Abstract
Background and Objectives
The Sapien 3 (S3) valve has not been compared to the Sapien XT (SXT) valve in Korea. We compared procedural and clinical outcomes between the 2 devices.
Methods
A total of 189 patients who underwent transcatheter aortic valve replacement (TAVR) with S3 (n=95) or SXT (n=94) valve was analyzed. The primary endpoint was cardiovascular mortality at 1 year. The median follow-up duration was 438 days.
Results
The Society of Thoracic Surgeons score was similar between the 2 groups. The device success rate (90.4% vs. 97.9%; p=0.028) was higher in the S3 than in the SXT. The S3 showed significantly fewer cases of moderate or severe paravalvular leakage (PVL) (16.7% vs. 0.0%; p=0.001) than the SXT. However, effective orifice area (EOA) (2.07±0.61 vs. 1.70±0.49 cm2; p<0.001) was smaller in the S3. Multivariable Cox regression analysis showed the S3 was associated with significantly fewer cardiovascular mortality at 1 year compared to the SXT (5.4% vs. 1.1%; hazard ratio, 0.031; 95% confidence interval, 0.001–0.951; p=0.047). Periprocedural complication rates, composite of disabling stroke or all-cause mortality, all-cause mortality, and disabling stroke at 1 year were similar between the 2 groups.
Conclusions
Cardiovascular mortality was lower in the S3 group than in the SXT group over 1 year of follow-up. The reduction in PVL was attributed to the higher device success rate of TAVR with the S3 valve. However, the benefit of S3 obtained at the expense of reduced EOA should be meticulously re-evaluated in larger studies during long-term follow-up.
Keywords: Transcatheter aortic valve replacement, Aortic valve stenosis
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) procedures have been performed more frequently worldwide as its indications have expanded. Historically, TAVR was performed in patients with prohibitive or high surgical risk. In contrast, it is now performed in low risk patients with severe symptomatic aortic stenosis (AS).1),2),3),4),5),6) In Korea, the TAVR procedure is poorly reimbursed by national insurance; regardless, the number of TAVR procedures is on the constant rise.7) The latest version of the balloon-expandable TAVR valve, or Sapien 3 (S3) transcatheter heart valve (THV), became available in Korea in 2014. The S3 valve is now widely used in most Korean TAVR centers.
The S3 THV was developed with several advanced features to overcome the drawbacks of its predecessor, the Sapien XT (SXT). The valve frame was coated with an outer polyethylene terephthalate skirt to reduce significant paravalvular leakage (PVL), as PVL predicts poor long-term prognosis.8) In addition, the S3 THV has a flared inflow morphology, which might contribute to the lower PVL in the S3 than that in the SXT.9) The required minimal luminal diameters of the femoral access site were further minimalized by the slender profile of the delivery system. Therefore, the transfemoral approach can be more generously applied for TAVR candidates with peripheral artery disease. Periprocedural bleeding complications are expected to be lower in the S3 THV than with SXT.
However, the overlaid outer skirt of the S3 THV might influence the effective orifice area (EOA) within the implanted THV due to its own thickness. Therefore, a prosthesis-patient mismatch may occur more frequently in patients treated with the S3 THV than it does with SXT. Asians tend to have a smaller annulus than do western people. Therefore, this theoretical prosthesis-patient mismatch is more likely to occur when THVs of the same size are implanted in Asian patients than it does in other populations. However, it is not well known how these prosthesis-patient mismatch problems relate to clinical results after TAVR. Therefore, we compared the procedural and clinical outcomes between Korean patients treated with S3 vs. SXT THV.
METHODS
Study population
A total of 189 patients with severe symptomatic AS treated with balloon-expandable TAVR at 4 medical centers (Korea University Anam Hospital, Samsung Medical Center, Seoul St. Mary's Hospital, and Sejong General Hospital) between July 2010 and March 2018 was reviewed retrospectively (Figure 1). Severe AS was defined as EOA≤1.0 cm2 or EOA index (EOAI)≤0.8 cm2, mean pressure gradient≥40 mmHg, and jet velocity≥4.0 m/s. Between July 2010 and March 2016, 94 patients were treated with the SXT device. The S3 was exclusively used in 95 patients until March 2018. This study was approved by the Institutional Review Board of Korea University Anam Hospital (2019AN0044). Informed consent was waived.
Figure 1. Flow chart of TAVR patient stratification according to balloon-expandable TAVR generation from a multicenter registry.
TAVR = transcatheter aortic valve replacement; VARC = Valve Academic Research Consortium.
Outcome measurements and definitions
The primary endpoint of the present study was cardiovascular mortality at 1 year.
The secondary endpoints were as follows: device success rate; PVL; and peri-procedural complications, including new permanent pacemaker (PPM) implantation, cardiac tamponade, coronary artery obstruction and major bleeding; hemodynamic outcomes assessed by echocardiography; and clinical outcomes including a composite of disabling stroke or all-cause mortality, all-cause mortality, and disabling stroke. Device success was defined as follows: 1) successful vascular access, delivery, and implantation of the device and successful removal of the delivery system; 2) correct device positioning in the proper anatomical location; 3) intended performance of the THV without moderate or severe PVL as assessed by intra-procedural echocardiography; 4) only 1 valve implanted in a single procedure.7),10),11) The endpoint definitions were adopted from the Valve Academic Research Consortium-2 criteria.12)
All of the clinical outcomes of interest were adjudicated and reported by the attending physician of each hospital.
Transcatheter aortic valve replacement procedures
The general indications for the TAVR procedure were as follows: severe symptomatic AS patients with 1) high surgical risk for surgical aortic valve replacement (SAVR); 2) contraindications to SAVR; 3) low to intermediate surgical risk (but refusal to be treated surgically).
The contraindications to SAVR were as follows: 1) advanced liver cirrhosis or chronic obstructive pulmonary disease; 2) porcelain aorta; 3) identification of the left internal mammary artery under the sternum during a prior coronary artery bypass grafting; 4) severe scoliosis; 5) severe frailty.
Patient surgical risk was evaluated with the Society of Thoracic Surgeons-Predicted Risk of Mortality (STS-PROM) score, which was calculated using the Online STS Adult Cardiac Surgery Risk Calculator V2.9 (http://riskcalc.sts.org/stswebriskcalc/calculate).
The final treatment decision (between TAVR and SAVR) was made by each hospital's multidisciplinary heart team, which consisted of an interventional cardiologist, imaging cardiologist, cardiac surgeon, and radiologist. The patients were denied for surgery based on presumed surgical risk (including advanced age) in combination with the following factors: STS-PROM scores, heart and valve function, previous open-heart surgery, severe pulmonary or liver disease, cognitive status, or history of stroke.
