Abstract
Context:
Contradictory data exist with regard to adjuvant vascular endothelial growth factor receptor (VEGFR)-targeted therapy in surgically managed patients for localized renal cell carcinoma (RCC).
Objective:
To systematically evaluate the current evidence regarding the therapeutic benefit (disease-free survival [DFS] and overall survival [OS]) and grade 3–4 adverse events (AEs) for adjuvant VEGFR-targeted therapy for resected localized RCC.
Evidence acquisition:
A critical review of PubMed/Medline, Embase, and the Cochrane Library in January 2018 according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement was performed. We identified reports and reviewed them according to the Consolidated Standards of Reporting Trials and Standards for the Reporting of Diagnostic Accuracy Studies criteria. Of eight full-text articles that were eligible for inclusion, five studies (two of five were updated analyses) were retained in the final synthesis. Study characteristics were abstracted and the number needed to treat (NNT) per trial was estimated.
Evidence synthesis:
The three randomized controlled phase III trials included the following comparisons: sunitinib versus placebo or sorafenib versus placebo (Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma [ASSURE] study, n = 1943), sunitinib versus placebo (S-TRAC, n = 615), and pazopanib versus placebo (Pazopanib As Adjuvant Therapy in Localized/Locally Advanced RCC After Nephrectomy study, n = 1135). The NNT ranged from 10 (S-TRAC) to 137 (ASSURE study). The pooled analysis showed that VEGFR-targeted therapy was not statistically significantly associated with improved DFS (hazard ratio [HRrandom]: 0.92, 95% confidence interval [CI]: 0.82–1.03, p = 0.16) or OS (HRrandom: 0.98, 95% CI: 0.84–1.15, p = 0.84) compared with the control group. The adjuvant therapy group experienced significantly higher odds of grade 3–4 AEs (ORrandom: 5.89, 95% CI: 4.85–7.15, p < 0.001). In exploratory analyses focusing on patients who started on the full-dose regimen, DFS was improved in patients who received adjuvant therapy (HRrandom: 0.83, 95% CI: 0.73–0.95, p = 0.005).
Conclusions:
This pooled analysis of reported randomized trials did not reveal a statistically significant effect between adjuvant VEGFR-targeted therapy and improved DFS or OS in patients with intermediate/high-risk local or regional fully resected RCC. Improvement in DFS may be more likely with the use of full-dose regimens, pending further results. However, adjuvant treatment was associated with high-grade AEs.
Patient summary:
Vascular endothelial growth factor receptor-targeted therapy after nephrectomy for localized kidney cancer is not associated with consistent improvements in delaying cancer recurrence or prolonging life and comes at the expense of potentially significant side effects.
Keywords: Metastatic renal cell carcinoma, Targeted therapy, Adjuvant therapy, Systemic therapy, Meta-analysis
1. Introduction
Kidney cancer is the 12th most common cancer in the world, with 338 000 new cases diagnosed in 2012. The highest incidence of kidney cancer is in North America and Europe, and the lowest in Africa and Asia [1]. Depending on tumor and patient characteristics at diagnosis, up to 40% of patients with locoregional renal cell carcinoma (RCC) recur after surgery and develop metastasis [2]. Given the increased incidence of patients with locoregional RCC, there is an immediate need to optimize treatment strategies for such patients.
Advances in our understanding of the pathogenesis of clear cell RCC, the most common subtype, such as alterations in the VHL gene driving the upregulation of the hypoxia inducible pathway including vascular endothelial growth factor receptor (VEGFR), prompted the development of targeted therapies against this frequent driver [3]. Since their debut, VEGFR-targeting drugs, such as the tyrosine kinase inhibitors sunitinib, pazopanib, and sorafenib, have demonstrated improved clinical outcomes in the metastatic setting for RCC [3].
Subsequently, studies have been undertaken to evaluate the efficacy of VEGF-directed therapies in patients with locoregional RCC at a high risk of relapse following nephrectomy. To date, three phase III randomized controlled trials (RCTs) [4–6] examining the effect of VEGFR-targeted therapies on disease-free survival (DFS) in patients with resected RCC tumors have been reported. As results of these studies have been inconsistent, we performed a pooled analysis of these studies to better understand the potential impact of adjuvant VEGFR-targeted therapy on patient outcomes and adverse events (AEs).
2. Evidence acquisition
2.1. Search strategy
A systematic PubMed/Medline, Embase, and Cochrane Library search was conducted in January 2018. The complete free-text search terms and the search strategy are detailed in Figure 1. Three studies were retained for the purpose of our pooled analysis [4–6]. Two additional published subset analyses from these studies were also included in the analyses [7,8]. Searches were limited to studies published from 2010 onward. No language restrictions were imposed. The search was complemented by additional sources, including relevant systematic reviews, and cited references from selected studies were also reviewed.
Fig. 1 -.
