Abstract
Clinical practice guidelines published by the European Society of Cardiology and the American College of Cardiology/American Heart Association summarize the available evidence and provide recommendations for health professionals to enable appropriate clinical decisions and improve clinical outcomes for patients with acute myocardial infarction (AMI). However, most current guidelines are based on studies in non-Asian populations in the pre-percutaneous coronary intervention (PCI) era. The Korea Acute Myocardial Infarction Registry is the first nationwide registry to document many aspects of AMI from baseline characteristics to treatment strategies. There are well-organized ongoing and published randomized control trials especially for antiplatelet therapy among Korean patients with AMI. Here, members of the Task Force of the Korean Society of Myocardial Infarction review recent published studies during the current PCI era, and have summarized the expert consensus for the pharmacotherapy of AMI.
Keywords: Myocardial infarction, Drug therapy
INTRODUCTION
Although the introduction of reperfusion strategy and effective medications for secondary prevention have improved the clinical outcomes for patients with acute myocardial infarction (AMI), the mortality and re-hospitalization rates after AMI remain high.1),2),3) Guidelines for AMI published by the European Society of Cardiology (ESC) and the American College of Cardiology/American Heart Association (ACC/AHA) summarize available evidence and provide recommendations for health professionals to enable appropriate clinical decisions.4),5),6),7) Recognition and application of guidelines are important factors for clinicians because medical treatments following such guidelines help reduce mortality rates during in-hospital and long-term follow-up periods.8),9)
However, there are some limitations and difficulties for the application of ESC and ACC/AHA guidelines to current clinical practices for treating Korean patients with AMI. First, most randomized studies on the pharmacotherapy of AMI were performed in the pre-percutaneous coronary intervention (PCI) era. Second, several drugs such as cangrelor and bivalirudin are not currently available in Korea. Third, there are some concerns about ethnic differences in pharmacologic responses.10) Therefore, members of the Task Force on Korean Society of Myocardial Infarction reviewed studies performed in Korea during the current PCI era, and have summarized the expert consensus on the pharmacotherapy of AMI.
FIBRINOLYSIS IN ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION
Summary of the major guidelines and recent publications
Early restoration of an effective forward coronary flow by reperfusion therapy reduces the incidences of myocardial damage, ischemic heart failure, and cardiac death. Reperfusion therapy includes pharmacologic fibrinolysis and invasive primary PCI in patients with ST-segment elevation myocardial infarction (STEMI). Primary PCI is preferred over fibrinolysis as a reperfusion therapy, because it is superior in terms of reducing death, reinfarction, and stroke if the delay in treatment is similar.11),12),13) However, the advantages of primary PCI over fibrinolysis diminish in proportion to PCI-related time delays. ACC/AHA and ESC guidelines set time limits for action plans.6),7) If patients with ST-segment elevation on electrocardiography are admitted to a non-PCI-capable center, fibrinolysis is recommended within 10 minutes when cases meet the following circumstances: 1) onset of ischemic symptoms within the previous 12 hours; 2) primary PCI cannot be performed within 2 hours; and 3) there are no contraindications for fibrinolysis. A fibrin-specific agent such as tenecteplase, alteplase, or reteplase is recommended for improving the prognosis.6),14) Transfer to a PCI-capable center is recommended for all patients after fibrinolysis for rescue (in case of failed fibrinolysis) or elective PCI (in case of successful fibrinolysis) (Table 1).
Table 1. Fibrinolysis for ST-segment elevation myocardial infarction.
| In non-PCI capable center (transfer all patients after or without fibrinolysis) | ||||
| Fibrinolysis is recommended when meet all 3 criteria | ||||
| 1. Ischemic symptom within 12 hours | ||||
| 2. Primary PCI cannot be performed within 2 hours | ||||
| 3. No contraindications for fibrinolysis | ||||
| Absolute | ||||
| - Previous intracranial hemorrhage or stroke of unknown origin at anytime | ||||
| - Ischemic stroke in the preceding 6 months (except acute stroke within 4.5 hours) | ||||
| - Central nervous system damage or neoplasm or arteriovenous malformation | ||||
| - Recent major trauma/surgery/head injury (within the preceding month) | ||||
| - Gastrointestinal bleeding within the past month | ||||
| - Known bleeding disorder (excluding menses) | ||||
| - Aortic dissection | ||||
| - Non-compressible puncture in the past 24 hours (e.g. liver biopsy, lumbar puncture) | ||||
| - For streptokinase, prior treatment within the 6 months | ||||
| Relative | ||||
| - Transient ischemic attack in the preceding 6 months | ||||
| - Oral anticoagulant therapy | ||||
| - Pregnancy or within 1 week postpartum | ||||
| - Refractory hypertension (SBP >180 mmHg and/or DBP >110 mmHg) | ||||
| - Advanced liver disease | ||||
| - Infective endocarditis | ||||
| - Active peptic ulcer | ||||
| - Prolonged or traumatic resuscitation | ||||
| In PCI capable center after fibrinolysis | ||||
| Emergent angiography (failed fibrinolysis) | ||||
| 1. <50% ST-segment resolution at 60–90 minutes after fibrinolysis | ||||
| 2. Hemodynamic or electrical instability | ||||
| 3. Worsening or recurred ischemia | ||||
| Elective angiography (2–24 hours after successful fibrinolysis) | ||||
DBP = diastolic blood pressure; PCI = percutaneous coronary intervention; SBP = systolic blood pressure.
Adjuvant pharmacotherapy is beneficial for reducing adverse cardiovascular events and mortality rates in patients treated with fibrinolysis. Aspirin and clopidogrel should be added to fibrinolysis to reduce adverse vascular events.15),16),17) There is no evidence for using glycoprotein (GP) type IIb/IIIa inhibitors or potent P2Y12 receptor inhibitors such as ticagrelor and prasugrel.6) Parenteral anticoagulation co-therapy with fibrinolysis is also recommended to be administered until PCI can be accomplished, or from at least 48 hours up to 8 days for medically treated patients with AMI. Enoxaparin and weight-adjusted unfractionated heparin are widely used in Korea. The net clinical benefit favored enoxaparin, but the risk of non-cerebral bleeding complications was increased in those treated with enoxaparin.18),19) The dosage of fibrinolytic and adjunctive antithrombotic agents should be adjusted according to the patient's age and renal clearance (Table 2).6),20),21)
Table 2. Dosage of fibrinolytic and adjunctive antithrombotic agents.
| Drug | Standard dosage | Comments | ||
|---|---|---|---|---|
| Fibrinolytic agents | ||||
| Tenecteplase | Single IV weight-based bolus | Fibrin specificity: strongest | ||
| 30 mg <60 kg | Patency rate 85% | |||
| 35 mg (7,000 IU) 60–69 kg | Age ≥75 years: 50% dose reduction | |||
| 40 mg 70–79 kg | ||||
| 45 mg (9,000 IU) 80–89 kg | ||||
| 50 mg ≥90 kg | ||||
| Reteplase | 10 units+10 units | Fibrin specificity: less strong | ||
| IV over 2 minutes (30 minutes apart) | Patency rate 84% | |||
| Alteplase | 15 mg IV bolus 1–2 minutes | Fibrin specificity: less strong | ||
| 0.75 mg/kg over 30 minutes | Patency rate 73–84% | |||
| 0.5 mg/kg over 60 minutes | Body weight >67 kg, 15 mg IV bolus, 50 mg over 30 minutes, 35 mg over 60 minutes | |||
| Antiplatelet agents | ||||
| Aspirin | 150–300 mg loading, 75–100 mg maintain | |||
| Clopidogrel | 300 mg loading, 75 mg maintain | Age ≥75 years | ||
| No loading, 75 mg maintain | ||||
| Anticoagulation | ||||
| Enoxaparin | 30 mg IV bolus | Age ≥75 years | ||
| 1 mg/kg SC every 12 hours (maximum 100 mg for the first 2 doses) | No bolus, 25% dose reduction | |||
| eGFR <30 mL/min/1.73m2 | ||||
| 1 mg/kg every 24 hours | ||||
| UFH | 60 IU/kg IV bolus (maximum 4,000 units) | No planned reperfusion | ||
| 12 IU/kg/hr (maximum 1,000 units/hr) | 50–70 IU/kg IV bolus (maximum 5,000 IU) | |||
| Target aPTT 1.5–2 times (approximately 50–70 seconds) | 12 IU/kg/hr | |||
| Target aPTT 1.5–2 times (approximately 50–70 seconds) | ||||
aPTT = activated partial thromboplastin time; eGFR = estimated glomerular filtration rate; IU = international units; IV = intravenous; SC = subcutaneous; UFH = unfractionated heparin.
