Abstract
Purpose
This study was conducted to evaluate the potential role of shear wave elastography (SWE) in the evaluation of testes in patients with varicocele.
Methods
A total of 116 testes of 58 patients with left-sided varicocele and 58 testes of 29 age-matched healthy controls were included in the study. The patients' testes were classified into groups A (normospermic) and B (oligospermic) based on the presence of oligospermia. The mean SWE values and volume of the testes with varicocele were compared with the contralateral normal testes of the patients and the testes of healthy controls.
Results
The mean SWE value of the left testes in groups A (4.77±1.16 kPa) and B (6.15±1.96 kPa) exceeded that of the healthy controls (3.79±0.94 kPa) (P<0.001). The mean SWE value of the left testes in group B (6.15±1.96 kPa) exceeded that of group A (4.77±1.16 kPa) (P=0.002). No correlation was observed between testicular stiffness and the grade of varicocele (r=0.102, P=0.423)
Conclusion
The testes with varicocele were stiffer than the contralateral testes and the testes of healthy controls, and SWE might play an auxiliary role to conventional ultrasonography in assessing pathologic alterations in the testis owing to varicocele.
Keywords: Shear wave elastography, Stiffness, Testis, Ultrasound, Varicocele
Introduction
Varicocele is a vascular abnormality characterized by dilated veins of the pampiniform plexus, and primarily results from incompetent or absent valves in the spermatic vein. The estimated prevalence of varicocele is approximately 15% in the adult male population [1]. Infertility, pain, and scrotal enlargement are common symptoms of the disease, and most cases occur on the left side given the drainage of the left spermatic vein into the left renal vein at a right angle [2]. Physical examination is the primary method for the diagnosis and grading of the disease. Imaging modalities are supplemental diagnostic methods, and ultrasonography (US) is the modality of choice for the radiologic evaluation of varicocele. Hypoechoic veins (with a "bag of worms" appearance), veins larger than 2 mm in diameter, and increased venous size during the Valsalva maneuver are the hallmark US findings of the disease [3]. Color Doppler US might be also used as an auxiliary method to identify retrograde venous flow into the testicular veins [3].
Shear wave elastography (SWE) is a novel method of elastography in which stress is generated by automatic shear waves rather than manual compression [4]. SWE provides quantitative information regarding the stiffness of the relevant tissue by tracking shear waves passing through them [5]. The diagnostic value of elastography has been explored in undescended testes, testicular tumors, hydrocele, and testicular microlithiasis, with favorable results [5-8]. Several studies have investigated the role of SWE in assessing testicular parenchyma and the associations between SWE values and sperm parameters in the presence of varicocele. However, despite some promising results, changes in testicular stiffness have been a subject of debate among studies [9-11].
In the present study, we had two main objectives: to evaluate the potential role of SWE in the evaluation of testicular parenchyma in patients with varicocele and to clarify inconsistent findings regarding testicular stiffness and varicocele reported in previous studies.
Materials and Methods
Male patients admitted to our infertility clinic between March 2017 and April 2018 who were unable to achieve pregnancy with their partner within 1 year despite not using any contraceptive methods were enrolled in the study. Informed written consent was obtained from each patient. The data of the patients were retrospectively scanned. The study was approved by the local ethics committee. The inclusion criteria of the study were as follows: (1) having been diagnosed with left-sided grade 1-3 varicocele on physical examination, (2) having Doppler and grayscale US findings that satisfied the criteria for varicocele, and (3) being between 18 and 50 years old. Patients with (1) chromosomal disorders, (2) a history of previous surgery, (3) present or previous malignancy, (4) bilateral or right-sided varicocele, (5) abnormal hormone levels, (6) chronic illness, (7) a history of radiation exposure, (8) hydrocele, (9) abnormal testicular parenchymal texture on US, and (10) teratozoospermia were excluded from the study. The healthy control group comprised randomly chosen patients who presented to our andrology clinic for infertility, but had normal testicular US, physical examinations, and laboratory results and were in couples that were ultimately diagnosed with female factor infertility. The same exclusion criteria were applied to the healthy controls and the patient group. Furthermore, the healthy controls must not have had any signs of varicocele on physical examination or US or infertility. Varicocele was graded following the recommendations of Dubin and Amelar [12]. Fig. 1 shows the flow chart of the inclusion and exclusion criteria of the study.
