ABSTRACT
Up to now, no proven effective medical therapy for surgery and radiation-refractory anaplastic meningioma (AM) exists. Patients with vascular endothelial growth factor receptor 2 (VEGFR-2) positive meningiomas showed significantly shorter progression-free survival. Apatinib is a small-molecule antiangiogenic agent that selectively inhibits VEGFR-2. We report three cases of recurrent AM (VEGFR-2 positive) treated with apatinib. After apatinib treatment, the best outcome for all three patients was the partial response. The Progression-free survival was 17.3 months, 10.3 months, and 14+ months, respectively. The third patient lost follow-up after the last review. The overall survival was 28.5 months and 18 months, respectively. The main adverse events were hypertension, hand-foot syndrome, and myelosuppression. Apatinib is active in recurrent AM patients and this is the first report in the world. It is promising to launch a Phase II clinical trial of apatinib to further evaluate its efficacy on AM.
Background
Anaplastic meningioma (AM) are rare and aggressive tumors with high recurrence rates despite optimal surgical or medical management. Up to now, no proven effective medical therapy, surgery, or radiation-refractory for AM exist. The progression-free survival (PFS) of patients with vascular endothelial growth factor receptor 2 (VEGFR-2)-positive meningiomas was significantly low. Apatinib (YN968D1) is a small-molecule antiangiogenic agent that selectively inhibits VEGFR-2.
Case presentation
Case #1
A 47-year-old Asian female patient with malignant meningioma underwent four operations and three radiotherapies. She was given a 500 mg apatinib daily oral treatment, and the dosage was halved to 250 mg 3 months into the treatment. According to the Response Assessment in Neuro-Oncology (RANO) evaluation criteria, the best outcome during treatment was the partial response (PR) 6 months after the treatment. The PFS was 17.3 months, whereas the overall survival (OS) was 28.5 months. The best change in the Karnofsky performance scale (KPS) was a 10-point increase. The main adverse events included anemia (grade II), thrombocytopenia (grade II), and proteinuria (grade I).
Case #2
A 71-year-old Asian woman with AM underwent two operations and two gamma knife stereotactic radiotherapies. She was given a 500 mg apatinib daily oral treatment with a follow-up period of 18 months. apatinib was taken orally for 10 months. According to the RANO evaluation criteria, the best outcome during treatment was PR. The PFS was 10.3 months, whereas the OS was 18 months. The best change in KPS was a 20-point increase. The main adverse events included hypertension (grade II), hand–foot syndrome (grade II), and fecal ocular blood (grade II).
Case #3: A 16-year-old Asian girl with AM underwent two operations and two radiotherapies. She was given a 250 mg apatinib daily oral treatment with a follow-up period of 16 months. Apatinib was taken orally for 8 months. The patient did not follow-up after the last review of the brain-enhanced magnetic resonance imaging. According to the RANO evaluation criteria, the best outcome during treatment was PR. The PFS was 14+ months, and the best change in KPS was a 10-point increase. The main adverse events included hypertension (grade I) and hand–foot syndrome (grade I).
Conclusion
Apatinib is actively used in treating patients with recurrent AM. A randomized trial and phase II clinical trial of this inhibitor should be performed to further evaluate its efficacy in treating malignant meningioma.
KEYWORDS: Apatinib, anaplastic meningioma, VEGFR-2, recurrence
Background
Meningiomas account for 33.8% of all primary brain tumors and are therefore the most common type of primary tumor in the central nervous system.1 Approximately 80% of meningiomas are classified according to the WHO grading system as WHO grade I. Atypical WHO grade II meningiomas account for 5% to 15% of all meningiomas. Anaplastic or malignant meningiomas are classified as WHO grade III and account for 1% to 3% of all cases.2,3 Atypical and anaplastic meningiomas (AM) usually recur after a maximum safe resection and radiation therapy. Few medical options are available for treating progressive/recurrent and atypical meningiomas/AM. The new chemotherapeutic options for treating meningiomas have been proven ineffective over the past decade.4 The vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptors (VEGFRs) play important roles in mediating angiogenesis and the formation of peritumoral edema. VEGFR-2-positive cranial meningiomas showed significantly higher recurrence and regrowth rates compared with other types of VEGFs. Several promising clinical trials for VEGFR inhibitors, such as sunitinib and bevacizumab, have been published.5 This paper reports the application of apatinib as a novel VEGFR inhibitor in three cases of recurrent AM.