Evidence from Korea
The pharmaco-invasive strategy is defined as fibrinolysis combined with rescue PCI or routine elective PCI. Currently, more than 90% of patients with AMI undergo primary PCI in Korea, and most patients receiving fibrinolysis are transferred to a primary PCI center. Therefore, a pharmaco-invasive strategy could be a useful option for reducing the total ischemic time in Korean patients. In the Korea Acute Myocardial Infarction Registry (KAMIR) data, early elective PCI at less than 24 or 48 hours after successful fibrinolysis reduced major adverse cardiac events during a 1-year follow-up.22) As with the Strategic Reperfusion Early after Myocardial Infarction (STREAM) study,20) pre-PCI antegrade flow of culprit epicardial artery was better in a pharmaco-invasive group.23) However, this advantage was not translated into better 1-year clinical outcomes.21),23) In the Comparison of Primary Angioplasty and Pre-hospital Fibrinolysis in Acute Myocardial Infarction (CAPTIM) trial, early presentation (treated within 2 hours) was associated with lower mortality rates when using a pharmaco-invasive strategy than primary PCI in the longer term (5-year) follow-up.24) Therefore, it is necessary to wait for more long-term follow-up data for such pharmaco-invasive strategies for patients with AMI.
Recommendations
• Fibrinolysis should be considered when primary PCI cannot be performed within 2 hours and ischemic symptoms appear within 12 hours.
• A fibrin-specific agent such as tenecteplase, alteplase, or reteplase is recommended. Co-therapy with dual antiplatelet agents and anticoagulants is also recommended. Dosages of these drugs should be adjusted according to the patient's age and creatinine clearance.
• Immediate transfer to a PCI-capable center is recommended in all patients after fibrinolysis. In PCI-capable center, emergency angiography is recommended in the presence of hemodynamic or electrical instability, and ischemic signs, and also in cases of failed fibrinolysis. Elective angiography is recommended at 2–24 hours after successful fibrinolysis.
BETA-BLOCKER THERAPY IN ACUTE MYOCARDIAL INFARCTION
Summary of the major guidelines and recent publications
Beta-blockers are the standard medications for patients with AMI based on evidence from the pre-thrombolytic era. The ClOpidogrel and Metoprolol in Myocardial Infarction (COMMIT) trial evaluated the effect of early intravenous (IV) and then oral metoprolol in over 40,000 patients with AMI, and half of the patients received fibrinolysis.25) Metoprolol lowered the rate of recurrent MI and ventricular fibrillation but increased the cardiogenic shock, reducing the clinical benefit. However, a meta-analysis of randomized controlled trials (RCTs) of patients with acute coronary syndrome (ACS) suggested the positive effect of early IV beta-blockers.26) For patients undergoing primary PCI, early IV metoprolol reduced infarct size, improved ejection fraction, and also reduced the incidence of major adverse cardiac events (MACE) in patients with anterior wall MI.27),28) However, metoprolol therapy failed to reduce the infarct size in another study enrolling all patients with STEMI, including those with non-anterior wall MI.29) A large-scale RCT to define the role of beta-blockers is needed in patients with STEMI treated by primary PCI. Oral beta-blockers should be initiated in the first 24 hours in the latest ACC/AHA guideline. Therapy with IV beta-blockers is recommended only in patients with STEMI with ongoing ischemia as Class IIa and evidence level B and is not recommended in cases of non-ST segment elevation myocardial infarction (NSTEMI). In the ESC guideline, an early beta-blocker is recommended in patients with NSTEMI with ongoing ischemia and without contraindications, and is also recommended in STEMI patients before primary PCI if the patients are stable.
Regarding the mid and long-term effect of beta-blockers, old studies suggested beta-blockers might have significant benefits for patient mortality after MI. However, those studies were done in the pre-PCI era, and such beneficial effect of beta-blockers was pronounced in patients with heart failure (HF) or ventricular arrhythmia after MI. The Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) study was the only RCT showing long-term benefits of beta-blockers for patients with AMI with reperfusion therapy.30) Patients of the CAPRICORN study had left ventricular ejection fraction (LVEF) ≤40%, were treated with an angiotensin converting enzyme (ACE) inhibitor, and half of the patients received reperfusion therapy. Carvedilol reduced all-cause mortality by 23% and all the other cardiac events. Recent articles using big data demonstrated no mortality benefits in patients aged >65 years and who survived >6 months after MI31) and in patients without HF after MI.32) However, many important confounding factors such as sex, and the location of infarction limit the interpretation of observational studies of beta-blockers.33) In the ACC/AHA guideline, a beta-blocker is recommended in patients with STEMI and NSTEMI regardless of ventricular function after discharge, but without mentioning the duration of administration. In the ESC guideline, Class IB in patients with NSTEMI, Class IA in patients with STEMI with HF, and Class IIa and evidence level B in all cases of STEMI regardless of HF are recommended without defining the duration of treatment.
Evidence from Korea
In a Korean observational study that recruited 3,019 PCI-treated patients with AMI with an LVEF ≥50%, the use of beta-blocker was associated with lower incidence of all-cause and cardiac deaths in patients with AMI during the 3 years of follow-up (hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.38–0.87 and HR, 0.38; 95% CI, 0.22–0.66, respectively). However, the incidence of MI (HR, 1.10; 95% CI, 0.45–2.69) and stroke (HR, 0.59; 95% CI, 0.26–1.35) were not significantly different between groups.34) Yang et al.35) used KAMIR data to identify 8,510 patients with STEMI who underwent primary PCI, and were discharged from hospital. Using a 2:1 propensity score-matching process for patients on a beta-blocker, 3,975 patients were included in the final analysis. After a median follow-up of approximately 1 year, the rate of death from any cause was lower in patients on beta-blocker therapy (adjusted HR, 0.46; 95% CI, 0.27–0.78). However, the subgroup of 3,073 patients (77%) with an LVEF >40% demonstrated no significant difference in the rate of all-cause death (unadjusted HR, 0.56; 95% CI, 0.29–1.07).35) Park et al enrolled all consecutive patients who presented with AMI in a single center. They evaluated all-cause mortality at 1, 3, and 5 years after AMI. Of 2,592 patients, the prescription rates of beta-blockers were 72%, 69%, 63%, and 60% at discharge and 1, 3, and 5 years after AMI, respectively. The patients who were receiving beta-blocker therapy were younger (62 vs. 65 years; p<0.001) and more likely to receive reperfusion therapy more often (92% vs. 80%; p<0.001) than those without beta-blocker prescriptions. The beta-blocker prescription at discharge was associated with a 29% reduced mortality risk (HR, 0.71; 95% CI, 0.55–0.90; p=0.006); however, beta-blocker prescriptions at 1 and 3 years after acute MI were not associated with reduced mortality.36)
The beta-blocker has been the state-of-the-art therapy for AMI based on survival benefits in studies conducted from the 1980s to 2000s. Changes in the treatment of such patients (higher rate of PCI and reperfusion rate and introduction of other potent medications including high-intensity statins, dual antiplatelet therapy, and RAAS inhibitors) call for a re-evaluation of the role of beta-blockers in patients with AMI. The conclusions obtained from contemporary observational studies using clinically available data tend to contradict older RCTs. However, there have been no RCTs aiming to define the role of beta-blocker therapy in patients with AMI. Early beta-blocker therapy might have had benefits such as reducing infarct size and the occurrences of ventricular arrhythmia, improvements in left ventricular (LV) function and patient survival but also have a risk of shock and unexpected death. In patients with HF (LVEF ≤40%), long-term beta-blocker treatment appears reasonable considering previous studies. However, the benefit of long-term beta-blocker therapy is controversial in patients without HF.
Recommendations
• Early beta-blocker therapy is recommended for patients with AMI irrespective of the revascularization status who do not have contraindication for its use (signs of HF, evidence of a low output state, cardiogenic shock, PR interval >0.24 seconds, second- or third-degree heart block, active asthma, or reactive airway disease).
• Long-term oral beta-blockers are indicated in patients with HF and/or LVEF ≤40% unless contraindicated.
RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM INHIBITORS IN ACUTE MYOCARDIAL INFARCTION
Summary of the major guidelines and recent publications
ACC/AHA guidelines recommend that an ACE inhibitor should be administered within the first 24 hours to all patients with STEMI with anterior location, HF, or LVEF ≤40%, unless contraindicated (Class I with level of evidence A).7) An angiotensin receptor blocker (ARB) should be given to patients with STEMI who have indications for, but are intolerant of, ACE inhibitors (Class I with level of evidence B). They also recommend that the use of ACE inhibitors is reasonable for all patients with STEMI and no contraindications to their use as Class IIa with level of evidence A. In patients with non-ST elevation acute coronary syndrome (NSTE-ACS), Class I recommendations are essentially identical to those for patients with STEMI. However, the ACC/AHA guidelines for patients with NSTE-ACS recommend that ARBs are reasonable in patients without Class I indications who are ACE inhibitor intolerant, as for Class IIa with level of evidence B and that the use of ACE inhibitors might be reasonable in all other patients with cardiac or other vascular disease as Class IIb with level of evidence B.5)
In general, the ESC guidelines are similar to the ACC/AHA guidelines, but there are subtle differences between the two major guidelines. The ESC guideline recommends that ACE inhibitors should be started within the first 24 hours of STEMI in patients with evidence of HF, LV systolic dysfunction, diabetes, or an anterior infarct as Class I with level of evidence A.6) An ARB, preferably valsartan, is an alternative to ACE inhibitors in patients with HF and/or LV systolic dysfunction, particularly those who are intolerant of ACE inhibitors (Class I with level of evidence B). They also recommend that ACE inhibitors should be considered in all patients in the absence of contraindications as Class IIa with level of evidence A. For patients with NSTE-ACS, the ESC guideline recommends the use of an ACE inhibitor in patients with LVEF ≤40% or HF, hypertension or diabetes, unless contraindicated and an ARB as an alternative, particularly if ACE inhibitors are not tolerated (Class I with level of evidence A).4) However, they do not make any comment on patients without LV systolic dysfunction or HF.
The ACC/AHA guidelines recommend therapy with an aldosterone receptor blocker in patients after AMI who are without significant renal dysfunction (serum creatinine level >2.5 mg/dL in men or >2.0 mg/dL in women) or hyperkalemia (K >5.0 mEq/L) and are receiving therapeutic doses of ACE inhibitors and beta-blockers and have LVEF ≤40% and HF or diabetes mellitus. The recommendations of the ESC guidelines are similar to those of the ACC/AHA guidelines.
Evidence from Korea
Several observational studies compared ACE inhibitors and ARBs using data from the KAMIR, but the results were inconsistent.37),38),39),40),41),42) Yang et al.37) reported that the risk of cardiac death or recurrent MI in patients with STEMI with preserved LV systolic function was similar in patients treated with ACE inhibitors and ARBs and lower than in those who receive no RAAS inhibitors. However, another study reported that the use of ACE inhibitors was associated with long-term survival benefits compared with ARBs in patients with AMI.42) Inclusion of patients with NSTE-ACS or LV systolic dysfunction, reperfusion strategy, and different end points might explain the inconsistency of results across previous studies. So far, there has been no RCT comparing ACE inhibitors and ARBs for Korean patients.
Data regarding the appropriate type and dose of RAAS inhibitors are very limited. One study compared insurmountable ARBs with surmountable ARBs and the former was associated with a reduced risk of a composite of cardiac death, nonfatal MI, and revascularization compared with the latter.43) In the Valsartan in Post-MI Remodeling (VALID) trial, the researchers investigated whether the recommended maximal tolerated dose of valsartan (320 mg/day or the maximum tolerated daily dose) was more efficacious than the low dose (80 mg/day) in retarding post-MI LV remodeling in 495 Korean patients with STEMI and LVEF ≤50%.44) In the VALID trial, treatment with the maximal tolerated dose of valsartan did not improve post-MI LV remodeling but caused adverse effects more frequently compared with low-dose treatment in Korean patients.
One observational study sought to investigate the effects of the aldosterone receptor blocker, spironolactone, on 1-year clinical outcomes in all-comer patients with AMI undergoing PCI.45) Spironolactone therapy was associated with a reduced risk of repeat revascularization after AMI in patients undergoing PCI. However, RCTs are needed to clarify the possible benefit of aldosterone receptor blockers in patients without LV systolic dysfunction or HF.
Recommendations
• ACE inhibitors are recommended for patients with AMI who have evidence of HF, LVEF ≤40%, or an anterior infarct.
• ARBs are alternatives to ACE inhibitors in patients AMI with HF or LVEF ≤40% or an anterior infarct, particularly those who are intolerant of ACE inhibitors.
• ACE inhibitors should be considered in all patients with STEMI in the absence of contraindications.
• Aldosterone receptor blockers are recommended in patients with an LVEF ≤40% and HF or diabetes, who are already receiving an ACE inhibitor and a beta-blocker, provided there is no renal failure or hyperkalemia.
ANTIPLATELET THERAPY FOR ACUTE MYOCARDIAL INFARCTION
Summary of the major guidelines and recent publications
Platelet activation as well as the coagulation cascade and inflammation play a crucial role in the initiation and evolution of an AMI event. Therefore, fast and sufficient platelet inhibition is essential among patients with AMI, especially during the early phase after PCI.
NSTEMI: peri-interventional treatment
Aspirin is recommended for all patients without contraindications at an initial oral loading dose of 150–300 mg, and at a maintenance dose of 75–100 mg daily long-term. In addition, dual antiplatelet therapy (DAPT) with aspirin and a potent P2Y12 receptor inhibitor (prasugrel or ticagrelor) is recommended. Clopidogrel should be permitted only when prasugrel or ticagrelor are not available or are contraindicated.
It is not recommended that prasugrel is administered in patients in whom coronary anatomy is not known. Because loading with ticagrelor was associated with an early benefit over clopidogrel, pre-treatment with ticagrelor can be used in these patients. The Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5 (ISAR-REACT 5) trial randomized patients with ACS (ACS=4,018, NSTEMI=1,855) into ticagrelor strategy (a loading dose of 180 mg as soon as possible and continued on 90 mg twice daily) or prasugrel strategy (a loading dose of 60 mg after coronary angiography/before PCI and continued on 10 mg daily: 5 mg daily for those aged ≥75 years or weight <60 kg).46) A composite of death, MI, or stroke was more frequently observed in patients receiving ticagrelor compared with prasugrel (HR, 1.36, 95% CI, 0.97–1.90), without any difference in the risk of major bleeding.
There is a lack of evidence for any clinical benefit in the routine upstream use of GP IIb/IIIa inhibitors in patients with NSTEMI planned for coronary angiography and receiving DAPT. In the setting of potent P2Y12 receptor inhibitor, where randomized data on GP IIb/IIIa inhibitor use has been limited, routine use of GP IIb/IIIa inhibitors cannot be recommended. However, this regimen should be considered for bailout situations or thrombotic complications (e.g., no-flow, large burden of thrombus), and might be applicable before PCI in patients with NSTEMI without pre-treatment with P2Y12 receptor inhibitors. The large-scale evidence regarding cangrelor suggests clinical benefits independent of index clinical presentation. Therefore, cangrelor might be considered in specific or high-risk settings in P2Y12-naïve patients undergoing PCI.4),5),47)
STEMI: peri-interventional treatment
Patients with STEMI undergoing primary PCI should receive aspirin and a P2Y12 receptor inhibitor as soon as the diagnosis of STEMI is established. In line with the recommendations for patients with NSTEMI, DAPT is the cornerstone of treatment and consists of aspirin and a potent P2Y12 receptor inhibitor. When a potent P2Y12 receptor inhibitor is contraindicated or is not available, clopidogrel may be given for primary PCI instead.
