ABSTRACT
Drug resistance limits the efficacy of chemotherapy in human cancers. Previous studies reported that long noncoding RNA colon cancer-associated transcript 1 (CCAT1) regulated progression of prostate cancer (PCa). However, the potential role of CCAT1 in the sensitivity of paclitaxel (PTX) in PCa and its mechanism remain largely unknown. The PTX-resistant PCa cells were established in PC3 and DU145 cells by increasing concentrations of PTX. The expressions of CCAT1, microRNA-24-3p (miR-24-3p) and fascin1 (FSCN1) were measured by quantitative real-time polymerase chain reaction. The viability and apoptosis were detected by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide assay, flow cytometry and western blot, respectively. The interaction among CCAT1, miR-24-3p and FSCN1 was explored by luciferase activity, RNA immunoprecipitation, RNA pull-down and western blot, respectively. Results showed that the expressions of CCAT1 were up-regulated and miR-24-3p was down-regulated in PCa and PTX-resistant PCa cells (PC3-TXR and DU145-TXR). Knockdown of CCAT1 or overexpression of miR-24-3p inhibited survival rate, half maximal inhibitory concentration (IC50) of PTX but increased apoptosis in PC3-TXR and DU145-TXR cells after treatment of PTX. miR-24-3p was bound to CCAT1 and its abrogation reversed knockdown of CCAT1-mediated increase of PTX sensitivity in PC3-TXR and DU145-TXR cells. Moreover, FSCN1 restoration attenuated miR-24-3p-mediated inhibition of PTX resistance. Besides, FSCN1 level was enhanced in PCa and PTX-resistant PCa cells and regulated by CCAT1 and miR-24-3p. Our data suggested interference of CCAT1 contributed to PTX sensitivity in PCa by regulating miR-24-3p and FSCN1, indicating a novel avenue for treatment of PCa through regulating chemoresistance.
KEYWORDS: Prostate cancer, CCAT1, miR-24-3p, FSCN1, paclitaxel sensitivity
Introduction
Prostate cancer (PCa) is one of the most common malignancies in men with the cause of 1-2% deaths worldwide.1 Despite great advance in the diagnosis and treatment of PCa, the morbidity is still increasing.2 The development of resistance is regarded as a main factor of limiting the efficacy of chemotherapy in PCa.3 Paclitaxel (PTX) is one of the major taxane-type anti-cancer drugs, which has been widely applied to chemotherapy for many human cancers, including PCa. Previous study has reported that the combined therapy is beneficial to reduce toxicity and improve the efficacy of PTX in cancer therapy.4 However, there is still an urgent need to explore novel avenue to attenuate the chemoresistance in human cancers.
Non-coding RNAs, including long non-coding RNAs (lncRNAs) and microRNAs (miRNAs), have been suggested to play essential roles in endocrine-related cancers, like breast, prostate, endometrial and thyroid cancers.5 Thereinto, lncRNAs are a class of functional RNAs with more than 200 nucleotides in length, which have been indicated as promising biomarkers for development, diagnosis, prognosis as well as drug-resistance in PCa.6,7 LncRNA colon cancer-associated transcript 1 (CCAT1) plays an important role in the prognosis and diagnosis of human cancers through regulating proliferation, migration, cell cycle progress, chemoresistance and other processes.8 Moreover, a number of investigators have demonstrated CCAT1 as an oncogene in multiple cancers, such as gastric adenocarcinoma, ovarian cancer, nasopharyngeal carcinoma and lung adenocarcinoma.9–12 Notably, Chen et al.13 have reported that CCAT1 contributes to migration and invasion in PCa cells. However, the role of CCAT1 in PTX sensitivity and its potential mechanism remain largely unknown.
miRNAs of 18–22 nucleotides in length lead to inhibition of target mRNAs by binding to their 3ʹ-untranslated regions (3ʹ-UTR), which have been suggested to interact with drug resistance in PCa.14 miR-24-3p, as a novel miRNA, serves as oncogene or tumor suppressor in varying human cancers, such as lung adenocarcinoma, hepatocellular carcinoma and colorectal cancer.15–17 Moreover, miR-24-3p is reported to be dysregulated in PCa tissues.18 While the evidence is limited in support of the interaction between miR-24-3p and chemoresistance in PCa. Fascins are a class of actin-binding proteins, which have pivotal impact on health and diseases.19 Among this family, fascin1 (FSCN1) has been emerging as a promising therapeutic target for carcinoma. Accruing works have suggested FSCN1 as an oncogene to promote tumorigenesis in PCa.20,21 Here we hypothesized miR-24-3p and FSCN1 might be implicated in the regulatory network of CCAT1 in PCa. In this study, we sought to investigate the effect of CCAT1 on PTX sensitivity and explore the potential interaction among CCAT1, miR-24-3p and FSCN1 in PCa cells.
Materials and methods
Clinical samples
A total of 30 PCa patients were recruited from Luoyang Central Hospital Affiliated to Zhengzhou University. The paired tumor tissues and corresponding adjacent normal samples were collected and stored at −80°C until used. The written informed consent was obtained from all participants in this research, which was permitted by the Ethics Committee of Luoyang Central Hospital Affiliated to Zhengzhou University.
Cell culture and treatment
The normal human prostate epithelial cells RWPE-1 and PCa cell lines (PC3 and DU145) were purchased from American Tissue Culture Collection (Manassas, VA, USA). All cells were cultured in Dulbecco’s Modified Eagle Medium (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco) at 37°C and 5% CO2. To construct PTX-resistant PCa cells (PC3-TXR and DU145-TXR), PC3 and DU145 cells were incubated with stepwise increased concentrations of PTX (Selleck, Houston, TX, USA) according to the previous report.22 PC3-TXR and DU145-TXR cells were cultured in normal medium with 10 nM PTX.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissues or cells using TRIzol reagent (Invitrogen) and reverse transcribed to cDNA by Taqman Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s instructions. Subsequently, cDNA was amplified with SYBR green (Applied Biosystems) using the ABI 7300 system (Applied Biosystems). The relative expressions of CCAT1, FSCN1 and miR-24-3p were measured with GAPDH or U6 small RNA as internal control using 2−ΔΔCt method.23 The primers were listed as follows: CCAT1 (Forward, 5ʹ-TCATTACCAGCTGCCGTGTT-3ʹ; Reverse, 5ʹ-TCATGTCTCGGCACCTTTCC-3ʹ), FSCN1 (Forward, 5ʹ-CTGGCTACACGCTGGAGTTC-3ʹ; Reverse, 5ʹ-CTGAGTCCCCTGCTGTCTCCT-3ʹ), miR-24-3p (Forward, 5ʹ-GATCCTGGCTCAGTTCAGCAGGAACAGC-3ʹ; Reverse, 5ʹ-TCGAGCTGTTCCTGCTGAACTGAGCCAG-3ʹ), U6 (Forward, 5ʹ-GCTTCGGCAGCACATATACTAAAAT-3ʹ; Reverse, 5ʹ-CGCTTCACGAATTTGCGTGTCAT-3ʹ), GAPDH (Forward, 5ʹ-CACGATGGAGGGGCCGGACTCATC-3ʹ; Reverse, 5ʹ-TAAAGACCTCTATGCCAACACAGT-3ʹ).