Case #1
Case description
In August 1999, a 47-year-old Asian female patient underwent a right temporal tumor resection. Her postoperative pathology was atypical meningioma WHO Ⅱ. The patient underwent radiotherapy 1 month after surgery. In January 2009 or 9.34 years later, the tumor recurred and a second tumor resection was performed. Her second postoperative pathology was AM WHO . The second radiation therapy (intensity-modulated radiation therapy or IMRT) was conducted 1 month after surgery. In December 2014 or 5.98 years after the second postoperative radiotherapy, malignant meningiomas recurred for the third time, and another tumor resection was performed. The postoperative pathology remained AM WHO . Postoperative local IMRT was conducted again. In May 2016, a brain-enhanced MRI examination revealed the recurrence of a fourth tumor (Figure 1). The patient was admitted to our hospital, and the VEGFR-2 was positive (Figure 2). The patient was given a 500 mg apatinib daily oral treatment.
Figure 1.
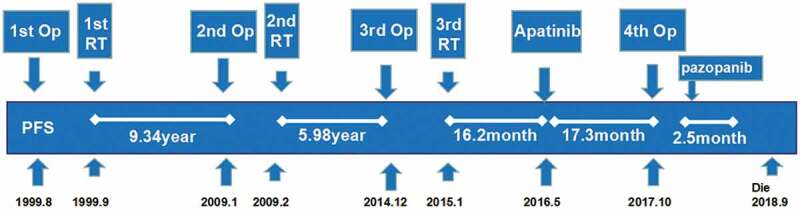
Treatment timeline (Op: operation, RT: radiotherapy).
Figure 2.
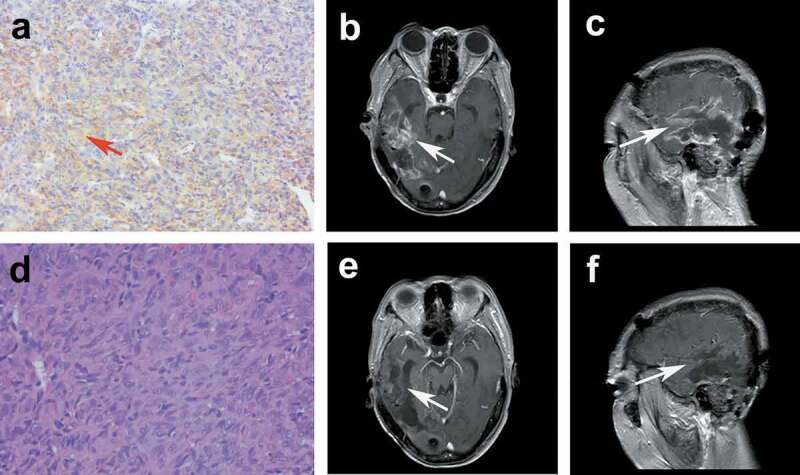
(a) The brown–yellow granules of the cell membrane and cytoplasm of vascular endothelial cells showed positive VEGFR2 (a × 100). Contrast-enhanced MRI (ceMRI) before treatment: transversal (b), and sagittal (c). ceMRI after treatment: transversal (e), and sagittal (f). e pathology of AE grade 3 (d × 200).
Follow-up and efficacy
The brain-enhanced MRI was reviewed at least once every 3 months during the apatinib treatment. Routine laboratory tests, including complete blood count, biochemical analysis, coagulation function test, urine analysis, and fecal occult blood test, were performed every 2 weeks. Apart from adverse reactions, the quality of life was recorded by using the Karnofsky performance scale (KPS). The efficacy was evaluated by using the Response Assessment in Neuro-Oncology (RANO) criteria, and the safety was assessed by using the National Cancer Institute-Common Terminology Criteria Adverse Events 4.0 (NCI-CTCAE 4.0).
