Abstract
PURPOSE:
Peptide receptor radionuclide therapy (PRRT) is effective for metastatic/inoperable neuroendocrine tumors (NETs). Imaging response assessment is usually efficient subsequent to treatment completion. Blood biomarkers such as PRRT Predictive Quotient (PPQ) and NETest are effective in real-time. PPQ predicts PRRT efficacy, NETest monitors disease. We prospectively evaluated: 1) NETest as a surrogate biomarker for RECIST; 2) The correlation of NETest levels with PPQ prediction.
METHODS:
Three independent 177Lu-PRRT-treated GEP-NET and lung cohorts (Meldola, Italy: n=72; Bad-Berka, Germany: n=44; Rotterdam, Netherlands: n=41). Treatment response: RECIST1.1 [Responder (stable, partial and complete response) vs Non-Responder]. Blood sampling: pre-PRRT, before each cycle and follow-up (2-12 months). PPQ (positive/negative) and NETest (0-100 score) by PCR. Stable≤40; progressive >40). CgA (ELISA) as comparator. Samples deidentified, measurement and analyses blinded. Kaplan-Meier survival and standard statistics.
RESULTS:
122 of the 157 were evaluable. RECIST stabilization or response in 67%; 33% progressed. NETest significantly (p<0.0001) decreased in RECIST-”responders” (−47±3%); in “non-responders” it remained increased (+79±19%) (p<0.0005). NETest monitoring accuracy was 98% (119/122). Follow-up levels >40 (progressive) vs stable (≤40) significantly correlated with mPFS (not reached vs. 10 months; HR 0.04 (95%CI: 0.02-0.07).
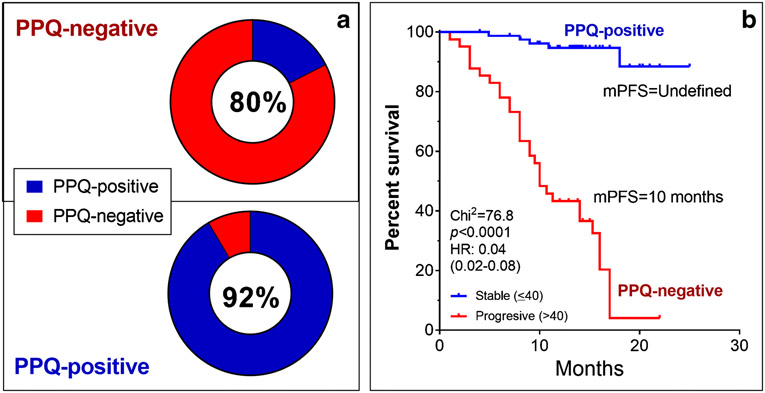
PPQ response prediction was accurate in 118 (97%) with a 99% accurate positive and 93% accurate negative prediction. NETest significantly (p<0.0001) decreased in PPQ-predicted responders (−46±3%) and remained elevated or increased in PPQ-predicted non-responders (+75±19%). Follow-up NETest categories stable vs progressive significantly correlated with PPQ prediction and mPFS (not reached vs. 10 months; HR 0.06 (95%CI: 0.03-0.12).
CgA did not reflect PRRT treatment: in RECIST-responders decrease in 38% and in non-responders 56% (p=NS).
CONCLUSIONS:
PPQ predicts PRRT response in 97%. NETest accurately monitors PRRT response and is an effective surrogate marker of PRRT radiological response. NETest decrease identified responders and correlated (>97%) with the pretreatment PPQ response predictor. CgA was non-informative.
Keywords: Biomarker, NETest, PPQ, liquid biopsy, Neuroendocrine, PRRT
Introduction
Peptide receptor radiotherapy (PRRT) is an effective treatment for metastatic or inoperable neuroendocrine tumors (NETs) [1] and 177Lu-DOTATATE is approved in USA and EU for the treatment of gastroenteropancreatic (GEP) NETs [2-4]. Standard protocols are typically administered over 6-8 months and have substantial costs in terms of exams and patient effort before the benefit can be reliably evaluated, typically 3 months after the treatment cycles are completed [5]. Although therapy is effective in the majority, approximately 15-30% of patients will progress during PRRT and can benefit from timely adjustment, such as therapy combinations, rapid sequencing or alternatives [2, 4, 6].
Biomarker guidance as to the efficacy of therapy is assessed either by imaging or the measurement of circulating tumor products. Predictive biomarkers identify individuals likely to experience a favorable response e.g., increase in PFS, prior to a treatment. Such biomarkers typically are based on evidence that a “target” is expressed and can be “engaged” [7]. Thus, the “intensity” of uptake at 68Ga-SSA-PET/CT, correlating with the amount of therapeutic radiation, can predict response to PRRT; the accuracy is, however, suboptimal (60% objective responses in Krenning grade 4). An alternative or supplement to a predictive biomarker is a pharmacodynamic or response biomarker [8]. The identification of alteration in blood levels can provide early evidence that a treatment positively or negatively impacts a clinical endpoint of interest e.g., PFS [8].
At this time, the response assessment for PRRT is based on locally implemented schemes that comprise varying combinations of intra- and post-therapy morphologic and functional imaging (CT, MRI [2], post-administration Lu-scan and/or interim and post-treatment 68Ga-SSA-PET/CT. Conventional 18FDG-PET/CT is considered a prognostic imaging biomarker. Sometimes, circulating biomarkers like chromogranin A (CgA) or specific hormones for secretory tumors (gastrin, glucagon, insulin), are studied [9, 10], although tumors with secretory products represent <10% of all neuroendocrine lesions. Spurious elevations in biomarkers, e.g., CgA, are well-recognized and obfuscate clinical assessment [5]. Key limitations in evaluative techniques include a failure to detect progression in a timely fashion or, with imaging, a difficulty in resolving pseudo-progression [5].
In 2018, we reported the efficacy of a circulating transcript assay (PPQ) that predicted tumor response to internal radiation in GEP- and bronchopulmonary NET with 95% accuracy [11]. Biomarker positive (PPQ-positive) predicts responders and biomarker negative (PPQ-negative) predicts non-responders, thereby stratifying treatment recipients [6]. A prediction of a non-responder therefore suggests that a revised clinical therapeutic strategy should be considered [12].