Cell transfection
Small interfering RNA (siRNA) against CCAT1 (siCCAT1), siRNA negative control (scramble), CCAT1 overexpression vector (CCAT1), FSCN1 overexpression vector (FSCN1), pcDNA empty vector (vector), miR-24-3p mimic (miR-24-3p), mimic negative control (NC), miR-24-3p inhibitor (anti-miR-24-3p) and inhibitor negative control (anti-NC) were synthesized by Genepharma (Shanghai, China). PC3-TXR and DU145-TXR cells were transfected with these oligonucleotides or vectors for 24 h by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Cell viability
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay was used to detect cell viability. PC3-TXR and DU145-TXR cells were seeded into 96-well plates at a density of 1 × 104 cells per well and treated with different concentrations of PTX for 24 h. Each sample was prepared in triplicate. Then, 10 μl MTT solution (5 mg/ml; Sigma, St. Louis, MO, USA) was added into each well and interacted with cells for another 4 h. Subsequently, 100 μl DMSO was added to solubilize the formazan after the removal of supernatant. The absorbance at 490 nm was measured using a microplate reader (Bio-Rad, Hercules, CA, USA). The relative survival rate was normalized to control group and the 50% survival rate group was regarded as half maximal inhibitory concentration (IC50) of PTX.
Colony formation
PC3, DU145, PC3-TXR and DU145-TXR cells were treated via different doses of radiation (0, 2, 4 and 8 Gy) using the x-ray apparatus (Rad Source Technologies, Alpharetta, GA, USA) with a dose rate of 200 cGy/min. After radiation for 24 h, cells were plated into 12-well plates with a density of 500 cells/well. After culture for 2 weeks, cells were fixed with methanol (Sigma) and then stained with 0.01% crystal violet (Sigma). The colony formation was observed under a microscope (Olympus, Tokyo, Japan). The survival fraction was calculated as number of colonies/number of cells plated by normalizing to the control cells.
Cell apoptosis
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Solarbio, Beijing, China) was used for cell apoptosis analysis via flow cytometry. After the transfection, PC3-TXR and DU145-TXR cells were exposed with 100 nM PTX for 24 h. The sample of each group was prepared in triplicate. At the ending point, cells were washed with PBS and then resuspended in binding buffer, followed by stained with 5 μl Annexin V-FITC for 10 min and 5 μl PI for 5 min in the dark. The apoptotic cells were analyzed by using a flow cytometer (Becton Dickinson, San Jose, CA, USA).
Western blot
Transfected PC3-TXR and DU145-TXR cells were treated with 100 nM PTX for 24 h and then collected and lysed with RIPA lysis buffer (Beyotime Biotech, Shanghai, China). The total proteins were obtained from supernatant via centrifugation at 12,000 g for 5 min and quantified by using a BCA protein assay kit (Thermo Fisher). Equal amounts of proteins (30 μg) were boiled at 100°C for 5 min and then separated via SDS-PAGE gel electrophoresis. After the electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) and blocked with 5% nonfat milk for 1 h at room temperature. Subsequently, the membranes were incubated overnight at 4°C with primary antibodies against BCL-2 (ab196495; Abcam, Cambridge, UK), BCL-XL (ab98143; Abcam), MCL-1(ab28147; Abcam), AKT (ab18785; Abcam), phosphorylated AKT (p-AKT) T308 (ab38449; Abcam), p-AKT S473 (ab81283; Abcam), mTOR (ab2732; Abcam), p-mTOR S2448 (ab131538; Abcam), S6K (ab9366; Abcam), p-S6K T389 (ab60948; Abcam), ERK1/2 (ab17942; Abcam), p-ERK1/2 T202/T185 (ab201015; Abcam), PTEN (ab137337; Abcam), Cleaved caspase 3 (C-caspase 3) (#9664; Cell Signaling Technology, Danvers, MA, USA), FSCN1 (#9269; Cell Signaling Technology) or GAPDH (#5174; Cell Signaling Technology) and then interacted for 2 h at room temperature with horseradish peroxidase-conjugated secondary antibody (#5127; Cell Signaling Technology). The protein signaling was visualized using enhanced chemiluminescence chromogenic substrate (Beyotime Biotech) and analyzed with GAPDH as loading control.
Luciferase activity assay
The putative binding sites of miR-24-3p and CCAT1 or FSCN1 were predicted by starBase and TargetScan online. The 3ʹ-UTR sequences of CCAT1 or FSCN1 carrying wild-type (wt) or mutant (mut) binding sites of miR-24-3p were amplified and cloned into the pmirGLO vectors (Promega, Madison, WI, USA) to obtain luciferase reporter vectors (CCAT1-wt, CCAT1-mut, FSCN1-wt or FSCN1-mut). PC3-TXR and DU145-TXR cells were co-transfected with CCAT1-wt, CCAT1-mut, FSCN1-wt or FSCN1-mut and miR-24-3p or NC using Lipofectamine 2000 according to the manufacturer’s protocols. After the transfection for 48 h, luciferase activity was measured with a luciferase assay kit (Promega) according to the manufacturer’s instructions.
RNA immunoprecipitation (RIP)
RIP assay was conducted by using Magna RNA immunoprecipitation kit (Millipore) according to the manufacturer’s protocols. In brief, PC3-TXR and DU145-TXR cells transfected with miR-24-3p or NC interacted with RIP immunoprecipitation buffer containing magnetic beads bound with Ago2 or IgG antibody. The enrichment of CCAT1 or FSCN1 was measured by qRT-PCR.
RNA pull-down assay
RNA-Protein Pull-Down Kit (Thermo Fisher, Wilmington, DE, USA) was used for RNA pull-down analysis in PC3-TXR and DU145-TXR cells. Cells were transfected with biotinylated miR-24-3p or NC for 48 h and incubated with lysis buffer. The cell lysates were incubated with streptavidin agarose beads (Invitrogen) for 3 h on ice, and then eluted with elution buffer. The complex was purified by TRIzol and used for measurement of CCAT1 level by qRT-PCR.
Statistical analysis
Data of each group were expressed as the mean ± standard deviation (S.D.) from three independent experiments. The statistical analysis was conducted by student’s t test or one-way analysis of variance (ANOVA) using GraphPad Prism 7 (GraphPad Inc., La Jolla, CA, USA). The difference was regarded as statistically significant when the P value was less than 0.05.