The follow-up period was 28.5 months, and apatinib was taken orally for 15 months. Three months into the treatment, the drug dose was reduced to 250 mg after the patient experienced grade II adverse reactions. According to the RANO evaluation criteria, the best outcome during treatment was the partial response (PR) 6 months after the treatment. The progression-free survival (PFS) was 17.3 months, and the overall survival (OS) was 28.5 months. The best change in KPS was a 10-point increase. The main adverse events included anemia (grade II), thrombocytopenia (grade II), and proteinuria (grade I). The patient underwent a fourth operation after the tumor recurred and was orally treated with pazopanib (400 mg votrient daily) after surgery. After 2.5 months, the tumor progressed and the patient received optimal supportive treatment. The patient eventually died in September 2018.
Case #2
Case description
In December 2014, a 71-year-old Asian woman underwent her first left parietal tumor resection, and the postoperative pathology was atypical meningioma (WHO grade II). The patient received gamma knife radiotherapy in May 2015. The tumor recurred in January 2016 or 13.2 months after the first operation, and a second tumor resection was performed. The postoperative pathology was AM (WHO grade III). Gamma knife stereotactic radiotherapy was performed again 1 month after surgery. In July 2016 or 5 months after the second radiotherapy, the brain MRI (Figure 5a–c) revealed the recurrence of the tumor (Figure 3). The patient was admitted to our hospital, and the VEGFR-2 was positive (Figure 4). The patient was given a 500 mg apatinib daily oral treatment.
Figure 5.
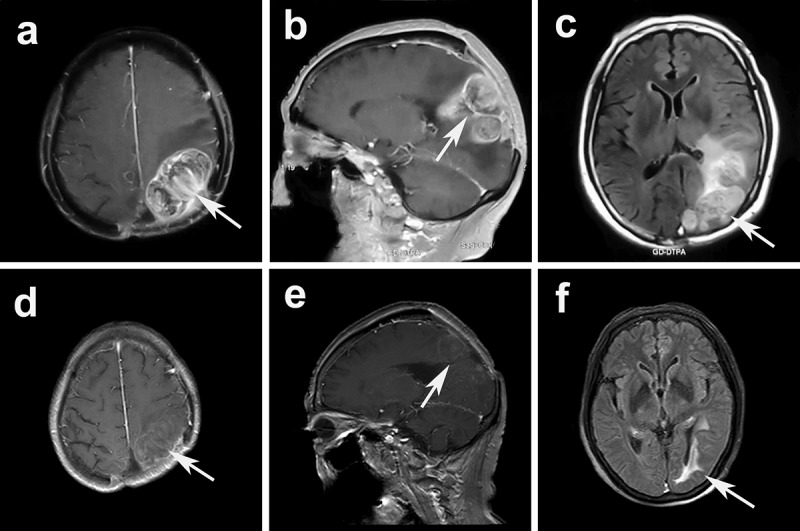
ceMRI before apatinib treatment: transversal (a), and sagittal (b). ceMRI after treatment: transversal (d), and sagittal (e). Flair images showed a significant relief of peritumoral edema (before treatment c vs. after treatment f).
Figure 3.
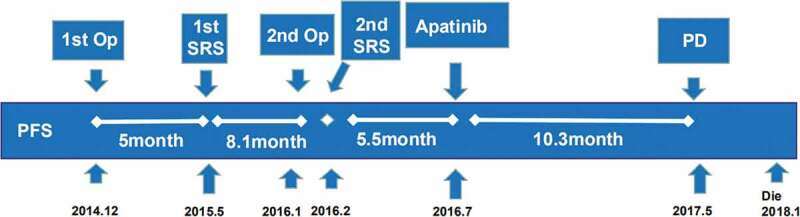
Treatment timeline (Op: operation; SRS: stereotactic radiosurgery).
Figure 4.
