Abstract
Globally, oral cancer is the sixth most common type of cancer with India contributing to almost one-third of the total burden and the second country having the highest number of oral cancer cases. Oral squamous cell carcinoma (OSCC) dominates all the oral cancer cases with potentially malignant disorders, which is also recognized as a detectable pre-clinical phase of oral cancer. Tobacco consumption including smokeless tobacco, betel-quid chewing, excessive alcohol consumption, unhygienic oral condition, and sustained viral infections that include the human papillomavirus are some of the risk aspects for the incidence of oral cancer. Lack of knowledge, variations in exposure to the environment, and behavioral risk factors indicate a wide variation in the global incidence and increases the mortality rate. This review describes various risk factors related to the occurrence of oral cancer, the statistics of the distribution of oral cancer in India by various virtues, and the socio-economic positions. The various conventional diagnostic techniques used routinely for detection of the oral cancer are discussed along with advanced techniques. This review also focusses on the novel techniques developed by Indian researchers that have huge potential for application in oral cancer diagnosis.
Keywords: Oral cancer, Diagnosis, India, Tobacco-related cancer, Smokeless tobacco
Graphical abstract
1. Introduction
Any uncontrolled growth of cells that invade and cause the adjacent tissue impairment is known as cancer. Oral cancer ensues with a small, unfamiliar, unexplained growth or sore in the mouthparts that include lips, cheeks, sinuses, tongue, hard and soft palate, the base of the mouth extended to the oropharynx. Globally, oral cancer ranks sixth among all types of cancer. India has the largest number of oral cancer cases and one-third of the total burden of oral cancer globally. Oral cancer poses a serious health challenge to the nations undergoing economic transition [1]. In India, around 77,000 new cases and 52,000 deaths are reported annually, which is approximately one-fourth of global incidences [2]. The increasing cases of oral cancer are the most important concern for community health as it is one of the common types of cancers in India [3]. As compared to the west, the concern of oral cancer is significantly higher in India as about 70% of the cases are reported in the advanced stages (American Joint Committee on Cancer, Stage III-IV). Because of detection in the late phase, the chances of cure are very low, almost negative; leaving five-year survival rates around 20% only [4].
Oral squamous cell carcinoma (OSCC) contributes remarkably i.e. 84-97% to oral cancer. OSCC commonly results from potentially malignant lesions or normal epithelium linings. Potentially malignant disorders (PMDs) such as inflammatory oral submucosa, fibrosis, erythroplakia, leukoplakia, candidal leukoplakia, dyskeratosis congenital, and lichen planus are indicators of the preclinical phase of oral cancer [5]. Tobacco consumption including smokeless tobacco (SLT), betel-quid chewing, excessive alcohol consumption, poor oral hygiene, nutrient-deficient diet, and sustained viral infections, i.e. human papillomavirus (HPV) are some of the risks associated with the occurrence of oral cancer. Lack of knowledge, exposure to extreme environmental conditions, and behavioral risk factors are indicators of a wide variation in the global incidence. Periodontal illnesses are also a high-risk consideration for oral malignancy, and it has a higher incidence among the Indian population, where oral cancer occurrence is mainly due to the habit of chewing paan [2]. Inflammation plays an important role in tumorigenesis and inflammation produced by viral and bacterial infections, and inflammatory bowel diseases may cause malignancy. Various socio-ecological and behavioral factors such as exposure to smoke, silica, asbestos, and other carcinogenic elements may lead to cancer [2]. Tobacco consumption (in any form) is a prime cause of cancer, prominently in developing nations. Apart from tobacco, chewing paan containing leaves of piper betel with areca nut, lime, catechu, cinnamon, etc., is a leading source of oral malignancy, especially in the north-eastern parts of India that contributes the highest incidence of cancer in India [6]. The continued activity of chewing paan causes prolonged exposure of oral mucosa along with abrasion of epithelium linings. SLT consumed both orally and nasally, shows association with potentially malignant oral disorders and oral cavity cancers.
Various conventional clinical techniques such as physical and histopathological examination, staining, biopsy, spectroscopic and radiological techniques, etc. are used routinely to detect oral cancer. The diagnosis of cancer in the early stage is a key factor to check further physical, psychological, and financial losses to the patient. Upon early diagnosis, timely and proper treatment can be initiated that may improve the survival rate up to 90%. With advancements in science and technology, numerous novel techniques are developed that have advantages as compared to the currently practiced conventional diagnostic methodologies. In this review, the current scenario of oral cancer diagnosis in India is described along with the recent statistics. The review also highlights various currently used diagnostic techniques for oral cancer detection as well as those in the development phase, which are easy-to-use, quicker, and painless. The research and development initiatives pursued by Indian researchers towards oral cancer detection are also highlighted herein.
2. Status of oral cancer in India
Oral cancer is an important health issue in India as it is one of the most common types of cancer affecting a large population. The low-income population is at the highest risk due to extensive exposure to various risk factors. Tobacco consumption has been the predominant factor causing oral cancer. The continual use of tobacco in various forms such as gutka, zarda, mawa, kharra, khaini, cigarettes, bidi, hookah, etc. is a major cause of tumor development in the oral cavity in both young as well as the adult Indian population [7]. The distribution of oral cancer across India is shown in Fig. 1 . The gender-based distribution of tobacco-related cancer cases and smokers are depicted in Fig. 2 . Various factors related to the occurrence of oral cancer in Maharashtra are shown in Fig. 3 . Apart from the direct use of tobacco, SLT is also found to be a major reason for oral and pharyngeal cancer with a higher risk in women [8]. A gender-based distinction has been found for oral cancer cases, where males show a high incidence of tobacco-related cancer [9].
Fig. 1.
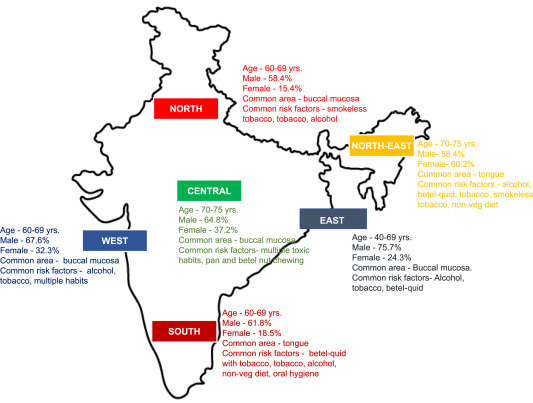
Distribution of oral cancer across the India.
Fig. 2.
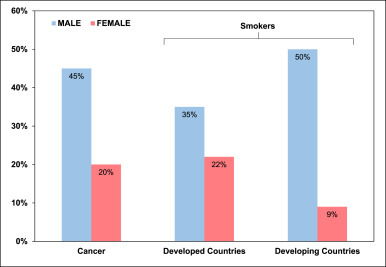
Gender-based distribution of tobacco related cancers and amongst smokers.
Fig. 3.
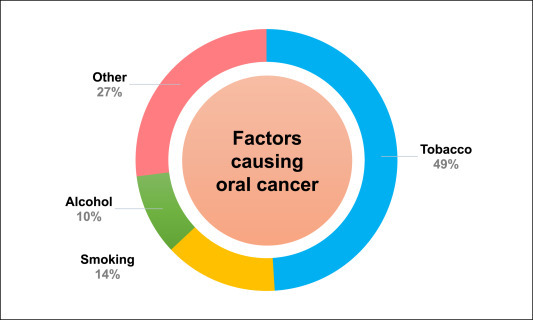
Distribution of various factors responsible for oral cancer in Maharashtra.
In India, epidemiologically, Kerala has the lowest incidence of oral cancer while West Bengal reports the highest. In the western regions of Maharashtra, the highest occurrence of oral malignancy is reported in the age group of ≥60 years, followed by between 40-59 years with a male-female ratio of 2:1 [5]. Several case-control studies, based on age, education, and socio-economic status, have been carried out to assess risk factors, effects, and the prevalence of PMDs and oral cancer. In one of the studies, it is reported that out of 100 patients suffering from PMDs and oral cancer, the highest number of cases were presented by individuals aging between 31 to ≥50 years [10]. In recent years, the occurrence of the tongue and buccal mucosa cancer has been increased in India with a higher number of buccal mucosa cancer cases [11]. A group from Chennai reported that oral cancer is most prevalent at the base of the tongue and the base of the mouth that enhances the metastasis [12]. Another group has reported that buccal mucosa, alveolus, and the base of the mouth are the most prevalent sites for the occurrence of oral cancer [13]. It is also reported that the nutritional diet is important for oral cancer patients to maintain the oral health-associated quality of life. Table 1 shows a comparative analysis of different sites in the oral cavity that has a high incidence of oral malignancy in the three regions of India, i.e. Kerala, Varanasi, and western Maharashtra.
Table 1.
A comparative analysis of the different sites of oral cancer in different regions of India.