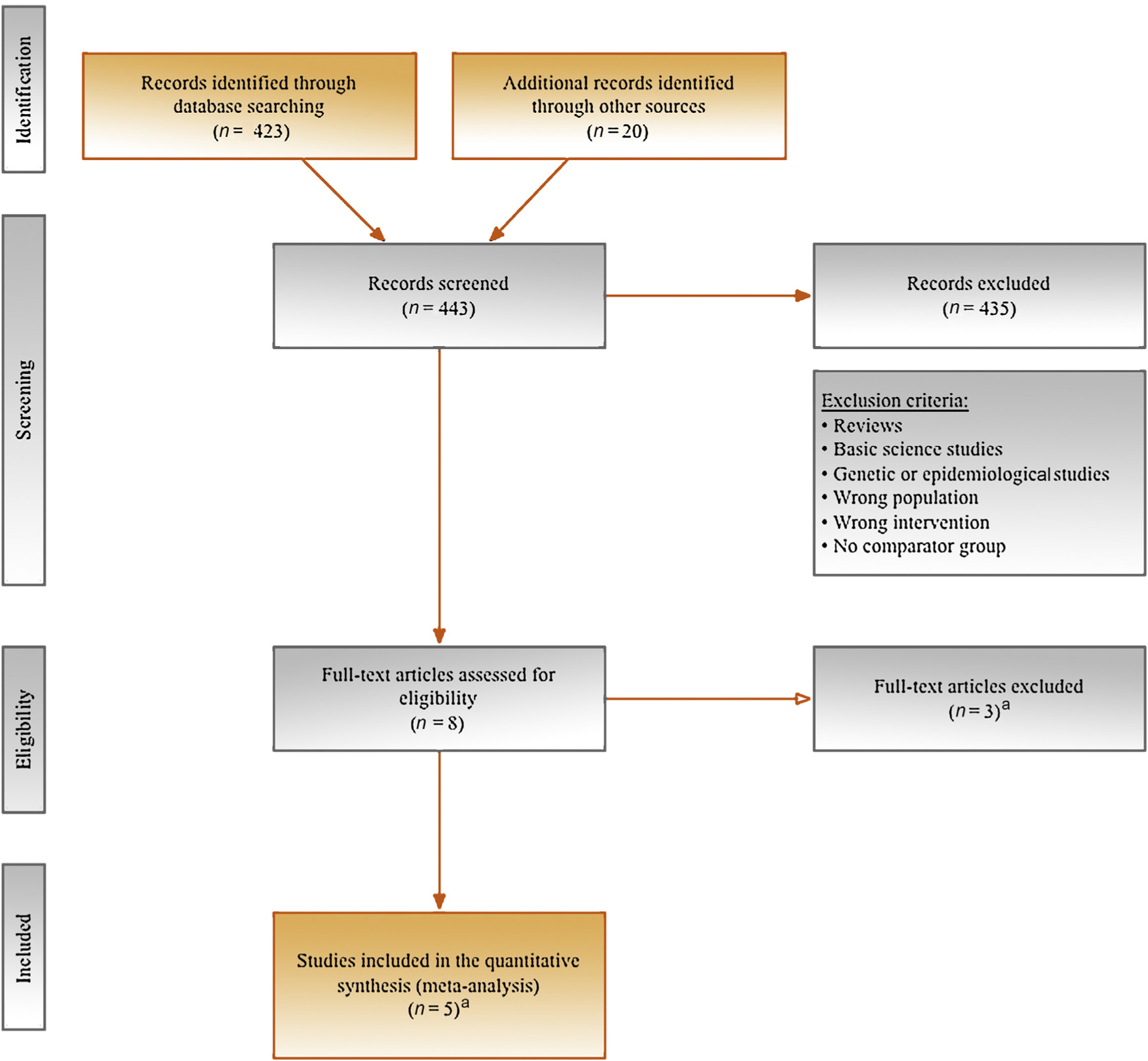
PRISMA flow diagram outlining search strategy and final included and excluded studies. PRISMA = Preferred Reporting Items for Systematic Review and Meta-Analysis. a Reasons for the three full-text articles that were excluded and the five full-text articles that were included are described in the Supplementary material.
2.2. Inclusion criteria
The review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [9]. The search strategy was conducted in accordance with the principles outlined in the Cochrane Handbook for Systematic Reviews of Interventions [10]. Risk of bias (RoB) was performed using the Cochrane Collaboration RoB tool for RCTs [11]. Two coauthors (L.M. and A.B.) screened all abstracts and full-text articles independently. The final list of included studies fulfilled the PICO (problem/patient/population, intervention/indicator, comparison, outcome and optional, time element or type of study) criteria.
2.3. Study eligibility
We defined study eligibility using the patient population, intervention, comparator, outcome, and study design approach [12]. We restricted our inclusion to RCT designs only. Case reports and nonrandomized designs were not permitted. The study population consisted of patients diagnosed with nonmetastatic RCC who received adjuvant VEGFR-targeted therapy or placebo after nephrectomy. Studies with ≤10 participants were not included. Those without a control group were also not considered. We excluded trials in which patients underwent cytoreductive nephrectomy or metastastectomy. Only trials that examined tyrosine kinase inhibitors were included (sunitinib, sorafenib, axitinib, pazopanib, dovitinib, cabozantinib, tivantinib, and erlotinib). Valid eligible comparators included any of the prespecified systemic therapy agents, placebo, or active surveillance.
2.4. Endpoints
The primary endpoints assessed within the current pooled analysis were DFS and overall survival (OS). DFS definitions differed per trial, as noted in the Supplementary material. As a secondary endpoint, we assessed all high-grade (3–4) AEs, which were graded using the National Cancer Institute Common Toxicity Criteria version 3.0 per clinical trial.
2.5. Statistical analysis
Study characteristics were abstracted and the number needed to treat (NNT) per trial was estimated using a previously reported method [13]. The pooled analysis and forest plots were produced using comprehensive meta-analysis. The overall effect was tested using Z scores, with the significance level set at p < 0.05. In the pooled analysis, both random-effect and fixed-effect models are presented. Statistical heterogeneity was quantified using the I2 statistic, and its significance evaluated based on the accompanying Cochran Q test p value (p < 0.10). Given the absence of individual patient data for these trials, we relied on additional methods to obtain the hazard ratio (HR) and associated statistics. Specifically, the log rank of observed minus expected events and the log-rank variance were derived from the number of events and the individual times to event on the intervention arm of each trial. In the case that this was not readily available, the inverse variance approach was used, which may be estimated given the upper and lower bounds of the HRs’ confidence intervals. These details have previously been detailed [14].
Within one study (Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma [ASSURE] study) [4], the same control group (placebo) was compared with different experimental groups (sorafenib and sunitinib), which produced two different HRs. In the overall analyses, we treated the two comparisons from that study as two independent studies, with a two-sided significance level of 2.5% as significant for comparisons between groups and a 5% level for two-way comparisons. However, we also performed sensitivity analyses by inflating the standard error by 20% in the pooled HR estimate [15]. In those analyses, the results did not change (data not shown). Finally, we calculated the statistical power for random-effects pooled analysis [16]. The statistical power was estimated to be adequate for the overall analysis and subanalyses at >90%.
