Supplemental Digital Content is available in the text.
Keywords: barotrauma, critical care, coronavirus disease 2019, mediastinal emphysema, pneumothorax, subcutaneous emphysema
Abstract
Importance:
Management of severe coronavirus disease 2019 relies on advanced respiratory support modalities including invasive mechanical ventilation, continuous positive airway pressure, and noninvasive ventilation, all of which are associated with the development of subcutaneous emphysema, pneumomediastinum, and pneumothorax (herein collectively termed barotrauma).
Objectives:
To assess the occurrence rate of barotrauma in severe coronavirus disease 2019 and to explore possible associated factors.
Design, Setting, and Participants:
A retrospective, single-center cohort study with nested case series, conducted at University Hospital Lewisham: a 450-bed general hospital in London, United Kingdom. All patients with confirmed coronavirus disease 2019 admitted to the critical care department from March 12, to April 12, 2020, were included.
Main Outcomes and Measures:
Patients were retrospectively screened for radiological evidence of barotrauma. Admission characteristics, modalities of respiratory support, and outcomes were compared between barotrauma and nonbarotrauma groups. Respiratory parameters in the period preceding barotrauma identification were recorded.
Results:
Of 83 admissions with coronavirus disease 2019, eight suffered barotrauma (occurrence rate 9.6%; 95% CI 4.3%–18.1%). Barotrauma cases had longer illness duration prior to critical care admission (10 vs 7 d; interquartile range, 8–14 and 6–10, respectively; p = 0.073) and were more often treated with continuous positive airway pressure or noninvasive ventilation as the initial modality of advanced respiratory support (87.5% vs 36.0%; p = 0.007). Patients managed with continuous positive airway pressure or noninvasive ventilation prior to the development of barotrauma had median minute ventilation of 16.2–19.9 and 21.3–22.7 L/min, respectively. Compared with the nonbarotrauma group, a higher proportion of patients with barotrauma had died (62.5% vs 43.2%), and a lower proportion of patients had been discharged (25.0% vs 53.3%) at 3-month follow-up.
Conclusions and Relevance:
Barotrauma appears to be a common complication of severe coronavirus disease 2019. Determining whether high minute ventilation while using continuous positive airway pressure or noninvasive ventilation predisposes patients to barotrauma requires further investigation.
The global spread of coronavirus disease 2019 (COVID-19) has caused significant pressure on healthcare systems, and critical care departments in particular. Countries that initially succeeded in controlling the infection face the prospect of further resurgences. With limited evidence for disease-modifying therapy, management of patients with severe disease has relied on respiratory support. When supplemental oxygen alone is insufficient, advanced respiratory support modalities, including invasive mechanical ventilation (IMV), continuous positive airway pressure (CPAP), and noninvasive ventilation (NIV), are required.
Recommendations for ventilation strategy are currently largely based on evidence from acute respiratory distress syndrome (ARDS) (1–3). For IMV, recommendations include using low tidal volume (VT), low plateau pressure, and high positive end-expiratory pressure (PEEP) ventilation in moderate to severe ARDS, along with prone positioning and judicious use of neuromuscular blocking agents. The best use of CPAP and NIV in severe COVID-19 is debated (4) but has been promoted in some guidelines (3, 5).
Subcutaneous emphysema, pneumomediastinum, and pneumothorax—herein collectively termed “barotrauma” (referring to the manifestation, rather than etiologic mechanism, of airway tract damage and resultant extra-alveolar air)—are known complications of all forms of positive pressure respiratory support and are associated with multiple organ failure and death (6). Barotrauma is a common complication of IMV in ARDS, with rates reported at 4.0–6.3% (7). Rates of CPAP- and NIV-associated barotrauma are not known but are reportedly rare (8–10). In initial reports, occurrence rate of barotrauma in COVID-19 has ranged from 1—2% in hospitalized patients (11, 12 to 5.9–15% in intubated patients (13, 14). Barotrauma in the absence of positive pressure respiratory support has also been reported in COVID-19 (15, 16), although such complications were more common during the severe acute respiratory syndrome outbreak of 2002–2004, where 19.5% of a hospitalized cohort developed pneumomediastinum (17, 18).
We performed a cohort study to assess the occurrence rate of barotrauma in critically ill COVID-19 patients admitted to the critical care department of University Hospital Lewisham in London, United Kingdom. We present this alongside comparisons of baseline characteristics and outcomes of patients with and without barotrauma. We additionally present a nested case series detailing the respiratory support provided to patients with barotrauma, highlighting factors of potential interest for further study.
