ABSTRACT
Heat shock proteins are known to be associated with a wide variety of human cancers including lung cancer. Overexpression of these molecular chaperones is linked with tumor survival, metastasis and anticancer drug resistance. In recent years, heat shock proteins are gaining much importance in the field of cancer research owing to their potential to be key determinants of cell survival and apoptosis. Lung cancer is one of the most common cancers diagnosed worldwide and the association of heat shock proteins in lung cancer diagnosis, prognosis and as drug targets remains unresolved. The aim of this review is to draw the importance of heat shock protein members; Hsp27, Hsp70, Hsp90, Hsp60 and their diagnostic and prognostic implications in lung cancer. Based on the available literature heat shock proteins can serve as biomarkers and anticancer drug targets in the management of lung cancer patients.
KEYWORDS: HSP, diagnosis, prognosis, biomarker, NSCLC, immunotherapy, EGFR
Introduction
Heat shock proteins (Hsps) are members of molecular chaperones ubiquitously expressed in cells and play a key role in protein folding, protein stability and in the maintenance of cellular homeostasis.1,2 Hsps were first discovered as a group of proteins induced by heat stress, but now are known to be expressed in response to UV light, wound healing, tissue remodeling, pathogens, cancers, etc.,3,4 In mammals, based on the molecular weight, Hsps are categorized into different classes; Hsp100, Hsp90, Hsp70, Hsp60 and small Hsps such as Hsp33 and Hsp27.5,6 An increased expression of Hsp is dependent on several sequential events including activation of stress-induced heat shock factor 1 (HSF1). But until now the exact mechanism of HSP gene activation is not clear. The activation of HSF1 is also not very clear, but signal pathways recruited in response to heat shock lead to the activation of certain protein kinases which in turn phosphorylate HSF1.7 Activated HSF1 binds to 5′ promoter sequences of HSP genes and initiates transcription.8
Hsps are overexpressed in various cancers including lung cancer and their increased expression is generally associated with tumor cell survival, invasion, metastasis and chemo-resistance.9 They interact with various oncogenes to drive the progression of cancer. Furthermore, cells with increased expression of Hsps have been protected against radiation-induced cell death.10 All these observations collectively imply the possible dependency of cancer cells on the expression of Hsps for their survival and proliferation even under lethal conditions.
Lung cancer is one of the most common cancers worldwide and is estimated to be responsible for 19.4% of cancer-related deaths.11 Lung cancer remained to be high in men as compared to women across the globe although a different geographical pattern has been reported. Countries with highest incidence of lung cancer are North America, Southern Europe, Central Eastern and East Asia while the lowest incidence was reported from West and Central Africa.12 In India, according to international agency for research on cancer, lung cancer accounts for 5.9% of new cancer cases and the risk of developing cancer before the age of 75 y is 9.3%.13 In spite of various strategies used for lung cancer patient management, the overall survival rate remains to be <20%.14,15 Lung cancer diagnosed in advanced stages is tougher to be treated by surgical interventions while metastasis to lymph nodes makes it further difficult to treat by radiation and chemotherapy. PET, PET-CT, X-ray, MRI, transthoracic needle aspiration (TTNA), CT scan, bronchoscopy and fine-needle biopsy are the different techniques used for early diagnosis and staging of lung tumors with varying diagnostic accuracy. Considering the increasing incidence of lung cancer worldwide and with the availability of a significant number of FDA approved drugs for treatment, there is a need for the development of reliable biomarkers for diagnosis, prognosis determination and for the prediction of treatment response. Case in point, detection of epidermal growth factor receptor (EGFR) mutations in its kinase domain remains to be an important test for non-small cell lung carcinoma (NSCLC) patients to predict their response to FDA approved EGFR targeted drugs. Nonetheless, some patients respond to the treatment and some develop drug resistance and how the resistance developed remains unclear, although some receptor kinase mutations have shown to confer resistance but it is not the case with all drug-resistant tumors.16 Therefore, it is necessary to identify the novel potential biomarkers to predict the response in NSCLC patients with EGFR-TKIs resistant mutations.17 The evidence of correlation between Hsp expression profile with lung tumors by transcriptomic and proteomic studies gives hope to clinicians that these molecular chaperones can serve as potential biomarkers for better patient management.18 Poor prognosis associated with elevated Hsp level in lung cancer patients prompts the consideration of these proteins as therapeutic targets as well to provide treatment options over/or with the existing lung cancer treatment strategies. Here, we reviewed the status of Hsp 27, 60, 70 and 90 and the possibility of these proteins to be considered as biomarkers for lung cancer patient management.
Hsp27
Hsp27 is a low molecular mass protein and is a member of subfamily of small Hsps. Its function is regulated by phosphorylation, a post-translational modification; aberrant phosphorylation of Hsp27 has been reported to be associated with cancer progression.19 Its expression is elevated in many cancers and was correlated with an increased risk of lung cancer in coal mine workers.20 Overexpression of Hsp27 is usually associated with poor prognosis; it confers chemo-resistance in NSCLC,21 laryngeal cancers22 and lung cancer stem-like cells.23 In support of this, several in vitro studies have demonstrated the metastatic behavior of cells with an increased level of Hsp2724,25 while the inhibition of Hsp27 with antisense drug OGX-427 sensitizes NSCLC tumors to chemotherapy and EGFR targeted drug Erlotinib, a TKI.26 It interacts with various proteins including components of intrinsic and extrinsic apoptotic pathways such as cytochrome c, pro-caspase-3, DAXX, Bax, Akt and mediates apoptosis.27-29 Together with 14-3-3ζ proteins, which are known to play a regulatory role in cancer initiation and progression, Hsp27 promotes the progression of NSCLC tumors.30 Furthermore, increased expression of Hsp27 in tumor tissues had a correlation with their levels in serum samples of NSCLC patients, as they had higher Hsp27 in the serum as compared to normal control patients and this difference was noted during an early stage of NSCLC tumors.31 This condition was observed to be associated with poorly differentiated cancer and advanced TNM staging. Furthermore, a single nucleotide polymorphism (SNP-1271 G > C) in the HSP27 gene encoded by heat shock protein beta-1 (HspB1) was found to be associated with poor survival of U.S. patients with NSCLC tumors.32 Interestingly, this allele was reported to be linked with an increased risk of lung cancer, but on the contrary, it was contributed to the survival of patients with advanced NSCLC tumors in two independent studies.33 Upon validation of this allele in vitro, a low level of Hsp27 was recorded in cancer tissues harboring SNP as compared to normal tissue. Thus, screening of patient samples for an SNP in the HSPB1 gene could be another discerning factor to predict the disease outcome and radiation/chemotherapy-associated severity especially in Caucasians but not in the Asian (Chinese) population. The difference in serum Hsp27 between a healthy and a patient population with early- and advanced-stage NSCLC tumors; cytoplasmic staining of Hsp27 in more than 70% of tumor tissues derived from NSCLC patients with a histology of squamous cell carcinoma,34,35 Hsp27 qualifies as a putative biomarker to diagnose and to determine TNM staging of NSCLC tumors. As Hsp27 low expression is associated with better prognosis in NSCLC patients, the determination of its expression level in NSCLC tumors may provide an effective clinical strategy to predict the treatment response.36 A meta-analysis demonstrates that Hsp27 expression can be a strong biomarker to predict the poor clinicopathological and prognostic characteristics as well in patients with NSCLC tumors.37 Considering the differential expression of Hsp27 in lung tumors including NSCLC; its association with the advancement of tumors and anti-cancer resistance to conventional therapies;38 targeting this chaperone can be a potential anti-cancer therapeutic strategy.