Fig. 1. Flow chart showing the inclusion and exclusion criteria of the study.
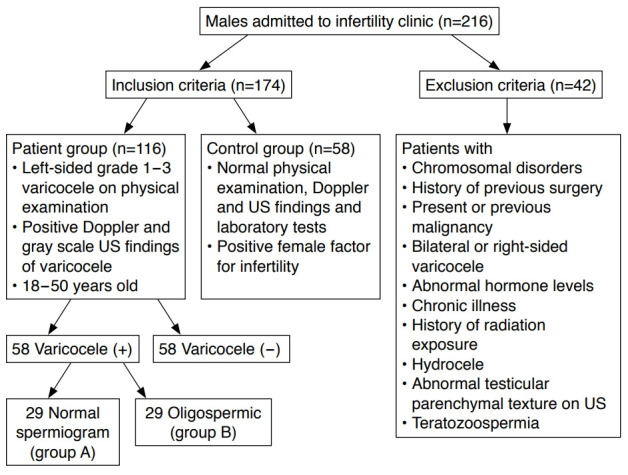
n, number of testes; US, ultrasonography.
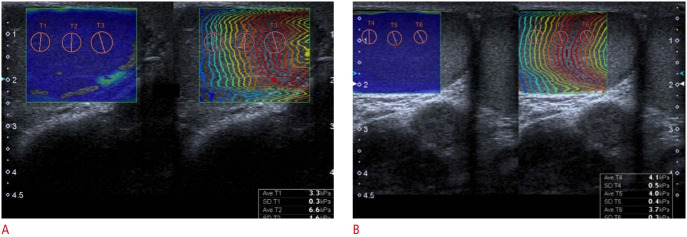
An observer with more than 8 years of experience with conventional US and elastography experience (O.T.) performed all examinations. The patients were asked to lie in the supine position before the measurements. All grayscale and SWE examinations were performed using an Aplio 500 Platinum (Canon, Tokyo, Japan) US unit. The measurements were conducted using a linear array high-frequency transducer (11-14 MHz). The testicular volumes were calculated from 2-dimensional images using the prolate ellipsoid method based on the formula: volume=length×width×height×0.523. During this phase of the measurements, patients were excluded from the study if the observer identified any abnormalities in the testicular parenchyma on grayscale US or did not confirm the presence of varicocele by a US assessment. After completing the conventional US evaluation, the observer initiated the SWE examination. While performing the SWE examination, the observer kept the transducer as stable as possible to avoid artifacts. Images that were taken longitudinally were chosen for the measurements. The observer placed three regions of interest with a diameter of 3 or 4 mm onto the chromatic box using propagation maps, and performed five consecutive measurements. The average of these measurements was calculated. The stiffness values were recorded in units of kilopascals (kPa). Fig. 2 shows the SWE evaluation of a testis with varicocele and a contralateral normal testis. The left testes of patients with varicocele were further categorized into groups A and B according to total sperm count, with group A constituting patients with normal spermiograms, while group B constituted patients with oligospermia (sperm count <15 million/mL). The mean SWE and volume values of the left testes of patients were compared with those of the contralateral testes and the testes of the healthy controls.
Fig. 2. A 25-year-old normospermic man with left-sided varicocele.
The average stiffness (4.86 kPa) value of the left testis (A) derived from three regions of interest was higher than that of the contralateral (B) testis (3.93 kPa).