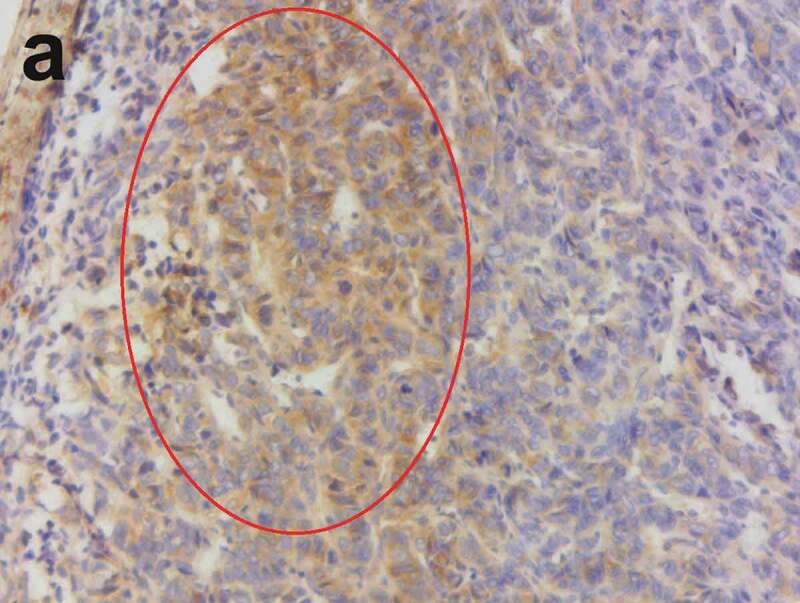
The brown–yellow granules of the cell membrane and cytoplasm of vascular endothelial cells showed a positive VEGFR2 (×200).
Follow-up and efficacy
The observed indicators and evaluation methods during the treatment were the same as those in case 1. The follow-up period was 18 months. Apatinib was taken orally for 10 months. According to the RANO evaluation criteria, the best outcome during treatment was PR. The PFS was 10.3 months, whereas the OS was 18 months. The best change in KPS was a 20-point increase. The main adverse events included hypertension (grade II), hand–foot syndrome (grade II), and fecal ocular blood (grade II). The patient eventually died in January 2018.
Case #3
Case description
A 16-year-old Asian girl was admitted to the hospital in July 2011 for epileptic seizures. The brain MRI examination revealed a tumor in her left temporal lobe. In July 2011, a left temporal lobe tumor resection was performed, and the tumor base was located on the cerebellar curtain. The postoperative pathology was atypical meningioma (grade II). In April 2016 or 4.76 years after the first operation, the tumor recurred and a second tumor resection was performed. The postoperative pathology was AM (WHO grade III), and the VEGFR-2 was positive (Figure 7). The tumor recurred a month after surgery. Therefore, IMRT (DT:60 Gy/30 f/6 w) was performed on the tumor bed. Unfortunately, the tumor did not shrink after radiation. The patient was given a 250 mg apatinib daily oral therapy (Figure 6).
Figure 7.
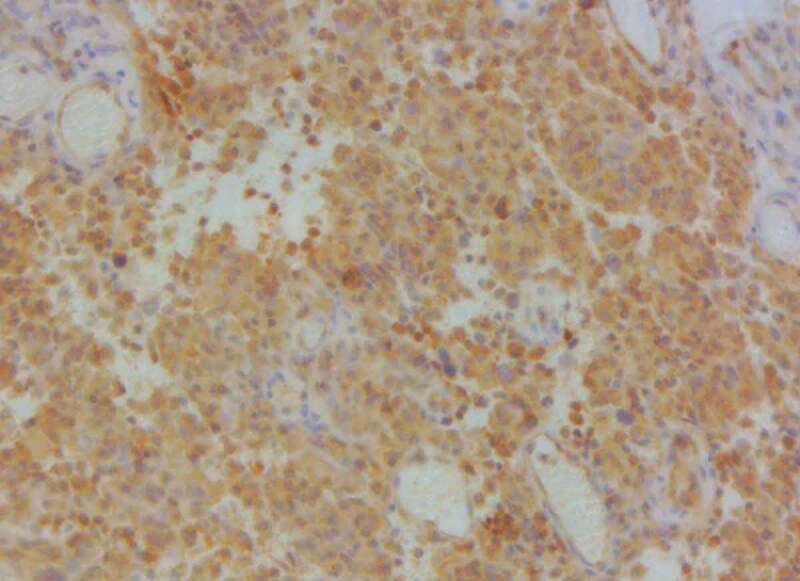
The brown–yellow granules of the cell membrane and cytoplasm of vascular endothelial cells showed a positive VEGFR2 (×200).