Hsp60
HSP60, also known as Cpn60 and HSPD1, belongs to a family of structurally related chaperonins. In humans, Hsp60 is encoded by the HSPD1 gene, which is located on chromosome 8. Hsp60 is largely located in the matrix of mitochondria and plays an extensive role in the folding of mitochondrial proteins. It prevents the aggregation of misfolded or partially folded proteins and targets them to degradation;39 also helps in translocation and assembly of native proteins. Functional Hsp60 requires Hsp10 that serves as a co-factor for its substrate binding and catalytic activity.
A significant number of studies have reported Hsp60 involvement in cancer development and cellular transformation. Previously, bronchial biopsies of human subjects suffering from chronic obstructive pulmonary disease and lung cancer have shown considerable down-regulation of Hsp60 and Hsp10. Based on these findings, it was hypothesized that the low expression or complete loss of Hsp60 could serve as a marker for the detection of lung cancer.40 In concurrence with the above report, a recent study demonstrated and confirmed in vitro that increased proliferation of renal carcinoma cell lines upon Hsp60 knockdown following their earlier observation of Hsp60 downregulation in renal cell carcinoma patients.41 On the other hand, screening of tumor tissues derived from lung cancer patients with adenocarcinoma histology; 68% of tissues were strongly stained positive for Hsp60 and their expression level was not only correlated with TNM staging but also with the prognosis significantly.42 In another proteomic study carried out with clinical samples collected from lung cancer patients with adeno or squamous cell, carcinoma histology had shown significantly increased Hsp60 and annexin 2, respectively, as compared to their matched normal controls. However, no difference in their levels was noted between early- and advanced-stage tumors.43 In light of their outcomes, authors of the above paper suggest that the use of these two proteins as biomarkers for diagnosis and prognosis of adeno and squamous cell carcinoma albeit no connection was built up in their investigation between Hsp60 expression and the progression of the tumor, and their response to existing lung cancer therapeutics. Studies mentioned above substantiate the significance of Hsp60 expression in lung cancer diagnosis and prognosis to some degree, but the expression of Hsp60 in various cancers is still ambiguous. In some cancers, the Hsp60 level was reported to be low while in some high. Therefore, a large-scale screening of lung cancer patients with different tumor histologies may authenticate its use in diagnosis, prognosis and as a drug target.
Hsp70
Hsp70, a protein with a molecular weight of 70 kDa, belongs to a family of highly conserved molecular chaperones that includes two major isoforms; constitutively expressed Hsc70 and stress-inducible Hsp70. Other members of Hsp70 family proteins show organelle and tissue-specific localization; glucose-regulated protein (Grp)78 is located in the endoplasmic reticulum and Grp75 is a resident of mitochondria.44 Hsp70 is involved in a large variety of protein folding processes, upregulated under heat stress and in response to toxic chemicals.45 These proteins also keep the cells viable under hypoxia, altered pH, oxidative stress, DNA damage response and many other stress conditions.46 Similar to Hsp27, function of Hsp70 is regulated through phosphorylation. Many studies have suggested the importance of Hsp70 phosphorylation in regulating cellular processes such as cell proliferation, apoptosis, protein degradation and resistance to anticancer drugs.47
Increased expression of Hsp70 is associated with a wide variety of cancers and tumor cell survival. Its expression had a correlation with the advancement of various tumors and poor prognosis. A substantial number of studies have reported a relatively higher risk of lung cancer with increased expression of Hsp70 and it was found to be high in Japanese population among male patients over females.48 However, it is neither correlated with drug resistance nor a histopathological type of NSCLC tumors.49,50 On the contrary, NSCLC patients with histology of adeno and squamous cell carcinoma with significant gross tumor volume had high serum Hsp70 as compared to normal healthy controls.51 Besides the difference of Hsp70 noted in serum samples between patients with early-stage and advanced-stage tumors. Based on the reports in NSCLC tumors; it was proposed that overexpression of Hsp70 can be a marker to predict the survival benefit from platinum-based adjuvant chemotherapy.52 More than a decade ago, it was observed that NSCLC patients had significantly higher serum Hsp70 antibody levels as compared to normal healthy controls with no marked difference in anti-Hsp90 antibodies between these two groups.53 However, a recent study had shown decreased level of circulating Hsp70 in plasma of NSCLC patients but higher levels of CEA when compared with healthy controls.54 This study suggests the consideration of Hsp70 levels in NSCLC patients along with well-established marker CEA as biomarkers for diagnosis of early-stage lung cancers. The combination of Hsp70 with other tumor markers might increase the specificity and sensitivity of lung cancer diagnosis. In addition to Hsp70, overexpression of Grp78 and Grp94 in lung tumors at RNA and protein levels correlated with the grade and stage of lung tumors. Poorly differentiated tumors had significantly increased Grp78 than moderately or well-differentiated lung tumors.55 Grp78 in association with mitochondrial protein Bax contributes to tumor progression and metastasis and advanced stage of NSCLC tumors had a higher Grp78 expression when compared to early pathological staging especially patients with positive lymph node metastasis than negative lymph node metastasis.56 In some cases, an intronic polymorphism (rs430397 G > A) in Grp78 affects the prognosis of NSCLC patients treated with platinum-based chemotherapy.57 NSCLC patients with A/A genotype showed Grp78 overexpression and poor response toward platinum-based agents, which indicate a potentially important role of this polymorphism in tumor progression and in treatment prediction. The above reports recommend these two chaperones; Hsp70 and Grp78 as potential biomarkers for lung cancer patient management. However, neither of these two proteins independent of each other determines the staging and prognosis of NSCLC patients.