3. Techniques used for diagnosis of oral cancer
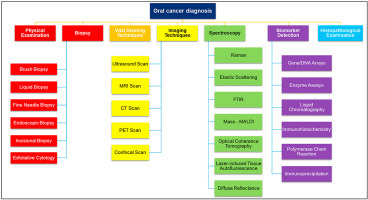
Early detection is very important to reduce the mortality rate of patients suffering from oral cancer. Thus, there is a huge demand for oral cancer diagnostic techniques that are non-invasive, rapid, and easy-to-use. For oral cancer diagnosis, traces of oral lesions in the mouth are first observed properly by a physician. Upon suspected malignancy, it is further referred to an oral or maxillofacial surgeon who conducts the specific tests. In the case of oral cancer, a dentist plays a pivotal role in the early examination of occurrence. Various routinely practiced techniques for oral cancer detection are shown in Fig. 4 and various parameters are compiled in Table 2 .
Fig. 4.
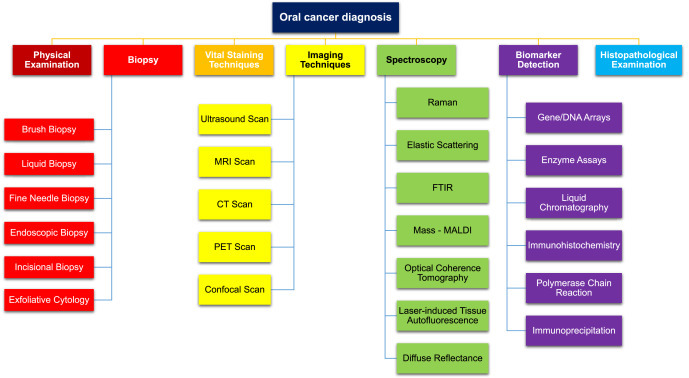
Techniques for oral cancer detection.
Table 2.
Various techniques used for the detection of oral cancer.
|
Sr. No. |
Technique | Principle/Mechanism | Specimen | Biomarker | Sensitivity | Advantages | Disadvantages | Cost (∼₹) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Autofluorescence spectroscopy | Autofluorescence spectroscopy and imaging | Oral tissue | NA | 91.1% | Non-invasive Patient compatible |
Non-specific | 400–1000 | [17] |
| 2. | Brush biopsy | Brushing and microscopic examination | Oral cells | Secretory leukocyte protease inhibitor | 77.0% | Non-invasive Relatively painless Low cost Requires minimum technical skills |
Inadequate sampling False negative results |
500–1500 | [18,19] |
| 3. | Computed tomography | Ionizing radiation (X-ray)-based tissue imaging | Soft tissue in head and neck region (in situ) | NA | NA | Rapid and painless Widely available Decent visualization |
The harm of radiation exposure Lower resolution Side effects of contrast medium |
500–5000 | [20] |
| 4. | Confocal microscopy | Optical imaging | Oral mucosal cells | NA | NA | Control of depth of field Collection of multiple optical sections Elimination of background data |
A limited number of excitation wavelength Harmful high-intensity laser irradiation Expensive |
4000 | [21] |
| 5. | Diffuse reflectance spectroscopy | Tissue reflectance optical spectroscopy | Oral tissue | Collagen, elastin, keratin, FAD, and NADH | 98.5% | Low cost Rapid Non-invasive Quantitative Good sensitivity and specificity Real-time data |
Complex Encompasses several broad and overlapping bands |
500–2000 | [22] |
| 6. | DNA microarray | Simultaneous measurement of multi-gene expression | Oral tissue | Genes i.e. CDH1, MMP3, SPARC, POSTN, TNC, TGM3, HMGA1, PABPC1, NT5E, FASN, FOS, P53, etc. | NA | High throughput technology Relatively affordable High sensitivity |
Laborious method Numerous printing devices are required |
700–5000 | [23,24] |
| 7. | Elastic scattering spectroscopy biopsy | Wavelength dependant light scattering | Oral tissue | NA | 87.0% | Used in deciding the extent of surgical margins No secondary procedures |
Time-consuming Limited angular resolution |
– | [25] |
| 8. | Enzyme assay | ELISA | Saliva | Proteases | NA | Less sample volume Non-invasive |
Laborious method Lower accuracy |
200–1000 | [26] |
| 9. | Exfoliative biopsy | Scraping and microscopic examination | Oral epithelial cell linings | Epidermal growth factor receptor (EGFR) | 100% | Patient Compliant Low cost Minimal skills required Minimal instrumentation |
Chances of contamination and false-negative results Low sensitivity Inadequate sampling |
600–2600 | [27,28] |
| 10. | Fine needle biopsy | Microscopic examination | Oral cells | NA | 75% | Simple Accurate Low cost |
Bleeding Infection Nerve Injury Swelling |
1000 | [29,30] |
| 11. | Fourier transform infrared spectroscopy | Absorption based optical spectroscopy | Sputum | Glycogen, keratin | NA | High sensitivity, specificity, and accuracy Non-invasive Early detection |
Potassium bromide reduces spectral quality | 1800 | [31] |
| 12. | High-performance liquid chromatography (HPLC) | Column chromatography | Serum | Serum proteins | 87.5% | Automated procedure Sample retrieval Quantitative investigation High sensitivity |
Require special detectors Expensive |
4000–5000 | [32] |
| 13. | Histopathological examination | Microscopic examination | Oral tissue | p53, ki-67 | NA | Large segments of tissues are studied Quick collection Little or no risk |
Time-consuming slide preparation Chances of human error Less specific Difficulty to identify specific types of cells |
800–6400 | [33] |
| 14. | Immunohistochemistry | Staining and microscopic examination | Oral tissue | Mutant p53 gene | NA | Widely used Rapid |
No availability of standardized stains Difficult quantification Chances of human error |
1750–3500 | [34] |
| 15. | Incisional biopsy | Microscopic examination | Oral tissue | Cytokeratin-19 | NA | A small piece of tissue is required Performed in suspected cases of malignancy and pre-malignancy Detailed, specific, and accurate analysis |
Increased risk of metastasis of malignant lesions Avoided in vascular cases |
1000–2000 | [35] |
| 16. | Laser capture microdissection | Cell extraction and molecular characterization | Oral tissue | Proteins (CK-14, CK-17) | NA | Accurate and quick cell separation Preserves tissue morphology |
Expensive Sample contamination High level of expertise required |
6000 | [36,37] |
| 17. | Laser-induced tissue auto-fluorescence | Fluorometry | Oral mucosa (in situ) | NADH, elastin, collagen | 100% | High sensitivity Low sample volume Rapid Suitable for in situ testing |
Limited depth of penetration | – | [38] |
| 18. | Liquid biopsy | Molecular biotechnology procedures (e.g. PCR) | Blood, saliva | Circulating tumor cells/DNA, exosomes for blood and Cd63, salivary exosomal miRNAs | 88.9% | Rapid Easy sampling Less invasive Comprehensive tissue profile Allows more frequent and serial samplings over time Allows molecular profiling of tissues |
Need for an initial histological diagnosis Chances of false-negative An expert analyst is required Expensive |
8000-20,000 | [39] |
| 19. | Magnetic resonance imaging | Non-ionizing radio frequency electromagnetic radiation-based analysis | Soft tissue in head and neck region (in situ) | NA | NA | Non-invasive and no radiation High-resolution images Multidimensional imaging Detects status of metastasis |
Expensive Probability of false-positive result Patients with metal implants cannot undergo MRI |
5000–17000 | [40] |
| 20. | Mass spectroscopy | The mass-to-charge ratio of ions is measured with respect to intensity | Oral tissue | Proteins and lipids | NA | Molecular profiling of tissues Accurate |
Expensive instrumentation | 2000–6000 | [41] |
| 21. | Micro total analysis system | Microfluidics | Saliva | Nucleic acids | NA | Rapid Convenient Low cost Portable Low sample volume Non-invasive Automated operation |
Complex fabrication method Insufficient interfaces for fluid transfer |
50–100 | [42] |
| 22. | Multispectral digital microscope | Fluorescence imaging | Oral tissue | NA | 98% | Highly sensitive Informative |
Slow Complex sample preparation |
500–2000 | [43] |
| 23. | Optical coherence tomography | White light Michelson interferometry | Oral mucosa | NA | 90% | High-resolution images Fast acquisition time Non-invasive Easy-to-use |
Limited depth of penetration (1.5–2 mm) Light interference reduces sensitivity Negative influence on image quality due to hyperkeratosis. |
1000–2500 | [44,45] |
| 24. | Physical examination | Systematic visual examination and palpation | Oral cavity (in situ) | NA | NA | Reduces morbidity, mortality, incidence of invasive cancers Identifies high-risk groups Low cost |
Detection in a late phase Requires skilled professional Chances of false positive and negative |
200–500 | [46] |
| 25. | Polymerase chain reaction | DNA amplification | Serum and saliva | HPV DNA, tetranectin | NA | Quantitative Greater sensitivity Reproducibility Better control of quality in the process Lower risk of contamination |
Expert analyst required Analysis depends on many complex, interrelated factors Expensive |
3000–4000 | [47] |
| 26. | Positron emission tomography | Imaging using radioactive agents | Head and neck region (in situ) | NA | NA | Measure cellular-level metabolic changes Early detection Non-invasive |
Less accurate sometimes Short decay-duration of a radioactive substance Expensive |
2000–27000 | [48] |
| 27. | Raman spectroscopy | Optical spectroscopic technique for chemical analysis | Oral tissue | Keratin | 95% | Minimal sample preparation Highly informative Non-destructive Non-invasive |
Signal inadequacy Bands overlap, difficulty in identifying individual component |
1000 | [49,50] |
| 28. | Spectral cytopathology | Micro-spectral measurement and multivariate data analysis | Oral cells | Proteins | NA | Accurate and reproducible Less human error |
Expensive | – | [51] |
| 29. | Ultrasound (Sonography) | High-frequency sound wave-based imaging | Head and neck region (in situ) | NA | NA | Non-invasive Non-ionizing radiation |
Operator dependent analysis Cannot image cyst duct |
600-20,000 | [52] |
| 30. | VELscope | Fluorometry | Oral tissue (in situ) | NA | 74.1% | Assist in biopsy site selection Low cost Non-invasive Easy and rapid Convenient Early diagnosis of lesions |