The NETest is a 51 multigene assay based on PCR analysis of specific NET circulating transcripts [13] which is reported as a score reflecting real-time tumor activity [14]. The molecular basis of the assay has been validated [15] and the correlation between tumor tissue and blood levels established [15]. Clinical utility (monitoring) has been documented in several independent clinical studies using diverse therapeutic strategies [16-18]. In addition, the NETest has been demonstrated as an effective (85-90% accuracy) surrogate biomarker for tumor progression measured with CT/MRI [18]. A short PFS is significantly correlated (>95%) with increased blood biomarker levels >40 (on a scale of 0-100) [18]. Similarly, RECIST progression to somatostatin analogues is also significantly associated (>90%) with score increases [18, 19]. In contrast, stabilization or response is associated with no change or score decrease (NETest levels ≤40) [18, 19]. These alterations (progression NETest score >40; disease stability ≤40) likely reflect the biological impact of treatment [14, 20, 21].
We have previously proposed that a fusion of a response biomarker and a predictive biomarker might have a significant clinical impact in PRRT patient stratification [22]. Patient benefit and health economic implications would be substantial, allowing early prediction and appropriate monitoring of efficacy with a decrease in diagnostic and therapeutic interventions for identified responders. Alternatively, the identification of non-responders would allow for early implementation of other strategies (e.g., combinations or rapid sequencing).
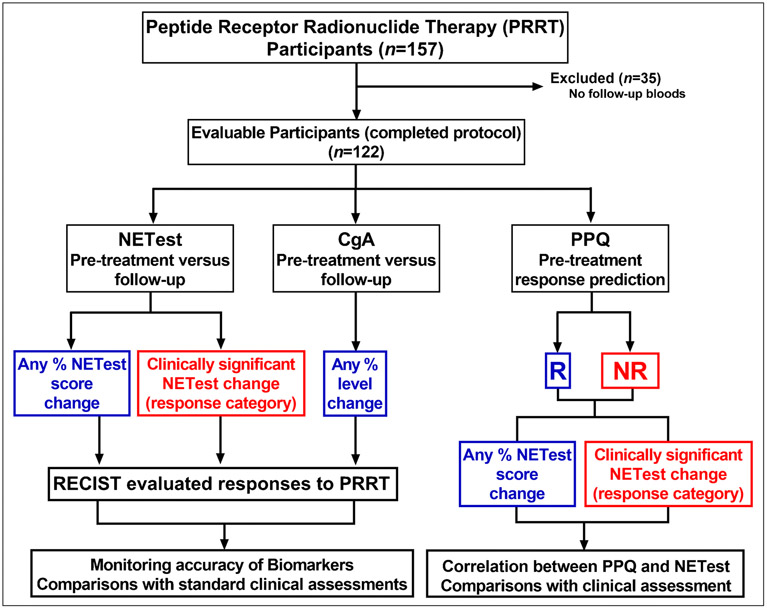
In this prospective study we examined two issues (Figure 1). Firstly, we evaluated the response to PRRT with the NETest to assess its function as a monitoring marker. For this, we compared a pre-PRRT measurement to follow-up levels. Secondly, we evaluated whether changes in NETest (prior vs. post-therapy) were concordant with RECIST and provided added value to PPQ biomarker prediction of response. As a comparator, we examined changes in the monoanalyte secretory peptide CgA, the current default NET biomarker, at the same time points.
Figure 1. STARD diagram outlining the study.
Three biomarkers were evaluated, the NETest, Chromogranin A (CgA) and the PPQ (PRRT predictive quotient). Changes in levels following therapy were assessed compared to pre-treatment levels for both the NETest and CgA. These were correlated with response to therapy. Changes in NETest category (i.e., stable/progressive following PRRT) were also evaluated. PPQ defines whether a patient is a responder (R) or non-responder (NR). Changes in NETest levels and categories were identified for both R and NR and compared to clinical assessments.
Methods:
Patients:
The three cohorts (including GEP- and bronchopulmonary NETs) that were recruited have previously been described [6].
In brief, 178 subjects at 3 sites were enrolled for 177Lu-octreotate-PRRT based on imaging SSR expression between 6/2012 and 6/2016: Nuclear Medicine and Radiometabolic Units, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori IRST IRCCS, Meldola, Italy; Theranostics Center for Molecular Radiotherapy and Imaging, Zentralklinik Bad Berka, Bad Berka, Germany; and the Radiology and Nuclear Medicine Department, Erasmus Medical Center, Rotterdam, The Netherlands. The current study is the summated analysis (total n=157) of evaluable patients from Meldola (n=72), Bad-Berka (n=44) and Rotterdam (n=41). All participants provided informed consent for PRRT and molecular genomic translational analysis, authorized by the respective ethics committees (PRRT: IRST 100.06, EudraCT: 2011–002891-18, 04/08/2011; transcripts: IRST B007 [70/12], 10/10//2012; WIRB: 20,150,174; MEC-2014–309, 24/07/2014, NL48623.078.14). The clinical characteristics and treatment protocols for each of these three cohorts have been previously described in detail [6]. This data is included in Supplemental Table 1 and 2.
Assessment of therapeutic response:
Response was assessed per RECIST 1.1 criteria. CT (or MRI) was performed at baseline (within 3 months of PRRT) and ~2-12 months after PRRT per protocol [6]. For this cohort, 105 (67%) were considered “responders” at follow-up compared to 52 (33%) as “non-responders”. Survival and progression times were calculated from PRRT commencement. A response was defined as either disease control (partial or complete response to therapy or stable disease – censored “0”). Progression (treatment failure – censored as “1”) at the date of confirmation of radiological progression. The latter included all patients who completed at least 1 PRRT cycle. Cases were censored at the limit of follow-up. All individuals who responded to PRRT had a complete follow-up of a median of 6 months (range 0-17) post-therapy. Patients who progressed or died due to progression during PRRT or follow-up were included (censored as “1”).
Blood sampling:
Samples were collected pre-PRRT and thereafter prior to each PRRT cycle (administered at intervals of ~2 months [2-4 cycles]) and then at follow-up (up to 9-22 months after the last PRRT cycle). At baseline whole blood (10 ml) was collected in 2×5 ml EDTA-K2 tubes and snap-frozen. Plasma CgA samples were collected at the same time point in PPT plasma preparations tubes. Tubes were anonymously coded and stored at −80°C within 2 hours of collection [22]. Randomly selected coded blood samples were sent de-identified to Wren Laboratories, Connecticut, USA.
NETest:
Details of the PCR methodology, mathematical analysis and validation have been published. In brief, it comprises a 2-step protocol (RNA isolation/cDNA production and qPCR) from EDTA-collected whole blood [14, 22]. Samples were assayed in a USA clinically certified laboratory (Wren Laboratories CL-0704, CLIA 07D2081388). Final results are expressed as an activity index (NETest score) from 0-100% [6, 22]. The normal score cut-off is 20 with two monitoring categories of score: ≤40% is considered “stable” disease (SD), and 41-100 reflect “progressive” disease (PD) [18].