The THV device size was chosen at the attending physician's discretion based on measurements on three dimensional (3D) reconstructed computed tomography imaging.
Unless contraindicated, the TAVR candidates were treated with dual antiplatelet therapy, including aspirin 100 mg once a day and clopidogrel 75 mg once daily. The TAVR procedures were either performed in a catherization laboratory or a hybrid operating room under general anesthesia or conscious sedation. A 14-20Fr sheath was introduced into the femoral artery or the left ventricular apex (in case of transapical approach) according to approaching site and device size. Pre-TAVR balloon aortic valvuloplasty was performed under rapid ventricular pacing if needed. After TAVR THV implantation, THV location, function, and PVL were assessed using transesophageal or transthoracic echocardiography. Post-dilation was performed when there was moderate or severe PVL or THV underexpansion. Dual antiplatelet therapy was administered for 3–6 months after the procedure. After this, a single antiplatelet therapy was considered at the attending physician's discretion. Routine transthoracic echocardiography was performed one month and one year after the TAVR.
Statistics
Continuous variables were expressed as mean±standard deviation. Categorical variables were expressed as frequency (percentage). Differences between groups were analyzed using Student's t-test for continuous variables and Pearson's χ2-test or Fisher's exact test for categorical variables. The cumulative incidence rate was analyzed based on the index procedure time to the occurrence of the first clinical outcome. This rate was estimated using the Kaplan-Meier method with the log-rank test. The Cox multivariable regression model was used to examine the hazard ratio (HR) and 95% confidence interval (CI) of each endpoint. The adjustment variables were age, sex, body mass index, diabetes mellitus, hypertension, chronic lung disease, peripheral arterial occlusive disease, bicuspid aortic valve, extent of coronary artery disease, atrial fibrillation, advanced chronic kidney disease (stage>3), device generation, and in-hospital moderate or severe PVL. Details regarding propensity score matching analysis and inverse probability of treatment weighting adjusted analysis were described elsewhere (Supplementary Data 1). All of the tests were 2-tailed. The p values<0.05 were considered statistically significant. Statistical analysis was carried out using SPSS version 24.0 (IBM Corporation, Armonk, NY, USA).
RESULTS
Baseline characteristics
The baseline patient demographics are shown in Table 1. A total of 94 patients underwent implantation of SXT THV and 95 with S3 THV. The mean patient age was 78.1±7.4 years, and 49.2% were male. The sex ratio was comparable between the 2 groups. Similarly, body mass index, annular diameter, and left ventricle ejection fraction were not significantly different between the 2 groups. The incidences of the following medical problems were also comparable between the 2 groups: diabetes mellitus, hypertension, chronic kidney disease, dialysis, peripheral arterial occlusive disease, previous stroke, previous myocardial infarction, left bundle branch block (LBBB), and right bundle branch block (RBBB). In contrast, the incidences of chronic lung disease, multi-vessel coronary artery disease, and atrial fibrillation were higher in the S3 group than they were in the SXT group. Most patients had New York Heart Association (NYHA) functional classification III or IV dyspnea. However, the incidence of NYHA class III/IV dyspnea was higher in the S3 group than in the SXT group. The median follow-up duration was significantly shorter in the S3 group than it was in the SXT group (444 vs. 357 days; p<0.001). The mean STS-PROM was 7.88±6.82. High surgical risk was present in 40.8% of patients. The STS score was not different between the 2 groups (7.25±5.96 vs. 8.51±7.55; p=0.204), but the incidence of inoperable conditions was higher in the SXT group than it was in the S3 group (21.3% vs. 8.4%; p=0.013). The mean pressure gradient was 50.0±18.6 mmHg. The mean peak velocity was 4.45±0.75 m/s, and the mean EOAI was 0.47±0.12 cm2/m2. There were no significant differences between the 2 groups with regard to the hemodynamic parameters measured using 2D echocardiography. The incidence of bicuspid AS and aortic regurgitation (>mild degree) was not different between the 2 groups. However, the incidence of mitral regurgitation (>mild) was higher in the S3 group than it was in the SXT group.
Table 1. Baseline patient demographics.
| Overall (n=189) | Sapien XT (n=94) | Sapien 3 (n=95) | p value | |||
|---|---|---|---|---|---|---|
| Male | 93 (49.2) | 47 (50.0) | 46 (48.4) | 0.828 | ||
| Age (years) | 78.1±7.4 | 77.6±7.4 | 78.5±7.5 | 0.380 | ||
| Follow-up duration (days) | 438 | 444 | 357 | <0.001 | ||
| BMI (kg/m2) | 23.8±3.5 | 24.0±3.6 | 23.6±3.5 | 0.453 | ||
| Diabetes mellitus | 63 (33.3) | 35 (37.2) | 28 (29.5) | 0.258 | ||
| Hypertension | 146 (77.2) | 73 (77.7) | 73 (76.8) | 0.893 | ||
| Chronic lung disease | 44 (23.3) | 14 (14.9) | 30 (31.6) | 0.007 | ||
| CCr (mL/min) | 47.49±21.52 | 49.26±23.34 | 45.71±19.47 | 0.259 | ||
| CKD>stage 3 | 38 (20.1) | 21 (22.3) | 17 (17.9) | 0.446 | ||
| Dialysis | 9 (4.8) | 5 (5.3) | 4 (4.2) | 0.747 | ||
| PAOD | 41 (21.7) | 19 (20.2) | 22 (23.2) | 0.623 | ||