2.6. Exploratory analyses
Additional analyses were performed by repeating the pooled analysis focusing on DFS in two subpopulations. The first comprised patients who started on full dose at trial initiation (ie, 50 mg/d of sunitinib for the ASSURE and S-TRAC studies, 400 mg twice per day of sorafenib for the ASSURE study, and 800 mg/d of pazopanib for the Pazopanib As Adjuvant Therapy in Localized/Locally Advanced RCC After Nephrectomy [PROTECT] study). The second comprised patients who were considered to be at a higher risk of recurrence, which varied according to trial. Specifically, in the ASSURE study, patients were at a higher risk if they had UCLA Integrated Staging System T3 without or with undetermined nodal involvement (pN0 or pNx), Fuhrman grade ≥2, Eastern Cooperative Oncology Group (ECOG) performance status ≥1, T4 tumor or any T stage with nodal involvement, any Fuhrman grade, any ECOG performance status within the S-TRAC study, pT3 or pT4, or node-positive disease. We did not include the PROTECT study in the subanalysis that focused exclusively on higher-risk patients, as the effect of adjuvant therapy in higher-risk populations was not specifically reported, not to mention the contamination of patients with full and reduced doses.
3. Evidence synthesis
3.1. Quantity and quality of evidence
A total of eight eligible full-text manuscripts were identified as of January 1, 2018. Of those, three were excluded because they did not fulfill at least one of the PICO criteria (Supplementary material). The included studies involved the VEGFR-targeted agents sorafenib, sunitinib, or pazopanib, and were placebo controlled [4–6]. In addition, two other studies that published additional analyses were also included in our current review [7,8]. Details of study characteristics and outcomes observed for DFS, OS, and serious AEs are described in Table 1. The estimated NNT ranged from 10 (S-TRAC) to 137 (ASSURE study with sunitinib; Supplementary material). This resulted in the selection of a total of 3693 patients for the meta-analysis. The RoB was considered low across all included studies (Supplementary material).
Table 1 -.
Detailed data of the three randomized controlled trials included in the pooled analysis
| Source | Trial phase | Disease stage | Follow-up(yr) | Additional criteria | Study groups | Treatment design | Dosagea | Median time to DFS | No. of patients | No. of events | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFS | Any death | G3/4 AE | ||||||||||
| Haas et al [4] (ASSURE) | III | Pt1b high-grade N0M0 or N + M0, clear cell and non-clear cell RCC | 4 | ECOG 0–1, normal liver and hematological function; creatinine clearance of >30 ml/min | Exp. | Sunitinib/ sorafenib | Sunitinib: 50 mg/d for the first 28 d of each 6-wk cycle Sorafenib: 400 mg twice per day throughout all cycles | 5.8/6.1 yr | 647/649 | 284/284 | 156/138 | 390/449 |
| Con. | Placebo | 6.6 yr | 647 | 287 | 141 | 159 | ||||||
| Ravaud et al [5] (S-TRAC) | III | Stage III-IV M0 (UISS modified criteria), clear cell RCC | 4 | ECOG 0–2 | Exp. | Sunitinib | 50 mg/d on a 4-wk-on, 2-wk-off schedule for 1 yr | 6.8 yr | 309 | 113 | 67 | 185 |
| Con. | Placebo | 5.6 yr | 306 | 144 | 74 | 59 | ||||||
| Motzer et al [6] (PROTECT) | III | pT2 high-grade, pT3–4 N0M0 or N + M0, clear cell RCC | 5 | KPS ≥80% | Exp. | Pazopanib | Starting 600 mg/d for 1 yr, with optional dose escalation to 800 mg/d after 8–12 wk | 30.4 mo | 571 | 188 | 65 | 338 |
| Con. | Placebo | 30.7 mo | 564 | 203 | 83 | 119 | ||||||
Con. = control; DFS = disease-free survival; ECOG = Eastern Cooperative Oncology Group; Exp. = experimental; G3/4 AE = grade 3/4 adverse events; KPS = Karnofsky performance status; RCC = renal cell carcinoma; UISS = UCLA Integrated Staging System.
Dosage: For ASSURE, given that high rates of toxicity-related discontinuation occurred after 1323 patients had enrolled (treatment discontinued by in 44% (193/438) and 45% (199/441) of sunitinib- and sorafenib-treated patients, respectively), the starting does for each drug was reduced compared with individually titrated up to the original full doses. Specifically, the starting dose was amended to 37.5 mg for sunitinib or 400 mg for sorafenib for the first one to cycles of therapy. For PROTECT, the trial was originally designed with pazopanib 800 mg once daily as the starting dose. An amendment to the protocol was introduced to reduce the starting dose to 600 mg once daily due to a higher than expected treatment discontinuation. This resulted in 568 patients being recruited, which served as the group for primary analysis. Previously, 198 patients received 800 mg as the starting dose, where 53% experienced adverse events and had their dosage reduced, and 51% discontinued treatment. For S-TRAC, dose interruptions occurred in 54% (166/309) of sunitinib patients, while dose reductions (to 37.5 mg/d) occurred in 46% (140/309).
3.2. DFS and OS
In the overall analysis, VEGFR-targeted therapy was not statistically significantly associated with improved DFS compared with the control group (HRrandom: 0.92, 95% confidence interval [CI]: 0.82–1.03, p = 0.16, Fig. 2A). Heterogeneity was present (I2 = 33%, p = 0.22). VEGFR-targeted therapy was not statistically significantly associated with improved OS compared with the control group (HRrandom: 0.98, 95% CI: 0.84–1.15, p = 0.84, Fig. 2B). Heterogeneity was absent (I2 = 21%, p = 0.28).
Fig. 2 -.

Forest plots for (A) meta-analyses of the adjusted effects between adjuvant vascular endothelial growth factor receptor (VEGFR)-targeted therapy and disease-free survival (DFS), (B) overall survival (OS), and (C) high-grade adverse effects (AEs; grade 3–4) in surgically managed patients for localized renal cell carcinoma. All data were pooled using both a fixed- and a random-effect model. CI = confidence interval; ITT = intention to treat.