PPQ:
The PPQ, a blood-based predictive classifier, has been previously described in detail [22]. In brief, circulating expression of genes involved in growth factor expression and metabolism are amplified by PCR and expression levels integrated with tumor grade to generate a prediction classifier summated using a Logistic Regression Model. Samples are scored as either biomarker “positive” or “negative”. PPQ-positive identifies those predicted to respond (disease stabilization or partial/complete response). PPQ-negative are predicted not to respond.
CgA assay:
CgA was measured using NEOLISA™ Chromogranin A kit (Euro Diagnostica AB, Malmö, Sweden). The upper limit of normal was 108 ng/ml [19]. A positive CgA is ≥109ng/ml.
Statistical Analysis:
Prism 6.0 for Windows (GraphPad Software, La Jolla CA USA, www.graphpad.com) and MedCalc Statistical Software version 12.7.7 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2013) were utilized [6, 22]. The efficacy of PRRT was defined by RECIST 1.1 as either disease control (PR+CR+SD) or progressive as described [6, 22]. The accuracy of changes (either an increase or decrease) in each of the two biomarkers (NETest, CgA) was directly assessed comparing baseline values to levels at follow-up. As a sub-analysis, we evaluated the number of patients that were “stable” (SD: NETest ≤40) or “progressive” (PD: NETest >40) score at follow-up and the correlation with RECIST outcome. Intergroup analyses were undertaken using 2-tailed non-parametric tests (Mann-Whitney U test) or Kruskal-Wallis testing (with Dunn’s corrections) as applicable. Kaplan-Meier survival curves (PFS) were generated and analyzed in Prism. Log-rank (Mantel–Cox) and hazard ratios (Mantel–Haenszel) were calculated. The utility of the NETest or CgA as a monitoring biomarker was evaluated by comparing mPFS between no change/decrease in the biomarker versus an increase in the biomarker. We also evaluated this for each of the two NETest categories at follow-up (relationship between mPFS and a “stable” or “progressive” score). RECIST-response (responder vs. non-responder) and PPQ-status (PPQ-positive vs. PPQ-negative) and mPFS were similarly analyzed. Diagnostic Odd’s ratios (DOR), the positive likelihood ratio (PLR) and the negative likelihood ratio (NLR) were calculated. A Fisher’s 2-tailed t-test was used to compare proportional changes. Statistical significance was defined at a p value < 0.05. Data are presented as mean±SD. Ninety-five percent confidence intervals are included where appropriate.
Results
The 3 cohorts comprised 178 of which 157 were evaluable for response [6]. Twenty-one were excluded: 9 withdrew consent; 5 pretreatment bloods were not collected; 5 medical reasons and 2 lost to follow-up. Of the 157, 35 were excluded from analysis as no follow-up bloods were available. The complete data are included in Supplemental Table 2.
1. Overall NETest changes in response to PRRT (n=122)
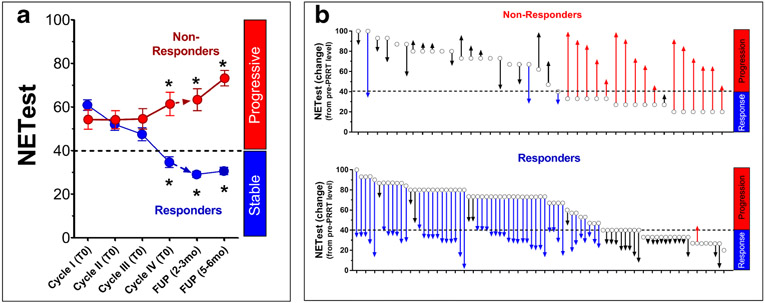
NETest scores significantly (Kruskal-Wallis statistic 97.22; p<0.0001) decreased after PRRT in RECIST-”responders” (n=83) (Table 1, Figure 2A). Pre-PRRT levels were 61±22. Prior to cycle IV, levels were 35±20. At both subsequent follow-ups, levels were further significantly (p<0.0001 vs. Cycle IV) decreased (29±26). In “non-responders” (n=39), pre-PRRT levels were 54±28. At Cycle IV, levels remained elevated (62±27) and increased further at follow-up (73±11, p<0.0005 vs. Pre-PRRT).
Table 1.
NETest scores, PRRT predictive quotient (PPQ) and Chromogranin A (CgA) values after PRRT in “RECIST”-responders and “non-responders”
| Total Patients (n=157) | Patients Assessed (n=122) | ||||
|---|---|---|---|---|---|
| RECIST Predicted Response | Responders (n=83) |
Non-Responders (n=39) |
|||
| NETest | |||||
| NETest alterations to PRRT | N | % | N | % | |
| NETest decrease | 82 | 99 | 10 | 26 | |
| NETest increase | 1 | 1 | 29 | 74 | |
| Accuracy (alterations) | 111/122 | 91% | |||
| Follow-up NETest after PRRT | N | % | N | % | |
| NETest ≤40 (“stable”) | 77 | 93 | 8 | 20 | |
| NETest >40 (“progressive”) | 11 | 7 | 31 | 80 | |
| Accuracy (category) | 108/122 | 89% | |||
| NETest overall accuracy | N | % | |||
| 119/122 | 98% | ||||
| PPQ Predicted Responders | PPQ +ve (n=81) |
PPQ -ve (n=41) |
|||
| NETest alterations to PRRT | %±SD | ||||
| Percentage change | −46±3 | ±75±19 | |||
| NETest levels | Mean±SD | ||||
| Pre-PRRT | 61±22 | 54±27 | |||
| Pre-IV cycle | 35±20 | 59±27 | |||
| At follow-up | 29±12 | 64±22 | |||
| Follow-up NETest after PRRT | N | % | N | % | |
| NETest ≤40 (“stable”) | 75 | 92 | 8 | 20 | |
| NETest >40 (“progressive”) | 7 | 8 | 32 | 80 | |
| PPQ Prediction Accuracy/RECIST | |||||
| RECIST-based responder | 80 | 99 | 2 | 5 | |
| RECIST-based no responder | 1 | 1 | 38 | 95 | |
| PPQ concordance | N | % | |||
| 118/122 | 97% | ||||
| Patients Assessed (n=112) | |||||
| CgA Predicted Responders/RECIST |
Responders (n=76) |
Non-Responders (n=36) |
|||
| CgA alterations during PRRT | N | % | N | % | |
| CgA decrease | 39 | 51 | 17 | 47 | |
| CgA increase | 37 | 49 | 19 | 53 | |
| CgA overall accuracy | N | % | |||
| All patients | 58/112 | 52 | |||
Fig. 2: NETest levels following PRRT administration (n=122).