MATERIALS AND METHODS
Setting and Context
University Hospital Lewisham is a 450-bed hospital in South-East London, which serves an ethnically and socioeconomically diverse local population. Pre pandemic, it had a 20-bed critical care unit with approximately 60 admissions per month. During the COVID-19 pandemic, critical care capacity was expanded to facilitate up to 36 patients receiving advanced respiratory support at any one time. A CPAP unit was also opened to which some critical care patients were stepped-down. All advanced respiratory support in the hospital was delivered in the critical care department and the CPAP unit.
Early in the COVID-19 pandemic, patients with a low Pao2 despite supplemental oxygen therapy were considered for early IMV, with an initial strategy of pressure-controlled ventilation aiming for a VT of 6 mL/kg of ideal body weight (IBW), peak inspiratory pressure (Pinsp) of less than 30 cm H2O, Pao2 of greater than 8 kPa, and arterial pH of greater than 7.25. Patients managed with IMV received sedation, with infusions of propofol and alfentanyl typically given first-line. Sedation was monitored according to the Richardson Agitation-Sedation Scale, aiming for –2 to –4. Where ventilatory targets were not met, responses included increasing sedation and the infusion of neuromuscular blocking agents, prone ventilation, infusion of sodium bicarbonate, or accepting arterial pH greater than 7.20 if the patient was hemodynamically stable.
From midway through the study period, CPAP or NIV was trialed when there were no contraindications. CPAP initiation pressures were typically 10 cm H2O. NIV was considered for type 2 respiratory failure or to assist with work of breathing, and initial PEEP and pressure support were typically both set at 5–10 cm H2O. Patients with deteriorating respiratory variables despite CPAP or NIV were escalated to IMV.
All patients admitted to critical care with suspected COVID-19 underwent testing with a uniplex reverse transcriptase polymerase chain reaction assay on nasopharyngeal swab, as per National Health Service England testing protocol (19). Where the initial COVID-19 test was negative but a reasonable index of suspicion remained, it was routine practice to obtain repeat sampling, including nondirected bronchial lavage where possible.
Inclusion Criteria
All patients admitted to the critical care department of University Hospital Lewisham with a molecular diagnosis of COVID-19 between March 12, 2020, and April 12, 2020, were included. These dates started from the first COVID-19 admission to critical care and spanned the peak of the local outbreak. Patients with possible COVID-19 but without a molecular diagnosis were excluded. Two CPAP unit admissions were never managed in a critical care environment and were excluded from this study.
Procedures
Clinical and laboratory data were collected retrospectively by reviewing electronic and written medical records. Age, sex, body mass index, self-reported ethnicity, Rockwood Clinical Frailty Scale, smoking history, history of underlying lung or connective tissue disease, critical care admission blood values, Pao2/Fio2 ratios, Acute Physiology and Chronic Health Evaluation (APACHE) II scores, and modes of respiratory support were collected. Electronic records were reviewed 3 months from the date of critical care admission to determine final outcomes (death, patient in critical care, patient on general ward, or discharge). All data were collected according to a standardized protocol to minimize recorder bias.
Imaging reports written by consultant radiologists as part of normal clinical practice in the 28 days following critical care admission were reviewed for all patients. Those with a radiological diagnosis of subcutaneous emphysema, pneumomediastinum, or pneumothorax were placed in the barotrauma group.
A radiological window was defined for barotrauma occurrence: the time between the last chest radiograph with no evidence of barotrauma and the first identification of barotrauma (Fig. 1). Respiratory variables were recorded hourly for the 48-hour period prior to barotrauma identification. Where the radiological window was wider than 48 hours, respiratory variables were recorded for the duration of this window. If the patient had been in critical care for less than 48 hours prior to barotrauma identification, then respiratory parameters were recorded from the point of critical care admission. Use of adjunctive therapies (e.g. proning, paralysis) and nonrespiratory organ support were also recorded.
Figure 1.