Statistical Analysis
SPSS version 22 (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses. The variables were investigated using analytical methods (Shapiro-Wilk test) and visual techniques (histograms, probability plots) to determine whether they were normally distributed. The Student's t test was used to compare these variables because the SWE and volume values showed a normal distribution. P-values of <0.05 were considered to indicate statistical significance. Correlations between testicular volume and varicocele grade and between testicular stiffness and varicocele grade for patients' left testes were calculated using the Spearman coefficient test.
Results
In total, 116 testes of patients with left-sided varicocele and 58 testes of 29 age-matched healthy controls were included in the study. Among the 58 testes with varicocele, 16 were assessed as grade 1 (27.6%), 16 as grade 2 (27.6%), and 26 as grade 3 (44.8%). The mean age of the patients was 32.81±9.07 years and the mean age of the healthy controls was 34.23±9.09 years (P=0.422).
There was no significant difference between the mean volume of the left testes (the ipsilateral side with varicocele) (12.84±2.68 mL) and the contralateral testes (13.44±4.07 mL) in group A (P=0.445). The mean SWE value of the left testes in group A was 4.77±1.16 kPa, which exceeded that of the contralateral testes (3.52±0.41 kPa) (P<0.001).
The mean volume of the left testes (the ipsilateral side with varicocele) in group B (12.44±2.82 mL) was significantly lower than that of the contralateral testes (15.01±5.44 mL) (P=0.022). The mean SWE value of the left testes in group B was 6.15±1.96 kPa, which exceeded that of the contralateral testes (3.81±0.71 kPa) (P<0.001). No significant difference was observed between groups A and B in terms of left testicular volume (P=0.587). Table 1 summarizes the elastographic and US findings of groups A and B.
Table 1.
Testicular SWE and volume findings of normospermic and oligospermic patients with left-sided varicocele and comparison with the contralateral testes
| Testes with varicocele (n=58) | Contralateral testes (n=58) | P-value | |
|---|---|---|---|
| Normospermic (n=29) | |||
| SWE (kPa) | 4.77±1.16 | 3.52±0.41 | <0.001 |
| Volume (mL) | 12.84±2.68 | 13.44±4.07 | 0.445 |
| Oligospermic (n=29) | |||
| SWE (kPa) | 6.15±1.96 | 3.81±0.71 | <0.001 |
| Volume (mL) | 12.44±2.82 | 15.01±5.44 | 0.022 |
Values are presented as mean±SD.
SWE, shear wave elastography; SD, standard deviation.
The mean SWE value of the left testes in group B (6.15±1.96 kPa) exceeded that of group A (4.77±1.16 kPa) (P=0.002). When we compared the mean volume (12.84±2.68 mL) and stiffness (4.77±1.16 kPa) of the left testes of patients in group A with healthy controls' testes (15.75±4.51 mL, 3.79±0.94 kPa), we identified a significant difference (P<0.001). When we compared the mean volume (12.44±2.82 mL) and stiffness (6.15±1.96 kPa) of the left testes of patients in group B with healthy controls’ testes (15.75±4.51 mL and 3.79±0.94 kPa, respectively), we also identified a significant difference (P<0.001). However, no significant difference was observed between the contralateral testes of the patients and the testes of the healthy controls with regard to volume or stiffness (P>0.05).
We detected no meaningful correlation between testicular stiffness and the grade of varicocele (r=0.102, P=0.423). However, a weak negative correlation was detected between the volume of the testes and varicocele grade (r=-0.321, P=0.014). No significant correlation was observed between stiffness and the volume of the testes (P=0.422). Moreover, no significant correlations were observed between volume and age or stiffness in the testes of the healthy controls.
Discussion
Testes with varicocele showed higher stiffness values and lower volume than the testes on the contralateral side in the same patients. Notably, the testes with varicocele in the oligospermic group had higher mean stiffness values than were observed in normospermic patients, despite a statistically similar volume in both groups. Furthermore, the testes with varicocele also had higher stiffness and lower volume than the testes of the healthy controls.