In a large variety of tumor cells including NSCLC tumors, often the plasma membrane stains positive for Hsp70 and is released into the blood circulation in lipid vesicles. Thus, the screening of lung tumor tissues harboring EGFR mutants for Hsp70 expression in different cellular compartments could be another criterion to link Hsp70 with EGFR in lung cancer. The combination of Hsp70 with other proteins discussed here might serve as markers for the detection of early stage of lung cancer, but the consideration of this chaperone as a cancer therapeutic drug target is worth investigating. A variety of cancers show abnormally high expression of Hsp70 associated with tumor progression, inhibition of apoptosis and autophagy perhaps conferring resistance to existing lung cancer targeted drugs and chemotherapy. Suppression of tumor size with Hsp70 inhibition indicates the cytoprotective nature of this chaperone. Thus, targeting Hsp70 possibly a good strategy to develop anti-lung cancer therapeutics and can be considered to give in combination with existing targeted drugs or with conventional chemotherapy for better lung cancer patient management.
Hsp90
Hsp90 (90kD) is an evolutionarily conserved molecular chaperone and most common among various stress-related proteins. Inducible Hsp90α and constitutive Hsp90β; two isoforms of Hsp90 have been reported in vertebrates. Mammalian cells express several homologues of Hsp90 viz. glucose-regulated protein 94 (Grp94), TNF receptor-associated protein1 (TRAP1/Hsp75) localized in the endoplasmic reticulum and mitochondrial matrix, respectively.58 Hsp90 in association with other chaperones maintains the structure and the stability of multiple protein kinases, membrane receptors, transcription factors including NF-kβ and myogenic differentiation protein MyoD.59 Cancer cells generally use the chaperone machinery of Hsp90 for their survival advantage. Therefore, Hsp90 is considered as an important facilitator for “oncogene addiction” and for the maintenance of malignant phenotype.60 Inducible Hsp90α in plasma was reported to be high in lung tumor patients as compared to normal healthy controls and the expression level was correlated with the development and progression of cancer.61 The level of plasma Hsp90α between patients with early- and advanced-stage lung tumors is also significantly different.62 Grp94, an Hsp90 like protein, also overexpressed in lung tumors of stages I, II and III. However, the studies on Grp94 in lung cancer are very limited, but in one study, higher Grp94 expression has been reported as an independent prognostic factor for poor survival of NSCLC patients and similar results were demonstrated in breast cancer patients as well.63,64
Approximately 10% of NSCLC cases harboring activating EGFR mutations are often associated with an increased expression of EGFR. Association of mutant EGFR with Hsp90 confers the conformational stability to the mutant receptor,65 and impairs the ligand-induced receptor downregulation.66 These two reports together suggest the essential role of Hsp90 in maintaining the mutant receptor expression. An increased expression of Hsp90B1, another homologue of Hsp90 contributes to the poor prognosis of NSCLC patients with adenocarcinoma histology. As Hsp90 may not serve as a standalone biomarker to predict the treatment outcome, other proteins that can serve as possible biomarkers in combination with this chaperone for accuracy need to be identified. Association of increased expression of Hsp70 in lung tumor progression and suppression of proliferation, migration and promotion of apoptosis in lung tumor cells and with the inhibition of Hsp90 demonstrates its significance as a drug target to treat lung tumors. The role of Hsps in lung tumor cell survival is illustrated in Figure 1.
Figure 1.
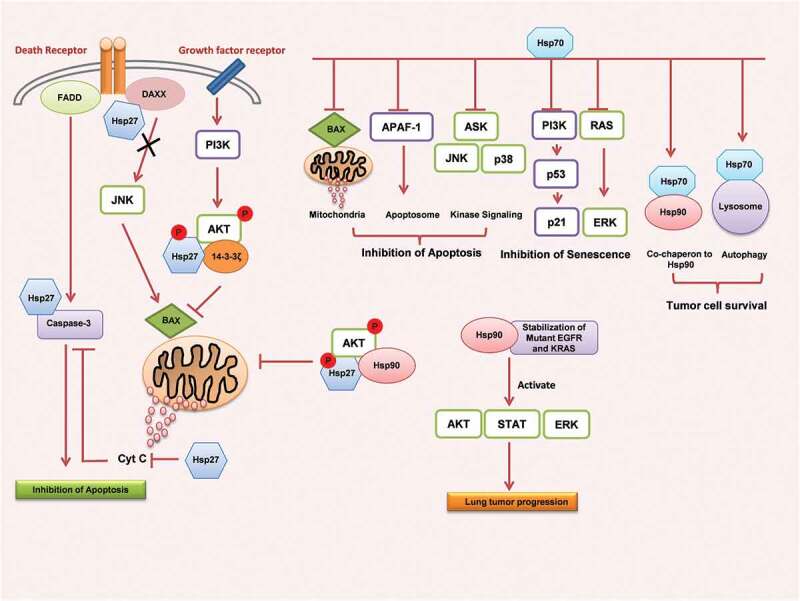
Heat shock proteins mediated cell survival and anti-apoptotic pathways in lung cancer. Hsp27 interacts with various apoptotic and cell survival regulators such as cytochrome c, caspase-3, DAXX, FADD, BAX, AKT, 14-3-3ζ and inhibits apoptosis and induces cell survival. Hsp70 also deregulates several signal proteins that are involved in the induction of apoptosis, senescence thus contributing to the tumor cell survival. Hsp90 interacts and stabilizes mutant receptors and enables them to recruit tumor cell proliferative signal pathways.