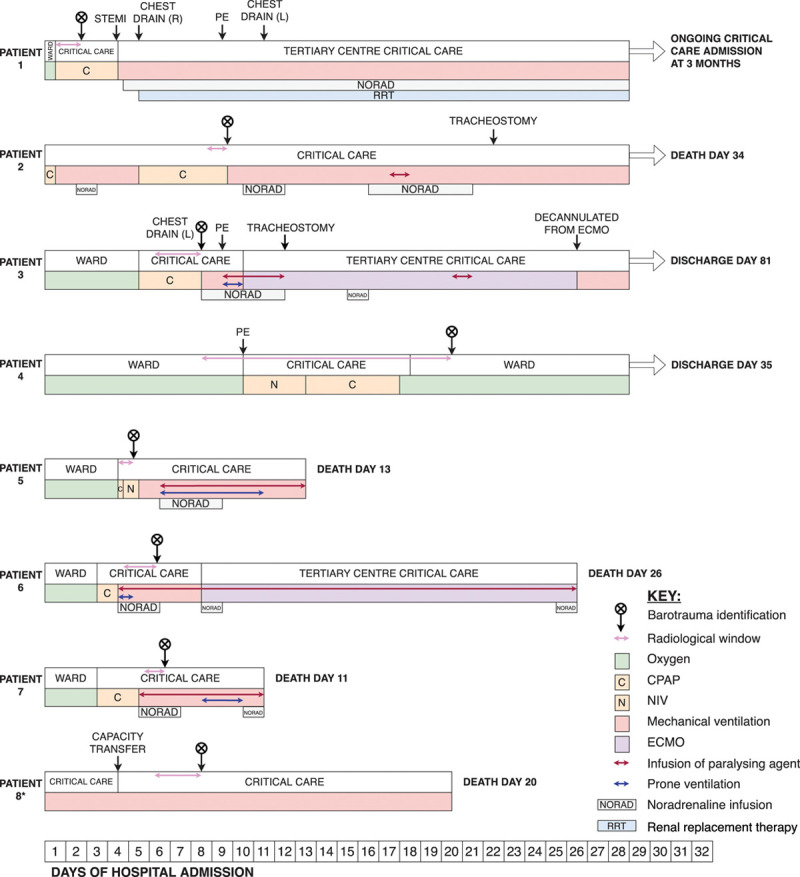
A timeline of the clinical course and interventions for patients suffering barotrauma. CPAP = continuous positive airway pressure, ECMO = extracorporeal membrane oxygenation, L = left, NIV = noninvasive ventilation, PE = pulmonary embolism/emboli, R = right, STEMI = ST elevation myocardial infarction. *Details regarding proning, paralysis, and nonrespiratory organ support are not known for patient 8.
For those in the barotrauma group, informed consent for publication was obtained from patients or, where the patient was deceased or lacked capacity to consent, their next of kin. The local ethics committee were consulted, and the need for ethical approval was waived.
Statistical Analyses
Occurrence rate was calculated with a 95% CI. Categorical and continuous variables were presented as number (%) and median (interquartile range [IQR]), respectively. p values were calculated using Fisher exact test or independent-samples Mann-Whitney U test for descriptive purposes. Analyses were performed by IBM SPSS Statistics for Macintosh, Version 26.0 (IBM Corp, Armonk, NY) and Stata Statistical Software: Release 14 (StataCorp LP, College Station, TX).
RESULTS
Between March 12, and April 12, 2020, there were 102 critical care admissions. Of these, 83 had confirmed COVID-19, 16 had possible COVID-19, and three had unrelated presentations. Of the 83 patients with confirmed COVID-19, eight patients (9.6%; 95% CI 4.3–18.1%) were given a radiological diagnosis of subcutaneous emphysema, pneumomediastinum, or pneumothorax (barotrauma). Of patients with barotrauma, eight (100.0%) had subcutaneous emphysema, seven (87.5%) had pneumomediastinum, and four (50·0%) had pneumothorax, including two (25.0%) with bilateral pneumothoraces (eTable 1, Supplemental Digital Content, http://links.lww.com/CCX/A313).
Table 1 compares patient characteristics at critical care admission and initial mode of advanced respiratory support. Compared with the nonbarotrauma group, all patients with barotrauma were male (100.0% vs 70.7%; p = 0.102) and of Black, Asian, and Minority Ethnic background (100.0% vs 61.6%; p = 0.046). The median duration of illness prior to critical care admission was 3 days longer in barotrauma patients (10 vs 7 d; IQR 8–14 and 6–10, respectively; p = 0.073). Other baseline characteristics, admission blood values, and indicators of acute disease severity were similar between the two groups. Seven of eight patients (87.5%) in the barotrauma group had received CPAP or NIV as the initial mode of advanced respiratory support, compared with 27 of 75 (36.0%) in the nonbarotrauma group (p = 0.007).
Table 1.