Randomized data regarding the clinical benefit of ticagrelor vs. prasugrel in patients with STEMI are limited. The Comparison of Prasugrel and Ticagrelor in the Treatment of Acute Myocardial Infarction (PRAGUE-18) trial with limited statistical power found similar safety and efficacy profiles of ticagrelor and prasugrel in a setting of primary PCI.48) The ISAR-REACT 5 trial (ACS=4,018, STEMI=1,653) randomized patients with STEMI into ticagrelor strategy or prasugrel strategy. The risk of death, MI, or stroke was higher in patients on ticagrelor compared with prasugrel (HR, 1.31; 95% CI, 0.94–1.81), without any significant difference in the risk of major bleeding.46)
GP IIb/IIIa inhibitors remain a considerable option as bailout therapy or in cases of high-risk PCI without pre-treatment with P2Y12 receptor inhibitors. In addition, cangrelor can be used in specific settings in P2Y12-naïve patients undergoing PCI.6),7)
AMI: maintenance treatment
Following PCI for AMI, DAPT consisting of a P2Y12 receptor inhibitor in addition to aspirin is generally recommended for 12 months. Furthermore, switching or de-escalation strategy of DAPT has been a topic of ongoing RCTs. The Anti-Xa Therapy to Lower cardiovascular events in Addition to Standard therapy in subjects with Acute Coronary Syndrome–Thrombolysis In Myocardial Infarction 51 (ATLAS-ACS 2–TIMI 51) trial (n=15,526) demonstrated that vascular-dose rivaroxaban (e.g., 2.5 mg twice daily) could be considered for patients without prior stroke, and at high ischemic risk and low bleeding risk, receiving aspirin and clopidogrel in patients with ACS.49)
In specific clinical scenarios, this standard DAPT duration can be shortened (e.g., 6 months in high-bleeding risk patients: PRECISE-DAPT ≥25) or extended (>12 months). Following DAPT, lifelong single antiplatelet therapy usually with aspirin is recommended and patients should be advised not to discontinue their antiplatelet regimen prematurely after AMI. However, in patients with high ischemic risk and without a high bleeding risk, adding a second antithrombotic drug to aspirin for long-term secondary prevention should be considered. Post-MI patients who have tolerated DAPT for 1 year with high ischemic risk (e.g., multi-vessel disease, diabetes mellitus, peripheral artery disease or HF) can be treated with long-term DAPT regimens to prevent recurrent MI.50),51)
Evidence from Korea
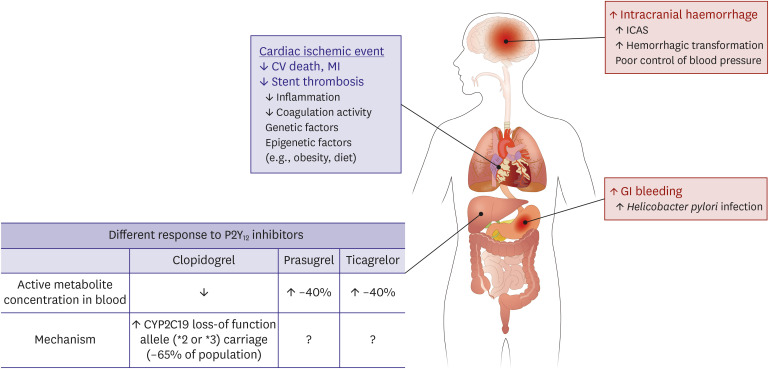
An increasing body of evidence suggests that East Asian patients have different risk-benefit ratio compared with Caucasian patients (Figure 1).10),52),53) Generally, East Asian patients have shown a lower risk of atherothrombotic events, and especially in coronary artery diseases. In addition, there are emerging concerns in this ethnic group regarding the increased risk of gastrointestinal bleeding and hemorrhagic stroke during antiplatelet treatment. This phenomenon has been partly explained with their different range in therapeutic window of platelet reactivity (the so-called “East Asian Paradox”).
Figure 1. Unique ischemic and bleeding tendency and pharmacokinetics of P2Y12 receptor inhibitors in East Asian population. This figure was modified from the original version.53).
CV = cardiovascular; ICAS = intracranial atherosclerosis; MI = myocardial infarction.
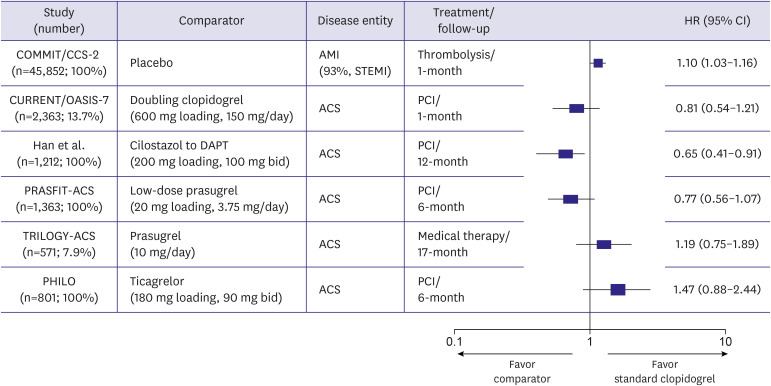
A subgroup analysis enrolling East Asian patients with ACS from RCTs provided important insight for this issue (Figure 2). Compared with standard-dose clopidogrel, moderate-intensity antiplatelet regimens (doubling clopidogrel, adjunctive cilostazol to DAPT and low-dose prasugrel therapy of 3.75 mg daily) showed an overall trend toward better clinical outcomes, whereas high-intensity antiplatelet regimens (standard-dose prasugrel of 10 mg daily and standard-dose ticagrelor of 90 mg twice daily) showed a trend for worse clinical outcomes. In Korean patients with AMI, potent antiplatelet regimens have shown the unique profile of efficacy and safety compared with DAPT with clopidogrel.52),53) In patients with STEMI undergoing primary PCI (n=4,203), triple antiplatelet therapy (TAPT; aspirin plus clopidogrel plus cilostazol) showed significantly lower incidences of cardiac death (adjusted odds ratio [OR], 0.52; 95% CI, 0.32–0.84; p=0.007), and total MACEs (adjusted OR, 0.74; 95% CI, 0.58–0.95; p=0.019) at 8 months than DAPT (aspirin plus clopidogrel).54) Older (>65 years old), female, and diabetic patients received more benefits from TAPT than from DAPT. Compared with DAPT, TAPT had a similar incidence of major bleeding events. DAPT with a potent P2Y12 receptor inhibitor has shown the limited benefit for ischemic events and increased risk of major bleeding compared with DAPT with clopidogrel. After propensity score-matching (1,377 pairs) from the KAMIR-National Institute of Health (NIH) registry, the incidences of in-hospital TIMI major and minor bleeding were higher with ticagrelor than with clopidogrel (2.6% vs. 1.2%, p=0.008; 3.8% vs. 2.5%, p=0.051, respectively).55) In-hospital mortality was higher in patients with than those without TIMI major bleeding (11.3% vs. 0.9%, p<0.001). A higher risk for in-hospital TIMI major bleeding with ticagrelor was observed in patients aged ≥75 years or with body weight <60 kg (OR, 3.21; 95% CI, 1.36–7.59) and in those receiving transfemoral intervention (OR, 2.00; 95% CI, 1.06–3.75). Analysis from KAMIR-NIH has shown that potent P2Y12 receptor inhibitors vs. clopidogrel provided a similar prevalence of ischemic events and increased risk of serious bleeding in patients with AMI.55),56),57)
Figure 2. Subgroup analyses of East Asians from randomized control trials about acute coronary syndrome. This figure was modified from the original version.53).
ACS = acute coronary syndrome; AMI = acute myocardial infarction; CI = confidence interval; DAPT = dual antiplatelet therapy; HR = hazard ratio; COMMIT/CCS-2 = Clopidogrel and Metoprolol in Myocardial Infarction Trial/Second Chinese Cardiac Study; CURRENT/OASIS-7 = Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent EveNTs/Optimal Antiplatelet Strategy for InterventionS-7; PCI = percutaneous coronary intervention; PHILO = Study to Assess Safety and Efficacy of Ticagrelor Versus Clopidogrel in Asian/Japanese Patients With Non-ST or ST Elevation Acute Coronary Syndrome; PRASFIT-ACS = PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI; STEMI = ST segment elevation myocardial infarction; TRILOGY-ACS = TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes.
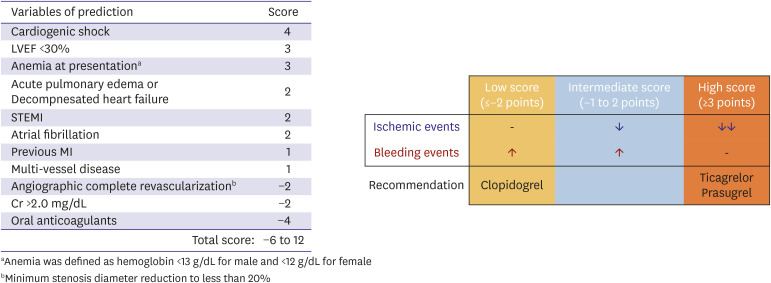
The clinical benefit of potent P2Y12 receptor inhibitors over clopidogrel can be different according to the ischemic and bleeding risk profiles. The KAMIR-NIH DAPT score was developed and is applicable to guide the selection of P2Y12 receptor inhibitor by evaluating combined ischemic (cardiac death, MI, and stent thrombosis) and bleeding (Bleeding Academic Research Consortium [BARC] 2, 3 and 5) endpoints during 12 months (Figure 3). The patients (n=10,687) were divided into three groups based on the distribution of the derived cohort by score: ≤−2 points for the low-score group (16.5%); −1 to 2 points for the intermediate-score group (65.7%); ≥3 points for the high-score group (17.8%). Among the low-score group, the observed bleeding risk (8.7% vs. 4.4%, p<0.001) on potent P2Y12 receptor inhibitors vs. clopidogrel exceeded the ischemic benefit (1.3% vs. 2.2%, p=0.185). Conversely, the high-score group showed an overall benefit from taking potent P2Y12 receptor inhibitors vs. clopidogrel from the standpoint of observed ischemic (8.6% vs. 17.1%, p<0.001) and bleeding events (10.1% vs. 6.8%, p=0.073).58) Another nationwide population-based cohort study (70,715 patients with ACS) showed similar results.59) In a propensity-matched cohort, compared with clopidogrel, prasugrel was associated with a higher risk of major bleeding (HR, 1.50; 95% CI, 1.01–2.21) but a similar risk for effectiveness outcomes. In addition, ticagrelor was associated with a higher risk of any bleeding (HR, 1.23; 95% CI, 1.14–1.33) but a lower risk of mortality (HR, 0.76; 95% CI, 0.63–0.91). No significant difference was observed between ticagrelor and prasugrel with respect to major safety and effectiveness outcomes. In the randomized Ticagrelor Versus Clopidogrel in Asian/Korean Patients with ACS Intended for Invasive Management (TICA-KOREA) trial (n=800), the incidence of clinically significant bleeding (PLATO major or minor bleeding) at 12 months was significantly higher in the ticagrelor compared with clopidogrel group (11.7% vs. 5.3%, HR, 2.26; 95% CI, 1.34–3.79; p=0.002). The incidence of cardiovascular (CV) death, MI, or stroke was not significantly different between the ticagrelor and clopidogrel groups (9.2% vs. 5.8%, HR, 1.62; 95% CI, 0.96–2.74; p=0.07).60)
Figure 3. Scoring system for predicting overall benefit from the use of potent P2Y12 receptor inhibitors. This figure was modified from the original version.58).