Heat shock proteins as therapeutic targets in lung cancer
Recent progress made in understanding the molecular mechanism associated with lung cancer development and progression has led to the identification of several novel molecular targets. Of which, Hsps have been identified as potential molecular targets for the development of anticancer therapeutics owing to their involvement in lung tumor progression. As Hsps play a regulatory role under physiological and pathological conditions, two approaches are in consideration to target Hsps; one is to regulate the expression level and their activity, while the second one is to develop Hsp-based immunotherapies. Until now, no drug has been approved clinically to regulate Hsp expression and its activity at the molecular level. However, there are several known Hsp90 inhibitors, which are under preclinical and clinical trials to treat various malignancies including lung cancer.67,68 The most potent Hsp90 inhibitors are natural products; geldanamycin, radicicol and their semi-synthetic derivatives. These drugs target the ATP binding site located in the N-terminal domain of Hsp90 and inhibit its ATPase activity thus preventing the binding of Hsp90 to its client proteins.69 NSCLC tumor-associated K-Ras and EGFR mutants are known to induce constitutively active proliferative signals and contribute to tumorigenicity. As EGFR depends on Hsp90 for its conformational maturation and inhibition of Hsp90 affects EGFR activity, Hsp90 inhibitors which are under preclinical trials reduce lung cancer by targeting overexpressed or mutated kinases viz. EGFR, ErbB2, B-Raf and c-Met.70,71
Hsp90 inhibitor, NVP-AUY922 showed antitumor activity in NSCLC cell lines harboring K-Ras mutations and in mouse xenograft models. It also sensitizes MEK inhibitor, trametinib resistant NSCLC tumors.72
As inhibition of Hsp90 leads to misfolding, ubiquitination and proteasomal degradation of client proteins; Hsp90 inhibitors and their analogues may act in a synergistic manner with other drugs or agents such as histone deacetylase inhibitors (HDACi). LBH59, known HDACi has shown to reduce the association of Hsp90 with mutant EGFR, Akt and STAT3 due to its increased acetylation73 and subsequently downregulates the survival proteins including Bcl-xL, Mcl-1, Bcl-2 followed by apoptosis in NSCLC cell lines. As phosphorylation of Hsp90 is important for its ability to chaperone its client proteins, recent studies have suggested that targeting kinases that phosphorylate Hsp90 may enhance the anticancer activity of Hsp90 inhibitors in cancer cells; thus providing a novel therapeutic approach to target various tumors.74
On the other hand, Hsp90 inhibitors do damage the chaperone machinery and inhibit the client proteins; c-Raf, CDK4. But it can activate heat shock response and induces Hsp70 expression which in turn attenuates the efficacy of Hsp90 inhibitors.75 Isoforms of Hsp70; constitutively expressed Hsc73 and inducible Hsp72 are overexpressed in advanced stages of many tumors including lung cancer and contribute to lymph node metastasis.76 However, inhibition of these isoforms using siRNA prior to Hsp90 inhibition increased the efficacy of Hsp90 inhibitors.77 Hence, “dual targeting” of Hsp90 and Hsp70, using antisense oligonucleotide therapies or other manipulations, offers an effective line of treatment against lung cancer. Approaches such as Hsp70 inhibitors78 and radio-sensitizing drugs are already at preclinical trials.79 So far, many Hsp70 inhibitors were tested in preclinical models, including MKT-077, PES (Pifithrin-μ), PES-Cl, VER-155008, JG-98, Aptamer A-17, apoptozole and others. These inhibitors have an extremely high affinity for certain domains of Hsp70. PES and PES-Cl bind to the substrate-binding domain; VER-155008 recognize ATP binding site; MKT-077 targets the allosteric site near the ATP-binding site and JG-98 binds to the region within the nucleotide-binding domain of Hsp70.80 Inhibition of Hsp70 by these molecules significantly inhibits proliferation and cell cycle progression in NSCLC cell lines.81,82 Suppression of Hsp70 by Ibuprofen and Quercetin sensitizes lung cancer cells to cisplatin by enhancing apoptosis at several stages of the mitochondrial cascade.83,84 A number of preclinical and clinical studies have supported the inhibition of Hsp90 and Hsp70 as a preventive approach against NSCLC treatment. Ongoing clinical trials further define the efficacy of Hsps inhibitors in NSCLC and are listed in Table 1. The given information in the Table 1 was retrieved from clinicaltrials.gov.
Table 1.
Ongoing and completed clinical trials with Hsp inhibitors in lung cancer patients
| Inhibitor | clinicaltrials.gov number | Treatment | Phase | Patients group |
|---|---|---|---|---|
| Hsp90 | ||||
| AUY922 |
NCT01259089 NCT01784640 NCT02276027 |
AUY922 monotherapy AUY922+erlotinib AUY922monotherapy |
PhaseI/II Phase1 Phase II |
Stage IIIB-IV NSCLC patients Previously treated stage IV NSCLC patients Chinese patients with advanced NSCLC |
| Ganetespib (STA-9090) |
NCT01579994 NCT01590160 |
Ganetespib + crizotinib Ganetespib +platinum |
Phase I Phase I/II |
Patients with ALK positive lung cancer Patients with malignant pleural mesothelioma (MESO-02) |
| Onalespib | NCT02535338 | Onalespib +erlotinib | Phase I/II | Patients with recurrent or metastatic EGFR-NSCLC |
| Hsp70 | ||||
| Targeted NK cell based adoptive immunotherapy | NCT02118415 | Hsp70 NK cell based adoptive immunotherapy | Phase II | Patients with NSCLC after radio-chemotherapy |
| Hsp27 | ||||
| Apatorsen (OGX-427) |
NCT02423590 | gemcitabine/carboplatin + Apatorsen vs. gemcitabine/carboplatin | Phase II | Patients with previously untreated advanced squamous cell lung cancer |
Besides being localized intracellularly, Hsps are also reported to be expressed on transformed cell membrane, and in extracellular space. Hsps located in extracellular space execute immunological events through modulating antigenic peptides and generate an immune response against tumor escape, its growth and metastasis.85,86 In addition, ever-increasing studies have established the involvement of a variety of receptors including toll-like receptors-2/4 (TLRs-2/4), lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), fascicle in EGF-like, laminin-type EGF-like and link domain-containing scavenger receptor-1 (FEEL-1) (FEEL-1) and several others in the uptake of Hsps and Hsp-processed exogenous peptides into antigen-presenting cells (APCs).87 Furthermore, Hsps have been found to stimulate the immunological activity of natural killer (NK) cells in combination with proinflammatory cytokines killing tumor cells.88 All the above studies support the importance of Hsp-based anti-cancer immunotherapeutics.
In another approach, the antitumor effect on mouse melanoma was generated by combining recombinant Hsps and hyperthermia using magnetite cationic liposomes (MCLs).89 Similarly, recombinant Hsp70 combined with magnetic nano-carriers like superparamagnetic iron oxide nanoparticles (SPIONs) were reported to be effective for the delivery of immunogenic peptide from tumor cell lysates to dendritic cells which was evident by activation of immunological response in experimental glioma models.90 Furthermore, an intra-tumoral vaccination with recombinant Hsp70 from oncolytic type-2 adenovirus inhibited tumor growth; metastasis and Hsp70 mediated immune responses.91 Recombinant Hsp70 with E7 protein from HPV or oncoprotein HER-2/neu induced antitumor immune responses.92-94 Similarly, a therapeutic vaccine consisting of tumor cell membrane Hsp70 and hydroxyapatite ceramic particles demonstrated positive response and tolerability in certain cancer patients.95 Several ongoing studies had reported promising results on immunization of cancer patients with autologous Hsp-based antitumor vaccine complexes. In a recent study, the efficacy of the Heat shock protein peptide complex–96 vaccine is determined in phase I and II clinical trials in patients with recurrent glioblastoma.96
Tumor/dendritic cell fusion technology also represents an alternative approach in the preparation of Hsp-based vaccines which has been shown to be effective against individual tumor cell-like cancer stem cells (CSCs).97 Recently, DC-CSC fusion vaccine-induced T cells have been shown to be killing selectively CSCs and radiation-resistant population of CSCs in ovarian cancer.98 Furthermore, an in vivo study with mice having preexisting lung metastasis reported survival effects of Hsp70-peptide complexes (Hsp70.PC-F) extracted from fusions of DCs and radiation-enriched tumor cells. Activation of T-cell-mediated immune response followed by inhibition of primary tumor growth and tumor cells metastasizing to lungs were concurrent findings of this study.99 A phase I clinical trial with HSP70 mRNA-transfected dendritic cell therapy for treating HCC has confirmed its safety and efficacy.100 Thus, tumor-directed therapy based on the use of Hsps in combination with chemo or radiotherapy and/or hyperthermia may provide an effective treatment regimen against many cancers including lung cancer.