Figure 6.
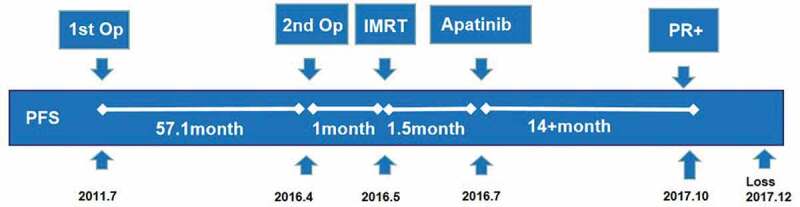
Treatment timeline (Op: operation; IMRT: intensity-modulated radiation therapy).
Follow-up and efficacy
The observed indicators and evaluation methods during the treatment were the same as those in case 1. The follow-up period was 16 months, and apatinib was taken orally for 8 months. The patient did not follow-up after the last review of the brain-enhanced MRI (Figure 8). According to the RANO evaluation criteria, the best outcome during treatment was PR. The PFS was 14+ months, and the best change in KPS was a 10-point increase. The main adverse events included hypertension (grade I) and hand–foot syndrome (grade I).
Figure 8.
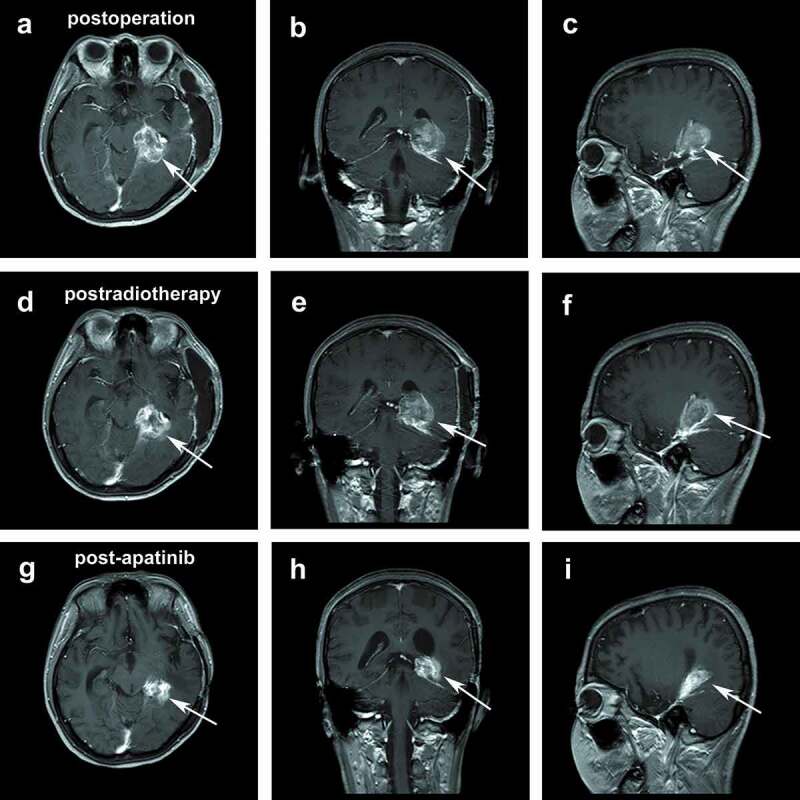
Postoperative ceMRI: transversal view (a), coronal view (b), and sagittal view (c). Postradiotherapy ceMRI: transversal view (d), coronal view (e), and sagittal view (f). ceMRI after treatment: transversal view (g), coronal view (h), and sagittal view (i).