Cr = serum creatinine; LVEF = left ventricular ejection fraction; MI = myocardial infarction; STEMI = ST segment elevation myocardial infarction.
Several clinical trials from Korea are ongoing or are being conducted to evaluate the efficacy and safety of a de-escalation antiplatelet strategy. These dedicated large-scale clinical trials could give deep insights into the optimal antiplatelet strategy for the Korean population. First, a reduced-dose strategy of potent P2Y12 receptor inhibitor could be considered after the acute phase in ACS patients. The Harmonizing optimal strategy for treatment of coronary artery diseases–comparison of REDUCtion of prasugrEl dose or POLYmer TECHnology in ACS patients (HOST-REDUCE-POLYTECH-ACS) trial finished its enrollment to explore the optimal maintenance dose of prasugrel beyond 1 month after PCI for ACS in the Korean population.61) After the standard dose of prasugrel (10 mg daily) for 1 month post-PCI, patients were randomized 1:1 either to the conventional (10 mg daily) or a reduced dose (5 mg daily). Second, a switching strategy from potent P2Y12 receptor inhibitor to clopidogrel could be another approach during the stabilized phase in patients with AMI. The TicAgrelor Versus CLOpidogrel in Stabilized Patients With Acute Myocardial Infarction (TALOS-AMI) trial has also finished its enrollment to compare the clinical efficacy and safety of clopidogrel vs. ticagrelor in Korean patients with stabilized MI. Third, another de-escalation strategy involves discontinuation of aspirin after the acute phase in patients with ACS. The Ticagrelor Monotherapy After 3 Months in the Patients Treated With New Generation Sirolimus Stent for Acute Coronary Syndrome (TICO) trial finished its enrollment to evaluate the clinical outcomes of DAPT with aspirin plus ticagrelor vs. ticagrelor monotherapy from 3 months post-PCI in patients with ACS. A landmark analysis at 3 months showed that the net adverse clinical event rate was 1.4% in the ticagrelor monotherapy group and 3.5% in the ticagrelor-based 12-month DAPT group (HR, 0.41; 95% CI, 0.25–0.68; p=0.001). The difference between the groups was mainly driven by a reduced risk of major bleeding in the ticagrelor monotherapy group (0.2% vs. 1.6%, HR, 0.13; 95% CI, 0.04–0.44; p=0.001).62) The Comparison Between P2Y12 Antagonist Monotherapy and Dual Antiplatelet Therapy After DES (SMART-CHOICE) trial compared clinical benefits of aspirin plus a P2Y12 receptor inhibitor for 3 months and thereafter P2Y12 receptor inhibitor alone or DAPT for 12 months in Korean patients treated with drug-eluting stent (DES) (n=2,993, 58.2% of patients with ACS).63) There were no significant differences in all-cause death (1.4% vs. 1.2%, HR, 1.18; 95% CI, 0.63–2.21; p=0.61), MI (0.8% vs. 1.2%, HR, 0.66; 95% CI, 0.31–1.40, p=0.28), or stroke (0.8% vs. 0.3%, HR, 2.23; 95% CI, 0.78–6.43, p=0.14) between the two groups. The rate of bleeding was significantly lower in the P2Y12 receptor inhibitor monotherapy group than in the DAPT group (2.0% vs 3.4%, HR, 0.58; 95% CI, 0.36–0.92; p=0.02). This study suggested the clinical application of 3-month DAPT and P2Y12 receptor inhibitor monotherapy in high bleeding risk patients with low ischemic risk. Finally, a shortened duration of DAPT (<12 months) can be a reasonable approach in patients with AMI with low ischemic risk or high bleeding risk. The randomized Safety of 6-month Duration of Dual Antiplatelet Therapy After Acute Coronary Syndromes (SMART-DATE) trial enrolled 2,712 patients with ACS undergoing PCI intervention with DES.64) Six months of DAPT was non-inferior to 12 months or longer of DAPT for preventing the composite of all-cause death, MI or stroke at 18 months (4.7% vs. 4.2%). MI occurred more frequently in the 6-month DAPT group than in the 12-month or longer DAPT group (1.8% vs. 0.8%, HR, 2.41; 95% CI, 1.15–5.05, p=0.02). However, the rate of BARC type 2–5 bleeding was 2.7% in the 6-month DAPT group and 3.9% in the 12-month or longer DAPT group (HR, 0.69; 95% CI, 0.45–1.05; p=0.09). This observation prevents us from concluding that short-term DAPT is safe in patients with ACS treated with current-generation DES.
Clinical evidence regarding adding a second antithrombotic drug to aspirin for long-term secondary prevention is still scarce in Korean patients with AMI. The Optimal Duration of Clopidogrel Therapy With DES to Reduce Late Coronary Arterial Thrombotic Event (DES-LATE) trial (n = 5,045, 61% patients with ACS) randomized DES-treated patients free of clinical events to receive aspirin monotherapy or DAPT with clopidogrel for at least 12 months.65) At 24 months, the composite of CV death, MI or stroke occurred in 2.4% and 2.6% of the aspirin-monotherapy group and the DAPT therapy group, respectively (HR, 0.94; 95% CI, 0.66–1.35; p=0.75). Major bleeding was observed in 1.1% and 1.4% of the aspirin-monotherapy group and the DAPT therapy group, respectively (HR, 0.71; 95% CI, 0.42–1.20; p=0.20). From the KAMIR-NIH database, patients who received DAPT beyond 12 months (n=4,795), compared with patients treated with 12-month DAPT (n=1,404), had a similar incidence of MACEs (1.3% vs. 1.0%, HR, 1.32; 95% CI, 0.71–2.45; p=0.378). The two groups did not differ significantly in the rates of death (0.1% vs. 0.1%), MI (0.8% vs. 0.6%), stent thrombosis (0.1% vs. 0.2%), and ischemic stroke (0.4% vs. 0.2%).66) The Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin (PEGASUS) trial (n=21,162) demonstrated that 60-mg ticagrelor vs. placebo in addition to aspirin reduced the rate of ischemic event (CV death, MI or stroke) (7.77% vs. 9.04%, HR, 0.84; 95% CI, 0.74–0.95; p=0.004) and increased the rate of TIMI major bleeding (2.30% vs. 1.06%, HR, 2.32; 95% CI, 1.68–3.21; p<0.001) at 3 years in patients with prior MI (1 to 3 years earlier).67) However, subgroup analysis with Asian patients showed no difference in the incidence of ischemic events (7.11% vs. 6.86%) but a considerable increase in the number of bleeding episodes (3.74% vs. 1.44%) at 3 years between the regimens.68) In addition, the Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS) trial (n=27,395) showed that adverse ischemic events (CV death, MI or stroke) occurred in fewer patients in the rivaroxaban-plus-aspirin group (rivaroxaban 2.5 mg twice daily) than in the aspirin-alone group (4.1% vs. 5.4%, HR, 0.76; 95% CI, 0.66–0.86; p<0.001), but major bleeding events occurred in more patients in the rivaroxaban-plus-aspirin group (3.1% vs. 1.9%, HR, 1.70; 95% CI, 1.40–2.05; p<0.001).69) However, the rivaroxaban-plus-aspirin regimen increased major bleeding by 1.18% in Western populations and 2.13% in Asians, compared with the aspirin-alone group.70) Therefore, dedicated trials including Korean patients are needed instead of blind application of Western recommendations for antithrombotic therapy in the Korean population.