3.3. Safety
Safety analyses were based on high-grade (grade 3–4) AEs reported within the three trials. Compared with placebo, adjuvant VEGFR-targeted therapy patients experienced higher odds of high-grade AEs (ORrandom: 5.89, 95% CI: 4.85–7.15, p < 0.001, Fig. 2C). Heterogeneity was present (I2 = 50%, p = 0.11). Specific kinds of AEs expected with this class of agents occurred more frequently with adjuvant VEGFR-targeted therapy compared with placebo (Table 2).
Table 2 -.
Pooled odds ratio estimates of serious adverse events (grade III–IV) within three randomized controlled trials of adjuvant targeted therapy for localized renal cell carcinoma
| Study | TKI | OR (95% CI) | p value |
|---|---|---|---|
| Decreased appetite | |||
| ASSURE | Sorafenib | 11.05 (0.61–200.312) | 0.104 |
| ASSURE | Sunitinib | 25.53 (1.51–432.13) | 0.025 |
| PROTECT | Pazopanib | 1.97 (0.18–21.77) | 0.581 |
| S-TRAC | Sunitinib | 5.00 (0.24–104.58) | 0.300 |
| Pooled ORfixed (95% CI) | 6.49 (1.63–25.78) | 0.008 | |
| Pooled ORrandom (95% CI) | 6.49 (1.63–25.78) | 0.008 | |
| Diarrhea | |||
| ASSURE | Sorafenib | 21.13 (6.58–67.81) | <0.001 |
| ASSURE | Sunitinib | 22.87 (7.14–73.26) | <0.001 |
| S-TRAC | Sunitinib | 12.37(1.60–95.71) | 0.016 |
| PROTECT | Pazopanib | 9.93 (3.52–28.01) | <0.001 |
| S-TRAC | Sunitinib | 12.37 (1.60–95.71) | 0.016 |
| Pooled ORfixed (95% CI) | 15.79 (8.53–29.19) | <0.001 | |
| Pooled ORrandom (95% CI) | 15.79 (8.53–29.19) | <0.001 | |
| Dyspepsia | |||
| ASSURE | Sorafenib | 6.03 (0.72–50.22) | 0.097 |
| ASSURE | Sunitinib | 15.37 (2.02–116.71) | 0.008 |
| PROTECT | Pazopanib | NR | - |
| S-TRAC | Sunitinib | 9.06 (0.49–168.99) | 0.140 |
| Pooled ORfixed (95% CI) | 9.67 (2.61–35.84) | 0.001 | |
| Pooled ORrandom (95% CI) | 9.67 (2.61–35.84) | 0.001 | |
| Fatigue | |||
| ASSURE | Sorafenib | 2.41 (1.39–4.17) | 0.002 |
| ASSURE | Sunitinib | 6.82 (4.14–11.26) | <0.001 |
| PROTECT | Pazopanib | 3.24 (1.05–10.01) | 0.041 |
| S-TRAC | Sunitinib | 3.87 (1.27–11.79) | 0.017 |
| Pooled ORfixed (95% CI) | 4.12 (2.94–5.76) | <0.001 | |
| Pooled ORrandom (95% CI) | 3.90 (2.14–4.44) | <0.001 | |
| Headache | |||
| ASSURE | Sorafenib | 6.03 (0.72–50.22) | 0.097 |
| ASSURE | Sunitinib | 8.10 (1.01–64.99) | 0.049 |
| PROTECT | Pazopanib | 1.97 (0.18–21.77) | 0.581 |
| S-TRAC | Sunitinib | 5.00 (0.24–104.58) | 0.300 |
| Pooled ORfixed (95% CI) | 4.94 (1.54–15.88) | 0.007 | |
| Pooled ORrandom (95% CI) | 4.94 (1.54–15.88) | 0.007 | |
| Hand-food syndrome | |||
| ASSURE | Sorafenib | 43.79 (20.41–93.95) | <0.001 |
| ASSURE | Sunitinib | 15.65 (7.20–34.03) | <0.001 |
| PROTECT | Pazopanib | NR | - |
| S-TRAC | Sunitinib | 57.77 (7.92–421.28) | <0.001 |
| Pooled ORfixed (95% CI) | 27.90 (16.50–47.17) | <0.001 | |
| Pooled ORrandom (95% CI) | 29.27 (12.87–66.57) | <0.001 | |
| Hypertension | |||
| ASSURE | Sorafenib | 4.48 (2.86–6.99) | <0.001 |
| ASSURE | Sunitinib | 4.66 (2.99–7.27) | <0.001 |
| PROTECT | Pazopanib | 4.65 (3.17–6.83) | <0.001 |
| S-TRAC | Sunitinib | 2.13 (0.72–6.28) | 0.172 |
| Pooled ORfixed (95% CI) | 4.43 (3.50–5.62) | <0.001 | |
| Pooled ORrandom (95% CI) | 4.43 (3.50–5.62) | <0.001 | |
| Mucositis/stomatitis | |||
| ASSURE | Sorafenib | 14.25 (1.87–108.70) | 0.010 |
| ASSURE | Sunitinib | 26.04 (3.52–192.79) | 0.001 |
| PROTECT | Pazopanib | 4.93 (0.24–102.91) | 0.304 |
| S-TRAC | Sunitinib | 45.86 (2.77–760.62) | 0.008 |
| Pooled ORfixed (95% CI) | 18.35 (5.68–59.29) | <0.001 | |
| Pooled ORrandom (95% CI) | 18.35 (5.68–59.29) | <0.001 | |
| Nausea | |||
| ASSURE | Sorafenib | 8.07 (1.01–64.67) | 0.049 |
| ASSURE | Sunitinib | 23.88 (3.22–177.37) | 0.002 |
| PROTECT | Pazopanib | 4.93 (0.24–102.91) | 0.304 |
| S-TRAC | Sunitinib | 13.17 (0.74–234.87) | 0.079 |
| Pooled ORfixed (95% CI) | 11.904 (3.63–39.06) | <0.001 | |
| Pooled ORrandom (95% CI) | 11.904 (3.63–39.06) | <0.001 | |
| Neutropenia/infection | |||
| ASSURE | Sorafenib | 2.39 (1.20–4.74) | 0.013 |
| ASSURE | Sunitinib | 1.52 (0.73–3.18) | 0.269 |
| PROTECT | Pazopanib | NR | NR |
| S-TRAC | Sunitinib | 57.54 (3.49–948.54) | 0.005 |
| Pooled ORfixed (95% CI) | 2.15 (1.31–3.53) | 0.002 | |
| Pooled ORrandom (95% CI) | 2.80 (0.98–7.97) | 0.054 | |
| Vomiting | |||
| ASSURE | Sorafenib | 3.52 (0.73–17.00) | 0.118 |
| ASSURE | Sunitinib | 7.15 (1.62–31.59) | 0.009 |
| PROTECT | Pazopanib | 0.98 (0.06–15.74) | 0.990 |
| S-TRAC | Sunitinib | 15.25 (0.87–268.21) | 0.063 |
| Pooled ORfixed (95% CI) | 4.76 (1.84–12.30) | 0.001 | |
| Pooled ORrandom (95% CI) | 4.76 (1.842–12.30) | 0.001 | |
CI = confidence interval; NR = not reported; OR = odds ratio; TKI = tyrosine kinase inhibitors.