| Extent of CAD* | 0.082 | |||||
| Not significant | 104 (55.0) | 52 (57.1) | 52 (55.3) | |||
| 1 vessel | 41 (21.7) | 26 (28.6) | 15 (16.0) | |||
| 2 vessels | 18 (9.5) | 7 (7.7) | 11 (11.7) | |||
| 3 vessels | 22 (11.6) | 6 (6.6) | 16 (17.0) | |||
| Atrial fibrillation | 26 (13.8) | 9 (9.6) | 17 (17.9) | 0.097 | ||
| RBBB | 15 (7.9) | 9 (9.6) | 6 (6.3) | 0.407 | ||
| LBBB | 3 (1.6) | 1 (1.1) | 2 (2.1) | 1.000 | ||
| Previous stroke or TIA | 20 (10.6) | 11 (11.7) | 9 (9.5) | 0.619 | ||
| Previous MI | 18 (9.5) | 9 (9.6) | 9 (9.5) | 0.981 | ||
| Previous procedure/surgery | ||||||
| PCI | 42 (22.2) | 24 (25.5) | 18 (18.9) | 0.276 | ||
| CABG | 10 (5.3) | 6 (6.4) | 4 (4.2) | 0.536 | ||
| SAVR | 2 (1.1) | 1 (1.1) | 1 (1.1) | 1.000 | ||
| TAVR | 3 (1.6) | 3 (3.2) | 0 (0.0) | 0.121 | ||
| PPM or ICD | 4 (2.1) | 3 (3.2) | 1 (1.1) | 0.368 | ||
| PPM | 3 (1.6) | 3 (3.2) | - | |||
| ICD | 1 (0.5) | - | 1 (1.1) | |||
| NYHA | 0.067 | |||||
| 1 | 2 (1.1) | 2 (2.1) | 0 (0.0) | |||
| 2 | 45 (23.8) | 21 (22.3) | 24 (25.3) | |||
| 3 | 99 (52.4) | 58 (61.7) | 41 (43.2) | |||
| 4 | 43 (22.8) | 13 (13.8) | 30 (31.6) | |||
| Syncope | 10 (5.3) | 10 (10.6) | 0 (0.0) | 0.001 | ||
| CCS | 0.001 | |||||
| None | 64 (33.9) | 44 (46.8) | 20 (21.1) | |||
| 1 | 32 (16.9) | 10 (10.6) | 22 (23.2) | |||
| 2 | 45 (23.8) | 22 (23.4) | 23 (24.2) | |||
| 3 | 34 (18.0) | 16 (17.0) | 18 (18.9) | |||
| 4 | 14 (7.4) | 2 (2.1) | 12 (12.6) | |||
| STS-PROM (%) | 7.88±6.82 | 7.25±5.96 | 8.51±7.55 | 0.204 | ||
| Low, STS<4 | 61 (32.3) | 31 (33.0) | 30 (31.6) | |||
| Intermediate | 51 (27.0) | 31 (33.0) | 20 (21.1) | |||
| High, STS≥8 | 77 (40.7) | 32 (34.0) | 45 (47.4) | |||
| Inoperable conditions | 28 (14.8) | 20 (21.3) | 8 (8.4) | 0.013 | ||
| Severe COPD | 17 (9.0) | 14 (14.9) | 3 (3.2) | 0.005 | ||
| Porcelain aorta | 3 (1.6) | 3 (3.2) | 0 (0.0) | 0.121 | ||
| Previous CABG, LIMA under the sternum | 5 (2.6) | 2 (2.1) | 3 (3.2) | 1.000 | ||
| Severe frailty | 4 (2.1) | 2 (2.1) | 2 (2.1) | 1.000 | ||
| Bicuspid | 10 (5.3) | 6 (6.4) | 4 (4.2) | 0.536 | ||
| Mean pressure gradient (mmHg) | 50.0±18.6 | 51.4±19.5 | 48.7±17.6 | 0.321 | ||
| Peak velocity (m/s) | 4.45±0.75 | 4.49±0.74 | 4.41±0.75 | 0.472 | ||
| EOA (cm2) | 0.74±0.20 | 0.74±0.18 | 0.73±0.21 | 0.758 | ||
| EOAI (cm2/m2) | 0.47±0.12 | 0.46±0.11 | 0.47±0.13 | 0.816 | ||
| Ejection fraction (%) | 55.8±11.7 | 56.8±11.9 | 54.8±11.5 | 0.237 | ||
| Annular diameter (mm) | 22.1±2.9 | 22.0±3.4 | 22.3±2.3 | 0.594 | ||
| Aortic regurgitation>mild | 49 (25.9) | 21 (22.3) | 28 (29.5) | 0.263 | ||
| Mitral regurgitation>mild | 34 (18.0) | 11 (11.7) | 23 (24.2) | 0.025 | ||
Follow-up duration is presented as median value. Other values are presented as number of patients (%) or mean±standard deviation.
BMI = body mass index; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCr = creatinine clearance; CCS = Canadian Cardiovascular Society grading of angina pectoris; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; EOA = effective orifice area; EOAI = effective orifice area index; ICD = implantable cardioverter defibrillator; LBBB = left bundle branch block; LIMA = left internal mammary artery; MI = myocardial infarction; NYHA = New York Heart Association; PAOD = peripheral arterial occlusive disease; PCI = percutaneous coronary intervention; PPM = permanent pacemaker; RBBB = right bundle branch block; SAVR = surgical aortic valve replacement; STS = Society of Thoracic Surgeons; STS-PROM = Society of Thoracic Surgeons-Predicted Risk Of Mortality; TAVR = transcatheter aortic valve replacement; TIA = transient ischemic attack.
*Left main coronary artery disease was considered 2-vessel disease.
Procedural outcomes
The procedural outcomes are summarized in Table 2. Most of the TAVR procedures were performed via transfemoral access (96.3%). All of the S3 procedures were performed via the transfemoral route (92.6 vs. 100.0%; p=0.007). Conscious sedation was applied for limited patients in the S3 group (0.0% vs. 4.2%; p=0.121). The device success rate was statistically higher in the S3 group than it was in the SXT group (90.4% vs. 97.9%; p=0.028). Among 11 patients who experienced device failure, 4 underwent 2nd THV implantation during TAVR (n=2 in the SXT group, n=2 in the S3 group). Inappropriate location of THV implantation was observed in 2 patients in the SXT group. Moderate or severe PVL developed in 7 patients in the SXT group, whereas no significant PVL was observed after TAVR implantation in the S3 group. Procedure time was significantly shorter in the S3 group than it was in the SXT group (107.7±66.7 vs. 77.1±38.4 minutes; p<0.001). Pre-dilation was more frequently performed in the SXT group than the S3 group (80.9% vs. 41.1%; p<0.001). However, there was no difference between the 2 groups with regard to need for post-dilation after THV implantation. Valve malpositioning and emergent conversion to surgery were only noted in the SXT group, although the difference was not statistically significant. The hospital duration tended to be shorter in the S3 group than it was in the SXT group (13.6±13.8 vs. 10.5±7.4 days; p=0.053). There was no difference between the groups with regard to complications of new PPM implantation, cardiac tamponade, major bleeding, and annular rupture. Notably, no coronary obstructions occurred in our study.
