekar AR. In vitro antiglycating effect and in vivo neuroprotective activity of Trigonelline in d-galactose induced cognitive impairment. Pharmacol Rep. 2018; 70:372–377 [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Tang C, Liao W, Zhu M, Liu M, Sun N. The antiapoptotic and antioxidative stress effects of Zhisanzhen in the Alzheimer’s disease model rat. Neuroreport. 2019; 30:628–636 [DOI] [PubMed] [Google Scholar]
- 24.Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005; 28:811–825 [DOI] [PubMed] [Google Scholar]
- 25.Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994; 61:59–64 [DOI] [PubMed] [Google Scholar]
- 26.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001; 36:60–90 [DOI] [PubMed] [Google Scholar]
- 27.Villemagne VL, Doré V, Bourgeat P, Burnham SC, Laws S, Salvado O, et al. Aβ-amyloid and Tau imaging in dementia. Semin Nucl Med. 2017; 47:75–88 [DOI] [PubMed] [Google Scholar]
- 28.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006; 66:1837–1844 [DOI] [PubMed] [Google Scholar]
- 29.Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA. 1989; 86:9574–9578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014; 71:505–508 [DOI] [PubMed] [Google Scholar]
- 31.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996; 17:1201–1207 [DOI] [PubMed] [Google Scholar]
- 32.Foidl BM, Humpel C. Differential hyperphosphorylation of Tau-S199, -T231 and -S396 in organotypic brain slices of Alzheimer mice. A model to study early Tau hyperphosphorylation using okadaic acid. Front Aging Neurosci. 2018; 10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quintanilla RA, von Bernhardi R, Godoy JA, Inestrosa NC, Johnson GV. Phosphorylated tau potentiates Aβ-induced mitochondrial damage in mature neurons. Neurobiol Dis. 2014; 71:260–269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