Conclusion
Increasing evidence based on in vitro and in vivo studies signifies the role of Hsps in the progression and migration of various tumors. Altered expression of these chaperones can be a danger signal of cancer. As Hsps do express under conditions other than cancers; it is hard to determine the staging of tumors based on their level of expression alone. Nonetheless, they can still be used as reliable biomarkers in combination with other established tumor-specific makers for accurate diagnostic and prognostic yield; also, to help define the optimal therapeutic strategy. Regardless of the fact that more studies are needed to establish the authenticity of molecular chaperone expression as diagnostic or prognostic markers or as drug targets in lung cancer patient management, there is a hope that these proteins can serve as potential biomarkers and anti-lung cancer drug targets.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- 1.Gething M-J, Sambrook J.. Protein folding in the cell. Nature. 1992;355:33. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 2.Netzer WJ, Ulrich Hartl F. Protein folding in the cytosol: chaperonin-dependent and -independent mechanisms. Trends Biochem Sci. 2018;23:68–73. doi: 10.1016/S0968-0004(97)01171-7. [DOI] [PubMed] [Google Scholar]
- 3.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 4.Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 5.Graf PCF, Martinez-Yamout M, VanHaerents S, Lilie H, Dyson HJ, Jakob U. Activation of the redox-regulated chaperone Hsp33 by domain unfolding. J Biol Chem. 2004;279:20529–20538. doi: 10.1074/jbc.M401764200. [DOI] [PubMed] [Google Scholar]
- 6.Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderwood SK, Xie Y, Wang X, Khaleque MA, Chou SD, Murshid A, Prince T, Zhang Y. Signal transduction pathways leading to heat shock transcription. Signal Transduction Insights. 2010;2:13–24. doi: 10.4137/STI.S3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 9.Calderwood SK, Gong J. Heat shock proteins promote cancer: it’s a protection racket. Trends Biochem Sci. 2016;41:311–323. doi: 10.1016/j.tibs.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2018;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Jacques F, Isabelle S, Rajesh D, Sultan E, Colin M, Marise R, Maxwell PD, David F, Freddie B. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Jacques F, Hai‐Rim S, Freddie B, David F, Colin M, Maxwell PD. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 13.GCO . Global Cancer Observatory. Malaysia Cancer Stat. 2019;593:1–2. Available from: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf. [Google Scholar]
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.v69.1. [DOI] [PubMed] [Google Scholar]
- 15.Parikh PM, Ranade AA, Govind B, Ghadyalpatil N, Singh R, Bharath R, Bhattacharyya GS, Koyande S, Singhal M, Vora A, et al. Lung cancer in India: current status and promising strategies. South Asian J Cancer. 2016;5:93–95. doi: 10.4103/2278-330X.187563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen K-SH, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Maity T, Kashyap MK, Bansal M, Venugopalan A, Singh S, Awasthi S, Marimuthu A, Charles Jacob HK, Belkina N, et al. Quantitative tyrosine phosphoproteomics of Epidermal Growth Factor Receptor (EGFR) tyrosine kinase inhibitor-treated lung adenocarcinoma cells reveals potential novel biomarkers of therapeutic response. Mol Cell Proteomics. 2017;16:891–910. doi: 10.1074/mcp.M117.067439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsogiannou M, Andrieu C, Rocchi P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front Genet. 2014;5:346. doi: 10.3389/fgene.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Xing J, Wang F, Han W, Ren H, Wu T, Chen W. Expression of Hsp27 and Hsp70 in lymphocytes and plasma in healthy workers and coal miners with lung cancer. J Huazhong Univ Sci Technol [Med Sci]. 2010;30:415–420. doi: 10.1007/s11596-010-0441-5. [DOI] [PubMed] [Google Scholar]
- 21.Berrieman HK, Cawkwell L, O’Kane SL, Smith L, Lind MJ. Hsp27 may allow prediction of the response to single-agent vinorelbine chemotherapy in non-small cell lung cancer. Oncol Rep. 2006;15:283–286. [PubMed] [Google Scholar]
- 22.Lee J-H, Sun D, Cho K-J, Kim M-S, Hong M-H, Kim I-K, Lee J-S, Lee J-H. Overexpression of human 27 kDa heat shock protein in laryngeal cancer cells confers chemoresistance associated with cell growth delay. J Cancer Res Clin Oncol. 2007;133:37–46. doi: 10.1007/s00432-006-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han‐Shui H, Jiun‐Han L, Wen‐Chien H, Tien‐Wei H, Kelly S, Shih‐Hwa C, Yo‐Ting T, Shih‐Chieh H. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer. 2010;117:1516–1528. doi: 10.1002/cncr.25599. [DOI] [PubMed] [Google Scholar]
- 24.Keshamouni VG, Michailidis G, Grasso CS, Anthwal S, Strahler JR, Walker A, Arenberg DA, Reddy RC, Akulapalli S, Thannickal VJ, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. J Proteome Res. 2006;5:1143–1154. doi: 10.1021/pr050455t. [DOI] [PubMed] [Google Scholar]
- 25.Yao H, Zhang Z, Xiao Z, Chen Y, Li C, Zhang P, Li M, Liu Y, Guan Y, Yu Y, et al. Identification of metastasis associated proteins in human lung squamous carcinoma using two-dimensional difference gel electrophoresis and laser capture microdissection. Lung Cancer. 2018;65:41–48. doi: 10.1016/j.lungcan.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Lelj-Garolla B, Kumano M, Beraldi E, Nappi L, Rocchi P, Ionescu DN, Fazli L, Zoubeidi A, Gleave ME. Hsp27 Inhibition with OGX-427 sensitizes non–small cell lung cancer cells to erlotinib and chemotherapy. Mol Cancer Ther. 2015;14:1107 LP– 1116. doi: 10.1158/1535-7163.MCT-14-0866. [DOI] [PubMed] [Google Scholar]
- 27.Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/A:1021601103096. [DOI] [PubMed] [Google Scholar]