3.4. Exploratory analyses
We repeated our pooled analysis focusing on (1) DFS in patients who started on the full-dose regimen in all three trials and (2) in patients who were considered at a higher risk of recurrent RCC. In the first set of analyses, patients who started on the full-dose regimen during any of the three trials experienced improved DFS compared with those who received placebo (HRrandom: 0.83, 95% CI: 0.73–0.95, p = 0.005, Fig. 3A). In the second set focusing on patients at a higher risk of recurrent RCC, our analyses did not find a statistically significant difference between adjuvant therapy and placebo (HRrandom: 0.88, 95% CI: 0.77–1.00, p = 0.06, Fig. 3B).
Fig. 3 -.
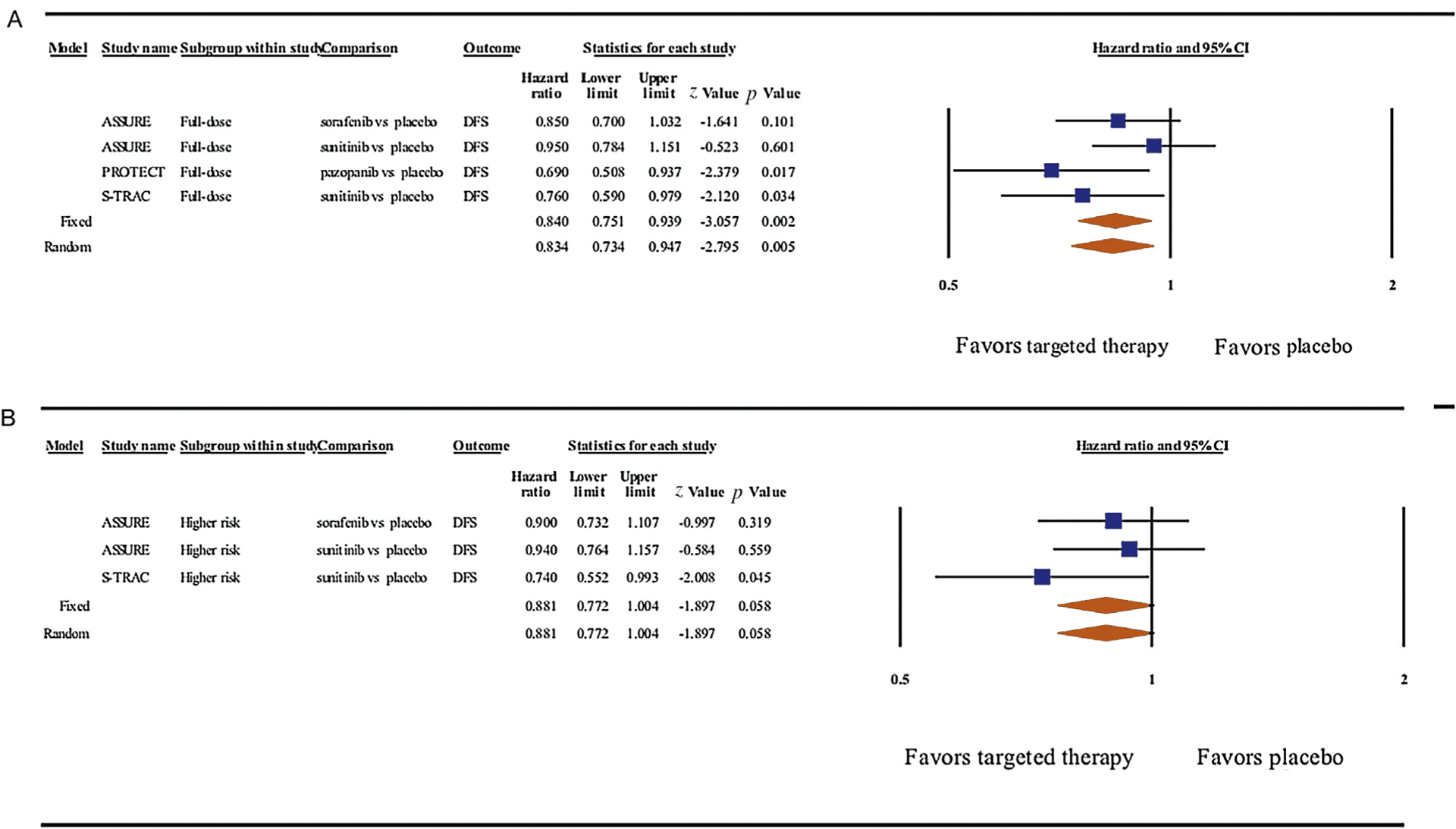
Forest plots for meta-analyses of the adjusted effects between adjuvant vascular endothelial growth factor receptor (VEGFR)-targeted therapy and disease-free survival (DFS) in (A) surgically managed patients for localized renal cell carcinoma who received the full-dose regimen during any of the three trials and (B) those with higher-risk disease. Data were pooled using both a fixed- and a random-effect model. Of note, the PROTECT study was not included in the analyses that pertained to higher-risk patients, as the original report did not specifically address this population. CI = confidence interval.