Results
CCAT1 is upregulated in PCa and PTX-resistant PCa cells
To explore the potential role of CCAT1 in PCa, its abundance was first measured in PCa tissues and cells. Compared with that in corresponding normal group, the expression of CCAT1 was abnormally enhanced in PCa tissues (Figure 1a). Similarly, high expression of CCAT1 was also displayed in PC3 and DU145 cells compared with that in RWPE-1 cells (Figure 1b,c). To investigate the correlation of CCAT1 and cancer resistance, the PTX-resistant PCa cells were constructed. Results showed that PC3-TXR and DU145-TXR cells showed higher level of CCAT1 than normal PC3 or DU145 cells, respectively (Figure 1b,c). To explore whether the PTX-resistant cells have the resistance to radiation, the cells were exposed to different doses of radiation. Results showed that PC3-TXR and DU145-TXR cells had higher resistance to radiation than PC3 and DU145 cells (Figure 1d). Moreover, BCL-2, BCL-XL and MCL-1 proteins were reported to be associated with the drug resistance. As shown in Figure 1e, the protein levels of BCL-2, BCL-XL and MCL-1 were enhanced in PTX-resistant cells compared with those in sensitive cells. Furthermore, the data of western blot displayed that PTEN protein was expressed in DU145 cells but not in PC3 cells (figure 1f). Besides, the AKT/mTOR and ERK pathways were activated in the resistant cells, revealed by the phosphorylation of AKT, mTOR, S6K and ERK1/2 (figure 1f).
Figure 1.
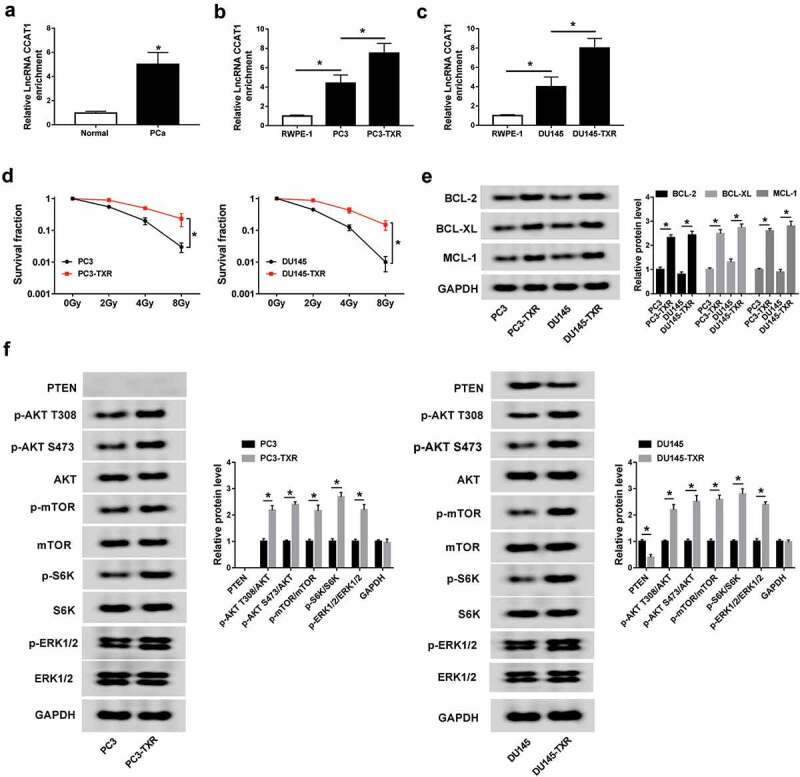
The expression of CCAT1 is enhanced in PCa and PTX-resistant PCa cells.
(a) The expression of CCAT1 was measured in PCa tissues and normal samples by qRT-PCR. (b and c) The level of CCAT1 was detected in PCa and PTX-resistant PCa cells by qRT-PCR. (d) The survival fraction of PCa cells and PTX-resistant cells was measured after the treatment of radiation by colony formation assay. (e) The expression levels of BCL-2, BCL-XL and MCL-1 protein were measured in PCa cells and PTX-resistant cells by western blot. (f) The abundances of proteins associated with AKT/mTOR and ERK pathways were measured in PCa cells and PTX-resistant cells by western blot. *P < .05.
Knockdown of CCAT1 suppresses PTX resistance in PTX-resistant PCa cells
To evaluate the effect of CCAT1 on chemoresistance in PCa, PC3-TXR and DU145-TXR cells were transfected with siCCAT1 or scramble and then treated with PTX for 24 h. As a result, the abundance of CCAT1 was effectively reduced in PC3-TXR and DU145-TXR cells transfected with siCCAT1 compared with that in scramble group (Figure 2a). After different concentrations of PTX treatment, down-regulation of CCAT1 significantly suppressed the survival rate and IC50 of PTX in PC3-TXR and DU145-TXR cells (Figure 2b). Moreover, cell apoptosis and viability were measured in PC3-TXR and DU145-TXR cells after the treatment of 100 nM PTX for 24 h. Annexin V-FITC/PI staining assay revealed that inhibition of CCAT1 led to strong increase of apoptosis in PTX-treated PC3-TXR and DU145-TXR cells (Figure 2c). However, knockdown of CCAT1 caused an opposite effect on cell viability in PC3-TXR and DU145-TXR cells after exposure of PTX (Figure 2d). In addition, the protein level of C-caspase 3 was markedly elevated in PC3-TXR and DU145-TXR cells transfected with siCCAT1 compared with that in the scramble group after treatment of PTX (Figure 2e). Besides, knockdown of CCAT1 significantly decreased the protein expression of BCL-2, BCL-XL and MCL-1 in PC3-TXR and DU145-TXR cells (figure 2f).
Figure 2.
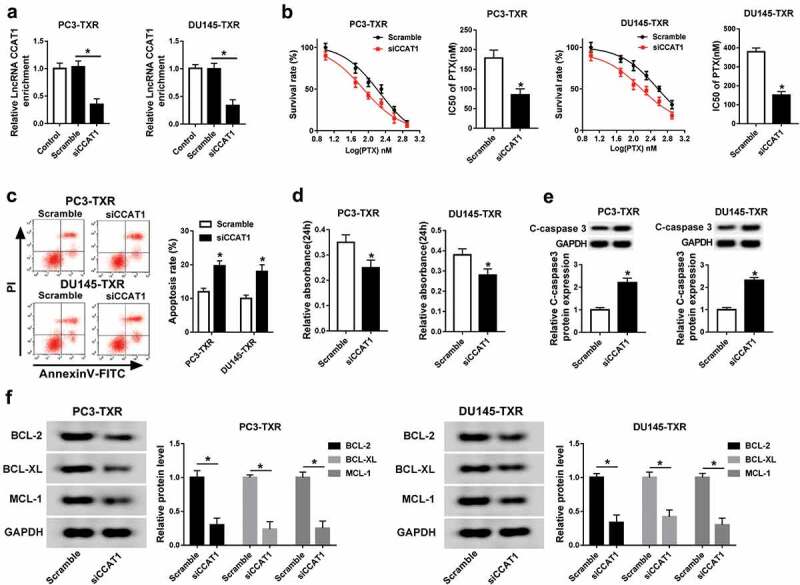
Interference of CCAT1 suppresses the resistance of PTX in PTX-resistant PCa cells.