- 28.Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008;283:12305–12313. doi: 10.1074/jbc.M801291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang SAI, HU Y, Huang Y, Xu H, WU G, Dai H. Heat shock protein 27 promotes cell proliferation through activator protein-1 in lung cancer. Oncol Lett. 2015;9:2572–2576. doi: 10.3892/ol.2015.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guang‐Yin Z, Jian‐Yong D, Jie G, Chun‐Lai L, Zong‐Wu L, Jing G, Di G. The overexpression of 14‐3‐3ζ and Hsp27 promotes non–small cell lung cancer progression. Cancer. 2014;120:652–663. doi: 10.1002/cncr.28452. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann M, Nickl S, Lambers C, Hacker S, Mitterbauer A, Hoetzenecker K, Rozsas A, Ostoros G, Laszlo V, Hofbauer H, et al. Discrimination of clinical stages in non-small cell lung cancer patients by serum HSP27 and HSP70: A multi-institutional case–control study. Clin Chim Acta. 2012;413:1115–1120. doi: 10.1016/j.cca.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Xu T, Wei Q, Guerra JLL, Wang L-E, Liu Z, Gomez D, O’Reilly M, Lin SH, Zhuang Y, Levy LB, et al. HSPB1 gene polymorphisms predict risk of mortality for U.S. patients after radio(chemo)therapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:e229–35. doi: 10.1016/j.ijrobp.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo H, Bai Y, Xu P, Hu Z, Liu L, Wang F, Jin G, Wang F, Deng Q, Tu Y, et al. Functional promoter −1271G>C variant of HSPB1 predicts lung cancer risk and survival. J Clin Oncol. 2010;28:1928–1935. doi: 10.1200/JCO.2009.24.4954. [DOI] [PubMed] [Google Scholar]
- 34.Malusecka E, Zborek A, Krzyċwska-Gruca S, Krawczyk Z. Heat shock proteins (HSP70) expression in primary non-small cell lung carcinomas. Lung Cancer. 2018;25:S27. doi: 10.1016/S0169-5002(99)90771-7. [DOI] [PubMed] [Google Scholar]
- 35.Qi H, Yukun Z, Xiangning F, Tangchun W. Expression of heat shock protein 70 and 27 in non-small cell lung cancer and its clinical significance. J Huazhong Univ Sci Technol [Med Sci]. 2005;25:693–695. doi: 10.1007/BF02896173. [DOI] [PubMed] [Google Scholar]
- 36.Huang ZC, Li H, Sun ZQ, Zheng J, Zhao RK, Chen J, Sun SG, Wu CJ. Distinct prognostic roles of HSPB1 expression in non-small cell lung cancer. Neoplasma. 2018;65:161–166. doi: 10.4149/neo_2018_102. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Zhang W, Fan J, Lai Y, Che G. Clinicopathological and prognostic significance of heat shock protein 27 (HSP27) expression in non-small cell lung cancer: a systematic review and meta-analysis. Springerplus. 2016;5:1165. doi: 10.1186/s40064-016-2827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oba M, Yano S, Shuto T, Suico MA, Eguma A, Kai H. IFN-gamma down-regulates Hsp27 and enhances hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo. Int J Oncol. 2008;32:1317–1324. doi: 10.3892/ijo_32_6_1317. [DOI] [PubMed] [Google Scholar]
- 39.Myung J-K, Afjehi-Sadat L, Felizardo-Cabatic M, Slavc I, Lubec G. Expressional patterns of chaperones in ten human tumor cell lines. Proteome Sci. 2004;2:8. doi: 10.1186/1477-5956-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappello F, Di Stefano A, D’Anna SE, Donner CF, Zummo G. Immunopositivity of heat shock protein 60 as a biomarker of bronchial carcinogenesis. Lancet Oncol. 2018;6:816. doi: 10.1016/S1470-2045(05)70393-4. [DOI] [PubMed] [Google Scholar]
- 41.Teng R, Liu Z, Tang H, Zhang W, Chen Y, Xu R, Chen L, Song J, Liu X, Deng H. HSP60 silencing promotes Warburg-like phenotypes and switches the mitochondrial function from ATP production to biosynthesis in ccRCC cells. Redox Biol. 2019;24:101218. doi: 10.1016/j.redox.2019.101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X, Wang W, Shao W, Yin W, Chen H, Qiu Y, Mo M, Zhao J, Deng Q, He J. Heat shock protein-60 expression was significantly correlated with the prognosis of lung adenocarcinoma. J Surg Oncol. 2011;104:598–603. doi: 10.1002/jso.v104.6. [DOI] [PubMed] [Google Scholar]
- 43.Ağababaoğlu İ, Önen A, Demir AB, Aktaş S, Altun Z, Ersöz H, Şanlı A, Özdemir N, Akkoçlu A. Chaperonin (HSP60) and annexin-2 are candidate biomarkers for non-small cell lung carcinoma. Medicine (Baltimore). 2017;96:e5903. doi: 10.1097/MD.0000000000005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/S0163-7258(98)00028-X. [DOI] [PubMed] [Google Scholar]
- 45.Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 2019;20:665–680. doi: 10.1038/s41580-019-0133-3. [DOI] [PubMed] [Google Scholar]
- 46.Knighton LE, Delgado LE, Truman AW. Novel insights into molecular chaperone regulation of ribonucleotide reductase. Curr Genet. 2019;65:477–482. doi: 10.1007/s00294-018-0916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nitika, Truman AW. Cracking the chaperone code: cellular roles for Hsp70 phosphorylation. Trends Biochem Sci. 2017;42:932–935. doi: 10.1016/j.tibs.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki K, Ito Y, Wakai K, Kawado M, Hashimoto S, Seki N, Ando M, Nishino Y, Kondo T, Watanabe Y, et al. Serum heat shock protein 70 levels and lung cancer risk: a case-control study nested in a large Cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:1733 LP– 1737. doi: 10.1158/1055-9965.EPI-06-0005. [DOI] [PubMed] [Google Scholar]
- 49.Volm M, Koomägi R, Mattern J, Stammler G. Heat shock (hsp70) and resistance proteins in non-small cell lung carcinomas. Cancer Lett. 1995;95:195–200. doi: 10.1016/0304-3835(95)03893-2. [DOI] [PubMed] [Google Scholar]