No recording Provide false positive and false negative No definitive diagnosis Low sensitivity Inadequate sampling |
1000–2000 | [53] |
| 31. | Vital staining techniques | Visual tissue staining (e.g. toluidine blue) and microscopic examination | Oral tissue | Sulfate, DNA, RNA |
97.8% | Simple Low cost Non-invasive Easy-to-do Widely available |
High percentage of false-positive cases | 20–200 | [54] |
| 32. | ViziLite | Tissue reflectance-based examination | Oral soft tissue (in situ) | NA | 77.3% | Non-invasive Easy and rapid Convenient Early detection Low cost |
No recording Low specificity for dysplasia Non-specific |
1000 | [55] |
| 33. | X-ray | Radiation-based imaging | Head and neck region (in situ) | NA | NA | Easy Rapid Non-invasive |
Unsafe for pregnant women No precise and detailed information for each tooth or soft tissues |
250–2000 | [56] |
In India, self-examination is considered one of the most effective methods for early diagnosis of oral malignancy. The feasibility of various methodologies such as visual examination, VelScope-aided investigation, and toluidine dye application has been assessed and compared by healthcare workers in Mumbai [15]. The visual screening was suggested to the patients suspected of suffering from oral cancer in Kerala. The patients found positive were further referred for clinical examination by physicians. The visual screening is a convenient method that helps in decreasing the mortality rate and can prevent around 37,000 oral cancer deaths across the globe [16].
3.1. Physical examination
The primary and the most crucial assessment for oral cancer is the physical examination, which usually consists of two steps – systematic visual examination and palpation. Primarily, the external parts such as lymph nodes, salivary glands, lips, etc. are inspected, and subsequently, an internal examination of the buccal cavity is performed. Abnormalities, irregularities, swelling, and fluctuance in superficial anatomy are recorded. Soft tissue thickening, lumps, soreness, trouble in jaw movement, chewing and swallowing, ear pain, etc. are some of the common observations. The parotid gland (largest salivary gland) is palpated both intra-orally as well as externally and further, the submandibular and sublingual glands are palpated [46]. The examiner compares physical observations with the patient's clinical presentations. The pathological changes are recorded along with abnormalities in the texture and color [57].
3.2. Histopathological examination
By the histopathological standpoint, the OSCC varies from indolent tumors to very aggressive tumors with high invasive potential. The gradual development of the carcinoma in the oral cavity starting from simple dysplasia to highly invasive tumors is revealed by the histological assays. The histopathological analysis is essential to verify the proliferation of cells and maturation abnormalities, cellular and cytoplasmic atypia, and alteration of the surface epithelium or deep tissue cytoarchitecture [33]. Identification and adequate sampling of the oral lesion is an important step in a histological investigation. Sometimes, histopathological changes may occur in the areas that do not show any evidence of oral lesions during physical examination. Molecular and genetic changes may take place in benign tissues before microscopic and clinical morphological changes occur. Thus, a procedure that detects both, histopathological and molecular changes, is preferred to diagnose benign or malignant tumors [58].
3.3. Vital staining techniques
Visual tissue staining is an adjunct technique used in the diagnosis of cancer [59]. Tolonium chloride (also known as toluidine blue) staining is used to detect the mucosal abnormalities in the oral cavity. Toluidine blue is a type of acidophilic metachromatic dye that stains acidic components of tissue such as sulfate, phosphate, and carboxylic moieties (i.e. DNA and RNA), selectively. Because of the ease of use and cost-effectiveness, this technique is suitable in developing countries like India. Lugol's iodine in combination with toluidine blue aids in the distinction of the inflammatory lesions. This combination predicts the degree of differentiation of malignant lesions, which makes it an important visual staining technique for the pre-therapeutic assessment of oral cancer [60]. In a study from Maharashtra, self-examination and clinical examination were carried out initially, after which, screening methods using toluidine blue and Lugol's iodine stains were performed followed by a biopsy. Lugol's iodine was found to be more sensitive in comparison to toluidine blue [61].
3.4. Biopsy
A tissue sample is removed surgically from the suspected region and sent to the pathological laboratory for the detailed microscopic examination. This is the only way to ascertain the presence of an oral cavity or oropharyngeal cancer [62]. For a biopsy, careful handling of the tissue is very critical for confident histological diagnosis. Improper handling of the sample may result in a defective biopsy and the procedure needs repetition. Depending on the specific requirement, biopsies such as exfoliative cytology and incisional biopsy are carried out.
3.4.1. Brush biopsy
In brush biopsy, the transepithelial cells from the oral lesion are obtained by scraping the surface mucosa. A brush biopsy is a simple, painless, chair-side, inexpensive, highly sensitive, and risk-free method for oral cancer screening. It is useful in identifying any suspicious lesion, which includes small red and white oral lesions, to rule out any dysplastic features. Brush biopsy has higher sensitivity and specificity of around 90% in comparison to other biopsy techniques [63]. The brush biopsy has been coupled with portable tablet-based microscopes for digital detection of abnormalities in stained tissues. The results are evaluated by clinicians remotely; this telemedicine-based oral cancer screening method has huge potential for wide application in India [64]. Cytological study of the oral cancer cells is a non-aggressive technique; recently, numerous advancements have been reported along with applications of scraped or exfoliated cytology for the detection of the cancerous lesions, and prediction of its progression and recurrence [65].
3.4.2. Exfoliative cytology
Exfoliated cytology is based on epithelial physiology and is a simple and non-invasive diagnostic technique for early detection of oral malignancy. Cellular cohesive forces are reduced due to the presence of benign disease or the formations of the malignant epithelial cells, leading to exfoliation; the exfoliated cells are collected for microscopic examination [66]. The oral exfoliative cytology (OEC) is an easy, non-invasive technique that is affable for the patient and suitable for preliminary detection of oral cancer. OEC focuses on the morphological and staining characteristics of the individual cell, which requires skilled cytopathologists [67]. Despite certain advantages, OEC is not specific and sensitive and thus, used primarily for periodic review of pre-malignant lesions, selection of suitable sites for biopsy in large lesions, and also in large-scale population screening [68].
3.4.3. Incisional biopsy
Upon malignancy indicated through OEC, the incisional biopsy is performed. In this type of biopsy, a representative sample of the tissue is carefully chosen for selective diagnosis. The incisional biopsy is relatively accurate as it does not apply to the entire lesion [69]. An incisional biopsy is used in situations when it is not possible to remove the whole lesion, such as lichen planus or a diffuse white patch. Also, the technique is preferred in cases where the clinical diagnosis is unidentified [70]. All the biopsy samples are collected and sent to laboratories for further examinations by the pathologists. Using microscopic techniques, the experts differentiate the various types of cancer cells based on structural modifications. Usually, various special types of staining agents are applied to the samples to identify and categorize the types of cancer cells [62]. Although biopsy is considered as the standard method for cancer diagnosis, it is invasive, painful, and require surgical intervention.
3.5. Imaging techniques
Several advanced imaging techniques are used for the diagnosis of oral cancer. Among these, the most routinely used scanning techniques are magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) [71].
3.5.1. Magnetic resonance imaging
MRI provides the details of the structures in the oral cavity along with adjacent parts. The soft-tissue discrimination by MRI aids in assessing the extent of local and regional spread of the tumor, invasion depth, and extent of its lymphadenopathy [40]. MRI is an accurate technique for determining the spread of oral cancer to its surrounding soft tissues as it has a very high-contrast resolution and multiplanar views. Thus, MRI is critical for the pre-treatment analysis of advanced oral and oropharyngeal cancer [72]. MRI also assists in recognizing the source, position, and margins of lesions. Thus, MRI may be considered as a supportive technique to biopsy for routine screening of oral cancer cases [73].