A meta-analysis including patients on DAPT (aspirin plus clopidogrel) (n=4,374) reported that proton pump inhibitor (PPI) treatment significantly decreased the risk of gastrointestinal (GI) bleeding compared with controls (OR, 0.36; 95% CI, 0.15–0.87).71) In addition, there was no significant difference in CV adverse events (OR, 1.00; 95% CI, 0.76–1.31). As PPIs that inhibit the CYP2C19 pathway can reduce the pharmacodynamic effect of clopidogrel, some PPI co-administration (e.g., omeprazole or esomeprazole) with clopidogrel is generally not recommended.50) From the Korean nationwide claims data (2007–2015; n=59,233), PPIs were associated with increased thrombotic risks (HR, 1.27; 95% CI, 1.12–1.45); PPIs with high CYP2C19-inhibitory potential (omeprazole, esomeprazole, and lansoprazole) were more relevant than those with low potential (HR, 1.28; 95% CI, 1.02–1.61).72) There were no randomized data comparing use vs. non-use of PPIs in patients with coronary artery disease receiving prasugrel or ticagrelor in addition to aspirin. However, the risk of GI bleeding was higher with DAPT in the form of prasugrel or ticagrelor compared with clopidogrel.50) Therefore, co-administration with PPI during DAPT may be mandatory in Korean patients with high-risk GI bleeding (such as a history of GI bleeding or peptic ulcer, Helicobacter pylori infection, age ≥65 years, concurrent use of anticoagulant, nonsteroidal anti-inflammatory drugs [NSAIDs] or steroids, or the use of prasugrel or ticagrelor).
The East Asian population may present a different profile (higher bleeding potential and lower ischemic event risk) compared with western population. Emerging evidence from East Asian patients have suggested that potent P2Y12 receptor inhibitors exhibit greater antiplatelet effect and consequently higher bleeding risk in this population compared with western patients.
Recommendations
• Use of standard-dose potent P2Y12 receptor inhibitors needs attention regarding the increased risk of bleeding especially in Korean patients with bleeding risk factors (e.g. prior stroke, old age, low body weight, female gender).
• Potent P2Y12 receptor inhibitors may be recommended over clopidogrel in the high ischemic risk patients with MI undergoing PCI, taking into account the combined ischemic and bleeding risks (e.g., KAMIR-NIH DAPT score ≥3 points).
• After considering the risk-benefit profile, a reduced maintenance dose of potent P2Y12 receptor inhibitors may be a considerable option for Korean patients with AMI.
• When discontinuation of a potent P2Y12 receptor inhibitor is required because of intolerance (e.g., bleeding or dyspnea), aspirin discontinuation or switching to clopidogrel may be a considerable option for Korean patients with ACS.
• When receiving DAPT in Korean patients, PPIs should be used in patients with a history of GI bleeding or peptic ulcer, or in those with risk factors for GI bleeding (Helicobacter pylori infection, age ≥65 years, the concomitant use of anticoagulants, NSAIDs or steroids, or the use of prasugrel or ticagrelor).
• The 12-month DAPT is reasonable for Korean patients with AMI. In patients with high ischemic risk (e.g., prior MI, diabetes mellitus, chronic kidney disease, multi-vessel disease, or complex intervention), prolonged use of DAPT over 12 months may be chosen.
• Shortened durations of DAPT can be considered for patients with a high risk of bleeding or who are intolerant to long-term DAPT treatment, even in a setting of AMI.
LIPID-LOWERING THERAPY
Summary of the major guidelines and recent publications
A meta-analysis of data from 170,000 participants in 26 randomized trials showed that the more intensive use of statins produced greater reductions in coronary death, nonfatal MI, ischemic stroke, and coronary revascularization.73) Each 1.0 mmol/L (40 mg/dL) reduction in low-density lipoprotein cholesterol (LDL-C) reduced the annual rate of major vascular events by just over a fifth. Accordingly, current ACC/AHA and ESC guidelines have stated to start high-intensity statin therapy as early as possible after AMI as a Class I recommendation.6),7) While the 2013 ACC Foundation/AHA guideline did not refer to a specific goal of LDL-C concentrations,7) the 2018 ACC guideline on the management of blood cholesterol adopted a recommendation of using a LDL-C threshold of 70 mg/dL in very high-risk patients including those with ACS.74) The most recent 2019 ESC guideline for the management of dyslipidemia went a step further and recommended reducing the goal of LDL-C level from 70 to 55 mg/dL in very high-risk patients, including those with MI, based on large-scale RCTs using ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.75),76),77),78) Furthermore, the guideline recommended that for patients with AMI who experience a second vascular event within 2 years (not necessarily of the same type as the first event) while taking maximally tolerated statin therapy, an LDL-C goal of <40 mg/dL may be considered, as Class IIb and with level of evidence B. However, most recent ACC/AHA or ESC guideline-directed prescription of PCSK9 inhibitors is not fully reimbursed by the Korean Health Insurance Review and Assessment Service (HIRA). Thus, we have to adjust two guidelines and Korean insurance regulation and keep a balance between them. In addition, there are no large-scale data on the association of PCSK9 inhibitors with clinical outcomes in Asian patients with very high cardiovascular risks including AMI. For the adoption of the 2019 ESC guidelines into Korean real-world practice, we need more data on 1) the efficacy and safety of PCSK9 inhibitors on clinical outcome and 2) whether lowering LDL-C levels below 55 mg/dL would be more beneficial than a LDL target of 70 mg/dL. Meanwhile, 2 of the guidelines recommend that a lipid profile should be obtained as early as possible after admission for AMI and should be re-evaluated 4–6 weeks after AMI to determine whether the target levels have been reached and for evaluating any side effects from statins. The intensity of statin therapy should be increased unless there are intolerance and safety issues.
The beneficial impact of statins on clinical outcome has been well established, exerted by dual mechanisms including plasma LDL-C lowering and pleiotropic anti-inflammatory effects on atherosclerotic plaques.79),80),81) The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) study is the first trial to show a benefit of adding a non-statin lipid-modifying agent to statin therapy.76) Thus, ezetimibe should be given to patients when the therapeutic goal is not achieved at the maximally tolerated statin dose or when statins are not tolerated because of serious side effects. Two major studies using PCSK9 inhibitors including evolocumab and alirocumab have shown significant benefits in reducing MACEs in patients with LDL-C levels of 70 mg/dL or higher despite maximally tolerated statin therapy.77),78) Thus, the large-scale, randomized, controlled studies using ezetimibe and two PCSK9 inhibitors has made 2019 ESC lipid guidelines revise the concept of LDL-C goals and include non-statin lipid therapy into the guidelines. While the recommendation that a high-intensity statin should be prescribed to the highest tolerated dose is still most important, ezetimibe and/or PCSK9 inhibitors can be considered in patients not achieving their lipid goal.
Evidence from Korea
The KAMIR data between 2005 and 2007 demonstrated that statin therapy significantly reduced the risk of the composite of all cause of death, recurrent MI, target vessel revascularization (TVR) and coronary artery bypass graft surgery even in patients with LDL-C below 70 mg/dL.82) In addition, further analysis of 1,048 patients with AMI with LDL-C <50 mg/dL in the KAMIR registry between 2005 and 2014 revealed statin therapy further reduced the risk of MACEs.83) Thus, Korean data strongly support the idea that serum LDL-C levels should be reduced as much as possible. Moreover, statin therapy should be given to all patients with AMI irrespective of their serum LDL-C levels, if tolerable. The COREA-AMI registry analyzing 3,921 statin-naïve patients with AMI undergoing PCI demonstrated that early statin therapy within 48 hours after admission reduced adverse long-term clinical outcomes compared with statin initiation later, which supports the concept that statin therapy should be started as soon as possible in patients with AMI.84) In terms of lipid therapy, Korean data also support the concepts of the recent ESC and ACC/AHA guidelines. However, high-intensity statins are sometimes prescribed to Korean patients with AMI in the real world. In the KAMIR data from 2005 to 2014, high-intensity statins were prescribed in only 6.8% of patients with AMI undergoing successful PCI (2,271/33,442 patients).85) In contrast, low- to moderate-intensity statins were prescribed in more than 75% of patients with AMI. Asian patients receive lower doses of statin in most clinical settings compared with their Western counterparts.86) In the 2013 ACC/AHA lipid guidelines, Asian ancestry is considered as a characteristic that might modify the decision to use high-intensity therapy.87) Further studies are needed on the issue of statin intensity in Korean patients with AMI.
Recommendations
• It is recommended to obtain a lipid profile in all patients with AMI as soon as possible after presentation.
• It is recommended to start high-intensity statin therapy as early as possible after AMI, unless contraindicated, and maintain it during the long term.