- 50.Michils A, Redivo M, Zegers de Beyl V, de Maertelaer V, Jacobovitz D, Rocmans P, Duchateau J. Increased expression of high but not low molecular weight heat shock proteins in resectable lung carcinoma. Lung Cancer. 2018;33:59–67. doi: 10.1016/S0169-5002(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 51.Gunther S, Ostheimer C, Stangl S, Specht HM, Mozes P, Jesinghaus M, Vordermark D, Combs SE, Peltz F, Jung MP, et al. Correlation of Hsp70 Serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non-small cell lung cancer. Front Immunol. 2015;6:556. doi: 10.3389/fimmu.2015.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park TS, Kim H-R, Koh JS, Jang SH, Hwang Y Il, Yoon H Il, Chung J-H, Kim CH, Kim -S-S, Kim WS, et al. Heat shock protein 70 as a predictive marker for platinum-based adjuvant chemotherapy in patients with resected non-small cell lung cancer. Lung Cancer. 2018;86:262–267. doi: 10.1016/j.lungcan.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Zhong L, Peng X, Hidalgo GE, Doherty DE, Stromberg AJ, Hirschowitz EA. Antibodies to HSP70 and HSP90 in serum in non-small cell lung cancer patients. Cancer Detect Prev. 2003;27:285–290. doi: 10.1016/S0361-090X(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 54.Tang T, Yang C, Brown HE, Huang J. Circulating heat shock protein 70 is a novel biomarker for early diagnosis of lung cancer. Dis Markers. 2018;2018:6184162. doi: 10.1155/2018/6184162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q, He Z, Zhang J, Wang Y, Wang T, Tong S, Wang L, Wang S, Chen Y. Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev. 2005;29:544–551. doi: 10.1016/j.cdp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Sun Q, Hua J, Wang Q, Xu W, Zhang J, Zhang J, Kang J, Li M. Expressions of GRP78 and Bax associate with differentiation, metastasis, and apoptosis in non-small cell lung cancer. Mol Biol Rep. 2012;39:6753–6761. doi: 10.1007/s11033-012-1500-8. [DOI] [PubMed] [Google Scholar]
- 57.Zhu X, Lin MCM, Fan W, Tian L, Wang J, Ng SS, Wang M, Kung H, Li D. An intronic polymorphism in GRP78 improves chemotherapeutic prediction in non-small cell lung cancer. Chest. 2018;141:1466–1472. [DOI] [PubMed] [Google Scholar]
- 58.Subbarao Sreedhar A, Kalmár É, Csermely P, Shen Y-F. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–15. doi: 10.1016/S0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- 59.Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816:89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang Y, Tong M, Chang G, Luo Y. The regulatory mechanism of Hsp90α secretion and its function in tumor malignancy. Proc Natl Acad Sci. 2009;106:21288 LP– 21293. doi: 10.1073/pnas.0908151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi Y, Liu X, Lou J, Han X, Zhang L, Wang Q, Li B, Dong M, Zhang Y. Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin Cancer Res. 2014;20:6016 LP– 6022. doi: 10.1158/1078-0432.CCR-14-0174. [DOI] [PubMed] [Google Scholar]
- 63.Kim S-H, Lee G, Lee J-S, Cho YJ, Jeong YY, Kim H-C, Lee JD, Lee JH, Lee EH, Lee HW. Association of high expression of Hsp90-beta and GRP 94 with poor survival in resected non-small cell lung cancer patients. J Clin Oncol. 2014;32:e22183–e22183. doi: 10.1200/jco.2014.32.15_suppl.e22183. [DOI] [Google Scholar]
- 64.Liu S, Li R, Zuo S, Luo R, Fang W, Xie Y. GRP94 overexpression as an indicator of unfavorable outcomes in breast cancer patients. Int J Clin Exp Pathol. 2018;11:3061–3067. [PMC free article] [PubMed] [Google Scholar]
- 65.Ahsan A, Ramanand SG, Whitehead C, Hiniker SM, Rehemtulla A, Pratt WB, Jolly S, Gouveia C, Truong K, Van Waes C, et al. Wild-type EGFR is stabilized by direct interaction with HSP90 in cancer cells and tumors. Neoplasia. 2012;14:670–677. doi: 10.1593/neo.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang S, Qu S, Perez-Tores M, Sawai A, Rosen N, Solit DB, Arteaga CL. Association with HSP90 inhibits Cbl-mediated down-regulation of mutant epidermal growth factor receptors. Cancer Res. 2006;66:6990 LP– 6997. doi: 10.1158/0008-5472.CAN-06-1042. [DOI] [PubMed] [Google Scholar]
- 67.Chatterjee S, Burns TF. Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci. 2017;18:1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chatterjee S, Bhattacharya S, Socinski MA, Burns TF. HSP90 inhibitors in lung cancer: promise still unfulfilled. Clin Adv Hematol Oncol. 2016;14:346–356. [PubMed] [Google Scholar]
- 69.Workman P. Pharmacogenomics in cancer drug discovery and development: inhibitors of the Hsp90 molecular chaperone. Cancer Detect Prev. 2018;26:405–410. doi: 10.1016/S0361-090X(02)00126-5. [DOI] [PubMed] [Google Scholar]
- 70.Xu W, Soga S, Beebe K, Lee M-J, Kim YS, Trepel J, Neckers L. Sensitivity of epidermal growth factor receptor and ErbB2 exon 20 insertion mutants to Hsp90 inhibition. Br J Cancer. 2007;97:741–744. doi: 10.1038/sj.bjc.6603950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimamura T, Li D, Ji H, Haringsma HJ, Liniker E, Borgman CL, Lowell AM, Minami Y, McNamara K, Perera SA, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res. 2008;68:5827–5838. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park K-S, Oh B, Lee M-H, Nam K-Y, Jin HR, Yang H, Choi J, Kim S-W, Lee DH. The HSP90 inhibitor, NVP-AUY922, sensitizes KRAS-mutant non-small cell lung cancer with intrinsic resistance to MEK inhibitor, trametinib. Cancer Lett. 2018;372:75–81. doi: 10.1016/j.canlet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 73.Edwards A, Li J, Atadja P, Bhalla K, Haura EB. Effect of the histone deacetylase inhibitor LBH589 against epidermal growth factor receptor–dependent human lung cancer cells. Mol Cancer Ther. 2007;6:2515 LP– 2524. doi: 10.1158/1535-7163.MCT-06-0761. [DOI] [PubMed] [Google Scholar]
- 74.Woodford MR, Dunn D, Miller JB, Jamal S, Neckers L, Mollapour M. Impact of posttranslational modifications on the anticancer activity of Hsp90 inhibitors. Adv Cancer Res. 2016;129:31–50. [DOI] [PubMed] [Google Scholar]
- 75.Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 76.Gabai VL, Budagova KR, Sherman MY. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene. 2005;24:3328. doi: 10.1038/sj.onc.1208495. [DOI] [PubMed] [Google Scholar]