(a) The expression of CCAT1 was measured in PC3-TXR and DU-145-TXR cells transfected with siCCAT1 or scramble by qRT-PCR. The survival rate, IC50 of PTX (b), apoptosis (c), viability (d), C-caspase 3 expression (e) and protein levels of BCL-2, BCL-XL and MCL-1 (f) were detected in PC3-TXR and DU-145-TXR cells transfected with siCCAT1 or scramble after treatment of PTX for 24 h by MTT assay, flow cytometry and western blot assays, respectively. *P < .05.
Addition of mir-24-3p enhances PTX sensitivity in PTX-resistant PCa cells
To explore the role of miR-24-3p in PCa, the expression of miR-24-3p was detected in PCa tissues and cells. the qRT-PCR analysis demonstrated low expression of miR-24-3p in PCa tissues and cells compared with that in their corresponding control group (Figure 3a–c). Notably, lower abundance of miR-24-3p was showed in PC3-TXR and DU145-TXR cells than that in PC3 and DU145 cells (Figure 3b,c). In order to investigate the effect of miR-24-3p on chemosensitivity in PCa, PC3-TXR and DU145-TXR cells were transfected with miR-24-3p or NC and then treated with PTX for 24 h. After the transfection, the level of miR-24-3p was greatly increased in PC3-TXR and DU145-TXR cells transfected with miR-24-3p compared with that in the NC group (Figure 3d). The survival rate of PC3-TXR and DU145-TXR cells was obviously inhibited in the miR-24-3p group compared with that in the NC group after treatment of different concentrations of PTX (Figure 3e). Meanwhile, overexpression of miR-24-3p significantly impeded the IC50 of PTX in PC3-TXR and DU145-TXR cells (Figure 3e). Besides, after treatment of 100 nM PTX for 24 h, abundant accumulation of miR-24-3p resulted in a strong increase of apoptosis and C-caspase 3 expression and reduction of viability in PTX-treated PC3-TXR and DU145-TXR cells (figure 3f–h).
Figure 3.
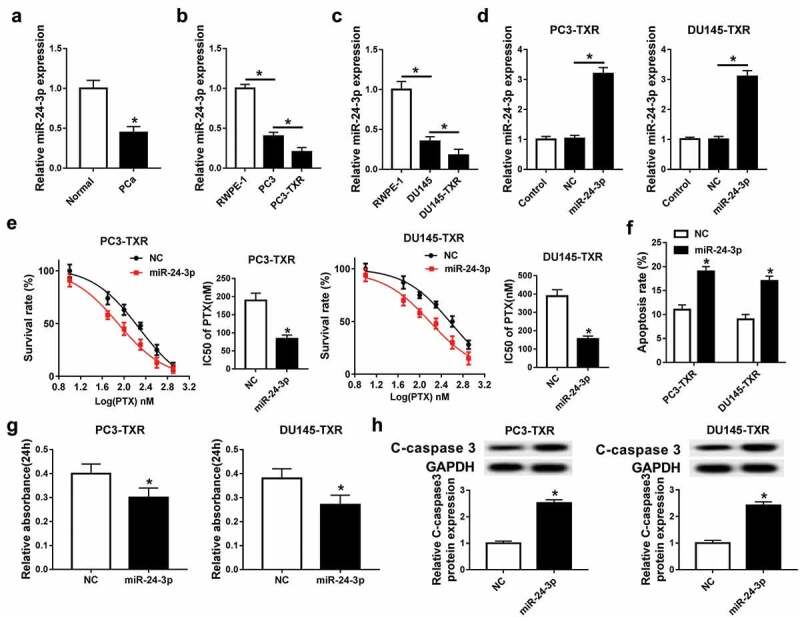
Overexpression of miR-24-3p inhibits the resistance of PTX in PTX-resistant PCa cells.
(a) The level of miR-24-3p was measured in PCa tissues and normal samples by qRT-PCR. (b and c) The abundance of miR-24-3p was detected in PCa and PTX-resistant PCa cells by qRT-PCR. (d) The expression of miR-24-3p was measured in PC3-TXR and DU-145-TXR cells transfected with miR-24-3p or NC by qRT-PCR. The survival rate, IC50 of PTX (e), apoptosis (f), viability (g) and C-caspase 3 expression (h) were examined in PC3-TXR and DU-145-TXR cells transfected with miR-24-3p or NC by MTT assay, flow cytometry and western blot assays, respectively. *P < .05.
miR-24-3p is bound to CCAT1
Having established the view of the function of CCAT1 and miR-24-3p, the potential association of CCAT1 and miR-24-3p was explored in PC3-TXR and DU145-TXR cells. To validate the potential interaction between CCAT1 and miR-24-3p, we probed the putative binding sites of CCAT1 and miR-24-3p by starBase and constructed the wt or mut luciferase reporter vector (Figure 4a). Luciferase reporter assay showed that luciferase activity was evidently decreased in PC3-TXR and DU145-TXR cells transfected with miR-24-3p compared with that in the treatment of NC in CCAT1-wt group, while it was not affected in CCAT1-mut group (Figure 4b). Moreover, overexpression of miR-24-3p led to more enrichment of CCAT1 in PC3-TXR and DU145-TXR cells after Ago2 RIP (Figure 4c). Similarly, RNA pull-down experiment revealed that the addition of bio-miR-24-3p induced more enrichment of CCAT1 in PC3-TXR and DU145-TXR cells than treatment of bio-NC (Figure 4d). Subsequently, the effect of CCAT1 on miR-24-3p expression was analyzed in PC3-TXR and DU145-TXR cells transfected with CCAT1, vector, siCCAT1 or scramble. Results exhibited that the abundance of miR-24-3p was obviously inhibited by the addition of CCAT1 and enhanced by the silencing of CCAT1 compared with their corresponding control (Figure 4e).
Figure 4.
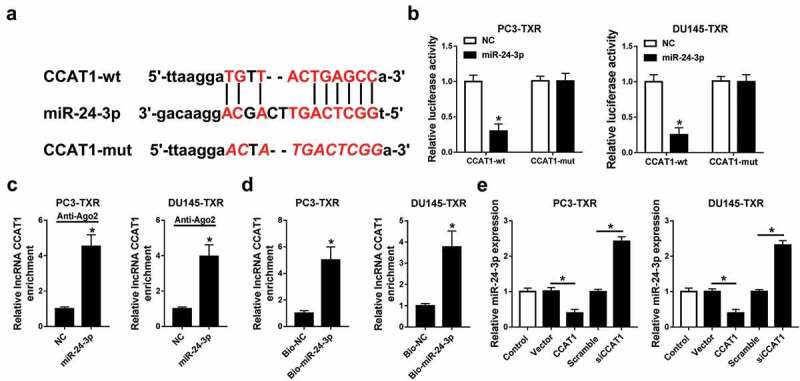
miR-24-3p is bound to CCAT1.
(a) The potential binding sites of CCAT1 and miR-24-3p were predicted by starBase. (b) Luciferase activity was measured in PC3-TXR and DU-145-TXR cells co-transfected with CCAT1-wt or CCAT1-mut and miR-24-3p or NC. (c and d) The enrichment of CCAT1 was detected in PC3-TXR and DU-145-TXR cells by RIP or RNA pull-down assay. (e) The abundance of miR-24-3p was measured in PC3-TXR and DU-145-TXR cells transfected with CCAT1, vector, siCCAT1 or scramble by qRT-PCR. *P < .05.