Comparison of Patients With and Without Barotrauma, at Admission to Critical Care
| Barotrauma Group (n = 8) | Nonbarotrauma Group (n = 75) | p | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, yr, median (IQR) | 54.5 (37.8–57.4) | 57.8 (50.4–65.2) | 0.134a |
| Sex, n (%) | 0.102b | ||
| Male | 8 (100.0) | 53 (70.7) | |
| Female | 0 (0.0) | 22 (29.3) | |
| Ethnicity, n (%) | 0.046b | ||
| Black, Asian, and Minority Ethnic | 8 (100.0) | 45 (61.6) | |
| White | 0 (0·0) | 28 (38.4) | |
| Body mass index, kg/m2, median (IQR) | 28 (27–29) | 30 (26–34) | 0.367a |
| Rockwood Clinical Frailty Scale, median (IQR) | 3 (2–3) | 2 (2–3) | 0.262a |
| Smoking history, n (%) | 2 (25.0) | 21 (28.0) | 1.000b |
| Underlying lung disease, n (%) | 1 (12.5) | 12 (16.0) | 1.000b |
| Chronic obstructive pulmonary disease | 0 (0.0) | 0 (0.0) | |
| Asthma | 0 (0.0) | 10 (13.3) | |
| Other | 1 (12.5) | 3 (4.0) | |
| Connective tissue disease, n (%) | 0 (0.0) | 4 (5.3) | 1.000b |
| Blood values at critical care admission (normal range), median (IQR) | |||
| Neutrophils (2.0–7.0 × 109/L) | 7.4 (6.5–7.7) | 7·8 (5·5–10·7) | 0.454a |
| Lymphocyte (1.0–3.0 × 109/L) | 0.7 (0.5–1.1) | 0·9 (0·6–1·1) | 0.369a |
| Creatinine (44–80 µmol/L) | 76 (70–97) | 94 (73–109) | 0.250a |
| C-reactive protein (0–5 mg/L) | 219 (163–232) | 180 (120–250) | 0.547a |
| Duration of symptoms prior to critical care admission, d, median (IQR) | 10 (8–14) | 7 (6–10) | 0.073a |
| Indicators of disease severity at critical care admission, median (IQR) | |||
| Acute Physiology and Chronic Health Evaluation II score | 13 (9–18) | 14 (12–17) | 0.557a |
| Ratio of Pao2 and Fio2, mm Hg | 76 (67–81) | 77 (65–96) | 0.886a |
| Initial mode of advanced respiratory support, n (%) | 0.007b | ||
| Continuous positive airway pressure or noninvasive ventilation | 7 (87.5) | 27 (36.0) | |
| Invasive mechanical ventilation | 1 (12.5) | 48 (64.0) |
IQR = interquartile range.
p values were calculated by independent samples Mann-Whitney U test (a) or Fisher exact test (b).
Patients with barotrauma were managed with a variety of respiratory support modalities during their radiological windows (Fig. 1). Detailed baseline characteristics, clinical course, radiological window length, and management of barotrauma for the eight barotrauma patients can be seen in eTable 1, eTable 2, and eText 1 (Supplemental Digital Content, http://links.lww.com/CCX/A313). Radiological examples of barotrauma manifestations are demonstrated in Figures 2 and 3.
Figure 2.
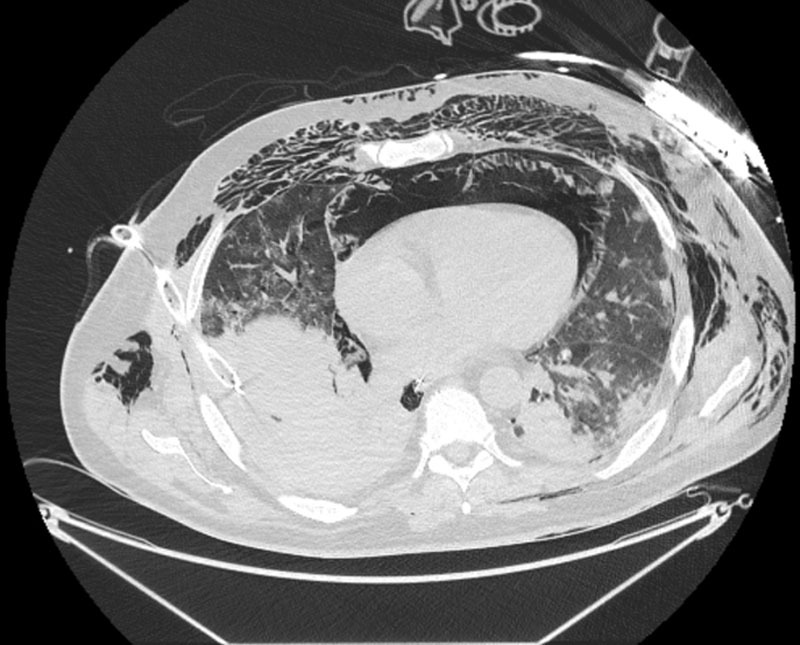
Axial CT image from patient 1 demonstrating pneumomediastinum, subcutaneous emphysema, ground glass opacification, and consolidation.