Table 2. Procedural characteristics and outcomes.
| Overall (n=189) | Sapien XT (n=94) | Sapien 3 (n=95) | p value | |||
|---|---|---|---|---|---|---|
| Approach | 0.007 | |||||
| TF | 182 (96.3) | 87 (92.6) | 95 (100.0) | |||
| TA | 7 (3.7) | 7 (7.4) | 0 (0.0) | |||
| Room | <0.001 | |||||
| Hybrid room | 143 (75.7) | 83 (88.3) | 60 (63.2) | |||
| Cath lab | 46 (24.3) | 11 (11.7) | 35 (36.8) | |||
| Anesthesia | 0.121 | |||||
| General | 185 (97.9) | 94 (100.0) | 91 (95.8) | |||
| Conscious sedation | 4 (2.1) | 0 (0.0) | 4 (4.2) | |||
| Valve size (mm) | 0.329 | |||||
| 23 | 76 (40.2) | 37 (39.4) | 39 (41.1) | |||
| 26 | 91 (48.1) | 48 (52.1) | 42 (44.2) | |||
| 29 | 22 (11.6) | 8 (8.5) | 14 (14.7) | |||
| Device success | 178 (94.2) | 85 (90.4) | 93 (97.9) | 0.028 | ||
| Pre-dilation | 115 (60.8) | 76 (80.9) | 39 (41.1) | <0.001 | ||
| Post-dilation | 24 (12.7) | 13 (13.8) | 11 (11.6) | 0.642 | ||
| Complications | ||||||
| PPM implantation | 3 (1.6) | 2 (2.1) | 1 (1.1) | 0.621 | ||
| Cardiac tamponade | 2 (1.1) | 2 (2.1) | 0 (0.0) | 0.246 | ||
| Coronary obstruction | - | - | - | - | ||
| MI | - | - | - | - | ||
| New onset AF | 8 (4.2) | 4 (4.3) | 4 (4.2) | 1.000 | ||
| LBBB | 10 (5.3) | 5 (5.3) | 5 (5.3) | 1.000 | ||
| Transient | 6 (3.2) | 3 (3.2) | 3 (3.2) | |||
| Persistent | 4 (2.1) | 2 (2.1) | 2 (2.1) | |||
| RBBB | 3 (1.6) | 1 (1.1) | 2 (2.1) | 1.000 | ||
| Major bleeding | 2 (1.1) | 2 (2.1) | 0 (0.0) | 0.246 | ||
| Mitral injury | - | - | - | - | ||
| Aortic dissection | - | - | - | - | ||
| Annular rupture | 1 (0.5) | 1 (1.1) | 0 (0.0) | 0.497 | ||
| LV perforation | - | - | - | - | ||
| TAV-in-TAV | 4 (2.1) | 2 (2.1) | 2 (2.1) | 1.000 | ||
| Valve malposition | 3 (1.6) | 3 (3.2) | 0 (0.0) | 0.121 | ||
| Emergent operation | 2 (1.1) | 2 (2.1) | 0 (0.0) | 0.246 | ||
| Procedure time (minutes) | 92.1±56.2 | 107.7±66.7 | 77.1±38.4 | <0.001 | ||
| Hospital duration (days) | 12.1±11.2 | 13.6±13.8 | 10.5±7.4 | 0.053 | ||
Values are presented as number of patients (%) or mean±standard deviation.
AF = atrial fibrillation; LBBB = left bundle branch block; LV = left ventricular; MI = myocardial infarction; PPM = permanent pacemaker; RBBB = right bundle branch block; TA = transapical; TAV = transcatheter aortic valve; TF = transfemoral.
In-hospital clinical outcomes
The incidence of cardiovascular mortality (SXT 3.2% vs. S3 1.1%; p=0.368), composite of disabling stroke or all-cause mortality, all-cause mortality, and disabling stroke during the in-hospital period did not differ between the 2 groups (Table 3). In 187 patients who were followed using in-hospital echocardiography, there were no cases of moderate or severe PVL in the S3 group (7.5% vs. 0.0%; p=0.007) (Table 4). However, the EOA (2.07±0.61 vs. 1.70±0.49 cm2; p<0.001) and EOAI (1.31±0.43 vs. 1.11±0.34 cm2/m2; p=0.002) were significantly larger in the SXT group than they were in the S3 group. Prosthesis-patient mismatch was more frequent in the S3 group in-hospital period echocardiographic follow-up (6.0% vs. 23.3%; p<0.004). Incidence of severe prosthesis-patient mismatch (EOAI<0.65 cm2/m2) was very rare in both groups (Table 4).
Table 3. Clinical outcomes after TAVR.
| Overall (n=189) | Sapien XT (n=94) | Sapien 3 (n=95) | p value* | HR (95% CI) | p value | ||
|---|---|---|---|---|---|---|---|
| In-hospital | |||||||
| Disabling stroke or all-cause mortality | 8 (4.2) | 4 (4.3) | 4 (4.2) | 1.000 | - | - | |
| All-cause mortality | 7 (3.7) | 3 (3.2) | 4 (4.2) | 1.000 | - | - | |
| Cardiovascular mortality | 4 (2.1) | 3 (3.2) | 1 (1.1) | 0.368 | - | - | |
| Disabling stroke | 2 (1.1) | 1 (1.1) | 1 (1.1) | 1.000 | - | - | |
| 1-month follow-up | |||||||
| Disabling stroke or all-cause mortality | 9 (4.8) | 5 (5.4) | 4 (4.3) | 0.731 | 0.460 (0.094–2.244) | 0.337 | |
| All-cause mortality | 8 (4.3) | 4 (4.3) | 4 (4.3) | 0.998 | 0.523 (0.099–2.761) | 0.445 | |
| Cardiovascular mortality | 5 (2.7) | 4 (4.3) | 1 (1.1) | 0.182 | 0.079 (0.005–1.303) | 0.076 | |
| Disabling stroke | 3 (1.7) | 2 (2.2) | 1 (1.1) | 0.564 | 0.074 (0.001–7.248) | 0.265 | |
| NYHA>2 | 1/153 (0.7) | 0/81 (0.0) | 1/72 (1.4) | 0.471 | - | - | |
| CCS>2 | 1/153 (0.7) | 0/81 (0.0) | 1/72 (1.4) | 0.471 | - | - | |
| 1-year follow-up | |||||||
| Disabling stroke or all-cause mortality | 21 (12.9) | 12 (13.6) | 9 (11.7) | 0.783 | 1.055 (0.376–2.960) | 0.919 | |
| All-cause mortality | 18 (11.1) | 10 (11.3) | 8 (10.5) | 0.931 | 0.977 (0.326–2.934) | 0.967 | |
| Cardiovascular mortality | 6 (3.3) | 5 (5.4) | 1 (1.1) | 0.107 | 0.031 (0.001–0.951) | 0.047 | |
| Disabling stroke | 5 (3.0) | 3 (3.5) | 2 (2.3) | 0.715 | 1.394 (0.107–18.196) | 0.800 | |
| NYHA>2 | 2/99 (2.0) | 2/73 (2.7) | 0/26 (0.0) | 1.000 | - | - | |
| CCS>2 | - | - | - | - | - | - | |
Values are presented as number of patients (%) or mean±standard deviation. Covariates for multivariable Cox regression analysis were age, sex, body mass index, diabetes mellitus, hypertension, chronic lung disease, peripheral arterial occlusive disease, bicuspid aortic valve, extent of coronary artery disease, atrial fibrillation, advanced chronic kidney disease (stage>3), device generation, and in-hospital moderate or severe paravalvular leakage.