• Treatment goals are recommended as an LDL-C reduction of ≥50% from baseline or an LDL-C level of <70 mg/dL in patients with AMI.
• In patients with AMI with LDL-C ≥70 mg/dL despite a maximally tolerated statin dose, combination with ezetimibe is recommended.
• In patients with AMI with LDL-C ≥70 mg/dL despite a maximally tolerated dose of a statin and ezetimibe, a combination with a PCSK9 inhibitor is recommended.
ACKNOWLEDGEMENTS
The Task Force on Expert Consensus Document of the Korean Society of Myocardial Infarction (KSMI): Myung Ho Jeong, MD, PhD, Shung Chull Chae, MD, PhD, Kyoo-Rok Han, MD, PhD, Hyo-Soo Kim, MD, PhD, Myeong Chan Cho, MD, PhD, Yang Soo Jang, MD, PhD, Chong Jin Kim, MD, PhD, Jin-Yong Hwang, MD, PhD, Doo Il Kim MD, PhD, Jong-Seon Park, MD, PhD, Hun Sik Park, MD, PhD, Byung Ok Kim, MD, PhD, Seung-Ho Hur, MD, PhD, Seung-Woon Rha, MD, PhD, Youngkeun Ahn, MD, PhD, Kiyuk Chang, MD, PhD, Seok-Kyu Oh, MD, PhD, Kwang Soo Cha, MD, PhD, Kyung-Kook Hwang, MD, PhD, Young-Hoon Jeong, MD, PhD, Joo-Yong Hahn, MD, PhD, Hyun-Jae Kang, MD, PhD, Weon Kim, MD, PhD, Ju Han Kim, MD, PhD, Jang-Whan Bae, MD, PhD, Eun Ho Choo, MD, PhD, Min Chul Kim, MD, PhD, and Hyun Kuk Kim, MD, PhD.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Jeong MH.
- Writing - original draft: Kim HK, Ahn Y, Chang K, Jeong YH, Hahn JY, Choo EH.
- Writing - review & editing: Kim HK, Ahn Y, Chang K, Jeong YH, Hahn JY, Choo EH, Kim MC, Kim HS, Kim W, Cho MC, Jang Y, Kim CJ, Jeong MH, Chae SC.
References
- 1.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 2.Lee SW, Kim HC, Lee HS, Suh I. Thirty-year trends in mortality from cardiovascular diseases in Korea. Korean Circ J. 2015;45:202–209. doi: 10.4070/kcj.2015.45.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim RB, Kim BG, Kim YM, et al. Trends in the incidence of hospitalized acute myocardial infarction and stroke in Korea, 2006–2010. J Korean Med Sci. 2013;28:16–24. doi: 10.3346/jkms.2013.28.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 5.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 7.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Yang DH, Park HS, et al. Suboptimal use of evidence-based medical therapy in patients with acute myocardial infarction from the Korea Acute Myocardial Infarction Registry: prescription rate, predictors, and prognostic value. Am Heart J. 2010;159:1012–1019. doi: 10.1016/j.ahj.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RH, Chen AY, Alexander KP, Ohman EM, Roe MT, Peterson ED. Doing the right things and doing them the right way: association between hospital guideline adherence, dosing safety, and outcomes among patients with acute coronary syndrome. Circulation. 2015;131:980–987. doi: 10.1161/CIRCULATIONAHA.114.013451. [DOI] [PubMed] [Google Scholar]
- 10.Kang J, Park KW, Palmerini T, et al. Racial differences in ischaemia/bleeding risk trade-off during anti-platelet therapy: individual patient level landmark meta-analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. doi: 10.1055/s-0038-1676545. [DOI] [PubMed] [Google Scholar]
- 11.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 12.Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 13.Thrane PG, Kristensen SD, Olesen KK, et al. 16-year follow-up of the Danish Acute Myocardial Infarction 2 (DANAMI-2) trial: primary percutaneous coronary intervention vs. fibrinolysis in ST-segment elevation myocardial infarction. Eur Heart J. 2020;41:847–854. doi: 10.1093/eurheartj/ehz595. [DOI] [PubMed] [Google Scholar]
- 14.GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 15.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 16.Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 18.Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet. 2001;358:605–613. doi: 10.1016/S0140-6736(01)05775-0. [DOI] [PubMed] [Google Scholar]
- 19.Antman EM, Morrow DA, McCabe CH, et al. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med. 2006;354:1477–1488. doi: 10.1056/NEJMoa060898. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379–1387. doi: 10.1056/NEJMoa1301092. [DOI] [PubMed] [Google Scholar]
- 21.Sinnaeve PR, Armstrong PW, Gershlick AH, et al. ST-segment-elevation myocardial infarction patients randomized to a pharmaco-invasive strategy or primary percutaneous coronary intervention: Strategic Reperfusion Early After Myocardial Infarction (STREAM) 1-year mortality follow-up. Circulation. 2014;130:1139–1145. doi: 10.1161/CIRCULATIONAHA.114.009570. [DOI] [PubMed] [Google Scholar]
- 22.Sim DS, Jeong MH, Ahn Y, et al. Safety and benefit of early elective percutaneous coronary intervention after successful thrombolytic therapy for acute myocardial infarction. Am J Cardiol. 2009;103:1333–1338. doi: 10.1016/j.amjcard.2009.01.339. [DOI] [PubMed] [Google Scholar]
- 23.Sim DS, Jeong MH, Ahn Y, et al. Pharmacoinvasive strategy versus primary percutaneous coronary intervention in patients with ST-segment-elevation myocardial infarction: a propensity score-matched analysis. Circ Cardiovasc Interv. 2016;9:e003508. doi: 10.1161/CIRCINTERVENTIONS.115.003508. [DOI] [PubMed] [Google Scholar]
- 24.Bonnefoy E, Steg PG, Boutitie F, et al. Comparison of primary Angioplasty and Pre-hospital fibrinolysis In acute Myocardial infarction (CAPTIM) trial: a 5-year follow-up. Eur Heart J. 2009;30:1598–1606. doi: 10.1093/eurheartj/ehp156. [DOI] [PubMed] [Google Scholar]
- 25.Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1622–1632. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 26.Sterling LH, Filion KB, Atallah R, Reynier P, Eisenberg MJ. Intravenous beta-blockers in ST-segment elevation myocardial infarction: a systematic review and meta-analysis. Int J Cardiol. 2017;228:295–302. doi: 10.1016/j.ijcard.2016.11.133. [DOI] [PubMed] [Google Scholar]
- 27.Ibanez B, Macaya C, Sánchez-Brunete V, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–1503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 28.Pizarro G, Fernández-Friera L, Fuster V, et al. Long-term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) J Am Coll Cardiol. 2014;63:2356–2362. doi: 10.1016/j.jacc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Roolvink V, Ibáñez B, Ottervanger JP, et al. Early intravenous beta-blockers in patients with ST-segment elevation myocardial infarction before primary percutaneous coronary intervention. J Am Coll Cardiol. 2016;67:2705–2715. doi: 10.1016/j.jacc.2016.03.522. [DOI] [PubMed] [Google Scholar]
- 30.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 31.Korhonen MJ, Robinson JG, Annis IE, et al. Adherence tradeoff to multiple preventive therapies and all-cause mortality after acute myocardial infarction. J Am Coll Cardiol. 2017;70:1543–1554. doi: 10.1016/j.jacc.2017.07.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dondo TB, Hall M, West RM, et al. β-blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol. 2017;69:2710–2720. doi: 10.1016/j.jacc.2017.03.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann A, Maura G, Weill A, Alla F, Danchin N. Clinical events after discontinuation of β-blockers in patients without heart failure optimally treated after acute myocardial infarction: a cohort study on the french healthcare databases. Circ Cardiovasc Qual Outcomes. 2018;11:e004356. doi: 10.1161/CIRCOUTCOMES.117.004356. [DOI] [PubMed] [Google Scholar]
- 34.Choo EH, Chang K, Ahn Y, et al. Benefit of β-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart. 2014;100:492–499. doi: 10.1136/heartjnl-2013-305137. [DOI] [PubMed] [Google Scholar]
- 35.Yang JH, Hahn JY, Song YB, et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2014;7:592–601. doi: 10.1016/j.jcin.2013.12.206. [DOI] [PubMed] [Google Scholar]