3.5.2. Computed tomography
The CT scan uses the x-ray radiations and a computer to create pictures of the body to locate the malignant lesion and determine its spread to the other parts of the body. CT scan is a widely accessible and comparatively less expensive procedure and thus, is considered as a standard imaging technique for the detection of head and neck tumors. But it is observed that lesions in their early stages cannot be recognized using a CT scan. Minor early-stage lumps existing in the buccal cavity can be detected by CT scan only upon enhancement using an intravenous contrast medium [20].
3.5.3. Positron emission tomography
The PET scan is used to determine the spread of tumor cells to the lymph nodes or other parts of the body. A radioactive dye is administered orally or intravenously and the gamma rays emitted from the positron decays are scanned. It is an accurate method that determines the staging of the lymph nodes. It does not implicate any change in the treatment approach even if additional lymph node metastasis is detected if the nodes are detected apart from the affected site [74]. The status of lymph nodes is determined before surgery by PET scan using fluorodeoxyglucose (FDG). Thus, PET scanning is crucial for the early detection of oral malignancy [75].
3.6. Optical and radiological techniques
X-ray is used to determine the spread of cancer to the other organs outside of the mouth and oropharynx. The x-ray for oral cancer is known as an orthopantomogram also known as the panorex scan [56]. X-ray images of the area surrounding the upper jawbone (maxilla) and the lower jawbone (mandible) demonstrate the presence of cancerous cells around these bones. Other radiological techniques that are used for the detection of cancer and staging of the cancer cells are fluoroscopy, ultrasound, etc. Various optical spectroscopy techniques such as laser-induced tissue autofluorescence and diffuse reflectance are recently recognized for the diagnosis of oral malignancy [76].
Optical imaging techniques such as optical coherence tomography and tissue fluorescence imaging can effectively differentiate between benign and malignant lesions. Laser capture microdissection (LCM) provides more specific detection ability and is used in establishing the molecular basis of malignancy. Micro total-analysis-system (μTAS) is an analytical laboratory on a single chip used in the examination of oral malignancy [77]. Multispectral digital microscope (MDM) is used to acquire images of oral tissues in various modes such as fluorescence, narrow-band reflectance, and orthogonal polarized reflectance, which aids in better visualization of the lesions [43]. Spectral cytopathology is another recent method for oral cancer diagnosis, it differentiates the disease status in individual exfoliated cells [78].
3.7. Biomarker detection and biosensors
The biological entities expressed in the serum or saliva that consequently leads to oral cancer may aid as biomarkers for oral cancer detection. Biomarkers in general are components of the cells present in body fluid or tumor cells that are overexpressed during the onset of the disease. Specifically, the advancement of cancer occurs in three major steps that include initiation, promotion, and progression. All the three steps correlate with major changes in the metabolome, transcriptome, and proteome of a cell [79,80]. These changes are due to alterations in particularly significant genes or proteins that lead to diversification or halting of critical metabolic and structural pathways. TSG p16, TSG p53, Ki-67 antigen, and DNA ploidy are some of the most common biomarkers associated with the progress of oral malignancy. The expression of p53 protein is considered as a primary indication related to the carcinogenesis in OSCC. The immunohistochemical staining procedure is used to check the appearance of the mutant p53 gene [81]. Salivary biomarkers such as circulating tumor DNA, miRNAs, and extracellular vesicles assist in the early detection of OSCC [82]. Numerous advanced methodologies such as ELISA, immunohistochemistry, immunoprecipitation, chromatography, polymerase chain reaction, mass spectroscopy, DNA arrays, nuclear magnetic resonance, gene-expression arrays, ribonucleoprotein immunoprecipitation gene chip, liquid chromatography, mass spectroscopy, etc. are used to detect the presence of biomarkers.
Recently, advancements are reported in biomarker detection such as the detection of cancer-derived exosomes. Exosomes are membrane-bound vesicles of endocytic origin that contain nucleic acids such as DNA, messenger RNA, micro RNA, or non-coding RNAs inside their lumen originating from the cytoplasm of the cell. These are secreted in abundance and are usually found in body fluids in high concentrations. The proteins found on the surface of exosomes can act as antigens for specific antibodies that can further be used for affinity-based methods for isolating desirable exosomes. The cancer-derived exosomes are reported to represent tumor micro-environment and thus, can effectively be used as a biomarker for tumor detection [83]. These membrane-bound vesicles are typically 30–150 nm in size and are present in densities of 1.13–1.19 g/ml in body fluids, which makes them excellent candidates for detection [84]. The lipid bilayer of exosomes is composed of transmembrane receptors, proteases, tetraspanins, and adhesion molecules and is enriched on cholesterol, phosphatidylserine, and ceramide. Some cancer-associated proteins are also highly expressed on the surface of exosomes that can be used as differentiation markers between various types of cancers. Proteins such as HER2, LMP1, and MUC18 can be used as targets for the development of biosensors for total exosomal detection. A particular nucleic acid, miR-24–3P (miRNA) found in the saliva, is highly expressed in patients with OSCC. Based on this, recently, a potential biosensor is developed that uses miRNA as a target for the diagnosis of oral cancer [85].
Nanobiosensors-based lateral flow immunosensing have revolutionized the field of diagnostics [[86], [87], [88], [89], [90], [91]] and present tremendous potential for enhancement in oral cancer diagnostics. The field of biosensing has emerged in the recent past for the development of techniques and devices that can precisely target cancer cells or its biomarkers. Moreover, biosensors utilizing nano-engineered particles have shown potential applications in the diagnosis of Coronavirus Disease – 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus – 2 (SARS-CoV-2) which is causing the ongoing pandemic [92,93]. Biosensors utilizing nanomaterials have also been employed for whole-cell detection which has been termed as cytosensing [94]. Cytosensing approaches have gained attention in cancer diagnosis and have been used for the detection of oral cancer. During the last few years, numerous biosensors have been developed that can be classified into various types such as electrochemical, optical, paper-based, etc. Electrochemical biosensors are the most advanced and have wide applicability due to their quantitation ability and low limit of detection and have majorly been used for cancer detection on a global scale [[95], [96], [97], [98]]. An electrochemical biosensor usually comprises an electrode system that relies on the physiochemical transducer to transform a biochemical signal into a quantifiable one. A significant advantage of an electrochemical biosensor is that it is not affected by the matrix in which the biochemical reaction takes place and thus, reduces signal noise due to interferences [98]. Consequently, this type of sensor can be used in a variety of colored medium and body fluids. An electrochemical biosensor typically consists of four major types of transducing elements including impedimetric (a measurement of charge transfer), voltammetric (a measurement of voltage change), potentiometric (a measurement of electric potential), and amperometric (a measurement of current change). Based on these transducers, electrochemical biosensors can be employed to detect multiple target analytes and consists of a range of transducing capability. A magnetically controllable electrochemical sensor has been reported by Wang et al., for the detection of microRNA correlated to oral cancer with sensitivity up to the attomolar level [99]. A novel ‘junction probe’ strategy is applied to capture the signal generated by the biochemical reaction and is detected through voltage change. Biological reactions such as nuclease-assisted target recycling of DNAzyme have been to develop electrochemical biosensors for the analysis of oral cancer-related DNA in saliva samples [100]. In another work, cancer biomarker cytokeratin-fragment-21-1 was detected using a cerium oxide nanocubes-reduced graphene oxide-based nanocomposite. This electrochemical detection technique comprised of the thin films of the nanocomposite spin-coated onto electrodes with a specific antibody against the biomarker and utilized pulse voltammetry for signal detection [101]. A similar biosensor was developed that detects the cytokeratin-fragment-21-1 biomarker using a nanostructured yttrium oxide, nY2O3 [102]. Saliva can be explored to develop point-of-care devices for the detection of oral cancer since it is comparatively easy to collect and analyze than any other body fluids. Advanced electrochemical platforms or simple paper-strip chromatographs are commercially available for clinical use that detects either a single salivary biomarker or a range of presented biomarkers. Few advanced detection formats have also been developed, which are commercially available as ‘Nano-biochips’ and ‘cytology-on-chips’. These devices integrate simple microfluidics with immunohistochemistry and fluorescence microscopy imaging for sensing atypical surface features in liquid biopsies [103].