2A. Dynamics of the biomarker and treatment. RECIST-responders (blue) were associated with a significant decrease (*p<0.05) in the NETest following therapy. Levels were lowest at follow-up falling into the “stable” NETest score category (SD ≤40), consistent with a treatment response (“Responders”). In non-responders (red), the mean NETest was initially unchanged but thereafter significantly increased (*p<0.05) in those who progressed (“Non-Responders”). NETest levels fell into the “progressive” NETest score category (PD >40).
2B. Modified waterfall Plot Analysis. Responders (n=83) are designated blue and non-responders (n=39) red. Open circles represent pre-PRRT NETest absolute levels. Changes in NETest levels are indicated by arrows (pre-treatment to biomarker level at follow-up). Red arrows identify a NETest increase and a category change to progressive (non-responsive). Blue arrows identify a NETest decrease in and a category change to responsive. In the responder group, 98% exhibited a decrease while in the non-responder group, 75% displayed a biomarker level increase (Fisher’s p<0.0001).
Modified waterfall plots for RECIST-responders and “non-responders” are in Figure 2B. Treatment failure group exhibited a NETest increase from baseline in 74% of patients. Ten patients exhibited a decrease in score from baseline (from 84±13 to 53±22) but the majority (n=7) still had elevated NETest scores (>40) at the time of progression (response category: “progressive”). Three patients had both a decrease in score and a response category change (progressive to stable) despite progressing with PRRT (Figure 2B – top: blue arrows). Patient #1: an 87-year old male with FDG-positive bronchial carcinoid; patient #2: a 55 year old male with rectal NET (G2, Ki67=15%) and patient #3: a 50 year old female with a small bowel NET (G2, Ki67=10%).
In the treatment response group, NETest scores decreased in the majority (99%). Score increase occurred in one patient and was associated with a score category change. This was a 67-year old female with an atypical lung carcinoid (Ki67=10%) (Figure 2B – bottom: red arrow).
Overall, with PRRT “responders”, the NETest decreased −47±3%. Progression during PRRT was associated with a mean +79±19% increase. The inclusive accuracy (biomarker/response category assessment) of the NETest as a monitor was 98% (119/122).
2. Association between changes in the NETest and outcome:
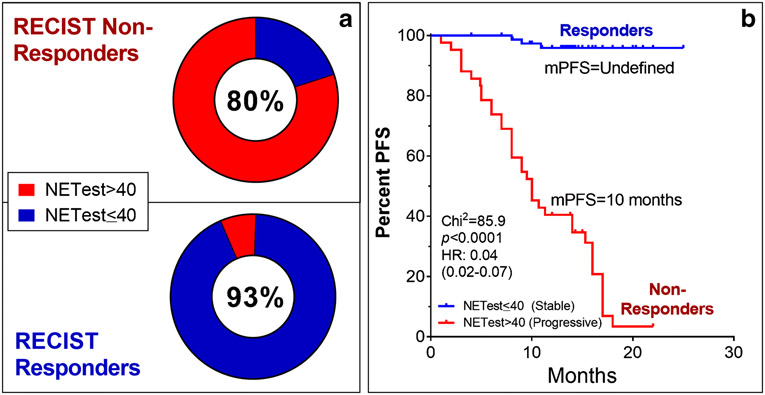
Of the RECIST-defined “responders” 77/83 (93%) had SD by NETest score while 80% (n=31/39) of “non-responders” had a PD score (Table 1, Figure 3A). This score-based response categorization was statistically significant (Fisher’s exact test: p<0.0001). The diagnostic accuracy was 93% (95%CI: 85-97%). The DOR was 49.7 (95%CI: 15.9-155, z-statistic 6.7, p<0.0001) confirming an accurate discriminative performance. The PLR was 5.6 (95%CI: 2.7-11.7) and negative likelihood ratio 0.11 (0.06-0.22) demonstrating that an increased test effectively “rules-out” a PRRT-response.
Fig. 3. Relationship between disease status change, NETest category and outcome.
3A. The proportion of patients categorized as SD (NETest ≤40) or PD (NETest >40) at follow-up for each of the two groups – responders and non-responders. A PD score at time of RECIST-defined progression was evident in 80% who progressed. The disease stabilization group had SD scores in 93% with (p<0.0001).
3B. Categorization of subjects into the two NETest categories (SD/PD) was significantly associated with outcome. Those classified as SD had an mPFS that was not reached. Those categorized as PD, had a significantly lower mPFS (10 months). The Hazard Ratio (HR) (0.04) was consistent with a significant (p<0.0001) association with PRRT efficacy.
A response categorization as either SD or PD was significantly (p<0.0001) associated with outcome to PRRT (Figure 3B). A prolonged mPFS (not reached) was identified in those with a stable NETest at follow-up compared to those who were categorized as “progressive” (mPFS: 10 months). The Hazard Ratio (HR) for the NETest as a monitoring biomarker was 0.04 (95%CI: 0.02-0.07) identifying a significant predictive effect (96%) for differentiating PRRT-responses.
3. Relationship between a Predictive biomarker – the PPQ – and the NETest as a Response Biomarker:
We next examined whether there was a relationship between PPQ-prediction and changes in the NETest.
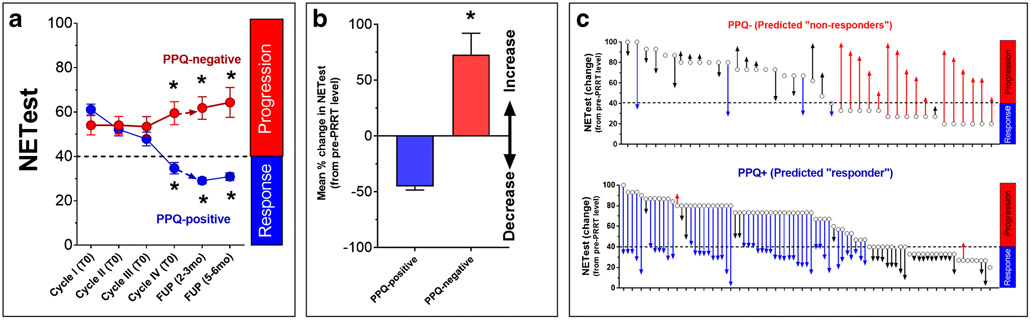
In the PPQ positive group (n=81), NETest scores significantly (Kruskal-Wallis statistic 95.27; p<0.0001) decreased after PRRT (Table 1, Figure 4A). Pre-PRRT levels were 61±22. Prior to cycle IV, levels were 35±20 and at both follow-ups, were further significantly (p<0.0001 vs. Cycle IV) decreased (29±12). In the PPQ-negative group (n=41), pre-PRRT levels were 53±28. They remained elevated (58±27) at Cycle IV and increased at follow-up (61±24. p<0.0005 vs. Pre-PRRT).