Knockdown of miR-24-3p reverses interference of CCAT1-mediated suppression of PTX resistance in PTX-resistant PCa cells
To further validate whether miR-24-3p is involved in CCAT1-addressed chemoresistance in PCa, PC3-TXR and DU145-TXR cells were co-transfected with siCCAT1 and anti-miR-24-3p or anti-NC and then exposed with PTX for 24 h. After the transfection, the expression level of miR-24-3p was evidently decreased in PC3-TXR and DU145-TXR cells transfected with siCCAT1 and anti-miR-24-3p compared within siCCAT1 and anti-NC group (Figure 5a). MTT assay showed that down-regulation of miR-24-3p attenuated knockdown of CCAT1-mediated inhibition of survival rate and IC50 of PTX in PC3-TXR and DU145-TXR cells after different concentrations of PTX treatment for 24 h (Figure 5b). Furthermore, deficiency of miR-24-3p relieved the regulatory effect of CCAT1 silencing on apoptosis, viability and C-caspase 3 level in PC3-TXR and DU145-TXR cells after 100 nM PTX treatment for 24 h (Figure 5c–e).
Figure 5.
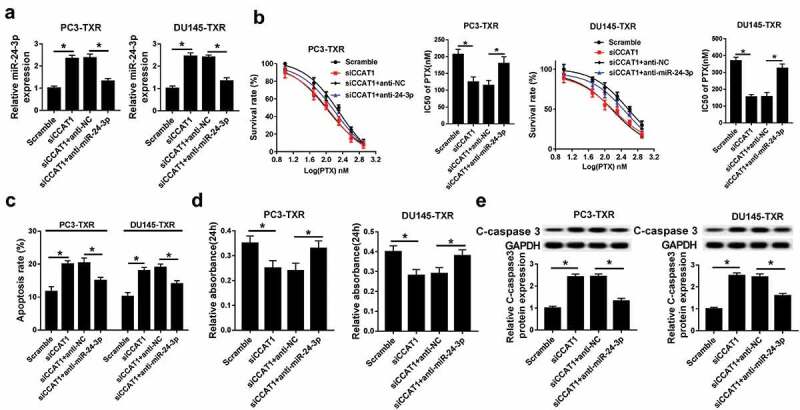
CCAT1 regulates PTX sensitivity by sponging miR-24-3p in PTX-resistant PCa cells.
(a) The expression of miR-24-3p was measured in PC3-TXR and DU-145-TXR cells co-transfected with siCCAT1 and anti-miR-24-3p or anti-NC. The survival rate, IC50 of PTX (b), apoptosis (c), viability (d) and C-caspase 3 expression (e) were detected in PC3-TXR and DU-145-TXR cells co-transfected with siCCAT1 and anti-miR-24-3p or anti-NC by MTT, flow cytometry and western blot assays, respectively. *P < .05.
FSCN1 is a target of miR-24-3p
To explore the underlying mechanism allows miR-24-3p participating in PCa chemoresistance, the potential targets of miR-24-3p were analyzed by TargetScan online. The data from bioinformatics analysis described the putative binding sites of miR-24-3p and FSCN1 (Figure 6a). In turn, luciferase activity and RIP assays were used to identify this prediction. Addition of miR-24-3p led to a great loss of luciferase activity of PC3-TXR and DU145-TXR cells in the FSCN1-wt group, whereas its efficacy was lost in response to FSCN1-mut group (Figure 6b). Moreover, overexpression of miR-24-3p resulted in more enrichment of FSCN1 in PC3-TXR and DU145-TXR cells after Ago2 RIP, while it failed to show any efficacy of enrichment in IgG RIP group (Figure 6c). Besides, the effect of miR-24-3p on FSCN1 protein level was evaluated in PC3-TXR and DU145-TXR cells. Western blot analysis revealed that the protein expression of FSCN1 was significantly inhibited by overexpression of miR-24-3p and increased by inhibition of miR-24-3p in PC3-TXR and DU145-TXR cells (Figure 6d).
Figure 6.
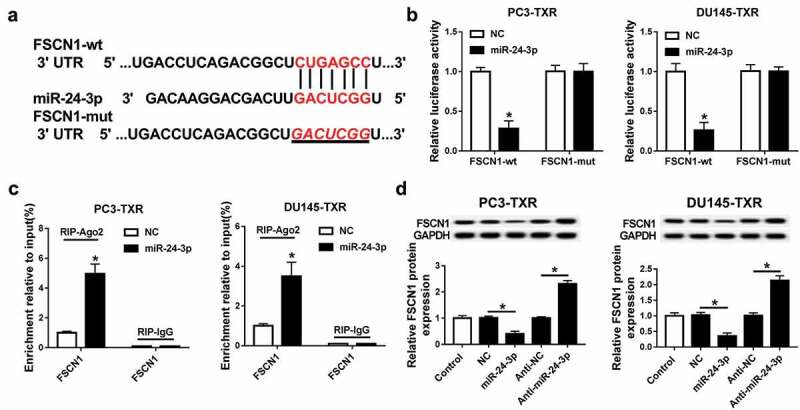
FSCN1 is a target of miR-24-3p.
(a) The putative binding sites of miR-24-3p and FSCN1 were provided by TargetScan. (b and c) The association between miR-24-3p and FSCN1 was analyzed by luciferase activity and RIP assays. (d) The abundance of FSCN1 protein was measured in PC3-TXR and DU-145-TXR cells transfected with miR-24-3p, NC, anti-miR-24-3p or anti-NC by western blot. *P < .05.
Restoration of FSCN1 alleviates miR-24-3p-induced increase of PTX sensitivity in PTX-resistant PCa cells
The potential role of FSCN1 in miR-24-3p-mediated chemosensitivity was further explored in this study. In PCa tissues and cells, the expression of FSCN1 mRNA was robustly enhanced compared with that in the normal group (Figure 7a–c). Moreover, PTX-resistance cells showed higher abundance of FSCN1 mRNA (Figure 7b,c). To investigate whether FSCN1 is required for miR-24-3p-mediated PTX sensitivity, PC3-TXR and DU145-TXR cells were co-transfected with miR-24-3p and FSCN1 or vector and then incubated with PTX for 24 h. After the transfection, the protein level of FSCN1 was obviously recovered in PC3-TXR and DU145-TXR cells transfected with miR-24-3p and FSCN1 compared within miR-24-3p and vector group (Figure 7d). Rescue experiment demonstrated that the introduction of FSCN1 abated addition of miR-24-3p-mediated loss of survival rate and IC50 of PTX in PC3-TXR and DU145-TXR cells after exposure with varying concentrations of PTX for 24 h (Figure 7e). In addition, restoration of FSCN1 weakened the regulatory effect of miR-24-3p on apoptosis, viability and C-caspase 3 expression in PTX-treated PC3-TXR and DU145-TXR cells (figure 7f–h).
Figure 7.