CCS = Canadian Cardiovascular Society grade of angina pectoris; CI = confidence interval; HR = hazard ratio; NYHA = New York Heart Association; TAVR = transcatheter aortic valve replacement.
*Events rates regarding mortality or stroke were estimated with Kaplan-Meier analysis. The p values were derived from the log-rank test.
Table 4. Hemodynamic parameters after TAVR.
| Overall (n=189) | Sapien XT (n=94) | Sapien 3 (n=95) | p value | |||
|---|---|---|---|---|---|---|
| In-hospital | ||||||
| Mean pressure gradient (mmHg) | 11.3±4.2 | 11.4±4.5 | 11.2±4.0 | 0.788 | ||
| Peak velocity (m/s) | 2.26±0.40 | 2.21±0.40 | 2.30±0.39 | 0.170 | ||
| EOA* (cm2) | 1.86±0.58 | 2.07±0.61 | 1.70±0.49 | <0.001 | ||
| 23 mm THV | 1.69±0.50 | 1.96±0.57 | 1.51±0.35 | 0.001 | ||
| 26 mm THV | 1.94±0.60 | 2.08±0.64 | 1.80±0.54 | 0.039 | ||
| 29 mm THV | 2.08±0.60 | 2.48±0.53 | 1.92±0.56 | 0.052 | ||
| EOAI* (cm2/m2) | 1.20±0.40 | 1.31±0.43 | 1.11±0.34 | 0.002 | ||
| 23 mm THV | 1.15±0.39 | 1.33±0.48 | 1.03±0.28 | 0.010 | ||
| 26 mm THV | 1.22±0.40 | 1.26±0.41 | 1.18±0.39 | 0.372 | ||
| 29 mm THV | 1.27±0.39 | 1.59±0.32 | 1.13±0.34 | 0.011 | ||
| Prosthesis-patient mismatch | 25/157 (15.9) | 4/67 (6.0) | 21/90 (23.3) | 0.004 | ||
| Severe | 1/157 (0.6) | 0/67 (0.0) | 1/90 (1.1) | 1.000 | ||
| PVL>mild | 7/187 (3.7) | 7/93 (7.5) | 0/94 (0.0) | 0.007 | ||
| 1-month follow-up | ||||||
| Mean pressure gradient (mmHg) | 12.5±4.5 | 12.7±4.8 | 12.2±4.3 | 0.615 | ||
| Peak velocity (m/s) | 2.39±0.41 | 2.39±0.43 | 2.40±0.38 | 0.911 | ||
| EOA* (cm2) | 1.72±0.49 | 1.86±0.57 | 1.60±0.37 | 0.010 | ||
| 23 mm THV | 1.54±0.42 | 1.67±0.50 | 1.43±0.32 | 0.083 | ||
| 26 mm THV | 1.81±0.46 | 1.92±0.58 | 1.70±0.26 | 0.069 | ||
| 29 mm THV | 2.04±0.76 | 2.63±0.44 | 1.80±0.75 | 0.216 | ||
| EOAI* (cm2/m2) | 1.07±0.30 | 1.13±0.36 | 1.02±0.22 | 0.069 | ||
| 23 mm THV | 1.01±0.30 | 1.05±0.36 | 0.98±0.25 | 0.510 | ||
| 26 mm THV | 1.10±0.27 | 1.15±0.34 | 1.06±0.16 | 0.219 | ||
| 29 mm THV | 1.18±0.46 | 1.66±0.18 | 0.99±0.40 | 0.081 | ||
| Prosthesis-patient mismatch | 22/101 (21.8) | 12/47 (25.5) | 10/54 (18.5) | 0.394 | ||
| Severe | 2/101 (2.0) | 1/47 (2.1) | 1/54 (1.9) | 1.000 | ||
| Ejection fraction (%) | 60.2±8.1 | 61.6±8.2 | 58.6±7.8 | 0.054 | ||
| PVL>mild | 10/114 (8.8) | 10/60 (16.7) | 0/54 (0.0) | 0.001 | ||
| Mitral regurgitation>mild | 4/113 (3.5) | 1/59 (1.7) | 3/54 (5.6) | 0.347 | ||
Values are presented as number of patients (%) or mean±standard deviation.
EOA = effective orifice area; EOAI = effective orifice area index; PVL = paravalvular leakage; TAVR = transcatheter aortic valve replacement; THV = transcatheter heart valve.
*Patients who underwent valve-in-valve procedure (n=5) were excluded from mean EOA/EOAI calculation.
Follow-up clinical outcomes and hemodynamic parameters
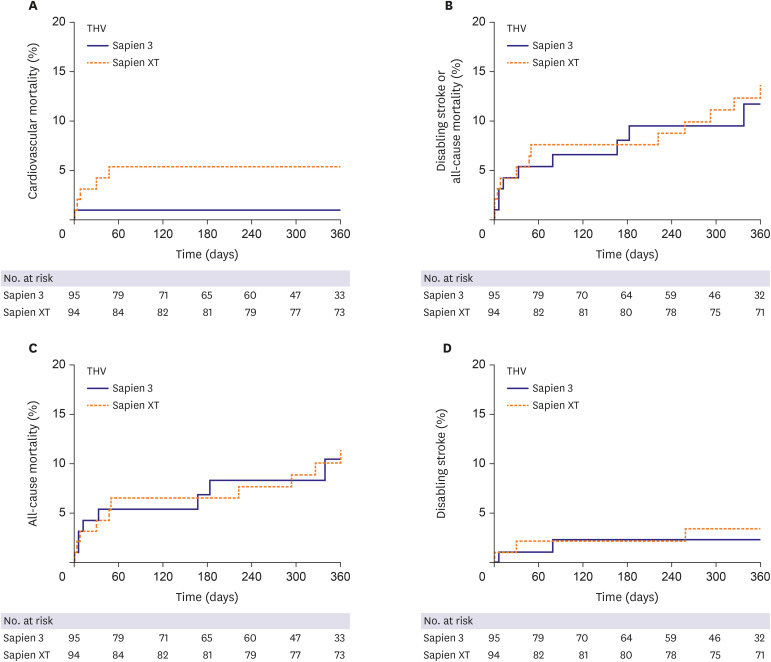
The clinical outcomes and hemodynamic parameters during a median 438 days of follow-up are summarized in Tables 3 and 4. In multivariable Cox regression analysis, there was a trend toward better survival (from cardiovascular mortality) in the S3 group at 1 month (4.3 vs. 1.1%; HR, 0.079; 95% CI, 0.005–1.303; p=0.076) compared to that of the SXT group. This survival rate became statistically higher in the S3 group at 1 year compared to that in the SXT group (5.4% vs. 1.1%; HR, 0.031; 95% CI, 0.001–0.951; p=0.047) (Figure 2A). The results of multivariable Cox regression analysis regarding cardiovascular mortality at 1 year are shown in Supplementary Table 1. Details regarding cases of cardiovascular mortality are shown elsewhere (Supplementary Table 2). There were no significant differences between the S3 and SXT groups in rates of disabling stroke or all-cause mortality at 1 month (SXT 5.4% vs. S3 4.3%; HR, 0.460; 95% CI, 0.094–2.244; p=0.337) or 1 year (13.6% vs. 11.7%; HR, 1.055; 95% CI, 0.376–2.960; p=0.919) (Figure 2B). In addition, the incidence of all-cause mortality (Figure 2C) and disabling stroke (Figure 2D) did not differ between the 2 groups during a median follow up of 438 days. Most patients had no significant symptoms for up to one year after TAVR.