3.5. Discussion
On November 16, 2017, the Food and Drug Administration (FDA) approved sunitinib for the adjuvant treatment of adult patients at a high risk of recurrent RCC following nephrectomy. This approval was based on a multicenter international double-blind placebo-controlled trial (S-TRAC) in 615 patients with high-risk recurrent RCC following nephrectomy. Individuals were randomized 1:1 to 50 mg sunitinib once daily, 4 wk on treatment followed by 2 wk off, or placebo. Median DFS for sunitinib and placebo was 6.8 versus 5.6 yr (HR: 0.76, 95% CI: 0.59–0.98, p = 0.03).
DFS benefit was not found in the other two studies of adjuvant VEGFR-targeted therapy reported to date. The ASSURE study of 1943 patients with RCC at intermediate or high risk of relapse did not demonstrate improvement in DFS or OS with 1 yr of adjuvant sunitinib or sorafenib compared with placebo [4,7]. The PROTECT study evaluated the efficacy of pazopanib as an adjuvant therapy for patients with locally advanced RCC at a high risk of relapse after surgery [6]. Initially, the study was designed with pazopanib 800 mg once daily as the starting dose. The primary objective of the study had to be amended to examine DFS in a cohort that received a reduced dose of pazopanib (600 mg) due to toxicity attrition. In the 600 mg cohort, a DFS benefit for pazopanib over placebo was not observed. Although a DFS benefit was observed in the sustained 800 mg dose cohort, it was not considered tolerable. Three other ongoing RCTs are evaluating the clinical benefit of adjuvant targeted therapies including SORCE (adjuvant sorafenib vs placebo; NCT00492258), EVEREST (adjuvant everolimus vs placebo; NCT01120249), and ATLAS (adjuvant axitinib vs placebo; NCT01599754).
Numerous reports have sought to characterize potential reasons why the S-TRAC study found a benefit, whereas the other two did not [17–19]. In an effort to consolidate the inconsistent findings of the studies reported thus far, we performed a pooled analysis of the three completed RCTs focusing on DFS and OS as the primary endpoints, as well as high-grade AEs as a secondary endpoint. The pooled analysis did not detect a statistically significant improvement in DFS or OS with adjuvant VEGFR-targeted therapy. Not unexpectedly, high-grade AEs were more frequent in those treated with adjuvant VEGFR-targeted therapy than controls.
Earlier generation trials of adjuvant therapy in RCC have studied cytokines, combined cytokines and chemotherapy, adoptive immunotherapy, vaccines, and monoclonal anti-bodies, none of which has demonstrated improvement in patient outcome compared with surgery alone [20]. While these results are not surprising given the minimal effectiveness of older therapies in treating metastatic disease, it was hoped that the significant benefit of VEGFR-targeted therapy in the metastatic setting would translate into meaningful clinical benefit when administrated in the adjuvant setting. The lack of dramatic effect of VEGFR-targeted therapy in the context of nonmetastatic RCC may align with the classical angiogenic switch model [21]. Blood-vessel formation will begin and continue as long as the tumor grows to a certain size, and only large tumor nodules may contain topological areas that are dependent on neoangiogenesis and confer sensitivity to VEGF-targeting agents. Consequently, the microscopic disease being targeted in the adjuvant setting may not be as susceptible or it may be simply that the mechanism of these drugs leads to disease stabilization or slowing of growth (cytostatic effect) rather than elimination (cytotoxic effect). When used in the adjuvant setting for a finite time period, this mechanism could result in a delay in radiographic progression.
Our exploratory pooled analysis evaluating the effects of dose intensity revealed that patients who started at full doses could experience improved DFS compared with placebo. This result could be interpreted as an argument in favor of adjuvant therapy for localized RCC, as it suggests that by sustaining the full-dose regimen, there is the possibility that this treatment strategy prolongs DFS. That interpretation, however, is not fully well understood as a recent post hoc analysis showed that within the PROTECT study, those achieving early (pazopanib exposure steady-state blood trough concentrations at week 3 or 5) or late Ctrough (at week 16 or 20) of >20.5 μg/ml had significantly longer DFS (not estimable) versus 29.5 mo, and not estimable versus 29.9 mo (both p ≤ 0.008), respectively [22]. Yet, dose was not correlated with higher Ctrough. This implies that potential benefit of adjuvant therapy is not completely driven by the starting dose of the drug but rather by blood concentration levels. A future pooled analysis with data on exposure as opposed to dosing and pharmacodynamics may be worthwhile.
In any case, an important clinical consideration is whether in the real-world setting full doses are unlikely to be tolerable and whether the majority of patients will receive a sustained full-dose adjuvant VEGFR-targeted therapy following nephrectomy. Notably, starting dose reductions were required for two of the three trials to address toxicity issues, and all three studies demonstrated marked amounts of drug discontinuations for drug-related toxicity (28–45%, regardless of dosing). Within the PROTECT study, the study protocol had to be amended from 800 to 600 mg of pazopanib due to intolerance and toxicity attrition. While full dosing of drug (800 mg of pazopanib) was associated with improved DFS efficacy, only 198 patients were included in that subanalysis, representing approximately one-third of the overall study group. Similarly, in the ASSURE study, the treatment regimen had to be amended to address toxicity issues for both sunitinib (from 50 to 37.5 mg) and sorafenib (from 400 mg twice per day to 200 mg twice per day). Although the reduction in starting dose ameliorated the toxicities, the proportion of patients who suffered a high-grade AE among those who started at a reduced dosage still exceeded 55% with both sunitinib and sorafenib. Contrary to positive DFS findings in the PROTECT full-dose cohort, post hoc subset analyses evaluating dose intensity in the ASSURE study did not reveal differences in DFS or OS outcomes [7].