- 77.Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2018;14:250–262. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Wen W, Liu W, Shao Y, Chen L. VER-155008, a small molecule inhibitor of HSP70 with potent anti-cancer activity on lung cancer cell lines. Exp Biol Med. 2014;239:638–645. doi: 10.1177/1535370214527899. [DOI] [PubMed] [Google Scholar]
- 79.Schilling D, Kühnel A, Konrad S, Tetzlaff F, Bayer C, Yaglom J, Multhoff G. Sensitizing tumor cells to radiation by targeting the heat shock response. Cancer Lett. 2015;360:294–301. doi: 10.1016/j.canlet.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 80.Shevtsov M, Multhoff G, Mikhaylova E, Shibata A, Guzhova I, Margulis B. Combination of anti-cancer drugs with molecular chaperone inhibitors. Int J Mol Sci. 2019;20:5284. doi: 10.3390/ijms20215284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sojka DR, Gogler-Piglowska A, Vydra N, Cortez AJ, Filipczak PT, Krawczyk Z, Scieglinska D. Functional redundancy of HSPA1, HSPA2 and other HSPA proteins in non-small cell lung carcinoma (NSCLC); an implication for NSCLC treatment. Sci Rep. 2019;9:14394. doi: 10.1038/s41598-019-50840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Budina-Kolomets A, Balaburski GM, Bondar A, Beeharry N, Yen T, Murphy ME. Comparison of the activity of three different HSP70 inhibitors on apoptosis, cell cycle arrest, autophagy inhibition, and HSP90 inhibition. Cancer Biol Ther. 2014;15:194–199. doi: 10.4161/cbt.26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Endo H, Yano M, Okumura Y, Kido H. Ibuprofen enhances the anticancer activity of cisplatin in lung cancer cells by inhibiting the heat shock protein 70. Cell Death Dis. 2014;5:e1027. doi: 10.1038/cddis.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee SH, Lee EJ, Min KH, Hur GY, Lee SH, Lee SY, Kim JH, Shin C, Shim JJ, In KH, et al. Quercetin enhances chemosensitivity to gemcitabine in lung cancer cells by inhibiting heat shock protein 70 expression. Clin Lung Cancer. 2018;16:e235–43. doi: 10.1016/j.cllc.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 85.Brian H, Graham PA. Molecular chaperones and protein‐folding catalysts as intercellular signaling regulators in immunity and inflammation. J Leukoc Biol. 2010;88:445–462. doi: 10.1189/jlb.1209779. [DOI] [PubMed] [Google Scholar]
- 86.Multhoff G, Pockley AG, Gaipl CS, US . Dual role of Heat Shock Proteins (HSPs) in anti-tumor immunity. Curr Mol Med. 2012;12:1174–1182. doi: 10.2174/156652412803306666. [DOI] [PubMed] [Google Scholar]
- 87.Shevtsov M, Multhoff G. Heat shock protein–peptide and HSP-based immunotherapies for the treatment of cancer. Front Immunol. 2016;7:171. doi: 10.3389/fimmu.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eer G, Kampinga HH, Laumbacher B, Johnson J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 2018;27:1627–1636. doi: 10.1016/S0301-472X(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 89.Ito A, Matsuoka F, Honda H, Kobayashi T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother. 2004;53:26–32. doi: 10.1007/s00262-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shevtsov MA, Nikolaev BP, Yakovleva LY, Parr MA, Marchenko YY, Eliseev I, Yudenko A, Dobrodumov AV, Zlobina O, Zhakhov A, et al. 70-kDa heat shock protein coated magnetic nanocarriers as a nanovaccine for induction of anti-tumor immune response in experimental glioma. J Control Release. 2015;220:329–340. doi: 10.1016/j.jconrel.2015.10.051. [DOI] [PubMed] [Google Scholar]
- 91.Li J-L, Liu H-L, Zhang X-R, Xu J-P, Hu W-K, Liang M, Chen S-Y, Hu F, Chu D-T. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2008;16:376. doi: 10.1038/gt.2008.179. [DOI] [PubMed] [Google Scholar]
- 92.Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin Exp Immunol. 2000;121:216–225. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zong J, Wang C, Liu B, Liu M, Cao Y, Sun X, Yao Y, Sun G. Human hsp70 and HPV16 oE7 fusion protein vaccine induces an effective antitumor efficacy. Oncol Rep. 2013;30:407–412. doi: 10.3892/or.2013.2445. [DOI] [PubMed] [Google Scholar]
- 94.Manjili MH, Wang X-Y, Chen X, Martin T, Repasky EA, Henderson R, Subjeck JR. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol. 2003;171:4054–4061. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 95.Ciocca DR, Frayssinet P, Cuello-Carrión FD. A pilot study with a therapeutic vaccine based on hydroxyapatite ceramic particles and self-antigens in cancer patients. Cell Stress Chaperones. 2007;12:33–43. doi: 10.1379/CSC-218R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bloch O, Crane CA, Fuks Y, Kaur R, Aghi MK, Berger MS, Butowski NA, Chang SM, Clarke JL, McDermott MW, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16:274–279. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Enomoto Y, Bharti A, Khaleque AA, Song B, Liu C, Apostolopoulos V, Xing P, Calderwood SK, Gong J. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell-tumor fusion cells. J Immunol. 2006;177:5946 LP– 5955. doi: 10.4049/jimmunol.177.9.5946. [DOI] [PubMed] [Google Scholar]
- 98.Desheng W, Baizheng S, John D, Valerie S, Zhengrong W, Shigeo KCS, Jianlin G. Induction of cytotoxic T lymphocytes against ovarian cancer‐initiating cells. Int J Cancer. 2011;129:1990–2001. doi: 10.1002/ijc.25851. [DOI] [PubMed] [Google Scholar]
- 99.Weng D, Song B, Koido S, Calderwood SK, Gong J. Immunotherapy of radioresistant mammary tumors with early metastasis using molecular chaperone vaccines combined with ionizing radiation. J Immunol. 2013;191:755–763. doi: 10.4049/jimmunol.1203286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maeda Y, Yoshimura K, Matsui H, Shindo Y, Tamesa T, Tokumitsu Y, Hashimoto N, Tokuhisa Y, Sakamoto K, Sakai K, et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: a phase 1 dose escalation clinical trial. Cancer Immunol Immunother. 2015;64:1047–1056. doi: 10.1007/s00262-015-1709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