Fig. 4: NETest level alterations based upon the PPQ status (n=122).
4A. Dynamics of the biomarker and treatment. PPQ-positive (n=81) (blue) were associated with a significant decrease (*p<0.05) in the NETest following PRRT. Levels at follow-up fell into the SD category. In those in whom PRRT was predicted to “fail” (PPQ-negative) (red), the mean NETest was initially unchanged and thereafter significantly increased (*p<0.05). Levels at RECIST-defined progression fell into the PD category.
4B. Mean changes in the NETest in PPQ-positive (n=81; decreased from pre-PRRT) compared to PPQ-negative subjects (n=41; net increase from baseline). Levels were significantly different (p<0.0001).
4C. Individual alterations in absolute NETest levels from pre-PRRT (open circles) to end of therapy/progression with red arrows indicative of an increase (and category change) and blue arrows a decrease (and category change).
The PPQ-positive biomarker (predict PRRT-”responder”) was associated with a mean percentage decrease in the NETest of −46±3% (Figure 4B). PPQ-negative subjects (predicted to fail PRRT) were associated with a mean increase of +75±19%. Individual changes are included in Figure 4C.
4. Association between NETest changes (clinical category) and the PPQ biomarker:
At follow-up, 74/81 (92%) PPQ-positive subjects were SD. Of the PPQ-negative group 80% (n=33/41) had PD score (Figure 5A). This was statistically significant (Fisher’s exact test: p<0.0001). The diagnostic accuracy was 87% (95%CI: 79-92%) with a PLR of 8.1 (95%CI: 4.1-15.8) and a NLR of 0.22 (0.12-0.41).
Fig. 5. Relationship between NETest, disease status and PPQ-predicted outcome.
5A. Proportion of patients categorized as SD or PD for each of the two groups – PPQ-positive and PPQ-negative.
The PPQ-negative group exhibited PD score at time of progression in 80%. The PPQ-positive group (predicted to respond to PRRT) exhibited SD scores in 92% (p<0.0001). NETest level changes correlated with PPQ-based prediction of PRRT response.
5B. Categorization into SD/PD groups was significantly associated with outcome. The PPQ-positive group with a SD score exhibited an mPFS that was not reached. The PPQ-negative group with a PD score, had a significantly lower mPFS (10 months). The Hazard Ratio (HR) (0.06) was consistent with a significant association with PRRT efficacy (p<0.0001).
Response score categorization was significantly associated with the PPQ biomarker prediction and mPFS (Figure 5B). A prolonged mPFS (not reached) was identified in those NETest-categorized as SD at follow-up compared to PD (mPFS: 10 months). The Hazard Ratio (HR) for the NETest in PPQ-defined patients was 0.06 (95%CI: 0.03-0.12).
5. Concordance between RECIST response, PPQ and NETest levels:
RECIST stabilization or response to PRRT occurred in 83/122 (68%) and 39 (32%) progressed. The accuracy of PPQ for predicting response was 118/122 (97%). Thus, 80/81 (99%) of PPQ-positive responded while 93% (38/41) of PPQ-negative, progressed on PRRT.
There was one false negative (predicted to respond but who progressed): a 59-year old male with an FDG-positive G2 pancreatic NET (stage IV disease). The pre-PRRT NETest was high (80) and increased (to 93) after cycle II. Three false negatives (i.e., those who responded to PRRT despite being PPQ-negative) were identified. Patient #1: 60 year old woman with a G3 gastric NET (liver metastases) who exhibited a partial response with disease stabilization at 19 months. The pre-PRRT NETest identified SD (40); the NETest decreased to 33 at cycle IV cycle and was 20 at 6-month follow-up. Patient #2: a 70-year old male with a G2 pancreatic NET initially RECIST-“stable”. The NETest was 80 and decreased to 33 at cycle IV with both follow-up NETests stable (both 27); the patient was followed for 4 months and considered stable at imaging. Patient #3: a 63-year old man with a G2 pancreatic NET. His NETest was 40 and decreased to 33 at cycle IV with both follow-up NETests stable (both 27); the patient was followed for 12 months and was stable at restaging.
6. Role of CgA as a biomarker:
Finally, we examined whether CgA functioned as a PRRT response biomarker. Data were available from 112 of the 122 patients.
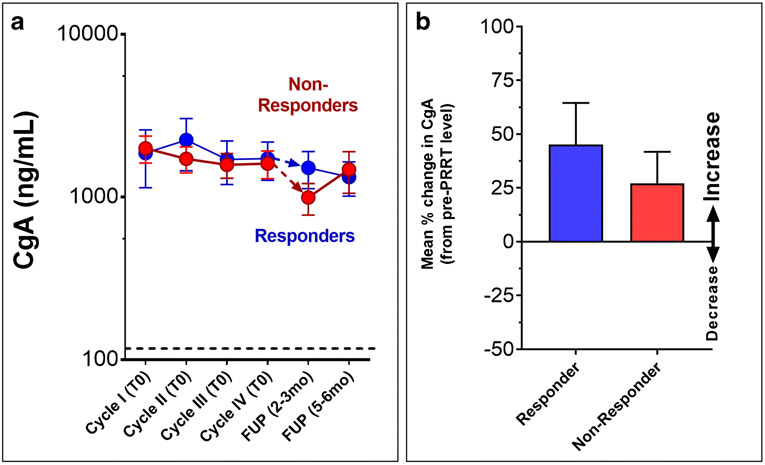
In RECIST-“responders” (n=76), pre-PRRT CgA levels were 1878±728ng/ml and at follow-up were 1735±458ng/ml. There was no significant decrease (mean: 1338-1525) (Kruskal-Wallis statistic 2.95; p=0.83) (Table 1, Figure 6A). In RECIST-“non-responders” (n=36), pre-PRRT levels were 1978±375ng/ml and remained elevated at follow-up (985-1465ng/ml) (p=NS vs. pre-PRRT). Overall, in “responders” a PRRT response was associated with a mean +45±19% CgA increase (from baseline in those who were CgA positive, Figure 6B). In progression on PRRT, CgA had a mean +27±13% increase from baseline.