Discussion and conclusion
Meningioma is the most common primary intracranial neoplasm. Grade III tumors have high frequencies of local invasion, recurrence, and metastasis. The outcome for patients with progressive grades II and III meningiomas remains poor, with most patients reporting 5-year survival rates of 28% to 61%. Despite maximal surgical resection and radiotherapy, a subset of these patients will recur and require additional treatment. Numerous conventional chemotherapies (including temozolomide, hydroxyurea, irinotecan, and triple therapy with cyclophosphamide+ doxorubicin+ vincristine) and hormonal therapies (including progesterone and estrogen modulators somatostatin analogs) have been proven ineffective.4–10 Targeted therapies should theoretically have better efficacy and safety profiles compared with systemic cytotoxic chemotherapy or radiotherapy. However, the inhibitors of platelet-derived growth factor receptors (PDGFRs; imatinib) and epidermal growth factor receptors (EGFRs; gefitinib and erlotinib) have been proven ineffective in clinical trials.11
VEGF is upregulated in almost all meningiomas and has been associated with neovascularization, tumor growth, and edema. VEGFR-2 is known as the main mediator of angiogenesis, and the inhibition of VEGFR-2 activity blocks the sarcoma cells in mice.12 VEGFR-2 also predicts a decreased survival rate among patients with soft tissue sarcomas, such as undifferentiated pleomorphic sarcoma, leiomyosarcoma, synovial sarcoma, liposarcoma, and angiosarcoma.13
Nakada S. et al.14 recently revealed that patients with VEGFR-2-positive meningiomas had a significantly short PFS. WHO grade II/III and the tumor location were significantly associated with recurrence. They suggested that VEGFR-2 inhibitors might be among the best candidates for the molecular therapy of recurrent meningiomas. Therefore, inhibitors of VEGFR-2 and the downstream molecules in its signaling pathway might be good candidate agents for the molecular therapy of recurrent meningiomas.
Two promising clinical trials on VEGFR inhibitors have been published.15 One of these trials evaluated the efficacy of sunitinib in 36 patients with malignant meningioma. The PFS rate was 42%, which satisfied the primary end-point of the study. Meanwhile, the median PFS and OS were 5.2 and 24.6 months, respectively, and the 2-year PFS and OS were 14.6% and 51.7%, respectively. One patient achieved a complete response, and another one achieved a partial response. VEGFR2 expression showed a strong association with response to sunitinib, thereby suggesting that VEGFR2 may be a good prognostic marker.16 The second trial evaluated the efficacy of bevacizumab in 14 patients with recurrent/progressive meningioma. The median PFS and PFS-6 were 17.9 months and 85.7%, respectively. Bevacizumab showed significant anti-edema advantages.17
On the basis of these trials, we conducted a clinical trial to evaluate the efficacy of apatinib in treating malignant meningiomas. Apatinib, also known as YN968D1, is a new small molecular tyrosine kinase inhibitor for VEGFR that was approved for sale in China in 2014 and was used for treating patients with advanced gastric cancer who had failed their second-line and the above treatments.18 Several phase Ⅱ trials showed that patients with advanced non-squamous, non-small-cell lung cancers, metastatic breast cancer, and hepatocellular carcinoma demonstrated significant improvements in their survival after taking apatinib.19–21 Therefore, apatinib shows potential in treating a broad range of advanced solid tumors. Apatinib is a small-molecule antiangiogenic agent (molecular weight: 493.58 Da) that selectively inhibits VEGFR-2 and mildly inhibits c-Kit and c-Src tyrosine kinases. Apatinib can also selectively compete for the adenosine triphosphate binding site of intracellular VEGFR-2 to block downstream signal transduction and inhibit tumor angiogenesis.22
The literature review identifies sunitinib, valatinib, and bevacizumab as VEGF/VEGFR inhibitors in recurrent/progressive meningioma. Sunitinib malate (SU011248, Sutent, Pfizer) is an orally administered tyrosine kinase inhibitor that targets VEGFR, PDGFR, and KIT. A phase Ⅱ study evaluated the efficacy of sunitinib in patients with malignant meningioma. A retrospective review by Lou et al. evaluated the use of bevacizumab-based therapy in treating recurrent/progressive meningiomas. In another phase Ⅱ clinical trial, valatinib showed some effects on recurrent or progressive radiation and surgery refractory meningiomas. These literature reviews are summarized in Table 1.16,17,23,24 Tian et al.25 found that apatinib had higher antitumor activity in vivo and in vitro compared to valatinib and sunitinib. Given these considerations, we carried out an apatinib therapeutic regimen, and the preliminary results showed the good anti-tumor and anti-edema effects of this inhibitor. However, if the good response data mainly based on the results of MRI may be an effect of pseudoresponse similar to what is known with bevacizumab. Further research must be confirmed to confirm this finding. The effect of apatinib may become more pronounced when combined with other drugs.