While the mid-trial dose reductions may have obscured the efficacy of adjuvant therapy in delaying radiographic progression, it may also be that any DFS efficacy may be too costly in terms of toxicity in the majority of patients. Using the S-TRAC results as an example, we determine here that, in the best-case scenario, the estimated number of patients needed to receive adjuvant therapy for one patient to experience a benefit in DFS is 10. Thus, we would need to subject nine individuals to the toxicity of VEGFR-targeted therapy for a single patient to benefit from a gain in DFS and without a definite OS benefit. Such quantitative observations are imperative to consider in the real-world clinical setting, potentially causing more harm than benefit and decreased quality of life. The balance between potential therapeutic benefit and associated toxicity and effects on quality of life with adjuvant VEGFR-targeted therapy is complex. The question remains whether clinicians and patients are willing to accept the toxicity while sustaining the full-dose effect, or continue treatment at a lower dose, but without the proven efficacy.
Another point of consideration is the relevancy of DFS as a primary endpoint in adjuvant studies, and whether an increase in DFS without a corresponding OS benefit should contribute to the decision to use these agents. This issue of whether DFS is a good surrogate for OS was recently investigated in a meta-analysis of 13 RCTs of adjuvant therapies in RCC, where only a moderate correlation between 5-yr DFS and OS rates was found [23]. Our pooled analysis currently failed to show superiority for OS in any of the studies, either individually or together. Although the follow-up time may be immature, some have argued that it will unlikely change in the future [18].
The results of this pooled analysis put into question whether routine use of adjuvant VEGFR-targeted therapy should be considered in fully resected RCC. Indeed, while the FDA has approved the use of adjuvant sunitinib for RCC, the European Medical Agency has recommended against such practice. Going forward, there are three important points to consider: (1) there remains the remote possibility that a longer follow-up will result in an effect on OS with adjuvant targeted therapy; (2) in addition to adjuvant VEGFR-targeted therapy studies, the role of mTOR pathway inhibition and immune checkpoint inhibition in the adjuvant setting is being evaluated; (3) the efficacy of immune checkpoint inhibition with anti-PD-1, PD-L1, and CTLA4 in metastatic RCC has generated enthusiasm for the potential utility of such therapies in the adjuvant setting [24]. Currently, five phase III RCTs examining the effect of immunotherapy in the adjuvant setting for locoregional high-risk RCC are ongoing (EA8143 PROSPER: NCT03055013; IMmotion 010:NCT03024996; KEYNOTE-564: NCT03142334; RAMPART: NCT03142334; and Check-Mate 914: NCT03138512).
The current report is not without limitations. First, there were only three studies to base our pooled analysis on, thereby reducing the generalizability of the results. Second, the studies contained a certain degree of heterogeneity. Heterogeneity across studies can be problematic for pooled analyses, beyond statistical interpretation. For example, the ASSURE study [4,7] included 21% non-clear cell RCC and the definition of high-risk patients differed across all three trials. Furthermore, the ASSURE study included lower stage (pT1b) albeit high-grade disease, which may have compromised the ability to detect a benefit of adjuvant therapy, as such tumors are less likely to recur. However, our exploratory analysis focusing on patients with higher-risk recurrent RCC failed to detect a difference for adjuvant therapy relative to placebo. In relation to that, it would have been noteworthy to compare adjuvant treatment with controls in patients with higher-risk RCC and/or sustained full dose. However, not all the three trials reported their results for this subgroup. Third, the ASSURE study includes two comparisons with the same control group (ie, the placebo group was the same for both sunitinib and sorafenib). From the statistical standpoint, the two estimates are presumed to be independent of each other, and therefore the point estimate computed across comparisons will likely be small, and the confidence intervals too narrow. To account for this, we have performed sensitivity analyses by artificially inflating the standard error by 20% to see how point estimates vary. The results did not change, and lack of statistical significance for adjuvant targeted therapy versus placebo remained in place despite this adjustment. Fourth, while the random-effect model was preferred, caution should be warranted given the small number of studies included in the meta-analysis (k = 3). Indeed when the number of studies is very small, it may be impossible to estimate the between-studies variance (tau square) with precision [15]. To address this, we also included results obtained by adopting a fixed-effect model. The results did not differ between these two approaches. Finally, the current study was not an individual patient data-based meta-analysis.
4. Conclusions
This pooled analysis of reported randomized trials did not reveal a statistically significant effect between adjuvant VEGFR-targeted therapy and improved DFS or OS in patients with intermediate/high-risk local or regional fully resected RCC. Improvement in DFS may be more likely with the use of full-dose regimens, pending further results. However, adjuvant treatment was associated with high-grade AEs. Our recommendation is that the existing randomized data do not collectively justify the use of routine adjuvant VEGFR-targeted therapy in resected RCC, pending the results of ongoing trials. Shared decision making between the patient and the physician should always be undertaken in considering adjuvant VEGFR-targeted therapy for patients with high-risk disease. The best potential treatment option continues to include a clinical trial in this setting.