Figure 3.
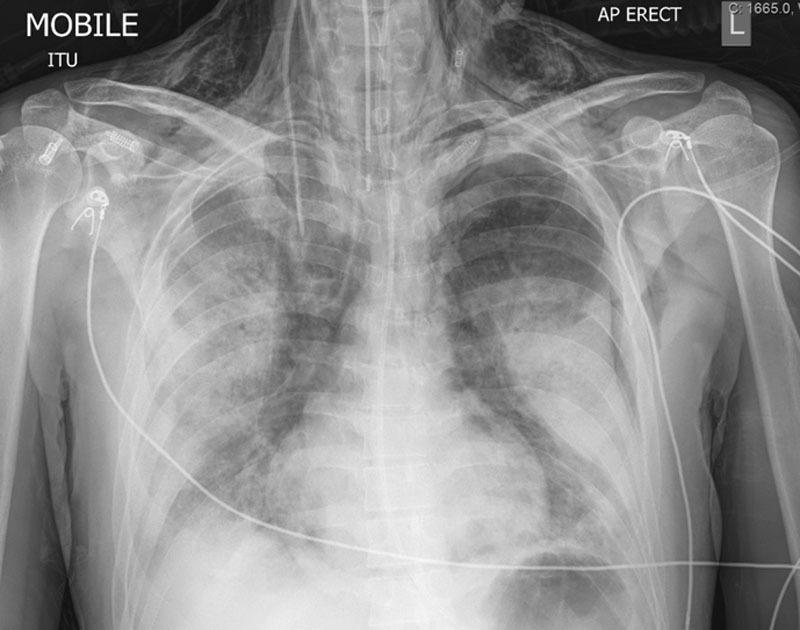
Chest radiograph from patient 3 demonstrating left-sided pneumothorax, subcutaneous emphysema, and bilateral consolidation. AP = anteroposterior, ITU = intensive treatment unit, L = left.
Table 2 outlines the respiratory variables for the patients who developed barotrauma. There was heterogeneity in respiratory support modality used prior to the identification of barotrauma.
Table 2.
Hourly Ventilatory Variables Prior to Barotrauma
| Patient | Mode of Respiratory Support | Proportion Time, % | PEEP, cm H2O, Median (IQR) | Peak Inspiratory Pressure (PEEP Plus Pressure Support), cm H2O, Median (IQR) | Fio2, %, Median (IQR) | Respiratory Rate, Breaths Per Minute, Median (IQR) | VT, mL, Median (IQR) | VT/Ideal Body Weight, mL/kg, Median (IQR) | Minute Ventilation, L/min, Median (IQR) | Peripheral Oxygen Saturation, %, Median (IQR) | Pao2, kPa, Median (IQR) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CPAP | 100 | 10 (10–10) | — | 50 (50–55) | 27 (25–29) | 604 (506–716) | 8.5 (7.1–10.0) | 16.2 (13.0–19.5) | 93 (92–95) | 10.1 (9.8–10.8) |
| 2 | CPAP | 100 | 10 (10–10) | — | 75 (70–75) | 36 (35–40) | 482 (414–549) | 7.4 (6.4–8.4) | 17.2 (15.1–21.0) | 94 (92–96 | 8.8 (7.8–9.9) |
| 3 | Facemask O2 | 60 | — | — | 80 (80–80) | 31 (28–33) | — | — | — | 92 (91–94) | 8.2 (8.0–9.1) |
| CPAP | 40 | 10 (10–10) | — | 70 (60–80) | 32 (30–33) | 487 (465–578) | 7.1 (6.8–8.4) | 18.1 (17.6–22.4) | 93 (92–94) | 9.3 (9.1–9.6) | |
| 4 | NIV | 30 | 10 (10–10) | 15 (15–15) | 53 (43–56) | 32 (26–33) | 715 (628–754) | 9.8 (8.6–10.3) | 21.3 (18.2–10.3) | 93 (92–94) | 9.1 (8.5–9.8) |
| CPAP | 35 | 8 (6–8) | — | 30 (30–30) | 24 (23–24) | 720a | 9.8a | 19.4a | 94 (91–94) | — | |
| Facemask O2 | 35 | — | — | 35 (35–35) | 20 (19–21) | — | — | — | 94 (93 - 94) | — | |
| 5 | CPAP | 30 | 12 (12–12) | — | 45 (45–49) | 51 (50–53) | 415 (401–431) | 5.7 (5.5–6.0) | 19.9 (19.4–23.0) | 96 (95–97) | 9.6 (9.0–10.1) |
| NIV | 70 | 15 (12–15) | 20 (17–24) | 50 (45–60) | 45 (44–47) | 501 (456–507) | 6.9 (6.3–7.0) | 22.7 (21.4–22.8) | 98 (90–98) | 8.4 (7.9–8.6) | |
| 6 | IMV (PC-BiPAP) | 100 | 12 (10–12) | 28 (28–30) | 55 (40–55) | 18 (18–18) | 475 (452–503) | 6.7 (6.4–7.1) | 8.6 (8.2–9.1) | 93 (90–94) | 9.1 (8.2–9.6) |
| 7 | CPAP | 35 | 10 (10–10) | — | 75 (70–75) | 40 (38–42) | 478 (455–522) | 5.9 (5.6–6.4) | 18.8 (18.2–21.0) | 92 (90–94) | 8.9 (8.2–9.1) |
| IMV (PC-BiPAP) | 65 | 12 (10–12) | 30 (30–31) | 55 (55–70) | 20 (20–20) | 562 (496–602) | 6.9 (6.1–7.4) | 11.1 (9.9–12.2) | 90 (89–92) | 8.5 (7.8–9.9) | |