3.8. Other methods
In recent years, significant advancements have been reported for the diagnostic techniques that are rapid and specific for the detection of cancer biomarkers. Raman spectroscopy probes the unique vibrational fingerprint of molecules assisting in analyte identification. Using Raman spectra, the differences in protein, amino acid, and beta carotene can be determined, which are excellent biomolecular differences markers for cancer detection [104]. Fourier transform infrared (FTIR) spectroscopy associated with computational systems has been used to distinguish between benign and malignant tumors accurately [105]. In another study from Gujarat, the micronuclei (MN) index, which is a good prognostic indicator or biomarker of the epithelial carcinogenesis, was studied. A step-wise increase in the MN index was observed from the control population to the pre-cancer patients to the cancer patients by 1.14 to 2.63 to 4.88, respectively [106]. In a recent study by Ghosh et al., Raman and FTIR data of exfoliated cells were used to distinguish the stages of oral cancer [107]. Few other spectroscopic methods used to detect the oral malignancy are elastic scattering spectroscopy [25], diffuse reflectance spectroscopy [22], and optical coherence tomography [44]. Light-based-detection systems (LBDS) have gained importance in the field of oral cancer diagnostics owing to its simplicity and ease of use. These devices are based on phenomena such as chemiluminescence, autofluorescence, etc. Devices such as ViziLite, Velscope, Identafi, Microlux/DL, etc. are commercially available and used in clinical settings. These devices utilize the quantum dots (semiconductor nanoparticle) that can confer advantages over conventional fluorescent dyes and fluorescent proteins [108].
Confocal microscopy is another light-based technique that provides images of many important cellular and architectural features of squamous cell carcinoma (SCC). This reflectance imaging technique is cost-effective, provides optical sections and high-resolution images. The confocal imaging of an oral cavity obtained by miniaturized fiber-optic confocal reflectance microscope shows the features such as nuclear irregularity changes, enlargement, crowding, the difference in nuclear to cytoplasmic ratio, changing capillary networks, and spacing, which is useful in differentiating the OSCC from the normal oral mucosa [109].
4. Summary and future perspectives
India is considered as the world capital for oral cancer cases as it shares one-third of the global burden. Southern parts of India present the highest incidence rate of oral cancer amongst the female population in India as well as worldwide. The genetic and epigenetic are the two major aspects that impact the occurrence of oral malignancy most. Various factors e.g. tobacco, radiation, immunosuppression, alcohol, diet and nutrition, oral thrust, use of mouthwash, dental issues, etc. play an important role in incidence. Poor oral health and HPV infection are two other major causes of the occurrence of oral cancer. About 60–80% of the patients in India suffering from oral cancer are detected in the advanced stages in comparison to 40% in developed countries, which contributes to an increased mortality rate. The financial burden towards the patient is very high during the treatment of oral cancer and most of the patients leave the treatment midway, which further adds to the mortality rate. The treatment of oral malignancy primarily depends on the location and size of the tumor, and the feasibility of organ preservation in patients. Radiotherapy and surgery are recommended modalities in the early stage of oral cancer. Prevention, early diagnosis, and timely treatment are critical aspects to tackle oral cancer-related burden in India. The awareness needs to be spread among the population about the causes and fatalities of oral cancer; the importance of quitting tobacco, alcohol, and maintaining oral hygiene.
Comprehending the importance, numerous research groups across the globe are working on techniques that could aid in early diagnosis of oral cancer. Apart from physical examination, the other techniques recommended are, 1) X-Rays, 2) CT, 3) PET, 4) MRI, and 5) Endoscopy. Moreover, histopathological examination, vital staining techniques, biopsies such as brush biopsy, biomarker detection with biosensors or immunohistochemistry, radiology, and optical imaging systems are the most commonly used methods for diagnosis of oral cancer in India. Medical and technological research institutions in India have been working in collaboration to develop and deliver innovative technologies to fulfill the requirement of a huge and diverse population. Various diagnostic techniques have been reported that could differentiate between benign and malignant tumors. The conventional diagnostic methodologies are expensive, time-consuming, need expert technicians, sometimes require surgical intervention, etc. Recently reported biosensor-based oral cancer biomarker sensing techniques to possess huge potential to be incorporated in diagnostic practices. Although numerous studies have been published, non-invasive, portable, easy-to-use, rapid, cost-effective techniques that do not require a skilled professional to process, analyze, and interpret the test results are still not available in India. Emerging advanced commercialized techniques need encouragement by professionals for integration in clinical diagnostic practices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Dr. Vivek Borse would like to acknowledge the Department of Science and Technology, Ministry of Science and Technology, Government of India for the INSPIRE Faculty Award (IFA18-ENG266, DST/INSPIRE/04/2018/000991).
References
- 1.Gupta B., Bray F., Kumar N., Johnson N.W. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: a case–control study from India. Cancer Epidemiol. 2017;51:7–14. doi: 10.1016/j.canep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Laprise C., Shahul H.P., Madathil S.A., Thekkepurakkal A.S., Castonguay G., Varghese I., Shiraz S., Allison P., Schlecht N.F., Rousseau M.C., Franco E.L., Nicolau B. Periodontal diseases and risk of oral cancer in Southern India: results from the HeNCe Life study. Int. J. Canc. 2016;139:1512–1519. doi: 10.1002/ijc.30201. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S., Satyanarayana L., Asthana S., Shivalingesh K.K., Goutham B.S., Ramachandra S. Oral cancer statistics in India on the basis of first report of 29 population-based cancer registries. J. Oral Maxillofac. Pathol. 2018;22:18–26. doi: 10.4103/jomfp.JOMFP_113_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veluthattil A., Sudha S., Kandasamy S., Chakkalakkoombil S. Effect of hypofractionated, palliative radiotherapy on quality of life in late-stage oral cavity cancer: a prospective clinical trial. Indian J. Palliat. Care. 2019;25:383. doi: 10.4103/IJPC.IJPC_115_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajay P., Ashwinirani S., Nayak A., Suragimath G., Kamala K., Sande A., Naik R. Oral cancer prevalence in Western population of Maharashtra, India, for a period of 5 years. J. Oral Res. Rev. 2018;10:11. doi: 10.4103/jorr.jorr_23_17. [DOI] [Google Scholar]
- 6.Singh M., Prasad C.P., Singh T.D., Kumar L. Cancer research in India: challenges & opportunities. Indian J. Med. Res. 2018;148:362–365. doi: 10.4103/ijmr.IJMR_1711_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varshitha A. Prevalence of oral cancer in India. J. Pharmaceut. Sci. Res. 2015;7:845–848. [Google Scholar]
- 8.Gupta S., Gupta R., Sinha D.N., Mehrotra R. Relationship between type of smokeless tobacco & risk of cancer: a systematic review. Indian J. Med. Res. Suppl. 2018;148:56–76. doi: 10.4103/ijmr.IJMR_2023_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asthana S., Patil R.S., Labani S. Tobacco-related cancers in India: a review of incidence reported from population-based cancer registries. Indian J. Med. Paediatr. Oncol. 2016;37:152–157. doi: 10.4103/0971-5851.190357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadashetti V., Shivakumar K., Chaudhary M., Patil S., Gawande M., Hande A. Influence of risk factors on patients suffering from potentially malignant disorders and oral cancer: a case-control study. J. Oral Maxillofac. Pathol. 2017;21:455–456. doi: 10.4103/jomfp.JOMFP_236_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik A., Mishra A., Garg A., Shetty R., Mair M., Chakrabarti S., Nair D., Balasubramaniam G., Chaturvedi P. Trends of oral cancer with regard to age, gender, and subsite over 16 years at a tertiary cancer center in India. Indian J. Med. Paediatr. Oncol. 2018;39:297. doi: 10.4103/ijmpo.ijmpo_6_17. [DOI] [Google Scholar]
- 12.Murugesan A. Sabarinath, Sivapathasundharam, Awareness of oral cancer among medical students in Chennai. J. Med. Radiol. Pathol. Surg. 2016;2:18–22. doi: 10.15713/ins.jmrps.58. [DOI] [Google Scholar]
- 13.Singh V., Singh A.K., Dutta K.D., Kumar N., Kumari A. Evaluation of quality of life and the nutritional status of oral cancer treated patients as compared with the control group in Varanasi district: a cross sectional study. Int. J. Community Med. Public Heal. 2019;6:4804. doi: 10.18203/2394-6040.ijcmph20195059. [DOI] [Google Scholar]
- 14.Thavarool S.B., Muttath G., Nayanar S., Duraisamy K., Bhat P., Shringarpure K., Nayak P., Tripathy J.P., Thaddeus A., Philip S., Satheesan B. Improved survival among oral cancer patients: findings from a retrospective study at a tertiary care cancer centre in rural Kerala, India. World J. Surg. Oncol. 2019;17:1–7. doi: 10.1186/s12957-018-1550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oral cancer screening in Mumbai, India by primary health care workers. https://clinicaltrials.gov/ct2/show/NCT00655421 Full Text View - ClinicalTrials.gov, (n.d.)