Supplementary Material
Financial disclosures:
Maxine Sun certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Bernard Escudier receives honoraria from Pfizer, Novartis, Bayer, Bristol-Myers Squibb, Ipsen, Exelixis, and Genentech; holds a consulting or advisory role for Bayer, Novartis, Pfizer, Exelixis, Bristol-Myers Squibb, and Ipsen; and has received travel and accommodation expenses from Novartis, Bristol-Myers Squibb, and Pfizer. Naomi B. Haas holds a consulting or advisory role for Cerulean Pharma, Exelixis, Pfizer, and Novartis; has been an expert testimony for Eli Lilly (I); and possesses stock or other ownership in Tetralogic. Lauren C. Harshman is the principal investigator/study chair of the National Clinical Trials Network PROSPER EA8143 study. She also has received research grants to her institution from, acted as a paid member of the advisory board for, and received travel expenses from Bayer; received research grants to her institution from and acted as a paid member of the advisory board for Genentech, Dendreon/Valeant, Pfizer, Medivation/Astellas, and Merck; acted as a paid member of the advisory board for Kew Group, Exelixis, and Corvus; acted in an uncompensated advisory role for Theragene Pharmaceuticals; received research grants to her institution from Bristol-Myers Squib, Sotio, Janssen, and Takeda; received fees for Continuing Medical Education from PER and Applied Clinical Education; and received travel fees from Sanofi for work performed outside of the current study. David I. Quinn reports personal fees from Exelixis, Genentech, Pfizer, Bayer, Peleton, and Merck outside the submitted work. James Larkin has received research funding from Pfizer, Merck Sharp & Dohme, Novartis, and Bristol-Myers Squibb, and travel or accommodations expenses paid by Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Eisai, GlaxoSmithKline, and Roche. Christopher W. Ryan reports personal fees from Pfizer and Genentech, and a research grant to institution from Onyx. Sumanta Pal receives honoraria from Novartis, Medivation, and Astellas Pharma; holds a consulting or advisory role for Pfizer, Novartis, AVEO, Myriad Pharmaceuticals, Genentech, Exelixis, Bristol-Myers Squibb, Astellas Pharma, Ipsen, and Eisai; and has received research funding from Medivation. Cora N. Sternberg received honoraria from Pfizer, Bristol-Myers Squibb, Novartis, Janssen, Bayer HealthCare Pharmaceuticals, Astellas Pharma, Sanofi, Eisai, Ipsen, GlaxoSmithKline, Merck Sharp & Dohme, and Eli Lilly, and has received research funding from Exelixis (Inst). Tim Eisen is employed by AstraZeneca and reports grants from Bayer, grants and personal fees from Pfizer, and personal fees from GlaxoSmithKline, Novartis, and Bristol-Myers Squibb. Thomas Powles is a company consultant for Novartis, Pfizer, and GlaxoSmithKline; has received company speaker honoraria from Novartis, Pfizer, GlaxoSmithKline, and Genentech; has participated in trials for GlaxoSmithKline, Pfizer, BMS, Genentech, and Genetech; and has received grants/research support from GlaxoSmithKline, Pfizer, and Novartis. Toni K. Choueiri declares receiving fees for consulting and for serving on advisory boards from GSK, Novartis, Pfizer, Merck, AstraZeneca, Bayer, Genentech, Exelixis, Eisai, Cerulean, Foundation Medicine Inc., Corvus, and Prometheus, and grant support through his institution from BMS, GSK, Novartis, Exelixis, Pfizer, Merck, Roche, AstraZeneca, TRACON Pharmaceuticals, and Peloton. Axel Bex has received company speaker honoraria from Pfizer; has participated in trials for Pfizer Europe; has participated in advisory boards for GlaxoSmithKline and Novartis; is a company consultant for Pfizer and Novartis; and has received grants/research support from Pfizer. All other authors declare no competing interests.
Funding/Support and role of the sponsor: None.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, athttps://doi.org/10.1016/j.eururo.2018.05.002.
References
- [1].Ferlay J, Soerjomataram I, Ervik M, et al. In: Cancer IAfRo, editor Cancer incidence and mortality worldwide: IARC Cancer Base No. 11 Lyon, France: IARC; 2014. [Google Scholar]
- [2].Janzen N, Kim H, Figlin R, Belldegrun A. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and managementof recurrent disease Urol Clin North Am 2003;30:843–52. [DOI] [PubMed] [Google Scholar]
- [3].Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017;376:354–66. [DOI] [PubMed] [Google Scholar]
- [4].Haas N, Manola J, Uzzo R, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387:2008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016;375:2246–54. [DOI] [PubMed] [Google Scholar]
- [6].Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol 2017;35:3916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Haas NB, Manola J, Dutcher JP, et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of ASSURE randomized trial. JAMA Oncol 2017;3:1249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Motzer RJ, Ravaud A, Patard J, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol 2017;73:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [10].Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- [11].Higgins J, Altman D, Cotzsche P, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [13].Altman D, Andersen P. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].TierneyJ, Stewart L, Ghersi D, Burdett S, Sydes M Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:2815–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Borenstein M, Hedges L, Higgins J, Rothstein H. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- [16].Hedges L, Pigott T. The power of statistical tests in meta-analysis. Psychol Methods 2001;6:203–17. [PubMed] [Google Scholar]
- [17].Casuscelli J, Hsieh J. Are we ready for adjuvant sunitinib in high-risk renal cell carcinoma? Eur Urol 2018;73:69–70. [DOI] [PubMed] [Google Scholar]
- [18].Gyawali B, Goldstein D. The US Food and Drug Administration’s approval of adjuvant sunitinib for renal cell cancer. A case of regulatory capture? JAMA Oncol 2018;4:623–4. [DOI] [PubMed] [Google Scholar]
- [19].Bex A, Albiges L, Ljunberg B, et al. Updated European Association of Urology guidelines regarding adjuvant therapy for renal cell carcinoma. Eur Urol 2017;71:719–22. [DOI] [PubMed] [Google Scholar]
- [20].Figlin RA, Leibovich BC, Stewart G, Negrier S. Adjuvant therapy in renal cell carcinoma: does higher risk for recurrence improve the chance for success? Ann Oncol 2018;29:324–31. [DOI] [PubMed] [Google Scholar]
- [21].Bergers G, Benjamin G. Angiogenesis: tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003;3:401–10. [DOI] [PubMed] [Google Scholar]
- [22].Sternberg CN, Donskov F, Haas NB, et al. Pazopanib exposure relationship with clinical efficacy and safety in the adjuvant treatment of advanced renal cell carcinoma. Clin Cancer Res. In press. 10.1158/1078-0432.CCR-17-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harshman LC, Xie W, Moreira RB, et al. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: a trial-level meta-analysis. Cancer 2018;124:925–33. [DOI] [PubMed] [Google Scholar]
- [24].Motzer RJ, Escudier B, McDermott D, et al. Nivolumab versus ever-olimus in advanced renal cell carcinoma. N Engl J Med 2015; 373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