Fig. 6: Changes in CgA with PRRT (n=112).
6A. Dynamics of the biomarker and treatment. Responders were not associated with a significant decrease in CgA levels following therapy. Similarly, no significant differences were identified in CgA levels in non-responders.
6B. Mean changes in CgA levels (between follow-up and baseline) in responders (n=76) compared to non-responders (n=36). CgA levels were increased and mean levels were not significantly different between responders and non-responders.
Discussion
Our study evaluated whether the NETest exhibited demonstrable clinical utility as a monitoring biomarker and could accurately evaluate response to PRRT as measured by PFS, a well-characterized clinical endpoint in NET studies. We performed an analysis of prospectively collected blood samples (a total of 157 treated patients) from three different treatment centers. NETest changes between baseline and follow-up were evaluated. We also investigated whether the circulating PRRT-predictive biomarker (the PPQ) [6] and its output i.e., PRRT-responder or non-responder, predicted clinically relevant alterations in the NETest during treatment and at follow-up. Finally, we also undertook an analysis of changes in CgA to evaluate whether this biomarker exhibited any utility as a marker of PRRT response.
Biomarker monitoring studies evaluate changes in biomarkers versus baseline levels to investigate whether a treatment is effective or not [7, 8]. Changes are typically correlated with a gold-standard measure such as imaging. Any stabilization (no change) or decrease in a biomarker is thus related to a treatment response. In an analogous fashion, any increase in the biomarker level would only be related to the tumor(s) growth, a reflection of no response or a failure to respond to a therapy e.g., PRRT.
Biomarker outputs can also be categorized into e.g., low and high scores or low/high risks [23]. Such categorizations, if they are associated with disease status e.g., activity of a tumor [14], provide a second and complimentary method to evaluate treatment efficacy. For example, a change from a “high” to “low” category, would suggest that the treatment is targeting the tumor and affecting its biology, e.g., decreasing expression of markers linked to growth or biological activity. An increase is presumed to reflect a lack of response to the therapy and continued tumor growth [18, 19]. The NETest output is both a score (0-100) and has two categories – low (≤40) or SD and high (>40) or PD [18, 21]. We examined the utility of both outputs to monitor responses to PRRT.
We first evaluated whether changes in the NETest score correlated with responses to PRRT in the cohort with complete image-based data and follow-up (n=122). This intention-to-treat cohort, while comprising 3 different centers and individualized treatment approaches, exhibited similar responses to PRRT irrespective of protocol or number of PRRT cycles (Supplemental Figure 1). Moreover, the NETest changes were not related to the type or extent of therapy (Supplemental Figures 2-3) or to the clinical status of the patient at baseline (Supplemental Figure 4) further supporting our approach to assessing grouped data.
The mean NETest scores were significantly lower (p<0.0001) in the disease stabilization or “responder” group (n=83; mean: 29) than in those who progressed while on PRRT (n=38, mean: 73). No change or a decrease in the NETest biomarker occurred in 98% when imaging determined therapy to be effective. Indeed, values were linearly reduced (Pearson r=0.65, p<3x10−11) after 2 and 3 cycles of PRRT, respectively (Supplemental Figures 5-6). This is consistent with our hypothesis that a decreased score reflected disease stabilization or response. Of the two patients with an increase in score, despite “disease stabilization”, one had a “low” score (<40) consistent with slow-growing disease [18, 24]. The second “responder” had an atypical carcinoid (Ki-67 10%) and her score was elevated (47 and 73) at both follow-up time points. We presume this patient, despite responding to PRRT, may be at an increased risk for subsequent progression and is being closely monitored. In this respect, it should be noted that small percentage increases in tumor diameter (i.e., 0-19%) would be considered RECIST-“stable”. We have previously reported that minimal growth, undetectable by imaging is associated with an increase in the NETest [25]. Under such circumstances, a patient might be incorrectly classified as an image-“responder”.
In the “non-responders”, ~75% exhibited an increase in the NETest consistent with our hypothesis (increased levels reflect disease progression) Indeed, values overall were linearly increased (Pearson r=0.71, p<3x10−6) after 2 and 3 cycles of PRRT, respectively (Supplemental Figures 5-6). In the 25% – 10 patients – with a decrease in score, the majority (7 of 10) exhibited a PD category response score at the time of imaging. This is consistent with the identification of “active” growing/progressing disease despite “some effect” of PRRT. We presume that the decrease in these seven “non-responders” reflects the therapeutic “effect” of PRRT on some lesions that are sensitive to the agent. It is well-known that PRRT does not uniformly affect all target lesions and it is reasonable to assume that the non-targeted lesions will continue to grow and are reflected in RECIST-evaluation as “progressive”. The NETest, because it is based on circulating transcripts, samples the entirety of a patient’s tumor(s) response, likely reflected by a score decrease. The overall decrease in score was not as profound in these “non-responders” (mean: −34%) compared to the “responders” (mean: −49%. p=0.04) supporting our supposition. A larger group would be necessary to further assess this issue more rigorously.
We then evaluated the association between disease category changes (e.g., does a responder exhibit an SD score at follow-up), and response to PRRT. In the PRRT-responder group 93% were SD while in the “non-responder” group 80% were PD. These data confirm that the cut-off of NETest of 40 is 93% accurate for determining PRRT response. This data is further supported by a DOR of ~50 and a PLR of 11 which are consistent with an accurate “rule-in” test [7]. Furthermore, the two categories were significantly related to outcome (defined as mPFS). Thus, SD was associated with a significantly (p=0.0001) longer mPFS (undefined) than a PD response category score (mPFs=10 months). The hazard ratio was 0.04. Overall, these observations support that the NETest provides clinically useful information relevant to outcome. Of note, the 35 patients with missing time points also exhibited comparable score changes, further supporting the NETest utility for PRRT monitoring (Supplemental Figure 7).