- 36.Park JJ, Kim SH, Kang SH, et al. Effect of β-blockers beyond 3 years after acute myocardial infarction. J Am Heart Assoc. 2018;7:e007567. doi: 10.1161/JAHA.117.007567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JH, Hahn JY, Song YB, et al. Angiotensin receptor blocker in patients with ST segment elevation myocardial infarction with preserved left ventricular systolic function: prospective cohort study. BMJ. 2014;349:g6650. doi: 10.1136/bmj.g6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song PS, Seol SH, Seo GW, et al. Comparative effectiveness of angiotensin II receptor blockers versus angiotensin-converting enzyme inhibitors following contemporary treatments in patients with acute myocardial infarction: results from the Korean Working Group in Myocardial Infarction (KorMI) registry. Am J Cardiovasc Drugs. 2015;15:439–449. doi: 10.1007/s40256-015-0140-5. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Bae MH, Yang DH, et al. Angiotensin II type 1 receptor blockers as a first choice in patients with acute myocardial infarction. Korean J Intern Med. 2016;31:267–276. doi: 10.3904/kjim.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi SY, Choi BG, Rha SW, et al. Angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers in acute ST-segment elevation myocardial infarction patients with diabetes mellitus undergoing percutaneous coronary intervention. Int J Cardiol. 2017;249:48–54. doi: 10.1016/j.ijcard.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Byun JK, Choi BG, Rha SW, Choi SY, Jeong MH Other Korea Acute Myocardial Infarction Registry (KAMIR) investigators. Comparison of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with diabetes mellitus and non-ST-segment elevation myocardial infarction who underwent successful percutaneous coronary intervention. Atherosclerosis. 2018;277:130–135. doi: 10.1016/j.atherosclerosis.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Choi IS, Park IB, Lee K, et al. Angiotensin-converting enzyme inhibitors provide better long-term survival benefits to patients with AMI than angiotensin II receptor blockers after survival hospital discharge. J Cardiovasc Pharmacol Ther. 2019;24:120–129. doi: 10.1177/1074248418795897. [DOI] [PubMed] [Google Scholar]
- 43.Jeong HC, Jeong MH, Ahn Y, et al. Comparative assessment of angiotensin II type 1 receptor blockers in the treatment of acute myocardial infarction: surmountable vs. insurmountable antagonist. Int J Cardiol. 2014;170:291–297. doi: 10.1016/j.ijcard.2013.07.146. [DOI] [PubMed] [Google Scholar]
- 44.Park K, Kim YD, Kim KS, et al. The impact of a dose of the angiotensin receptor blocker valsartan on post-myocardial infarction ventricular remodelling. ESC Heart Fail. 2018;5:354–363. doi: 10.1002/ehf2.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song PS, Kim DK, Seo GW, et al. Spironolactone lowers the rate of repeat revascularization in acute myocardial infarction patients treated with percutaneous coronary intervention. Am Heart J. 2014;168:346–353.e3. doi: 10.1016/j.ahj.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 47.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 48.Motovska Z, Hlinomaz O, Miklik R, et al. Prasugrel versus ticagrelor in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: multicenter randomized PRAGUE-18 Study. Circulation. 2016;134:1603–1612. doi: 10.1161/CIRCULATIONAHA.116.024823. [DOI] [PubMed] [Google Scholar]
- 49.Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 50.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 51.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 52.Bae JS, Ahn JH, Tantry US, Gurbel PA, Jeong YH. Should antithrombotic treatment strategies in east Asians differ from Caucasians? Curr Vasc Pharmacol. 2018;16:459–476. doi: 10.2174/1570161116666180117103238. [DOI] [PubMed] [Google Scholar]
- 53.Huo Y, Jeong YH, Gong Y, et al. 2018 update of expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Sci Bull (Beijing) 2019;64:166–179. doi: 10.1016/j.scib.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 54.Chen KY, Rha SW, Li YJ, et al. Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2009;119:3207–3214. doi: 10.1161/CIRCULATIONAHA.108.822791. [DOI] [PubMed] [Google Scholar]
- 55.Park KH, Jeong MH, Ahn Y, et al. Comparison of short-term clinical outcomes between ticagrelor versus clopidogrel in patients with acute myocardial infarction undergoing successful revascularization; from Korea Acute Myocardial Infarction Registry-National Institute of Health. Int J Cardiol. 2016;215:193–200. doi: 10.1016/j.ijcard.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 56.Park KH, Jeong MH, Kim HK, et al. Comparison of prasugrel versus clopidogrel in Korean patients with acute myocardial infarction undergoing successful revascularization. J Cardiol. 2018;71:36–43. doi: 10.1016/j.jjcc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Kang J, Han JK, Ahn Y, et al. Third-generation P2Y12 inhibitors in East Asian acute myocardial infarction patients: a nationwide prospective multicentre study. Thromb Haemost. 2018;118:591–600. doi: 10.1055/s-0038-1626697. [DOI] [PubMed] [Google Scholar]
- 58.Lee SH, Kim HK, Jeong MH, et al. Practical guidance for P2Y12 inhibitors in acute myocardial infarction undergoing percutaneous coronary intervention. Eur Heart J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Yun JE, Kim YJ, Park JJ, et al. Safety and effectiveness of contemporary P2Y12 inhibitors in an East Asian population with acute coronary syndrome: a nationwide population-based cohort study. J Am Heart Assoc. 2019;8:e012078. doi: 10.1161/JAHA.119.012078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park DW, Kwon O, Jang JS, et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140:1865–1877. doi: 10.1161/CIRCULATIONAHA.119.041766. [DOI] [PubMed] [Google Scholar]
- 61.Lee JM, Jung JH, Park KW, et al. Harmonizing Optimal Strategy for Treatment of coronary artery diseases--comparison of REDUCtion of prasugrEl dose or POLYmer TECHnology in ACS patients (HOST-REDUCE-POLYTECH-ACS RCT): study protocol for a randomized controlled trial. Trials. 2015;16:409. doi: 10.1186/s13063-015-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim BK, Hong SJ, Cho YH, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323:2407–2416. doi: 10.1001/jama.2020.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hahn JY, Song YB, Oh JH, et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019;321:2428–2437. doi: 10.1001/jama.2019.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hahn JY, Song YB, Oh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018;391:1274–1284. doi: 10.1016/S0140-6736(18)30493-8. [DOI] [PubMed] [Google Scholar]
- 65.Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014;129:304–312. doi: 10.1161/CIRCULATIONAHA.113.003303. [DOI] [PubMed] [Google Scholar]
- 66.Sim DS, Jeong MH, Kim HS, et al. Dual antiplatelet therapy beyond 12 months versus for 12 months after drug-eluting stents for acute myocardial infarction. J Cardiol. 2020;75:66–73. doi: 10.1016/j.jjcc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 68.Jeong YH, Smith SC, Jr, Gurbel PA. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;373:1273–1274. doi: 10.1056/NEJMc1508692. [DOI] [PubMed] [Google Scholar]
- 69.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 70.Jeong YH, Bae JS, Gurbel PA. Rivaroxaban in stable cardiovascular disease. N Engl J Med. 2018;378:396. doi: 10.1056/NEJMc1714934. [DOI] [PubMed] [Google Scholar]
- 71.Mo C, Sun G, Lu ML, et al. Proton pump inhibitors in prevention of low-dose aspirin-associated upper gastrointestinal injuries. World J Gastroenterol. 2015;21:5382–5392. doi: 10.3748/wjg.v21.i17.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim MS, Song HJ, Lee J, Yang BR, Choi NK, Park BJ. Effectiveness and safety of clopidogrel co-administered with statins and proton pump inhibitors: a Korean National Health Insurance Database Study. Clin Pharmacol Ther. 2019;106:182–194. doi: 10.1002/cpt.1361. [DOI] [PubMed] [Google Scholar]
- 73.Cholesterol Treatment Trialists' (CTT) Collaboration. Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 76.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 77.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 79.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 80.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 81.Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–1862. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 82.Lee KH, Jeong MH, Kim HM, et al. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2011;58:1664–1671. doi: 10.1016/j.jacc.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 83.Piao ZH, Jin L, Kim JH, et al. Benefits of statin therapy in patients with acute myocardial infarction with serum low-density lipoprotein cholesterol ≤ 50 mg/dl. Am J Cardiol. 2017;120:174–180. doi: 10.1016/j.amjcard.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Kim MC, Ahn Y, Cho JY, et al. Benefit of early statin initiation within 48 hours after admission in statin-naïve patients with acute myocardial infarction undergoing percutaneous coronary intervention. Korean Circ J. 2019;49:419–433. doi: 10.4070/kcj.2018.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ji MS, Jeong MH, Ahn YK, et al. Clinical outcome of statin plus ezetimibe versus high-intensity statin therapy in patients with acute myocardial infarction propensity-score matching analysis. Int J Cardiol. 2016;225:50–59. doi: 10.1016/j.ijcard.2016.09.082. [DOI] [PubMed] [Google Scholar]
- 86.Wang P. Statin dose in Asians: is pharmacogenetics relevant? Pharmacogenomics. 2011;12:1605–1615. doi: 10.2217/pgs.11.98. [DOI] [PubMed] [Google Scholar]
- 87.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]