Figure 2. Kaplan-Meier analysis of the cumulative incidence of clinical outcomes according to balloon-expandable TAVR valve generation. (A) The time-to-event Kaplan-Meier curves for the cumulative incidence of cardiovascular mortality. (B) The cumulative incidence of a composite of disabling stroke or all-cause mortality. (C) The cumulative incidence of all-cause mortality. (D) The cumulative incidence of disabling stroke.
TAVR = transcatheter aortic valve replacement.
The results of inverse probability of treatment weighting adjusted analysis and propensity score matching analysis for primary and secondary endpoints are shown in Supplementary Tables 3-6. Unlike the analysis result of multivariable Cox regression analysis, there was no significant association between device generation and cardiovascular mortality at 1 year on the analysis result through inverse probability of treatment weighting adjusted analysis and propensity score matching analysis.
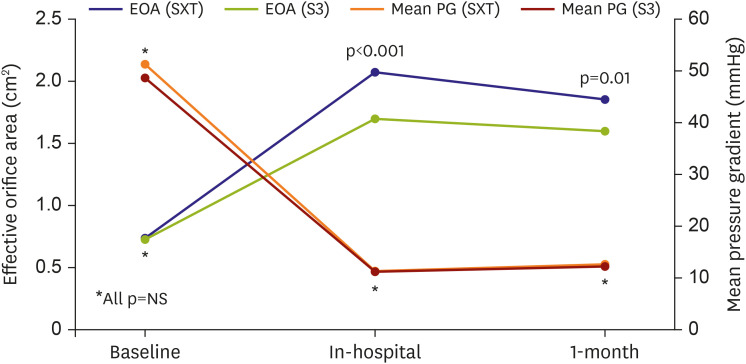
The echocardiographic follow-up data at 1 month were available in 114 of the patients. There was no moderate or severe PVL observed in the S3 group (16.7% vs. 0.0%; p=0.001). The post-TAVR mean pressure gradient and peak velocity were similar between the 2 groups. However, the EOA was smaller in the S3 group than it was in the SXT group (1.86±0.57 vs. 1.60±0.37 cm2; p=0.010) (Figure 3). As the size of THV increased, both EOA and EOAI tended to increase. Most EOA and EOAIs were larger in the SXT group, irrespective of THV size. Notably, in the 29-mm-sized THV subgroup, EOA and EOAI were substantially smaller in the S3 group than the SXT group (Table 4).
Figure 3. Echocardiographic findings regarding the EOA and mean PG in the SXT group and the S3 group.
EOA = effective orifice area; NS = not significant; SXT = Sapien XT; S3 = Sapien 3; PG = pressure gradient.
DISCUSSION
In this study, we compared the procedural and clinical outcomes between the SXT group and the S3 group. The major findings of the present study are as follows: 1) cardiovascular mortality rate at 1 year was significantly lower in the S3 group than it was in the SXT group (5.4% vs. 1.1%; HR, 0.031; 95% CI, 0.001–0.951; p=0.047); 2) TAVR using the S3 THV produced a significantly higher device success rate than did the SXT THV (SXT 90.4% vs. S3 97.9%; p=0.028); 3) the higher device success rate in the S3 group was mainly driven by a reduction in moderate or severe PVL (7.5% vs. 0.0%; p=0.007); 4) the EOA was significantly smaller in the S3 group during the in-hospital period (2.07±0.61 vs. 1.70±0.49 cm2; p<0.001) and at 1 month (1.86±0.57 vs. 1.60±0.37 cm2; p=0.010) than it was in the SXT group; 5) hospital stay (12.1±11.2 days) was longer than in the previously reported K-TAVI registry (7.5±6.1 days), probably due to different study periods (2010–2018 in the present study and 2015–2017 in K-TAVI registry) and difference in severity of patient characteristics (STS PROM≥8, 40.7% in the present study, 34.7% in K-TAVI registry).
TAVR performed using the S3 THV showed a significantly higher device success rate than that of the SXT THV. This success rate was mainly driven by a remarkable reduction in the PVL in the S3 group. These findings are consistent with those of previous reports.13),14) Significant PVL is a well-known, long-term prognostic factor after TAVR implantation.15),16) In the early period of TAVR, the prevalence of moderate to severe PVL was reported to be >10%.17) The risk factors for PVL are not fully understood; however, some of the known contributing factors include prosthesis under-sizing, device malpositioning, and uneven aortic annular and valve leaflet calcifications. Recent studies have shown that 3D-reconstructed computed tomography allows for accurate measurement of aortic root structures for TAVR procedures.18) In addition to the precise measurement of aortic structures and enhanced experience with the TAVR procedure, a polyethylene terephthalate outer skirt of the S3 THV contributed to the remarkable reduction of PVL in this study.
Also, in this study, the rate of pre-TAVR balloon valvuloplasty was much lower in the S3 group than the SXT group (SXT 80.9% vs. S3 41.1%; p<0.001). One explanation for this phenomenon is that balloon sizing was more frequently utilized in the early days of TAVR experience. As 3D-reconstructed computed tomography becomes more precise, the need for pre-TAVR balloon valvuloplasty appears to have diminished.