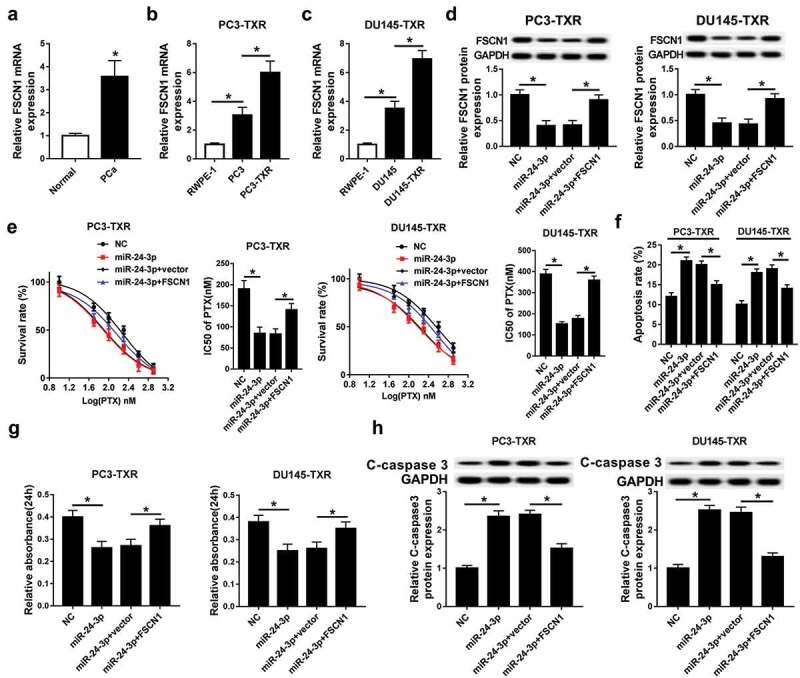
FSCN1 is required for miR-24-3p-mediated increase of PTX sensitivity in PTX-resistant PCa cells.
(a-c) The expression of FSCN1 mRNA was measured in PCa and PTX-resistant PCa cells by qRT-PCR. (d) The protein level of FSCN1 was detected in PC3-TXR and DU-145-TXR cells co-transfected with miR-24-3p and FSCN1 or vector by western blot. The survival rate, IC50 of PTX (e), apoptosis (f), viability (g) and C-caspase 3 expression (h) were analyzed in PC3-TXR and DU-145-TXR cells co-transfected with miR-24-3p and FSCN1 or vector by MTT, flow cytometry and western blot assays, respectively. *P < .05.
FSCN1 is regulated by CCAT1 and miR-24-3p
Seeing that ceRNA network is the main pathway in many conditions, we next want to evaluate whether CCAT1 may function as a competing endogenous RNA (ceRNA) of miR-24-3p to regulate FSCN1. To identify the promising hypothesis, PC3-TXR and DU145-TXR cells were transfected with scramble, siCCAT1, siCCAT1 and anti-NC or anti-miR-24-3p. Western blot analysis exhibited that knockdown of CCAT1 led to an obvious reduction of FSCN1 protein expression in PC3-TXR and DU145-TXR cells compared with scramble group, while abrogation of miR-24-3p significantly counteracted this effect (Figure 8).
Figure 8.
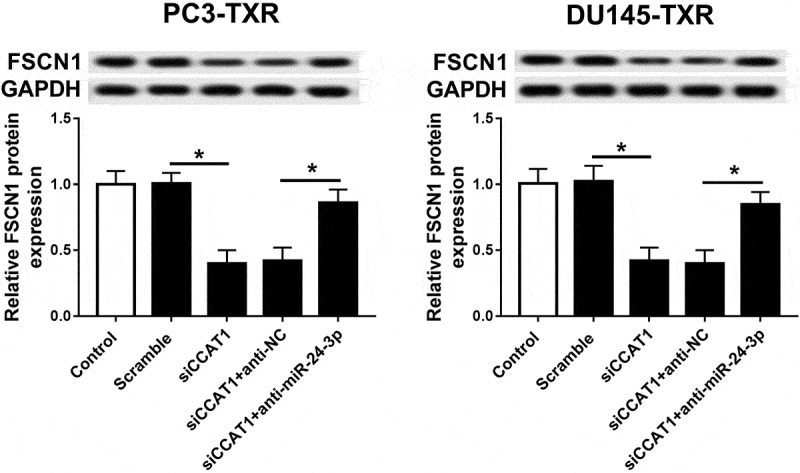
FSCN1 is regulated by CCAT1 and miR-24-3p.
The protein expression of FSCN1 was measured in PC3-TXR and DU-145-TXR cells transfected with scramble, si-CCAT1, si-CCAT1 and anti-NC or anti-miR-24-3p by western blot. *P < .05.
Discussion
In recent years, PCa chemoresistance and drug-related genes have gained so much attentions.24 The regulatory networks of lncRNA and miRNA have been reported as important targets for the progression and therapeutics of PCa.25 CCAT1 has been suggested to serve as an oncogenic lncRNA in the development of human cancers by various pathways.26 However, there is no direct evidence in support of the association of CCAT1 and PTX resistance in PCa. In this study, we first provided the view on the effect of CCAT1 on PTX sensitivity in PCa and explored the underlying mechanism.
This study showed that CCAT1 was highly expressed in PCa tissues and cells, which is also consistent with former work that suggested that CCAT1 expression was reduced in PCa tissues compared with that in adjacent tissues.13 Moreover, Chen et al.13 have revealed that knockdown of CCAT1 inhibited proliferation, migration and invasion in PCa cells. This suggested that CCAT1 might play as an oncogene in PCa development, while the effect of CCAT1 on PTX resistance in PCa remains poorly understood. Here we established two PTX-resistant PCa cells and found that the IC50 of PTX was lower in PC3-TXR than that in DU145-TXR cells. This suggested that DU145 cells exhibited more PTX resistance, which is also in agreement with previous study.22 Notably, PTX-resistance analysis provided that interference of CCAT1 decreased the IC50 of PTX and cell viability but increased apoptosis in PC3-TXR and DU145-TXR cells. Moreover, the previous study suggested that the Bcl-2 family was associated with chemo-sensitization in prostate cancer.27 Here we also found that the protein levels of Bcl-2, Bcl-xl and MCL-1 were increased in PTX-resistant cells, which was attenuated by CCAT1. These data uncovered that down-regulation of CCAT1 enhanced PTX sensitivity in PCa, which is similar to the increasing reports that elucidated CCAT1 promoted chemoresistance in other cancers, such as non-small-cell lung cancer, lung adenocarcinoma and nasopharynx cancers.28–30 To further explore the regulatory process of CCAT1 in PCa, the potential downstream targets were probed. Here we validated that miR-24-3p might be bound to CCAT1 by luciferase activity, RIP and RNA pull-down assays.