- 16.Sankaranarayanan R., Ramadas K., Thomas G., Muwonge R., Thara S., Mathew B., Rajan B. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365:1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 17.De Veld D.C.G., Witjes M.J.H., Sterenborg H.J.C.M., Roodenburg J.L.N. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41:117–131. doi: 10.1016/j.oraloncology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Babshet M., Pervatikar S., Nandimath K., Naikmasur V. Efficacy of oral brush cytology in the evaluation of the oral premalignant and malignant lesions. J. Cytol. 2011;28:165. doi: 10.4103/0970-9371.86342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y., Rhodus N.L., Ondrey F.G., Wuertz B.R.K., Chen X., Zhu Y., Griffin T.J. Quantitative proteomic analysis of oral brush biopsies identifies secretory leukocyte protease inhibitor as a promising, mechanism-based oral cancer biomarker. PloS One. 2014;9 doi: 10.1371/journal.pone.0095389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueiredo P.T.D.S., Leite A.F., Freitas A.C., Nascimento L.A., Cavalcanti M.G., Melo N.S., Guerra E.N. Comparison between computed tomography and clinical evaluation in tumour/node stage and follow-up of oral cavity and oropharyngeal cancer. Dentomaxillofacial Radiol. 2010;39:140–148. doi: 10.1259/dmfr/69910245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark A.L., Gillenwater A.M., Collier T.G., Alizadeh-Naderi R., El-Naggar A.K., Richards-Kortum R.R. Confocal microscopy for real-time detection of oral cavity neoplasia. Clin. Canc. Res. 2003;9:4714–4721. [PubMed] [Google Scholar]
- 22.Jayanthi J.L., Nisha G.U., Manju S., Philip E.K., Jeemon P., Baiju K.V., Beena V.T., Subhash N. Diffuse reflectance spectroscopy: diagnostic accuracy of a non-invasive screening technique for early detection of malignant changes in the oral cavity. BMJ Open. 2011;1 doi: 10.1136/bmjopen-2011-000071. e000071–e000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi P., Jordan C.D., Mendez E., Houck J., Yueh B., Farwell D.G., Futran N., Chen C. Examination of oral cancer biomarkers by tissue microarray analysis. Arch. Otolaryngol. Neck Surg. 2008;134:539. doi: 10.1001/archotol.134.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suhr Lunde M.L., Warnakulasuriya S., Sand L., Hirsch J.M., Vasstrand E.N., Ibrahim S.O. Gene expression analysis by cDNA microarray in oral cancers from two western populations. Anticancer Res. 2010;30:1083–1091. [PubMed] [Google Scholar]
- 25.Jerjes W., Swinson B., Johnson K.S., Thomas G.J., Hopper C. Assessment of bony resection margins in oral cancer using elastic scattering spectroscopy: a study on archival material. Arch. Oral Biol. 2005;50:361–366. doi: 10.1016/j.archoralbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Feng Y., Li Q., Chen J., Yi P., Xu X., Fan Y., Cui B., Yu Y., Li X., Du Y., Chen Q., Zhang L., Jiang J., Zhou X., Zhang P. Salivary protease spectrum biomarkers of oral cancer. Int. J. Oral Sci. 2019;11:7. doi: 10.1038/s41368-018-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigum S.E., Floriano P.N., Redding S.W., Yeh C.K., Westbrook S.D., McGuff H.S., Lin A., Miller F.R., Villarreal F., Rowan S.D., Vigneswaran N., Williams M.D., McDevitt J.T. Nano-Bio-Chip sensor platform for examination of oral exfoliative cytology. Canc. Prev. Res. 2010;3:518–528. doi: 10.1158/1940-6207.CAPR-09-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaitley S., Agarwal P., Upadhyay R. Role of oral exfoliative cytology in predicting premalignant potential of oral submucous fibrosis: a short study. J. Canc. Res. Therapeut. 2015;11:471–474. doi: 10.4103/0973-1482.151421. [DOI] [PubMed] [Google Scholar]
- 29.Saleh H.A., Clayman L., Masri H. Fine needle aspiration biopsy of intraoral and oropharyngeal mass lesions. CytoJournal. 2008;5:4. doi: 10.1186/1742-6413-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.dos Santos A.P.C., Sugaya N.N., Pinto Junior D. dos S., Junior C.A.L. Fine needle aspiration biopsy in the oral cavity and head and neck region. Braz. Oral Res. 2011;25:186–191. doi: 10.1590/S1806-83242011000200015. [DOI] [PubMed] [Google Scholar]
- 31.Menzies G.E., Fox H.R., Marnane C., Pope L., Prabhu V., Winter S., Derrick A.V., Lewis P.D. Fourier transform infrared for noninvasive optical diagnosis of oral, oropharyngeal, and laryngeal cancer. Transl. Res. 2014;163:19–26. doi: 10.1016/j.trsl.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Patil A., Prabhu V., Choudhari K.S., Unnikrishnan V.K., George S.D., Ongole R., Pai K.M., Shetty J.K., Bhat S., Kartha V.B., Chidangil S. Evaluation of high-performance liquid chromatography laser-induced fluorescence for serum protein profiling for early diagnosis of oral cancer. J. Biomed. Optic. 2010;15 doi: 10.1117/1.3523372. [DOI] [PubMed] [Google Scholar]
- 33.Dascălu I.T. Histopathological aspects in oral squamous cell carcinoma. Open Access J. Dent. Sci. 2018;3 doi: 10.23880/oajds-16000173. [DOI] [Google Scholar]
- 34.Ulaganathan G., Mohamed Niazi Kt, Srinivasan S., Balaji V., Manikandan D., Shahul Hameed K.K., Banumathi A. A clinicopathological study of various oral cancer diagnostic techniques. J. Pharm. BioAllied Sci. 2017;9:S4–S10. doi: 10.4103/jpbs.JPBS_110_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusukawa J., Suefuji Y., Ryu F., Noguchi R., Iwamoto O., Kameyama T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J. Oral Pathol. Med. 2000;29:303–307. doi: 10.1034/j.1600-0714.2000.290703.x. [DOI] [PubMed] [Google Scholar]
- 36.Muruganandhan J., Sujatha G., Patil S., Raj A.T. Laser capture microdissection in oral cancer. J. Contemp. Dent. Pract. 2018;19:475–476. doi: 10.5005/jp-journals-10024-2286. [DOI] [PubMed] [Google Scholar]
- 37.Wang R., Yuan Y., Zhou Y., Zhang D., Zhang L., Zeng X., Ji N., Zhou M., Liang X., Chen Y., Geng N., Li J., Chen Q. Screening diagnostic biomarkers of OSCC via an LCM-based proteomic approach. Oncol. Rep. 2018;40:2088–2096. doi: 10.3892/or.2018.6610. [DOI] [PubMed] [Google Scholar]
- 38.Mallia R.J., Thomas S.S., Mathews A., Kumar R R., Sebastian P., Madhavan J., Subhash N. Laser-induced autofluorescence spectral ratio reference standard for early discrimination of oral cancer. Cancer. 2008;112:1503–1512. doi: 10.1002/cncr.23324. [DOI] [PubMed] [Google Scholar]
- 39.Lousada-Fernandez F., Rapado-Gonzalez O., Lopez-Cedrun J.-L., Lopez-Lopez R., Muinelo-Romay L., Suarez-Cunqueiro M. Liquid biopsy in oral cancer. Int. J. Mol. Sci. 2018;19:1704. doi: 10.3390/ijms19061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh A., Thukral C.L., Gupta K., Sood A.S., Singla H., Singh K. Role of MRI in evaluation of malignant lesions of tongue and oral cavity. Pol. J. Radiol. 2017;82:92–99. doi: 10.12659/PJR.899352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bednarczyk K., Gawin M., Chekan M., Kurczyk A., Mrukwa G., Pietrowska M., Polanska J., Widlak P. Discrimination of normal oral mucosa from oral cancer by mass spectrometry imaging of proteins and lipids. J. Mol. Histol. 2019;50:1–10. doi: 10.1007/s10735-018-9802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandya D., Nagarajappa A., Kumar, Reddy S., Bhasin M. Lab-on-a-Chip – oral cancer diagnosis at your door step. J. Int. Oral Health. 2015;7:122–128. [Google Scholar]
- 43.Roblyer D., Richards-Kortum R., Sokolov K., El-Naggar A.K., Williams M.D., Kurachi C., Gillenwater A.M. Multispectral optical imaging device for in vivo detection of oral neoplasia. J. Biomed. Optic. 2008;13 doi: 10.1117/1.2904658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidari A.E., Suresh A., Kuriakose M.A., Chen Z., Wilder-Smith P., Sunny S.P., James B.L., Lam T.M., Tran A.V., Yu J., Ramanjinappa R.D., K U., Birur P. Optical coherence tomography as an oral cancer screening adjunct in a low resource settings. IEEE J. Sel. Top. Quant. Electron. 2019;25:1–8. doi: 10.1109/JSTQE.2018.2869643. [DOI] [Google Scholar]
- 45.Reddy R.S., Sai Praveen K.N. Optical coherence tomography in oral cancer: a transpiring domain. J. Canc. Res. Therapeut. 2017;13:883–888. doi: 10.4103/0973-1482.180684. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg M.A., Estefan D.J. Assessing oral malignancies. Am. Fam. Physician. 2002;65:1–5. [PubMed] [Google Scholar]