| 8b | IMV | 100 | — | — | — | — | — | — | — | — | — |
CPAP = continuous positive airway pressure, IMV = invasive mechanical ventilation, IQR = interquartile range, NIV = noninvasive ventilation, PC-BiPAP = pressure-control biphasic positive airway pressure setting, PEEP = positive end-expiratory pressure, VT = tidal volume.
aSingle reading.
bData unavailable due to patient transfer > 48 hr prior to barotrauma.
Recorded during the radiological window or the 48 hr prior to barotrauma—whichever was longer or for the entire critical care admission if shorter than 48 hr.
For the six patients managed with CPAP prior to barotrauma, median settings were 8–12 cm H2O and Fio2 ranged between 30% and 75%. Median respiratory rates were 24–51 breaths/min, with median VT of 5·7—9·8 milliliters per kilogram of IBW (mL/kg IBW). Median minute ventilation (VE) ranged from 16.2 to 19.9 liters per minute (L/min).
For the two patients managed with NIV prior to barotrauma, median positive end-expiratory pressure (PEEP) and pressure support were 10—15 and 5 cm H2O respectively, giving median Pinsp of 15—20 cm H2O. Median Fio2 ranged between 50% and 53%. Median respiratory rates were 32–45 breaths/min, with median VT of 6.9—9.8 mL/kg IBW, giving median VE of 21.3—22.7 L/min.
For the two patients managed with pressure-controlled IMV prior to barotrauma, median PEEP and Pinsp were 12 and 28—30 cm H2O, respectively. Median Fio2 was 55%. Median respiratory rate was 18–20 breaths/min, and median VT was 6.7–6.9 mL/kg IBW, giving median VE of 8.6–11.1 L/min.
Regardless of mode of respiratory support, all patients maintained median peripheral oxygen saturation greater than or equal to 90% and median Pao2 greater than or equal to 8 KPa for the duration of the period studied. It was not possible to retrospectively establish whether patients received recruitment maneuvers, but these were not routinely performed in stable patients established on IMV. In four cases, there were temporally and anatomically relevant procedures for which subcutaneous emphysema, pneumomediastinum, or pneumothorax are recognized complications (eTable 1, Supplemental Digital Content, http://links.lww.com/CCX/A313).
Table 3 describes patient outcomes at 3 months from critical care admission. Compared with the nonbarotrauma group, a higher proportion of patients with barotrauma had died (62.5% vs 43.2%), and a lower proportion of patients had been discharged (25.0% vs 53.3%). One patient with barotrauma was still in critical care, and one patient without barotrauma was still on a general ward.
Table 3.