Next, we examined the changes in the NETest in relationship to the PPQ. This biomarker has a ~95% accuracy for predicting those in whom PRRT would be efficacious [6]. We tested the hypothesis that those predicted to respond to PRRT (i.e., PPQ-positive) would exhibit a significant NETest decrease compared to PPQ-negative. The PPQ-positive group numbered 81 and the PPQ-negative 41. In the PPQ-positive cohort, there was either no change or a decreased NETest score in 98%. Similarly, in the PPQ-positive cohort, 92% exhibited a SD score at follow-up and this was associated with a mPFS not reached. Conversely, in the PPQ-negative patients, ~75% exhibited an increased score of whom 80% were PD at follow-up. This was associated with a mPFS of 10 months. Overall, the accuracy of the PPQ to determine treatment response was 97%. These data are consistent with the PPQ biomarker accurately reflecting NETest delineation of the PRRT response. It is of interest to consider whether a PRRT outcome index (combination of the PPQ measured before PRRT is initiated and NETest monitoring during PRRT and follow-up) could be developed as a mathematical strategy for predicting and evaluating PRRT response. Our assessment in this study identified that while NETest measurements after 3 cycles was 81% accurate in predicting response, the combination of the PPQ and the NETest (3 cycles) was 99.2% accurate.
CgA has also been used to measure PRRT response, but there are significant concerns [5], such as variable detection (highest in small bowel ~70%, lowest in lung ~35% [26]), and possible utility only in extensive disease and secretory tumors [27]. A subset of patients therefore is typically only appropriate for study. One study identified that a ≥50% decrease in CgA was not associated with a response to PRRT [10]. Another identified increases in CgA (≥20% compared to baseline) in both responders (27%) and non-responders (34%), a clear limitation [5]. Our initial study identified a low correlation (24% accuracy) between alterations in CgA and PRRT response [22]. In the current study, we compared no change/any decrease in CgA versus an increase in CgA following PRRT using identical time-points as the NETest. CgA increased in 49% of PRRT responders, while 53% of those who failed therapy (progressive disease) also exhibited an increase. This data is compelling in implying that CgA is not a useful monitoring biomarker. Previous reports have proposed that a 30% decrease in CgA is indicative of a NET therapeutic response [28]. In our study, decreases of ≥30% were identified in 36% of PRRT responders. However, 22% of those with progressive disease also exhibited a ≥30% decrease. This was not significant (p=0.15). These data further indicate that CgA has no utility as a clinically effective PRRT biomarker.
A strength of our study is the prospective use of liquid biopsy molecular genomics to assess PRRT efficacy. It investigated a substantial number of patients (n=157) in three different centers and evaluated both predictive and prognostic multigene biomarkers. However, there are some limitations. Firstly, we utilized PFS measured by imaging as an endpoint. Although the relationship between biomarker changes and this endpoint was evident, we noted some discrepancies. These differences could reflect that imaging and the NETest may measure different magnitudes of the same phenomenon. While image-determined PFS is “gold-standard”, it is well-known that accurate morphological assessments of PRRT responses are typically only reliable at ≥3 months after cessation of PRRT [5]. In our prior study [6], 15% of RECIST-responses required revision during long-term follow-up after PRRT. Since the NETest evaluates cellular changes with a high sensitivity (>90%) compared to image-based analysis which assesses billions of cells, some discordance is predictable. To obviate such issues, it has been suggested that somatostatin receptor imaging be used as a monitoring tool as opposed to anatomic imaging [29, 30]. However, post-administration Lu-Scan protocols have limited resolution. Measuring alterations in SSA-PET-CT uptake pre- and post-therapy, may have better sensitivity. At this time, this is not considered standard and remains sub-optimal [31].
To conclude, our results identify a role for the NETest in monitoring responses to PRRT of NETs (Supplemental Table 3). A decrease in score identifies responders and correlates well with an independent biomarker prediction, the PPQ, for response. Increases in the NETest readily identified non-responders well in advance of any current imaging protocols. This could allow a prompt change in strategy (e.g., direct sequencing of treatments right after PRRT, augmentation of PRRT with other treatments or even an early discontinuation of PRRT). Our data indicate that the NETest is an effective and accurate surrogate marker that reflects radiological responses in GEP- and bronchopulmonary NETs treated with PRRT. The results were independent of the number of PRRT cycles, were not limited to a type of treatment approach and functioned effectively whether a patient undergoes a standard regimen of 4 PRRT cycles or was included in an individualized protocol. We also confirmed the biological and therapeutic validity of the PPQ biomarker. Both assays are available and can be applied to clinical practice. We anticipate that such real-time information can be used to reassure both the patient and the clinician that PRRT is the appropriate treatment strategy and that therapy is effective. The use of these biomarkers in PRRT will, in addition, have likely best practice medical and health economic implications.
Supplementary Material
Footnotes
Ethical approval: All human participant procedures were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all participants in the study.
Conflict of Interest: LB, RPB and GP – consultancy fees from Ipsen and Advanced Accelerator Applications unrelated to the submitted work (AAA). EPK and DJK – support from AAA unrelated to the submitted work. RPB – support from Ipsen, ROTOP Pharmaka and OctreoPharm Sciences unrelated to the work. MK – employed by Wren Laboratories. IMM & IAD – consultants for Wren Laboratories. No conflicts for AS, WAvZ, SS or AM.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Kwekkeboom DJ, Krenning EP. Peptide Receptor Radionuclide Therapy in the Treatment of Neuroendocrine Tumors. Hematol Oncol Clin North Am. 2016;30(1):179–91. doi: 10.1016/j.hoc.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodei L, Cremonesi M, Grana CM, Fazio N, lodice S, Baio SM, et al. Peptide receptor radionuclide therapy with (1)(7)(7)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38(12):2125–35. Epub 011 Sep 3. [DOI] [PubMed] [Google Scholar]