- 47.Sajan T., Murthy S., Krishnankutty R., Mitra J. A rapid, early detection of oral squamous cell carcinoma: real time PCR based detection of tetranectin. Mol. Biol. Res. Commun. 2019;8:33–40. doi: 10.22099/mbrc.2019.31544.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohsako T., Shimamoto H., Tomioka H., Hirai H., Kuroshima T., Mochizuki Y., Kugimoto T., Tsushima F., Nakamura S., Kurabayashi T., Harada H. Detection of extraoral primary cancers by positron emission tomography/computed tomography in patients with oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020;129:272–276. doi: 10.1016/j.oooo.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Barroso E.M., ten Hove I., Bakker Schut T.C., Mast H., van Lanschot C.G.F., Smits R.W.H., Caspers P.J., Verdijk R., Noordhoek Hegt V., Baatenburg de Jong R.J., Wolvius E.B., Puppels G.J., Koljenović S. Raman spectroscopy for assessment of bone resection margins in mandibulectomy for oral cavity squamous cell carcinoma. Eur. J. Canc. 2018;92:77–87. doi: 10.1016/j.ejca.2018.01.068. [DOI] [PubMed] [Google Scholar]
- 50.Malik A., Sahu A., Singh S.P., Deshmukh A., Chaturvedi P., Nair D., Nair S., Murali Krishna C. In vivo Raman spectroscopy–assisted early identification of potential second primary/recurrences in oral cancers: an exploratory study. Head Neck. 2017;39:2216–2223. doi: 10.1002/hed.24884. [DOI] [PubMed] [Google Scholar]
- 51.Papamarkakis K., Bird B., Schubert J.M., Miljković M., Wein R., Bedrossian K., Laver N., Diem M. Cytopathology by optical methods: spectral cytopathology of the oral mucosa. Lab. Invest. 2010;90:589–598. doi: 10.1038/labinvest.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joshi P., Pol J., Sudesh A. Ultrasonography - a diagnostic modality for oral and maxillofacial diseases. Contemp. Clin. Dent. 2014;5:345. doi: 10.4103/0976-237X.137942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawan D., Mashlah A. Evaluation of premalignant and malignant lesions by fluorescent light (VELscope) J. Int. Soc. Prev. Community Dent. 2015;5:248. doi: 10.4103/2231-0762.159967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sreeshyla H., Sudheendra U., Shashidara R. Vital tissue staining in the diagnosis of oral precancer and cancer: stains, technique, utility, and reliability. Clin. Cancer Investig. J. 2014;3:141. doi: 10.4103/2278-0513.130156. [DOI] [Google Scholar]
- 55.Sambandham T., Masthan K.M.K., Sathish Kumar M., Jha A. The application of Vizilite in oral cancer. J. Clin. Diagn. Res. 2013;7:184–185. doi: 10.7860/JCDR/2012/5163:2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diagnosis of oral cancer - Canadian cancer society. https://www.cancer.ca/en/cancer-information/cancer-type/oral/diagnosis/?region=on (n.d.)
- 57.Mangalath U., Mikacha M.K., Abdul Khadar A.H., Aslam S., Francis P., Kalathingal J. Recent trends in prevention of oral cancer. J. Int. Soc. Prev. Community Dent. 2014;4:131. doi: 10.4103/2231-0762.149018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasan S., Elongovan S. Conventional and advanced diagnostic aids in oral cancer screening – the journey so far. Int. J. Pharm. Pharmaceut. Sci. 2015;7:29–33. [Google Scholar]
- 59.Sreeshyla H., Sudheendra U., Shashidara R. Vital tissue staining in the diagnosis of oral precancer and cancer: stains, technique, utility, and reliability. Clin. Cancer Investig. J. 2014;3:141. doi: 10.4103/2278-0513.130156. [DOI] [Google Scholar]
- 60.Nagaraju K., Prasad S., Ashok L. Diagnostic efficiency of toluidine blue with Lugol's iodine in oral premalignant and malignant lesions. Indian J. Dent. Res. 2010;21:223. doi: 10.4103/0970-9290.66633. [DOI] [PubMed] [Google Scholar]
- 61.Chaudhari A., Hegde-Shetiya S., Shirahatti R., Agrawal D. Comparison of different screening methods in estimating the prevalence of precancer and cancer amongst male inmates of a jail in Maharashtra, India. Asian Pac. J. Cancer Prev. APJCP. 2013;14:859–864. doi: 10.7314/APJCP.2013.14.2.859. [DOI] [PubMed] [Google Scholar]
- 62.Tests for oral cavity and oropharyngeal cancers. https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/detection-diagnosis-staging/how-diagnosed.html n.d. accessed March 23, 2020.
- 63.Mehrotra R., Mishra S., Singh M., Singh M. The efficacy of oral brush biopsy with computer-assisted analysis in identifying precancerous and cancerous lesions. Head Neck Oncol. 2011;3:39. doi: 10.1186/1758-3284-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skandarajah A., Sunny S.P., Gurpur P., Reber C.D., D'Ambrosio M.V., Raghavan N., James B.L., Ramanjinappa R.D., Suresh A., Kandasarma U., Birur P., Kumar V.V., Galmeanu H.C., Itu A.M., Modiga-Arsu M., Rausch S., Sramek M., Kollegal M., Paladini G., Kuriakose M., Ladic L., Koch F., Fletcher D. Mobile microscopy as a screening tool for oral cancer in India: a pilot study. PloS One. 2017;12 doi: 10.1371/journal.pone.0188440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehrotra R., Gupta A., Singh M., Ibrahim R. Application of cytology and molecular biology in diagnosing premalignant or malignant oral lesions. Mol. Canc. 2006;5 doi: 10.1186/1476-4598-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Chandra A., Gupta S., Verma R., Verma R., Singh A., Badni M. Evaluation of exfoliative cytology in the diagnosis of oral premalignant and malignant lesions: a cytomorphometric analysis. Dent. Res. J. 2015;12:83. doi: 10.4103/1735-3327.150339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaitley S., Agarwal P., Upadhyay R. Role of oral exfoliative cytology in predicting premalignant potential of oral submucous fibrosis: a short study. J. Canc. Res. Therapeut. 2015;11:471–474. doi: 10.4103/0973-1482.151421. [DOI] [PubMed] [Google Scholar]
- 68.Shaila M., Shetty P., Pai P. A new approach to exfoliative cytology: a comparative cytomorphometric study. Indian J. Canc. 2016;53:193. doi: 10.4103/0019-509X.180830. [DOI] [PubMed] [Google Scholar]
- 69.Louise Sylvie Avon, Hagen Klieb B.E. Oral soft tissue biopsy:an overview. J. Can. Dent. Assoc. 2012;78:c75. [PubMed] [Google Scholar]
- 70.Zargaran M. A review of biopsy in dentistry: principles, techniques, and considerations. J. Dent. Mater. Tech. 2014;3:47–54. [Google Scholar]
- 71.Pałasz P., Adamski Ł., Górska-Chrząstek M., Starzyńska A., Studniarek M. Contemporary diagnostic imaging of oral squamous cell carcinoma – a review of literature. Pol. J. Radiol. 2017;82:193–202. doi: 10.12659/PJR.900892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolzoni A., Cappiello J., Piazza C., Peretti G., Maroldi R., Farina D., Nicolai P. Diagnostic accuracy of magnetic resonance imaging in the assessment of mandibular involvement in oral-oropharyngeal squamous cell carcinoma: a prospective study. Arch. Otolaryngol. Head Neck Surg. 2004;130:837–843. doi: 10.1001/archotol.130.7.837. [DOI] [PubMed] [Google Scholar]
- 73.Moreira M.A., Lessa L.S., Bortoli F.R., Lopes A., Xavier E.P., Ceretta R.A., Faustini Sônego F.G., Tomasi C.D., Pires P.D.S., Ceretta L.B., Schweigert Perry I.D., Simões P.W. Meta-analysis of magnetic resonance imaging accuracy for diagnosis of oral cancer. PloS One. 2017;12 doi: 10.1371/journal.pone.0177462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schöder H., Carlson D.L., Kraus D.H., Stambuk H.E., Gönen M., Erdi Y.E., Yeung H.W.D., Huvos A.G., Shah J.P., Larson S.M., Wong R.J. 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J. Nucl. Med. 2006;47:755–762. [PubMed] [Google Scholar]
- 75.Masthan K.M.K., Aravindha Babu N., Dash K.C., Elumalai M. Advanced diagnostic aids in oral cancer. Asian Pac. J. Cancer Prev. APJCP. 2012;13:3573–3576. doi: 10.7314/APJCP.2012.13.8.3573. [DOI] [PubMed] [Google Scholar]
- 76.Jayanthi J.L., Subhash N., Stephen M., Philip E.K., Beena V.T. Comparative evaluation of the diagnostic performance of autofluorescence and diffuse reflectance in oral cancer detection: a clinical study. J. Biophot. 2011;4:696–706. doi: 10.1002/jbio.201100037. [DOI] [PubMed] [Google Scholar]