Compared with that in the normal group, the expression of miR-24-3p was decreased in PCa tissues and cells, which is also in agreement with former study.18 Moreover, our study revealed that PTX-resistant cells displayed lower abundance of miR-24-3p. The available evidences have indicated that miR-24-3p knockdown might contribute to chemoresistance in head and neck squamous cell carcinoma and small-cell lung cancer.31,32 Similarly, in vitro experiments also demonstrated that miR-24-3p blocked the PTX resistance in PCa, reflected by overexpression of miR-24-3p led to inhibition of IC50 of PTX and cell viability as well as increase of apoptosis in PTX-treated PCa cells. Furthermore, we found that miR-24-3p expression was negatively regulated by CCAT1 and its exhaustion relieved the suppressive effect of CCAT1 knockdown on PTX resistance, indicating that CCAT1 regulated PTX resistance in PCa by sponging miR-24-3p. Functional miRNA is known to regulate potential targets in many conditions. Li et al.33 have reported that miR-24 inhibited cell proliferation, migration, invasion and tumor growth by targeting FSCN1 in nasopharyngeal carcinoma. Thus, this work identified FSCN1 as a functional target of miR-24-3p in PCa cells by luciferase activity and RIP analyses.
FSCN1 has been reported to be associated with the increasing risk of progression of cancers.34 Moreover, FSCN1 was regarded as an oncogene in PCa progression. For example, Xu et al.20 reported that high expression of FSCN1 was positively associated with proliferation, migration and invasion in PCa. Fuse et al.21 revealed that inhibition of FSCN1 suppressed cell growth, migration and invasion in PCa. In this study, we also revealed that FSCN1 mRNA level was elevated in PCa tissues and cells. Several such reports implicated that FSCN1 might contribute chemoresistance in some cancers.35,36 Nevertheless, the exact role of FSCN1 alone in chemoresistance in PCa was not investigated in the present study. To figure out whether FSCN1 was involved in miR-24-3p-mediated regulation of PTX sensitivity in PCa, the rescue experiments were conducted in PC3-TXR and DU145-TXR cells. Restoration of FSCN1 mitigated miR-24-3p-induced suppression of PTX resistance in PCa cells, which uncovered that miR-24-3p addressed PTX resistance in PCa by targeting FSCN1. What’s more, western blots analysis revealed that FSCN1 abundance was inhibited by knockdown of CCAT1 and rescued by the deficiency of miR-24-3p in PCa cells, which presented CCAT1 might act as a ceRNA of miR-24-3p to mediate FSCN1 level. Interestingly, we found that PC3-TXR cells had higher resistance to radiation than PC3 cells. We hypothesized that the development of radiation resistance in chemo-resistant cells might be induced due to the expression of some proteins associated with DNA repair. More details on the cross-resistance should be explored in future.
However, there are some limitations in the present study. First, it is expected for more clinical samples to explore the clinical significance of CCAT1 in PCa. Moreover, the pre-clinical experiments should be conducted for better understanding the role of CCAT1. In addition, we found that AKT/mTOR and ERK pathways were activated in the PTX-resistant cells, indicating that these pathways might be associated with the sensitivity of paclitaxel in prostate cancer. However, whether CCAT1/miR-24-3p/FSCN1 mediated these signaling remains further study in future.
In conclusion, the expressions of CCAT1 and FSCN1 were enhanced and miR-24-3p was reduced in PCa tissues and cells. miR-24-3p was bound to CCAT1 and its inhibition reversed knockdown of CCAT1-mediated promotion of PTX sensitivity in PC3-TXR and DU145-TXR cells. Moreover, FSCN1 was a target of miR-24-3p and its restoration abated miR-24-3p-mediated increase of PTX sensitivity in PC3-TXR and DU145-TXR cells. Besides, FSCN1 was positively regulated by CCAT1 and weakened by miR-24-3p. Collectively, the silencing of CCAT1 facilitated PTX sensitivity in PCa by functioning as a ceRNA for miR-24-3p to regulate FSCN1. This may indicate a promising strategy to mediating chemoresistance, leading to the improvement of treatment of PCa.
Supplementary Material
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental material
Supplemental data for this article can be accessed publisher’s website.
Ethical Statement
The written informed consent was obtained from all participants in this research, which was permitted by the Ethics Committee of Luoyang Central Hospital Affiliated to Zhengzhou University.
References
- 1.Attard G, Parker C, Eeles R, Schröder F, Tomlins S, Tannock I, Drake C, de Bono J.. 2016. Prostate cancer. Lancet. 387(10013):70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Tan HJ.. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532–2542. doi: 10.1001/jama.2017.7248. PMID:28655021. [DOI] [PubMed] [Google Scholar]
- 3.Wade CA, Kyprianou N. Profiling prostate cancer therapeutic resistance. Int J Mol Sci. 2018;19(3):E904. doi: 10.3390/ijms19030904. PMID:29562686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Y, Pu X, Zhao L. Preclinical studies for the combination of paclitaxel and curcumin in cancer therapy (Review). Oncol Rep. 2017;37(6):3159–3166. doi: 10.3892/or.2017.5593. PMID:28440434. [DOI] [PubMed] [Google Scholar]
- 5.Klinge CM. Non-coding RNAs: long non-coding RNAs and microRNAs in endocrine-related cancers. Endocr Relat Cancer. 2018;25(4):R259–R82. doi: 10.1530/ERC-17-0548. PMID:29440232. [DOI] [PubMed] [Google Scholar]
- 6.Xu T, Lin CM, Cheng SQ, Min J, Li L, Meng XM, Huang C, Zhang L, Deng ZY, Li J. Pathological bases and clinical impact of long noncoding RNAs in prostate cancer: a new budding star. Mol Cancer. 2018;17(1):103. doi: 10.1186/s12943-018-0852-7. PMID:30037351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolle MA, Bauernhofer T, Pummer K, Calin GA, Pichler M. Current insights into long non-coding RNAs (LncRNAs) in prostate cancer. Int J Mol Sci. 2017;18(2):E473. doi: 10.3390/ijms18020473. PMID:28241429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang N, Yu Y, Xu B, Zhang M, Li Q, Miao L. Pivotal prognostic and diagnostic role of the long noncoding RNA colon cancerassociated transcript 1 expression in human cancer (Review). Mol Med Rep. 2019;19(2):771–782. doi: 10.3892/mmr.2018.9721. PMID:30535444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang H, Liu HM, Wu WH, Liu H, Pan Y, Li WJ. Upregulation of long noncoding RNA CCAT1-L promotes epithelial-mesenchymal transition in gastric adenocarcinoma. Onco Targets Ther. 2018;11:5647–5655. doi: 10.2147/OTT.S170553. PMID:30254457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai XJ, Cheng HF. LncRNA colon cancer-associated transcript 1 (CCAT1) promotes proliferation and metastasis of ovarian cancer via miR-1290. Eur Rev Med Pharmacol Sci. 2018;22(2):322–328. doi: 10.26355/eurrev_201801_14175. PMID:29424889. [DOI] [PubMed] [Google Scholar]