- 4.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26(13):2124–30. [DOI] [PubMed] [Google Scholar]
- 5.Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, de Herder WW, Feelders RA, et al. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [(177)Lu-DOTA(0),Tyr(3)]octreotate. Endocr Relat Cancer. 2017;24(5):243–51. doi: 10.1530/ERC-16-0524. Epub 2017 Mar 20. [DOI] [PubMed] [Google Scholar]
- 6.Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, et al. PRRT Genomic Signature in Blood for Prediction of 177Lu-octreotate Efficacy. EJNMMI. 2018;45(7):1155–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X, Modur V, Carayannopoulos LN, Laterza OF. Biomarkers in Pharmaceutical Research. Clin Chem. 2015;61(11): 1343–53. doi: 10.73/clinchem.2014.231712. Epub 2015 Sep 25. [DOI] [PubMed] [Google Scholar]
- 8.Amin S, Bathe OF. Response biomarkers: re-envisioning the approach to tailoring drug therapy for cancer. BMC Cancer. 2016;16(1):850. doi: 10.1186/s12885-016-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezziddin S, Lohmar J, Yong-Hing CJ, Sabet A, Ahmadzadehfar H, Kukuk G, et al. Does the pretherapeutic tumor SUV in 68Ga DOTATOC PET predict the absorbed dose of 177Lu octreotate? Clin Nucl Med. 2012;37(6):e141–7. doi: 10.1097/RLU.0b013e31823926e5. [DOI] [PubMed] [Google Scholar]
- 10.Haug AR, Auernhammer CJ, Wangler B, Schmidt GP, Uebleis C, Goke B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;51(9):1349–56. Epub 2010 Aug 18. [DOI] [PubMed] [Google Scholar]
- 11.Kidd M, Modlin IM. Therapy: The role of liquid biopsies to manage and predict PRRT for NETs. Nat Rev Gastroenterol Hepatol. 2017;14(6):331–2. [DOI] [PubMed] [Google Scholar]
- 12.Bodei L, Herrmann K, Baum RP, Kidd M, Malczewska A, Modlin IM. Caveat Emptor: Let Our Acclaim of the Apotheosis of PRRT Not Blind Us to the Error of Prometheus. J Nucl Med. 2019;60(1):7–8. doi: 10.2967/jnumed.118.219386. [DOI] [PubMed] [Google Scholar]
- 13.Modlin I, Drozdov I, Kidd M. The Identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. Plos One. 2013;e63364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidd M, Drozdov I, Modlin I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat Cancer. 2015;22(4):561–75. doi: 10.1530/ERC-15-0092. Epub 2015 Jun 2. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Zhang Y, Gibbons DL, Deneen B, Kwiatkowski DJ, Ittmann M, et al. Pan-Cancer Molecular Classes Transcending Tumor Lineage Across 32 Cancer Types, Multiple Data Platforms, and over 10,000 Cases. Clin Cancer Res. 2018;24(9):2182–93. doi: 10.1158/078-0432.CCR-17-3378. Epub 2018 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Treijen MJC, Korse CM, van Leeuwaarde RS, Saveur LJ, Vriens MR, Verbeek WHM, et al. Blood Transcript Profiling for the Detection of Neuroendocrine Tumors: Results of a Large Independent Validation Study. Front Endocrinol (Lausanne). 2018;9:740.(doi): 10.3389/fendo.2018.00740.eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malczewska A, Oberg K, Bodei L, Aslanian H, Lewczuk A, Filosso PL, et al. NETest liquid biopsy is Diagnostic of Lung Neuroendocrine Tumors and Identifies Progressive Disease. Neuroendocrinology. 2019;108(3):219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu E, Paulson S, Gulati A, Freudman J, Kafer S, Wickremesinghe P, et al. Assessment of NETest Clinical utility in a US Registry-based study. The Oncologist. 2019;24(6):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cwikla JB, Bodei L, Kolasinska-Cwikla A, Sankowski A, Modlin IM, Kidd M. Circulating transcript analysis (NETest) in GEP-NETs treated with Somatostatin Analogs defines Therapy. J Clin Endocrinol Metab. 2015;100(11):E1437–45. [DOI] [PubMed] [Google Scholar]
- 20.Kidd M, Modlin I, Drozdov I, Aslanian H, Bodei L, Matar S, et al. A Liquid Biopsy for Bronchopulmonary/lung Carcinoid Diagnosis. Oncotarget. 2017;9(6):7182–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malczewska A, Makulik K, Witkowska M, Bocian A, Walter A, Zajęcki W, et al. NETest Liquid Biopsy is Diagnostic of Small Intestine and Pancreatic Neuroendocrine Tumors and Accurately Correlates with Anatomical and Functional Imaging Endocrine Connections. 2019;March 1. pii: EC-19-0030.R1. doi: 10.1530/EC-19-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodei L, Kidd M, Modlin IM, Severi S, Drozdov I, Nicolini S, et al. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2016;43(5):839–51. doi: 10.1007/s00259-015-3250-z. Epub 2015 Nov 23. [DOI] [PubMed] [Google Scholar]
- 23.You YN, Rustin RB, Sullivan JD. Oncotype DX((R)) colon cancer assay for prediction of recurrence risk in patients with stage II and III colon cancer: A review of the evidence. Surg Oncol. 2015;24(2):61–6. doi: 10.1016/j.suronc.2015.02.001. Epub Feb 14. [DOI] [PubMed] [Google Scholar]
- 24.Pavel M, Jann H, Prasad V, Drozdov I, Modlin IM, Kidd M. NET Blood Transcript Analysis defines the Crossing of the Clinical Rubicon: When Stable Disease becomes Progressive. Neuroendocrinology. 2017;104(2):170–82. [DOI] [PubMed] [Google Scholar]
- 25.Malczewska A, Bodei L, Kidd M, Modlin IM. Blood mRNA Measurement (NETest) for Neuroendocrine Tumor Diagnosis of Image-Negative Liver Metastatic Disease. J Clin Endocrinol Metab. 2019;104(3):867–72. doi: 10.1210/jc.2018-01804. [DOI] [PubMed] [Google Scholar]
- 26.Oberg K, Modlin I, DeHerder W, Pavel M, Klimstra D, Frilling A, et al. Biomarkers for Neuroendocrine Tumor Disease: A Delphic Consensus assessment of Multianalytes, Genomics, Circulating Cells and Monoanalytes. Lancet Oncol. 2015;16:e435046. [Google Scholar]
- 27.Kidd M, Bodei L, Modlin IM. Chromogranin A: any relevance in neuroendocrine tumors? Curr Opin Endocrinol Diabetes Obes. 2016;23(1):28–37. doi: 10.1097/MED.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 28.Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22(23):4762–71. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Oksuz MO, Winter L, Pfannenberg C, Reischl G, Mussig K, Bares R, et al. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)Y-DOTATOC: is treatment response predictable by pre-therapeutic uptake of (68)Ga-DOTATOC? Diagn Interv Imaging. 2014;95(3):289–300. doi: 10.1016/j.diii.2013.07.006. Epub Sep 12. [DOI] [PubMed] [Google Scholar]
- 30.Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grunwald F, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55(2):183–90. doi: 10.2967/jnumed.113.125336. Epub 2014 Jan 16. [DOI] [PubMed] [Google Scholar]
- 31.Oberg K Molecular Imaging Radiotherapy: Theranostics for Personalized Patient Management of Neuroendocrine Tumors (NETs). Theranostics. 2012;2(5):448–58. Epub 2012 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.