Table 1.
Studies of VEGF/VEGFR inhibitors in treating refractory recurrent meningioma.
| Treatment | Study type | Number patients | Median PFS (months) | PFS-6 (%) | Citation |
|---|---|---|---|---|---|
| Sunitinib | Phase Ⅱ | 36(Ⅱ, n = 30;,n = 6) | 5.2 | 42 | Kaley et al.16 |
| Valatinib | Phase Ⅱ | 25 meningioma:24(Ⅰ,n = 2;Ⅱ,n = 14;,n = 8) nemangiopericytoma:1 |
3.6 | 37.50 | Raizer et al.23 |
| Bevacizumab+TMZ or VP16 |
Retrospective | 14(Ⅰ,n = 5;Ⅱ,n = 5;,n = 3,Unknown:1) | 17.9 | 85.7 | Lou et al.17 |
| Bevacizumab | Retrospective | 15(Ⅱ,n = 6;,n = 9) | 6.5 | 43.8 | Nayak et al.24 |
| Apatinib | Retrospective | 3(,n = 3) | 17.3, 10.3, 14+ | 100 | Wang et al (this reseach) |
This report is the first to investigate the treatment of malignant meningiomas by using apatinib. The results suggest that a phase Ⅱ clinical trial should be conducted to further evaluate the efficacy of apatinib in treating malignant meningioma.
Acknowledgments
The authors thank Baoyan Liu of the Shandong Medical Biotechnological Center, School of Medicine and Life Science, Shandong Academy of Medical Sciences, University of Jinan for his help in editing this manuscript.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
No specific funding was used for the data collection, analyses, results reporting, or manuscript writing.
Authors’ contributions
RT conceived the idea for this case study, YW wrote the manuscript, YHL, XJM, and SZZ were involved in the diagnostic flow, and JX followed the patients along with the author. All authors read and gave their final approval of the version to be published.
Availability of data and materials
The datasets used and/or analyzed during this study are available upon request from the corresponding author.
Consent for publication
Written informed consent was obtained from each patient for the publication of this case report and any accompanying images.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS.. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fathi AR, Roelcke U. Meningioma. Curr Neurol Neurosci Rep. 2013;13(4):337. doi: 10.1007/s11910-013-0337-4. [DOI] [PubMed] [Google Scholar]
- 3.System Tumors . 2016 World Health Organization classification of central nervous. Continuum (Minneap Minn). 2017;23(6,Neuro–oncology):1531–1547. doi: 10.1212/CON.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 4.Le Rhun E, Taillibert S, Chamberlain MC. Systemic therapy for recurrent meningioma. Expert Rev Neurother. 2016;16(8):889–901. doi: 10.1080/14737175.2016.1184087. [DOI] [PubMed] [Google Scholar]
- 5.Caruso G, Elbabaa SK, Gonzalez-Lopez P, Barresi V, Passalacqua M, Caffo M. Innovative therapeutic strategies in the treatment of meningioma. Anticancer Res. 2015;35(12):6391–6400. [PubMed] [Google Scholar]
- 6.Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomidefor treatment-resistant recurrentmeningioma. Neurology. 2004. 13;62(7):1210–1212. doi: 10.1212/01.WNL.0000118300.82017.F4. [DOI] [PubMed] [Google Scholar]
- 7.Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, Rogers L, Schiff D, Vogelbaum M, Weber D, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. doi: 10.1093/neuonc/not330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neurooncol. 2006;78(3):271–276. doi: 10.1007/s11060-005-9093-x. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain MC. Adjuvant combined modality therapy for malignant meningiomas. J Neurosurg. 1996. May;84(5):733–736. doi: 10.3171/jns.1996.84.5.0733. [DOI] [PubMed] [Google Scholar]