Outcomes at 3 Months From the Date of Critical Care Admission
| Death, n (%) | Patient in Critical Care, n (%) | Patient on General Ward, n (%) | Discharge, n (%) | Total, n (%) | |
|---|---|---|---|---|---|
| Barotrauma group | 5 (62.5) | 1 (12.5) | 0 (0.0) | 2 (25.0) | 8 (100.0) |
| Nonbarotrauma group | 34 (45.3) | 0 (0.0) | 1 (1.3) | 40 (53.3) | 75 (100.0) |
DISCUSSION
In this study, we describe a cohort of patients with COVID-19 admitted to a critical care department in London between March 12, and April 12, 2020, during the peak of the local COVID-19 outbreak. Of 83 patients, eight patients (9.6%) suffered barotrauma. This is higher than the occurrence rate reported in initial COVID-19 critical care cohorts (12).
Although our study was not designed to infer causation, our findings identify two potential associations with barotrauma requiring further study. First, we observed that a high proportion of patients with barotrauma were managed with CPAP or NIV, rather than IMV, as the initial mode of advanced respiratory support. Second, barotrauma patients also tended to have a longer disease course prior to critical care admission. Markers of disease severity (including blood values, APACHE II score, and ratio of Pao2 and Fio2), in comparison, were similar between the barotrauma and nonbarotrauma groups at the point of critical care admission.
Outcomes in the barotrauma group were worse than in the nonbarotrauma group, with higher rates of death and lower rates of hospital discharge. This observation is consistent with the findings of other investigators in COVID-19 (14) and with the known association between barotrauma and multiple organ failure and death (6). However, causality between barotrauma and poor outcomes cannot be inferred from these data.
Several mechanisms may explain barotrauma in COVID-19. Pathologic processes such as interstitial pneumonia (20), consolidation (21), and in-situ thrombosis (22), all seen in the barotrauma cohort, could increase the friability of lung parenchyma and pleura, thus increasing the risk of fistulation between the distal airways and pleural space or hilum. Radiological and postmortem studies describe the disease’s peripheral predominance (21, 23) and propensity to cause cystic change (24, 25), which may further increase this risk.
In ARDS, edema and atelectasis in dependent regions lead to reduced lung volume. This can result in damage from regional overdistension in recruited lung (volutrauma), increased shear strain in ventilated alveolar tissue (atelectrauma), high transpulmonary pressures (barotrauma), and surfactant dysfunction and inflammation (biotrauma) (6, 26, 27). Each of these processes may contribute to the occurrence of subcutaneous emphysema, pneumomediastinum, and pneumothorax in critically ill COVID-19 patients and highlights the limitation of the term “barotrauma” to describe all cases of extra-alveolar gas in this context.
In mechanically ventilated patients, these factors collectively contribute to ventilator-induced lung injury. Lung-protective ventilation is an established strategy that reduces mortality in ARDS (28) and has formed the basis of guidelines and consensus for mechanical ventilation in COVID-19 (2, 29). Ventilation data for the intubated barotrauma patients in this study show lung-protective ventilation variables were largely achieved: patients received high PEEP, and VT was maintained below 8 mL/kg IBW. Although plateau pressures were not routinely documented, median Pinsps were 30 cm H2O or below, suggesting lung-protective plateau pressures of 30 cm H2O were maintained. This shows that, in our cohort, adopting a lung-protective ventilation strategy was not sufficient in preventing the development of subcutaneous emphysema, pneumomediastinum, or pneumothorax.
In CPAP and NIV, neither VE nor the large swings in transpulmonary pressure resulting from spontaneous respiratory effort can be limited. This can compound the reduced functional lung volume seen in ARDS, resulting in patient self-inflicted lung injury (P-SILI) (22, 30). In this study, patients managed with CPAP or NIV sustained markedly high VE prior to the development of barotrauma, which may have put them at risk of P-SILI. Notably, this occurred despite patients maintaining acceptable oxygen saturations and arterial partial pressures of oxygen.
This was a retrospective, single-center cohort study with modest patient numbers, limiting the generalizability of our findings. The estimated occurrence rate of barotrauma may be influenced by the sensitivity of chest radiography for its detection, as well as the exclusion of patients without a molecular diagnosis of COVID-19. Ventilation data were documented by critical care staff as part of their usual clinical care and were therefore potentially liable to inaccuracy, bias, or missing data. Detailed ventilation data were only collected for patients in whom barotrauma was identified due to the lack of a relevant time point of interest in the nonbarotrauma group; therefore, comparison of ventilation data between barotrauma and nonbarotrauma groups is not possible. Plateau pressure was not routinely recorded, such that we were unable to calculate driving pressure and lung compliance. Finally, although potentially relevant to the occurrence of barotrauma, data on the use of recruitment maneuvers and bag-valve mask ventilation could not be obtained on retrospective review of clinical documentation.