It is well recognized that the presence of varicocele substantially impacts the microstructure and macrostructure of the testis [13]. Several mechanisms underlying the effect of varicocele on the testis have been proposed, of which testicular hyperthermia is the most widely acknowledged [14]. The pampiniform plexus serves as a critical thermoregulatory mechanism, since the ultrastructural integrity of the testis and the continuation of healthy spermatogenesis require a proper environment, which should be several degrees cooler than normal body temperature [1,14,15]. However, varicocele affects and disturbs this thermoregulation mechanism, which eventually leads to the impairment of testicular function and histology [14-16]. It is well-known that varicocele commonly results in testicular hypotrophy, and many US studies have demonstrated that testes with varicocele had lower volume than testes without varicocele [16]. In our study, testicular volume was also lower on the side with varicocele. However, volumetric changes in testes with varicocele might manifest at a later stage of the disease, and testicular volume might not be an ideal surrogate marker of underlying ultrastructural changes in the testes.
The feasibility and efficiency of SWE in detecting ultrastructural changes in testicular parenchyma in various diseases have been demonstrated in previous reports [5-7]. Furthermore, several studies have focused on the impact of varicocele on the testes using elastography. Dede et al. [9] published one of the first reports that introduced the use of SWE to evaluate testes with varicocele. Contrary to our results, they observed lower stiffness values in testes with varicocele, which they claimed was linked to apoptosis in germ cells. They did not obtain any histologic data, but supported their findings by asserting that testes with varicocele were also softened on physical examinations [9]. Conversely, a further report by Camoglio et al. [10], in which strain elastography was used to evaluate testes with varicocele, reported that the testes with varicocele had higher stiffness values than normal testes, in accordance with our results. No correlation was found between varicocele grade and the stiffness values of the testes, in contrast to previous works by Camoglio et al. [10] and Dede et al. [9].
Previous histologic reports have indicated that testes with varicocele are characterized by thickened tubular basement membranes and an increased deposition of interstitial fibrous tissue in addition to germ cell atrophy [17,18], and increased stiffness secondary to fibrosis is widely recognized feature of elastographic examinations [19]. Since we did not obtain any histologic data, we could not be able to extrapolate a possible explanation for the discrepancy between the results of our study and those of the study by Dede et al. [9]. However, with all due respect, it is clear that the aforementioned histologic changes of testes with varicocele-particularly peritubular fibrosis-should inevitably lead to increased stiffness values on SWE. Additionally, a negative correlation between peritubular fibrosis and sperm count has been demonstrated in the previous reports, corroborating our results showing that the testicular stiffness of oligospermic patients with varicocele was higher than that of normospermic patients with varicocele [20].
Other radiologic methods such as Doppler US, diffusion weighted magnetic resonance imaging (MRI), and MRI spectroscopy have been used and discussed in previous studies for assessing male infertility [21-26]. In a Doppler US study using testicular artery flow parameters for assessing male infertility, Biagiotti et al. [21] stated that the resistive index and peak systolic velocity were significantly higher in patients with obstructive azoospermia than in those with nonobstructive azoospermia. They suggested that these parameters might be helpful for differentiating obstructive from nonobstructive azoospermia.
Han et al. [25] reported that the testicular apparent diffusion coefficient (ADC) and normalized ADC were significantly lower in patients with obstructive azoospermia than in those with nonobstructive azoospermia, and stated that the ADC was useful for predicting the histopathologic grade of azoospermia and differentiating obstructive from nonobstructive azoospermia. Karakas et al. [26] reported that the ADC of the testicular parenchyma in patients with varicocele was lower than that in healthy subjects. They proposed that the ischemic and fibrotic process in patients with varicocele was related to this decrease.
Several drawbacks in our study need to be acknowledged. First and foremost, we did not calculate interobserver variability since only a single observer made all the examinations, but SWE has shown good interobserver variability in previous reports. Additionally, we did not obtain any histologic data, but our results are substantially compatible with those of previous histopathologic studies.