- 10.Cea-Soriano L, Blenk T, Wallander MA, Rodríguez LA. Hormonal therapies and meningioma: is there a link? Cancer Epidemiol. 2012;36(2):198–205. doi: 10.1016/j.canep.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Norden AD, Raizer JJ, Abrey LE, Lamborn KR, Lassman AB, Chang SM, Yung WKA, Gilbert MR, Fine HA, Mehta M, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2010;96(2):211–217. doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaman S, Leppänen VM, Alitalo K. Vascular endothelial growth factor signaling in development and disease. Development. 2018;145:14. doi: 10.1242/dev.151019. [DOI] [PubMed] [Google Scholar]
- 13.Kampmann E, Altendorf-Hofmann A, Gibis S, Lindner LH, Issels R, Kirchner T, Knösel T. VEGFR2 predicts decreased patients survival in soft tissue sarcomas. Pathol Res Pract. 2015;211(10):726–730. doi: 10.1016/j.prp.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Nakada S, Sasagawa Y, Tachibana O, Iizuka H, Kurose N, Shioya A, Guo X, Yamada S, Nojima T. The clinicopathological analysis of receptor tyrosine kinases in meningiomas: the expression of VEGFR-2 in meningioma was associated with a higher WHO grade and shorter progression-free survival. Brain Tumor Pathol. 2019;36(1):7–13. doi: 10.1007/s10014-018-0332-1. [DOI] [PubMed] [Google Scholar]
- 15.Nassehi D. Intracranial meningiomas, the VEGF-A pathway, and peritumoral brain oedema. Dan Med J. 2013;60(4):B4626. Review. [PubMed] [Google Scholar]
- 16.Kaley TJ, Wen P, Schiff D, Ligon K, Haidar S, Karimi S, Lassman AB, Nolan CP, DeAngelis LM, Gavrilovic I, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17(1):116–121. doi: 10.1093/neuonc/nou148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lou E, Ashley L, Sumrall ST, Peters KB, Desjardins A, Vredenburgh JJ, McLendon RE, Herndon JE, McSherry F, Norfleet J, et al. Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neurooncol. 2012;109(1):63–70. doi: 10.1007/s11060-012-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51(4):223–229. doi: 10.1358/dot.2015.51.4.2320599. [DOI] [PubMed] [Google Scholar]
- 19.Xichun H, Cao J, Wenwei H, Changping W, Pan Y, Cai L, Tong Z, Wang S, Li J, Wang Z, et al. Multicenter phase II study ofApatinibin non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14:820. doi: 10.1186/1471-2407-14-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roviello G, Ravelli A, Fiaschi AI, Cappelletti MR, Gobbi A, Senti C, Zanotti L, Polom K, Reynolds AR, Fox SB, et al. Apatinib for the treatment of gastric cancer. Expert Rev Gastroenterol Hepatol. 2016;10(8):887–892. [DOI] [PubMed] [Google Scholar]
- 21.Xie L, Guo W, Wang Y, Yan T, Ji T, Xu J. Apatinibfor advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Cancer. 2018;18:396. doi: 10.1186/s12885-018-4303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, double-blind, placebo-controlled phase III trial ofapatinibin patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. [DOI] [PubMed] [Google Scholar]
- 23.Raizer JJ, Grimm SA, Rademaker A, Chandler JP, Muro K, Helenowski I, Rice L, McCarthy K, Johnston SK, Mrugala MM, et al. A phase II trial of PTK787/ZK222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neurooncol. 2014;117(1):93–101. doi: 10.1007/s11060-014-1358-9. [DOI] [PubMed] [Google Scholar]
- 24.Nayak L, Iwamoto FM, Rudnick JD, Norden AD, Lee EQ, Drappatz J, Omuro A, Kaley TJ. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. 2012;109(1):187–193. doi: 10.1007/s11060-012-0886-4. [DOI] [PubMed] [Google Scholar]
- 25.Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. doi: 10.1111/cas.2011.102.issue-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available upon request from the corresponding author.