- 11.Dong Y, Yuan H, Jin G. Identification of long non-coding RNA CCAT1 as an oncogene in nasopharyngeal carcinoma. Oncol Lett. 2018;16(2):2750–2756. doi: 10.3892/ol.2018.8969. PMID:30013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H, Cheng W, Yan H, Zhang X. Overexpression of the long noncoding RNA CCAT1 promotes metastasis via epithelial-to-mesenchymal transition in lung adenocarcinoma. Oncol Lett. 2018;16(2):1809–1814. doi: 10.3892/ol.2018.8813. PMID:30008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, He Y, Hou YS, Chen DQ, He SL, Cao YF, Wu XM. Long non-coding RNA CCAT1 promotes the migration and invasion of prostate cancer PC-3 cells. Eur Rev Med Pharmacol Sci. 2018;22(10):2991–2996. doi: 10.26355/eurrev_201805_15055. PMID:29863242. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Mahato RI. MicroRNAs and drug resistance in prostate cancers. Mol Pharm. 2014;11(8):2539–2552. doi: 10.1021/mp500099g. PMID:24742219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olbromski M, Rzechonek A, Grzegrzolka J, Glatzel-Plucinska N, Chachaj A, Werynska B, Podhorska-Okolow M, Dziegiel P. Influence of miR-7a and miR-24-3p on the SOX18 transcript in lung adenocarcinoma. Oncol Rep. 2018;39(1):201–208. doi: 10.3892/or.2017.6077. PMID:29115529. [DOI] [PubMed] [Google Scholar]
- 16.Fan JC, Zeng F, Le YG, Xin L. LncRNA CASC2 inhibited the viability and induced the apoptosis of hepatocellular carcinoma cells through regulating miR-24-3p. J Cell Biochem. 2018;119(8):6391–6397. doi: 10.1002/jcb.26479. PMID:29091305. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Liu Y, Du L, Li J, Qu A, Zhang X, Wang L, Wang C. Down-regulation of miR-24-3p in colorectal cancer is associated with malignant behavior. Med Oncol. 2015;32(1):362. doi: 10.1007/s12032-014-0362-4. PMID:25502080. [DOI] [PubMed] [Google Scholar]
- 18.Fredsoe J, Rasmussen AKI, Thomsen AR, Mouritzen P, Hoyer S, Borre M, Orntoft TF, Sorensen KD. Diagnostic and prognostic MicroRNA biomarkers for prostate cancer in cell-free urine. Eur Urol Focus. 2018;4(6):825–833. doi: 10.1016/j.euf.2017.02.018. PMID:28753866. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol. 2011;224(3):289–300. doi: 10.1002/path.2894. PMID:21618240. [DOI] [PubMed] [Google Scholar]
- 20.Xu W, Chang J, Du X, Hou J. Long non-coding RNA PCAT-1 contributes to tumorigenesis by regulating FSCN1 via miR-145-5p in prostate cancer. Biomed Pharmacother. 2017;95:1112–1118. doi: 10.1016/j.biopha.2017.09.019. PMID:28922730. [DOI] [PubMed] [Google Scholar]
- 21.Fuse M, Nohata N, Kojima S, Sakamoto S, Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, Naya Y, Ichikawa T, et al. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol. 2011;38(4):1093–1101. doi: 10.3892/ijo.2011.919. PMID:21258769. [DOI] [PubMed] [Google Scholar]
- 22.Takeda M, Mizokami A, Mamiya K, Li YQ, Zhang J, Keller ET, Namiki M. The establishment of two paclitaxel-resistant prostate cancer cell lines and the mechanisms of paclitaxel resistance with two cell lines. Prostate. 2007;67(9):955–967. doi: 10.1002/pros.20581. PMID:17440963. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. PMID:11846609. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Meng Y, Liu N, Wen XF, Yang T. Insights into chemoresistance of prostate cancer. Int J Biol Sci. 2015;11(10):1160–1170. doi: 10.7150/ijbs.11439. PMID:26327810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma G, Tang M, Wu Y, Xu X, Pan F, Xu R. LncRNAs and miRNAs: potential biomarkers and therapeutic targets for prostate cancer. Am J Transl Res. 2016;8(12):5141–5150. PMID:28077991. [PMC free article] [PubMed] [Google Scholar]
- 26.Guo X, Hua Y. CCAT1: an oncogenic long noncoding RNA in human cancers. J Cancer Res Clin Oncol. 2017;143(4):555–562. doi: 10.1007/s00432-016-2268-3. PMID:27638771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karnak D, Xu L. Chemosensitization of prostate cancer by modulating Bcl-2 family proteins. Curr Drug Targets. 2010;11(6):699–707. PMID: 20298153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu B, Zhang H, Wang Z, Zhang F, Wei H, Li L. LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance in non-small-cell lung cancer cell line by targeting SOX4. Cancer Biol Ther. 2017;18(12):974–983. doi: 10.1080/15384047.2017.1385679. PMID:29020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Zhang K, Song H, Wang R, Chu X, Chen L. Long noncoding RNA CCAT1 acts as an oncogene and promotes chemoresistance in docetaxel-resistant lung adenocarcinoma cells. Oncotarget. 2016;7(38):62474–62489. doi: 10.18632/oncotarget.11518. PMID:27566568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Zhang W, Hao S. LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle. 2017;16(8):795–801. doi: 10.1080/15384101.2017.1301334. PMID:28358263. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Sun X, Xiao D, Xu T, Yuan Y. miRNA-24-3p promotes cell proliferation and regulates chemosensitivity in head and neck squamous cell carcinoma by targeting CHD5. Future Oncol. 2016;12(23):2701–2712. doi: 10.2217/fon-2016-0179. PMID:27513190. [DOI] [PubMed] [Google Scholar]
- 32.Pan B, Chen Y, Song H, Xu Y, Wang R, Chen L. Mir-24-3p downregulation contributes to VP16-DDP resistance in small-cell lung cancer by targeting ATG4A. Oncotarget. 2015;6(1):317–331. doi: 10.18632/oncotarget.2787. PMID:25426560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li YQ, Lu JH, Bao XM, Wang XF, Wu JH, Hong WQ. MiR-24 functions as a tumor suppressor in nasopharyngeal carcinoma through targeting FSCN1. J Exp Clin Cancer Res. 2015;34:130. doi: 10.1186/s13046-015-0242-6. PMID:26503504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan VY, Lewis SJ, Adams JC, Martin RM. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: a systematic review and meta-analysis. BMC Med. 2013;11:52. doi: 10.1186/1741-7015-11-52. PMID:23442983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda O, Ando T, Ohmiya N, Ishiguro K, Watanabe O, Miyahara R, Hibi Y, Nagai T, Yamada K, Goto H. Alteration of gene expression and DNA methylation in drug-resistant gastric cancer. Oncol Rep. 2014;31(4):1883–1890. doi: 10.3892/or.2014.3014. PMID:24504010. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Lu Y, Zhang C, Huang D, Wu W, Zhang Y, Shen J, Cai Y, Chen W, Yao W. FSCN1 increases doxorubicin resistance in hepatocellular carcinoma through promotion of epithelial-mesenchymal transition. Int J Oncol. 2018;52(5):1455–1464. doi: 10.3892/ijo.2018.4327. PMID:29568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