In conclusion, our study showed higher stiffness values in testes with varicocele than in the contralateral testes and the testes of healthy male controls, and also determined that the testes of oligospermic patients had higher stiffness values than those of normospermic patients. We suggest that SWE, as a non-invasive and quantitative method, might play an auxiliary role to conventional US in assessing the parenchymal damage of testes resulting from varicocele and might be helpful in differentiating oligospermic patients from normospermic patients.
Footnotes
Author Contributions
Conceptualization: Turna O. Data acquisition: Turna O. Data analysis or interpretation: Aybar MD. Drafting of the manuscript: Turna O. Critical revision of the manuscript: Aybar MD. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
References
- 1.Fretz PC, Sandlow JI. Varicocele: current concepts in pathophysiology, diagnosis, and treatment. Urol Clin North Am. 2002;29:921–937. doi: 10.1016/s0094-0143(02)00075-7. [DOI] [PubMed] [Google Scholar]
- 2.Nagler HM, Grotas AB. Varicocele. In: Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the male. 4th ed. New York: Cambridge University Press; 2009. pp. 331–361. [Google Scholar]
- 3.Chiou RK, Anderson JC, Wobig RK, Rosinsky DE, Matamoros A Jr, Chen WS, et al. Color Doppler ultrasound criteria to diagnose varicoceles: correlation of a new scoring system with physical examination. Urology. 1997;50:953–956. doi: 10.1016/S0090-4295(97)00452-4. [DOI] [PubMed] [Google Scholar]
- 4.Sigrist RM, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7:1303–1329. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ucar AK, Alis D, Samanci C, Aslan M, Habibi HA, Dikici AS, et al. A preliminary study of shear wave elastography for the evaluation of unilateral palpable undescended testes. Eur J Radiol. 2017;86:248–251. doi: 10.1016/j.ejrad.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Kocaoglu C, Durmaz MS, Sivri M. Shear wave elastography evaluation of testes with non-communicating hydrocele in infants and toddlers: a preliminary study. J Pediatr Urol. 2018;14:445. doi: 10.1016/j.jpurol.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Bayramoglu Z, Kandemirli SG, Comert RG, Akpinar YE, Caliskan E, Yilmaz R, et al. Shear wave elastography evaluation in pediatric testicular microlithiasis: a comparative study. J Med Ultrason (2001) 2018;45:281–286. doi: 10.1007/s10396-017-0837-y. [DOI] [PubMed] [Google Scholar]
- 8.Fang C, Huang DY, Sidhu PS. Elastography of focal testicular lesions: current concepts and utility. Ultrasonography. 2019;38:302–310. doi: 10.14366/usg.18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dede O, Teke M, Daggulli M, Utangac M, Bas O, Penbegul N. Elastography to assess the effect of varicoceles on testes: a prospective controlled study. Andrologia. 2016;48:257–261. doi: 10.1111/and.12440. [DOI] [PubMed] [Google Scholar]
- 10.Camoglio FS, Bruno C, Peretti M, Bianchi F, Bucci A, Scire G, et al. The role of sonoelastography in the evaluation of testes with varicocele. Urology. 2017;100:203–206. doi: 10.1016/j.urology.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Kucukdurmaz F, Sarica MA, Emre O, Baykara M, Kizildag B, Resim S. Evaluation of the diagnostic efficacy of strain elastography in infertile population with normal and abnormal semen parameters. Turk J Urol. 2017;43:261–267. doi: 10.5152/tud.2017.34793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubin L, Amelar RD. Amelar RD, Dubin L, Walsh PC. Male infertility. Philadelphia, PA: W.B. Saunders; 1977. The varicocele and infertility; pp. 57–68. [Google Scholar]