Conducted in the midst of a pandemic, this study spanned a period where consensus regarding the optimal management of critically ill patients with COVID-19 was rapidly evolving, which was reflected in local practice. Subsequently, the threshold for admission to the critical care department, medical management, and rates of interhospital transfer varied throughout the study period.
CONCLUSIONS
Barotrauma appears to be a common complication of severe COVID-19, with an occurrence rate of 9.6% among our cohort. Clinicians should be alert to the risk of barotrauma in any critically ill patient with COVID-19. Determining whether high VE while using CPAP or NIV predisposes patients to barotrauma requires further investigation.
Supplementary Material
Footnotes
Drs. Jones and Gould are joint-first authors and contributed equally to the work.
This work was performed in University Hospital Lewisham, Lewisham High Street, London, SE13 6LH, without financial support.
Drs. Jones, Gould, and Pillay were responsible for study conception and design. Drs. Jones, Gould, Khorasanee, Sykes, Cox, Shurovi, and Isted were responsible for data collection. Drs. Jones, Gould, Khorasanee, and Bazo-Alvarez were responsible for data analysis. Drs. Jones, Gould, Pillay, Khorasanee, Simpson, Jennings, Breeze, and Khaliq were responsible for data interpretation. Drs. Jones, Gould, Pillay, Khorasanee, and Bazo-Alvarez drafted the initial article, and all authors contributed to critically revising this draft. All authors read and approved the final version of the article and agree to be accountable for the accuracy and integrity of the work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhazzani W, Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020. May 15. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization:. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. 2020. Available at: https://apps.who.int/iris/handle/10665/332196. Accessed July 20, 2020
- 4.Arulkumaran N, Brealey D, Howell D, et al. Use of non-invasive ventilation for patients with COVID-19: A cause for concern? Lancet Respir Med. 2020; 8:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NHS England, NHS Improvement. Guidance for the Role and Use of Non-Invasive Respiratory Support in Adult Patients With Coronavirus (Confirmed or Suspected). 2020. Available at: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-NIV-respiratory-support-and-coronavirus-v3.pdf. Accessed May 10, 2020
- 6.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013; 369:2126–2136 [DOI] [PubMed] [Google Scholar]
- 7.Moss M, Huang DT, Brower RG, et al. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network: Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019; 380:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carron M, Freo U, BaHammam AS, et al. Complications of non-invasive ventilation techniques: A comprehensive qualitative review of randomized trials. Br J Anaesth. 2013; 110:896–914 [DOI] [PubMed] [Google Scholar]
- 9.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001; 163:540–577 [DOI] [PubMed] [Google Scholar]
- 10.Wild M, Alagesan K. PEEP and CPAP. BJA CEPD Rev. 2001; 1:89–92 [Google Scholar]
- 11.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020; 395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao W, Wang T, Jiang B, et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: Lessons learnt and international expert recommendations. Br J Anaesth. 2020; 125:e28–e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuinness G, Zhan C, Rosenberg N, et al. High incidence of barotrauma in patients with COVID-19 infection on invasive mechanical ventilation. Radiology. doi: 10.1148/radiol.2020202352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou C, Gao C, Xie Y, et al. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020; 20:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Gao R, Zheng Y, et al. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020; 27:taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peiris JS, Chu CM, Cheng VC, et al. ; HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003; 361:1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu CM, Leung YY, Hui JY, et al. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004; 23:802–804 [DOI] [PubMed] [Google Scholar]
- 19.NHS. 2020 Available at: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/guidance-and-sop-covid-19-virus-testing-in-nhs-laboratories-v1.pdf. Accessed May 1, 2020.
- 20.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8:420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020; 215:87–93 [DOI] [PubMed] [Google Scholar]
- 22.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020; 323:2329–2330 [DOI] [PubMed] [Google Scholar]
- 23.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir Med. 2020; 8:681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020; 21:541–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K, Zeng Y, Xie P, et al. COVID-19 with cystic features on computed tomography. Medicine. 2020; 99:e20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016; 37:633–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L, Marini JJ, Pesenti A, et al. The “baby lung” became an adult. Intensive Care Med. 2016; 42:663–673 [DOI] [PubMed] [Google Scholar]
- 28.Brower RG, Matthay MA, Morris A, et al. ; Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. WHO Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected. 2020. Available at: https://apps.who.int/iris/handle/10665/331446. Accessed May 10, 2020
- 30.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017; 195:438–442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