All TAVR procedures in the S3 group were performed via the transfemoral route, because the low profile of the e-sheath (of the S3) allows for the use of small vessels as the access site. However, there were no differences between the 2 groups with regard to major bleeding event rates or vascular access site complications. Other peri-procedural complications, such as PPM implantation rate, were similar between the 2 groups. Several recent studies have reported that S3 THV was associated with a higher rate of PPM implantation and new-onset intraventricular conduction abnormalities than is SXT, which is in contrast to our results. This discrepancy may be explained by low implantation due to the different foreshortening length of the valve platform, which develops during valve deployment. Based on increasing experience using the S3 TAVR, there does not appear to be a higher incidence of conduction abnormalities than that using the SXT. Also, in our study, PPM implantation rate (1.6%) and incidence of new-onset LBBB (5.3%) after TAVR are lower than those of previous studies (PPM rate in PARTNER trials 3.4–8.5%, CoreValve trials 11.7–25.9%) and of K-TAVI registry data (PPM rate 5.6%, new-onset LBBB data not available) 3),4),7),19),20). One possible explanation for these differences is that the present study only included balloon-expandable TAVR devices, which are known to have lower likelihood of atrioventricular block after TAVR. In K-TAVI registry data, about half of the study population was treated with self-expandable valve. Also, lower incidence of pre-existing bundle branch block (LBBB 1.6%, RBBB 7.9%) than that of a previous study (LBBB 3.0%, RBBB 10.3% in PARTNER 3 trial) may result in lower incidence of new PPM implantation.5) In addition, smaller sizes of THV used in TAVR might contribute to the low incidence of conduction disorder.21) In present study, up to 90% of patients were treated with THV size no greater than 26 mm. Among 10 patients who developed LBBB, 4 had persistent LBBB, while 6 recovered from bundle branch block. This was similar to the results of previous studies, which have shown that more than half of these conduction abnormalities disappear within days to months following TAVR with a balloon-expandable valve.22)
The one-month and one-year clinical outcomes were acceptable and similar to those previously described in high surgical risk patients.23) There was no significant difference in incidence of all-cause mortality or disabling stroke between the 2 groups. This result is in line with previous findings.23) Cardiovascular mortality rate at 1 year was significantly lower in the S3 group than it was in the SXT group. Multivariable Cox regression analysis demonstrated that S3 was the only protective factor for cardiovascular mortality at 1 year (HR, 0.031; 95% CI, 0.001–0.951; p=0.047), while pre-existing atrial fibrillation was a risk factor (HR, 22.933; 95% CI, 2.153–244.270; p=0.009; Supplementary Table 1). The S3 group had a higher incidence of NYHA IV heart failure, Canadian Cardiovascular Society grade IV angina, atrial fibrillation, and multi-vessel coronary artery disease than did the SXT group. The mean STS-PROM score was also numerically higher in the S3 group than it was in the SXT group. Nevertheless, cardiovascular mortality was lower in the S3 group than it was in the SXT group. The difference in cardiovascular mortality occurred within 60 days of the procedure and was maintained for one year (Figure 2A). Possible explanations for this early difference in cardiovascular mortality include cumulative TAVR experience, a higher device success rate, enhanced cooperation of the heart team, and post-procedural management in the S3 group.
On the other hands, inverse probability of treatment weighting adjusted analysis and propensity score matching analysis showed no significant difference in incidence of cardiovascular mortality between the 2 group. The most reliable explanation for these conflicting results between multivariable Cox regression analysis and other analysis methods is believed to be due to the small number of study subjects, large number of covariates to match or adjust, and lack of sufficient clinical events due to relatively short-term follow-up. Therefore, the association between device generation and cardiovascular mortality observed in this study should be reconfirmed in large-scale future studies with longer-term follow-up period.
In present study, the EOA and EOAI after TAVR were significantly smaller in the S3 group than they were in the SXT group. In contrast, body mass index, annular diameter, and size of THV were not different between the 2 groups. One of the best explanations for the differences in EOA and EOAI between 2 groups is the difference in thickness of a polyethylene terephthalate outer skirt of the S3 THV. This finding provides some insight regarding the possibility of a prosthesis-patient mismatch and longevity of newer generation balloon-expandable THV.
Prosthesis-patient mismatches occur when the EOA of the prosthesis is smaller than patient body surface area.24) A smaller EOA may lead to a patient-prosthesis mismatch, which could give rise to hemodynamic alterations and have a negative effect on valve durability. A severe prosthesis-patient mismatch might also impact patient long-term survival and functional recovery.25) Prosthesis-patient mismatches are considered to be severe when EOAI is <0.65 cm2/m2, while an EOAI >0.85 cm2/m2 is not significant.12) One small prospective study in France showed that the S3 group had small EOA and EOAI, and that there was more frequent prosthesis-patient mismatch rate in the S3 group compared to that with SXT.26) Prosthesis-patient mismatch was most frequent in the 23-mm-sized S3 THV in the study.
In this study, the mean EOAI in the S3 group was generally larger than the definition of a severe prosthesis-patient mismatch (in-hospital mean EOAI 1.11±0.34 cm2/m2, 1 month 1.02±0.22 cm2/m2 in the S3 group). However, patients with significant prosthesis-patient mismatch must be carefully monitored for signs of poor valve longevity and functional aggravation. The post-TAVR mean pressure gradient of S3 valve was very low and not different from that of the SXT valve. Following from this, a smaller EOA in the S3 group does not seem to deteriorate hemodynamics after TAVR. Although a smaller EOA is unlikely to affect short-term clinical outcomes, we are unable to predict long-term outcomes due to the absence of relevant data. However, we tolerate the (not clinically significant) small EOA of the S3 group because the S3 group otherwise demonstrated a higher device success rate, lower PVL rate, and lower cardiovascular mortality rate than did the SXT group.
The number of valve-in-valve TAVR procedures for failed bioprosthetic surgical aortic valves has increased with acceptable efficacy and safety.27),28) In valve-in-valve procedures, prosthesis-patient mismatch issues are very important, because this procedure creates a relatively smaller EOA than does conventional TAVR.29) This is particularly true with small valve sizes. Valve-in-valve procedures with SXT may provide a larger EOA than do those with S3. Therefore, patients with a prosthesis-patient mismatch may be better served with valve-in-valve procedures using SXT instead of S3. Therefore, SXT may have a role in this particular setting if PVL risk is not high.
Clinical implications in present study we would like to emphasize are as follows. 1) The benefit of reducing PVL due to the outer skirt may be accompanied by a trade-off of reduced EOA/EOAI. 2) A THV such as SXT without an outer skirt may be advantageous in patients with low risk of PVL, that is, with less calcium in the aortic structure or those undergoing a valve-in-valve procedure. 3) Therefore, we believe that diversifying the portfolio of balloon-expandable valves will help optimize the clinical outcome of various patients with severe AS. For example, in a patient group where the risk of PVL is low and the securing of EOA is important, such as valve-in-valve, THV without outer skirt may be more advantageous.
This study has several limitations. First, it has all of the inherent limitations of a small, retrospective study design. Since the sample size and the number of events in this study were relatively small, all confounding factors could not be adequately corrected. A second limitation is that there was a time difference in the SXT and the S3 eras. Therefore, intra-operator experience between early period and late period could not be corrected adequately and these might have influenced the clinical outcomes. Third, we are lacking data regarding oversizing % and implantation depth of THV.