- 13.Lipshultz LI, Corriere JN Jr. Progressive testicular atrophy in the varicocele patient. J Urol. 1977;117:175–176. doi: 10.1016/s0022-5347(17)58387-1. [DOI] [PubMed] [Google Scholar]
- 14.Dahl EV, Herrick JF. A vascular mechanism for maintaining testicular temperature by counter-current exchange. Surg Gynecol Obstet. 1959;108:697–705. [PubMed] [Google Scholar]
- 15.Zorgniotti AW, Macleod J. Studies in temperature, human semen quality, and varicocele. Fertil Steril. 1973;24:854–863. [PubMed] [Google Scholar]
- 16.Zucchi A, Mearini L, Mearini E, Fioretti F, Bini V, Porena M. Varicocele and fertility: relationship between testicular volume and seminal parameters before and after treatment. J Androl. 2006;27:548–551. doi: 10.2164/jandrol.05200. [DOI] [PubMed] [Google Scholar]
- 17.Johnsen SG, Agger P. Quantitative evaluation of testicular biopsies before and after operation for varicocele. Fertil Steril. 1978;29:58–63. doi: 10.1016/s0015-0282(16)43038-4. [DOI] [PubMed] [Google Scholar]
- 18.Abdelrahim F, Mostafa A, Hamdy A, Mabrouk M, el-Kholy M, Hassan O. Testicular morphology and function in varicocele patients: pre-operative and post-operative histopathology. Br J Urol. 1993;72(5 Pt 1):643–647. doi: 10.1111/j.1464-410x.1993.tb16225.x. [DOI] [PubMed] [Google Scholar]
- 19.Guibal A, Renosi G, Rode A, Scoazec JY, Guillaud O, Chardon L, et al. Shear wave elastography: an accurate technique to stage liver fibrosis in chronic liver diseases. Diagn Interv Imaging. 2016;97:91–99. doi: 10.1016/j.diii.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Uygur MC, Arik AI, Erol D, Ozer E, Ustun H. Quantitative evaluation of biopty gun testis needle biopsy: correlation between biopsy score of varicocele-bearing testis and sperm count. J Reprod Med. 1999;44:445–449. [PubMed] [Google Scholar]
- 21.Biagiotti G, Cavallini G, Modenini F, Vitali G, Gianaroli L. Spermatogenesis and spectral echo-colour Doppler traces from the main testicular artery. BJU Int. 2002;90:903–908. doi: 10.1046/j.1464-410x.2002.03033.x. [DOI] [PubMed] [Google Scholar]
- 22.Battaglia C, Giulini S, Regnani G, Madgar I, Facchinetti F, Volpe A. Intratesticular Doppler flow, seminal plasma nitrites/nitrates, and nonobstructive sperm extraction from patients with obstructive and nonobstructive azoospermia. Fertil Steril. 2001;75:1088–1094. doi: 10.1016/s0015-0282(01)01770-8. [DOI] [PubMed] [Google Scholar]
- 23.Chew WM, Hricak H, McClure RD, Wendland MF. In vivo human testicular function assessed with P-31 MR spectroscopy. Radiology. 1990;177:743–747. doi: 10.1148/radiology.177.3.2243981. [DOI] [PubMed] [Google Scholar]
- 24.Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod. 2010;25:847–852. doi: 10.1093/humrep/dep475. [DOI] [PubMed] [Google Scholar]
- 25.Han BH, Park SB, Seo JT, Chun YK. Usefulness of testicular volume, apparent diffusion coefficient, and normalized apparent diffusion coefficient in the MRI evaluation of infertile men with azoospermia. AJR Am J Roentgenol. 2018;210:543–548. doi: 10.2214/AJR.17.18276. [DOI] [PubMed] [Google Scholar]
- 26.Karakas E, Karakas O, Cullu N, Badem OF, Boyaci FN, Gulum M, et al. Diffusion-weighted MRI of the testes in patients with varicocele: a preliminary study. AJR Am J Roentgenol. 2014;202:324–328. doi: 10.2214/AJR.13.10594. [DOI] [PubMed] [Google Scholar]