- 77.Mehrotra R., Gupta D.K. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head Neck Oncol. 2011;3:33. doi: 10.1186/1758-3284-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papamarkakis K., Bird B., Schubert J.M., Miljković M., Wein R., Bedrossian K., Laver N., Diem M. Cytopathology by optical methods: spectral cytopathology of the oral mucosa. Lab. Invest. 2010;90:589–598. doi: 10.1038/labinvest.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Padmavathi G., Bordoloi D., Banik K., Kunnumakkara A.B. Next Gener. Point-of-Care Biomed. Sensors Technol. Cancer Diagnosis. Springer Singapore; 2017. Cancer biomarkers: important tools for cancer diagnosis and prognosis; pp. 1–29. [DOI] [Google Scholar]
- 80.Chandra P., Tan Y.N., Singh S.P. Springer Singapore; 2017. Next Generation Point-Of-Care Biomedical Sensors Technologies for Cancer Diagnosis. [DOI] [Google Scholar]
- 81.Ulaganathan G., Mohamed Niazi K., Srinivasan S., Balaji V., Manikandan D., Shahul Hameed K., Banumathi A. A clinicopathological study of various oral cancer diagnostic techniques. J. Pharm. BioAllied Sci. 2017;9:S4–S10. doi: 10.4103/jpbs.JPBS_110_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cristaldi M., Mauceri R., Di Fede O., Giuliana G., Campisi G., Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: current status and perspectives. Front. Physiol. 2019;10:1476. doi: 10.3389/fphys.2019.01476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma S., Zuñiga F., Rice G.E., Perrin L.C., Hooper J.D., Salomon C. Tumor-derived exosomes in ovarian cancer - liquid biopsies for early detection and real-time monitoring of cancer progression. Oncotarget. 2017;8:104687–104703. doi: 10.18632/oncotarget.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng N., Du D., Wang X., Liu D., Xu W., Luo Y., Lin Y. Recent advances in biosensors for detecting cancer-derived exosomes. Trends Biotechnol. 2019;37:1236–1254. doi: 10.1016/j.tibtech.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 85.He L., Ping F., Fan Z., Zhang C., Deng M., Cheng B., Xia J. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed. Pharmacother. 2020;121:109553. doi: 10.1016/j.biopha.2019.109553. [DOI] [PubMed] [Google Scholar]
- 86.Konwar A.N., Borse V. Current status of point-of-care diagnostic devices in the Indian healthcare system with an update on COVID-19 pandemic. Sensors Int. 2020;1:100015. doi: 10.1016/j.sintl.2020.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borse V.B., Konwar A.N., Jayant R.D., Patil P.O. Perspectives of characterization and bioconjugation of gold nanoparticles and their application in lateral flow immunosensing. Drug Deliv. Transl. Res. 2020;10:878–902. doi: 10.1007/s13346-020-00771-y. [DOI] [PubMed] [Google Scholar]
- 88.Borse V., Srivastava R. Nanobiomaterial Eng. Springer Singapore; Singapore: 2020. Nanobiotechnology advancements in lateral flow immunodiagnostics; pp. 181–204. [DOI] [Google Scholar]
- 89.Borse V., Srivastava R. Process parameter optimization for lateral flow immunosensing. Mater. Sci. Energy Technol. 2019;2:434–441. doi: 10.1016/j.mset.2019.04.003. [DOI] [Google Scholar]
- 90.Borse V., Srivastava R. Fluorescence lateral flow immunoassay based point-of-care nanodiagnostics for orthopedic implant-associated infection. Sensor. Actuator. B Chem. 2019;280:24–33. doi: 10.1016/j.snb.2018.10.034. [DOI] [Google Scholar]
- 91.Borse V.B., Konwar A.N., Srivastava R. Handb. Miniaturization Anal. Chem. Elsevier; 2020. Nanobiotechnology approaches for miniaturized diagnostics; pp. 297–333. [DOI] [Google Scholar]
- 92.Chandra P. Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis. Sensors Int. 2020;1:100019. doi: 10.1016/j.sintl.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mahapatra S., Chandra P. Clinically practiced and commercially viable nanobio engineered analytical methods for COVID-19 diagnosis. Biosens. Bioelectron. 2020;165:112361. doi: 10.1016/j.bios.2020.112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Purohit B., Kumar A., Mahato K., Roy S., Chandra P. Nanotechnol. Mod. Anim. Biotechnol. Concepts Appl. Elsevier; 2019. Cancer cytosensing approaches in miniaturized settings based on advanced nanomaterials and biosensors; pp. 133–147. [DOI] [Google Scholar]
- 95.Mahato K., Kumar S., Srivastava A., Maurya P.K., Singh R., Chandra P. Handb. Immunoass. Technol. Approaches, Performances, Appl. Elsevier; 2018. Electrochemical immunosensors: fundamentals and applications in clinical diagnostics; pp. 359–414. [DOI] [Google Scholar]
- 96.Kumar A., Purohit B., Maurya P.K., Pandey L.M., Chandra P. Engineered nanomaterial assisted signal-amplification strategies for enhancing analytical performance of electrochemical biosensors. Electroanalysis. 2019;31:1615–1629. doi: 10.1002/elan.201900216. [DOI] [Google Scholar]
- 97.Purohit B., Mahato K., Kumar A., Chandra P. Sputtering enhanced peroxidase like activity of a dendritic nanochip for amperometric determination of hydrogen peroxide in blood samples. Microchim. Acta. 2019;186:1–10. doi: 10.1007/s00604-019-3773-2. [DOI] [PubMed] [Google Scholar]
- 98.Mahato K., Kumar A., Maurya P.K., Chandra P. Shifting paradigm of cancer diagnoses in clinically relevant samples based on miniaturized electrochemical nanobiosensors and microfluidic devices. Biosens. Bioelectron. 2018;100:411–428. doi: 10.1016/j.bios.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Wang Z., Zhang J., Guo Y., Wu X., Yang W., Xu L., Chen J., Fu F. A novel electrically magnetic-controllable electrochemical biosensor for the ultra sensitive and specific detection of attomolar level oral cancer-related microRNA. Biosens. Bioelectron. 2013;45:108–113. doi: 10.1016/j.bios.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 100.Chen J., Zhang J., Guo Y., Li J., Fu F., Yang H.-H., Chen G. An ultrasensitive electrochemical biosensor for detection of DNA species related to oral cancer based on nuclease-assisted target recycling and amplification of DNAzyme. Chem. Commun. 2011;47:8004. doi: 10.1039/c1cc11929j. [DOI] [PubMed] [Google Scholar]
- 101.Pachauri N., Dave K., Dinda A., Solanki P.R. Cubic CeO 2 implanted reduced graphene oxide-based highly sensitive biosensor for non-invasive oral cancer biomarker detection. J. Mater. Chem. B. 2018;6:3000–3012. doi: 10.1039/C8TB00653A. [DOI] [PubMed] [Google Scholar]
- 102.Kumar Panwar, Kumar Augustine, Malhotra Biofunctionalized nanostructured yttria modified non-invasive impedometric biosensor for efficient detection of oral cancer. Nanomaterials. 2019;9:1190. doi: 10.3390/nano9091190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Panta P., Wong D.T.W. Oral Cancer Detect. Springer International Publishing; Cham: 2019. Saliva-based point-of-care in oral cancer detection: current trend and future opportunities; pp. 297–314. [DOI] [Google Scholar]
- 104.Jeng M.-J., Sharma M., Sharma L., Chao T.-Y., Huang S.-F., Chang L.-B., Wu S.-L., Chow L. Raman spectroscopy analysis for optical diagnosis of oral cancer detection. J. Clin. Med. 2019;8:1313. doi: 10.3390/jcm8091313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su K.Y., Lee W.L. Fourier transform infrared spectroscopy as a cancer screening and diagnostic tool: a review and prospects. Cancers. 2020;12 doi: 10.3390/cancers12010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagh A., Raval J., Aiyer R.G., Amin S. Micronuclei in exfoliated oral epithelial cells in tobacco users and controls with various oral lesions: a study from Gujarat, India. Indian J. Otolaryngol. Head Neck Surg. 2019;71:109–114. doi: 10.1007/s12070-018-1260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghosh A., Raha S., Dey S., Chatterjee K., Roy Chowdhury A., Barui A. Chemometric analysis of integrated FTIR and Raman spectra obtained by non-invasive exfoliative cytology for the screening of oral cancer. Analyst. 2019;144:1309–1325. doi: 10.1039/c8an02092b. [DOI] [PubMed] [Google Scholar]
- 108.Mascitti M., Orsini G., Tosco V., Monterubbianesi R., Balercia A., Putignano A., Procaccini M., Santarelli A. An overview on current non-invasive diagnostic devices in oral oncology. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vagish Kumar L. Microlux and in vivo confocal microscopy in the diagnosis of potentially malignant and malignant lesions of the oral cavity. Clin. Cancer Investig. J. 2015;4:478–479. doi: 10.4103/2278-0513.148934. [DOI] [Google Scholar]