In conclusion, cardiovascular mortality was lower in the S3 group than in the SXT group in patients with severe symptomatic AS treated with balloon-expandable TAVR over 1 year of follow-up. The S3 THV demonstrated a higher device success rate and lower significant PVL rate than did the SXT THV. The long-term impact of a smaller EOA of S3 THV on clinical outcomes and the longevity of valve function remain to be determined in future studies.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kook H, Joo HJ, Park JH, Hong SJ, Choi SH, Yu CW.
- Data curation: Kook H, Jang DH, Yang KS, Joo HJ, Park JH, Lim DS, Choi SH, Choi YJ, Chang K.
- Formal analysis: Kook H, Yang KS, Chang K.
- Investigation: Jang DH, Lim DS, Choi YJ.
- Methodology: Joo HJ, Hong SJ, Choi YJ.
- Supervision: Park JH, Hong SJ, Lim DS, Choi SH, Chang K.
- Validation: Yu CW.
- Writing - original draft: Kook H, Jang DH, Chang K, Yu CW.
SUPPLEMENTARY MATERIALS
Supplementary methods
The results of multivariable Cox regression analysis regarding cardiovascular mortality at 1 year
Details of cardiovascular mortality in the study population
The results of various analysis models regarding cardiovascular mortality at 1 year
The results of various analysis models regarding composite of disabling stroke or all-cause mortality at 1 year
The results of various analysis models regarding all-cause mortality at 1 year
The results of various analysis models regarding disabling stroke at 1 year
References
- 1.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 5.Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 6.Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 7.Yu CW, Kim WJ, Ahn JM, et al. Trends and outcomes of transcatheter aortic valve implantation (TAVI) in Korea: the results of the first cohort of Korean TAVI registry. Korean Circ J. 2018;48:382–394. doi: 10.4070/kcj.2018.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Belle E, Juthier F, Susen S, et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 registry. Circulation. 2014;129:1415–1427. doi: 10.1161/CIRCULATIONAHA.113.002677. [DOI] [PubMed] [Google Scholar]
- 9.Kazuno Y, Maeno Y, Kawamori H, et al. Comparison of Sapien 3 and Sapien XT transcatheter heart valve stent-frame expansion: evaluation using multi-slice computed tomography. Eur Heart J Cardiovasc Imaging. 2016;17:1054–1062. doi: 10.1093/ehjci/jew032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50:69–76. doi: 10.1016/j.jacc.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Sawa Y, Saito S, Kobayashi J, et al. First clinical trial of a self-expandable transcatheter heart valve in Japan in patients with symptomatic severe aortic stenosis. Circ J. 2014;78:1083–1090. doi: 10.1253/circj.cj-14-0162. [DOI] [PubMed] [Google Scholar]
- 12.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 13.Vendrik J, van Kesteren F, van Mourik MS, et al. Procedural outcome and midterm survival of lower risk transfemoral transcatheter aortic valve implantation patients treated with the Sapien XT or Sapien 3 device. Am J Cardiol. 2018;121:856–861. doi: 10.1016/j.amjcard.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Nijhoff F, Abawi M, Agostoni P, Ramjankhan FZ, Doevendans PA, Stella PR. Transcatheter aortic valve implantation with the new balloon-expandable Sapien 3 versus Sapien XT valve system: a propensity score-matched single-center comparison. Circ Cardiovasc Interv. 2015;8:e002408. doi: 10.1161/CIRCINTERVENTIONS.115.002408. [DOI] [PubMed] [Google Scholar]
- 15.Sinning JM, Hammerstingl C, Vasa-Nicotera M, et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59:1134–1141. doi: 10.1016/j.jacc.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 16.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 17.Lefèvre T, Kappetein AP, Wolner E, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–157. doi: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leipsic J, Gurvitch R, Labounty TM, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging. 2011;4:416–429. doi: 10.1016/j.jcmg.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 20.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad M, Patel JN, Kim M, Baman T, Barzallo M, Mungee S. Larger valve size is associated with permanent pacemaker implantation in edwards Sapien 3™ transcatheter aortic valves. Cureus. 2019;11:e4370. doi: 10.7759/cureus.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bax JJ, Delgado V, Bapat V, et al. Open issues in transcatheter aortic valve implantation. Part 2: procedural issues and outcomes after transcatheter aortic valve implantation. Eur Heart J. 2014;35:2639–2654. doi: 10.1093/eurheartj/ehu257. [DOI] [PubMed] [Google Scholar]
- 23.Binder RK, Stortecky S, Heg D, et al. Procedural results and clinical outcomes of transcatheter aortic valve implantation in Switzerland: an observational cohort study of Sapien 3 versus Sapien XT transcatheter heart valves. Circ Cardiovasc Interv. 2015;8:e002653. doi: 10.1161/CIRCINTERVENTIONS.115.002653. [DOI] [PubMed] [Google Scholar]
- 24.Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation. 1978;58:20–24. doi: 10.1161/01.cir.58.1.20. [DOI] [PubMed] [Google Scholar]
- 25.Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart. 2006;92:1022–1029. doi: 10.1136/hrt.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theron A, Pinto J, Grisoli D, et al. Patient-prosthesis mismatch in new generation trans-catheter heart valves: a propensity score analysis. Eur Heart J Cardiovasc Imaging. 2018;19:225–233. doi: 10.1093/ehjci/jex019. [DOI] [PubMed] [Google Scholar]
- 27.Tuzcu EM, Kapadia SR, Vemulapalli S, et al. Transcatheter aortic valve replacement of failed surgically implanted bioprostheses: the STS/ACC registry. J Am Coll Cardiol. 2018;72:370–382. doi: 10.1016/j.jacc.2018.04.074. [DOI] [PubMed] [Google Scholar]
- 28.Webb JG, Murdoch DJ, Alu MC, et al. 3-year outcomes after valve-in-valve transcatheter aortic valve replacement for degenerated bioprostheses: the PARTNER 2 registry. J Am Coll Cardiol. 2019;73:2647–2655. doi: 10.1016/j.jacc.2019.03.483. [DOI] [PubMed] [Google Scholar]
- 29.Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–170. doi: 10.1001/jama.2014.7246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods
The results of multivariable Cox regression analysis regarding cardiovascular mortality at 1 year
Details of cardiovascular mortality in the study population
The results of various analysis models regarding cardiovascular mortality at 1 year
The results of various analysis models regarding composite of disabling stroke or all-cause mortality at 1 year
The results of various analysis models regarding all-cause mortality at 1 year
The results of various analysis models regarding disabling stroke at 1 year