Abstract
The targeted alpha therapy radium-223 (223Ra) can prolong survival in men with castration-resistant prostate cancer (CRPC) who have symptomatic bone metastases and no known visceral metastases. Preclinical studies demonstrate that 223Ra preferentially incorporates into newly formed bone matrix within osteoblastic metastatic lesions. The emitted high-energy alpha particles induce DNA double-strand breaks that might be irreparable and lead to cell death in nearby exposed tumour cells, osteoblasts and osteoclasts. Consequently, tumour growth and abnormal bone formation are inhibited by these direct effects and by the disruption of positive-feedback loops between tumour cells and the bone microenvironment. 223Ra might also modulate immune responses within the bone. The clinical utility of 223Ra has encouraged the development of other anticancer targeted alpha therapies. A thorough understanding of the mechanism of action could inform the design of new combinatorial treatment strategies that might be more efficacious than monotherapy. On the basis of the current mechanistic knowledge and potential clinical benefits, combination therapies of 223Ra with microtubule-stabilizing cytotoxic drugs and agents targeting the androgen receptor axis, immune checkpoint receptors or DNA damage response proteins are being explored in patients with CRPC and metastatic bone disease.
Prostate cancer is the second most common cancer in men, with 1.3 million new diagnoses worldwide in 2018 (REF.1), and is the fifth leading cause of cancer death globally2. Age is a major risk factor for prostate cancer, and most diagnoses are made in men aged >65 years3. The incidence of prostate cancer has increased globally by 40% since 2006, owing to an increased number of diagnoses because of prostate-specific antigen (PSA) screening4. An increasingly ageing population also contributes to rising prostate cancer rates across the world4.
The distribution of metastatic prostate cancer is primarily to bone. Specifically, the bones of the axial skeleton, which contains the body’s bone marrow, are most commonly involved. Treatment of newly diagnosed metastatic disease involves androgen deprivation therapy (ADT), for example, gonadotropin-releasing hormone agonists and antagonists, given either alone or in combination with a next-generation agent, such as abiraterone, that targets the androgen receptor (AR) signalling axis, or the chemotherapeutic agent docetaxel5–7. Despite an initial response to this treatment, the disease eventually progresses to metastatic castration-resistant prostate cancer (mCRPC). Various treatments improve survival in men with mCRPC, including cytotoxic therapies, AR-directed therapies, an immune checkpoint inhibitor for patients with microsatellite instability (MSI)-high or DNA mismatch repair-deficient tumours, targeted alpha-particle therapy (TAT) and cellular adoptive immunotherapy8–20.
Radium-223 (223Ra), an alpha-particle-emitting radionuclide approved for patients with mCRPC and bone metastases by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA)21,22, prolongs overall survival (median 14.9 versus 11.3 months receiving placebo; HR 0.70, 95% CI 0.58–0.83; P < 0.001) in men with mCRPC18. 223Ra is incorporated into newly formed abnormal bone within metastatic lesions23,24. Because alpha emitters efficiently and lethally break DNA, 223Ra might be less susceptible to the development of resistance mechanisms observed with other anticancer therapies, such as chemotherapy and pathway-directed therapy, against which multiple escape and resistance mechanisms exist24–26. 223Ra is also under investigation in other cancers that metastasize to bone23,27–37.
In this Review, we summarize the mode of action of 223Ra and discuss new preclinical studies aimed at elucidating the mechanism through which 223Ra exerts its biological effects. Our current understanding of this mechanism and those of other anticancer agents generates hypotheses for potential 223Ra combination therapies in patients with bone metastases, which are now being tested in clinical trials. We also discuss future perspectives for TAT to improve outcomes for patients with bone-predominant disease.
Clinical experience with 223Ra
Clinical studies.
In the ALSYMPCA (ALpharadin in SYMPtomatic Prostate CAncer) trial, patients with mCRPC and symptomatic bone metastases were treated with the best standard of care and either six injections of 223Ra at 55 kBq/kg every 4 weeks or placebo18. Patients in the 223Ra arm had longer overall survival (median 14.9 versus 11.3 months; HR 0.70, 95% CI 0.58–0.83; P < 0.001) and a longer time to first symptomatic skeletal event (SSE) (median 15.6 versus 9.8 months; HR 0.66, 95% CI 0.52–0.83; P < 0.001) than those in the placebo arm18,38. The short-term and long-term safety profiles were favourable, with improvements in quality-of-life measures and low rates of myelosuppression18,39,40. Subsequent analyses have not demonstrated that more doses or higher doses of 223Ra confer an additional benefit to patients with mCRPC41. The ALSYMPCA trial led to the approval of 223Ra for patients with mCRPC and bone metastases by the FDA in 2013 (REF.21) and the EMA in 2018 (REF.22). Subsequently, in 2018, the EMA restricted the use of 223Ra to exclude combination with abiraterone acetate and prednisone or prednisolone owing to increased rates of death and skeletal fractures in the ERA-223 trial and recommended 223Ra for patients who have had at least two previous treatments and who cannot receive other treatments42,43.
The National Comprehensive Cancer Network (NCCN) guidelines recommend treatment with 223Ra in patients with mCRPC, symptomatic bone metastases and no metastases in the viscera44. The American Urological Association (AUA) guidelines suggest offering 223Ra to patients with mCRPC and symptomatic bone metastases, good performance status and no previous docetaxel chemotherapy or known visceral disease45. The European Association of Urology (EAU) recommends 223Ra as one of the life-prolonging treatment options for patients with mCRPC and progression following docetaxel chemotherapy46.
Long-term safety.
The use of any radiopharmaceutical engenders concerns about long-term potential toxicities, such as effects on marrow integrity and viability, as well as the potential inception of second malignancies. Long-term follow-up monitoring of men with mCRPC and symptomatic bone metastases in the ALSYMPCA trial evaluated the safety of 223Ra treatment for up to 3 years from the first injection47. The 3-year follow-up analysis did not find an association between 223Ra treatment and occurrence of second malignancies, such as acute myelogenous leukaemia, myelodysplastic syndrome or new primary bone cancer. Non-treatment-related malignancies occurred at a low incidence at a similar rate in patients treated with 223Ra or placebo47. 223Ra was associated with a low incidence of myelosuppression and low cumulative incidence rates for haematological and non-haematological adverse events47.
REASSURE is an ongoing global, prospective, single-arm, observational study of patients with mCRPC and bone metastases who receive 223Ra in routine clinical practice48. The objective of this study is to evaluate long-term safety and the occurrence of primary second malignancies during a 7-year follow-up period. At this time, the rate and degree of severity of treatment-emergent adverse events revealed by REASSURE48 or follow-up data from ALSYMPCA47 are not substantially different from those observed in the original ALSYMPCA trial18. The primary safety concern raised by 223Ra regards the use of the drug with novel AR-targeted drugs such as abiraterone for first-line mCRPC, which is associated with bone fractures.
Mode of action of 223Ra
Characteristics of bone metastases.
The health and structural integrity of normal bone is maintained by a balanced, continuous cycle of bone resorption by osteoclasts and bone formation by osteoblasts49. Metastatic prostate cancer cells interact, via signalling pathways, with different cellular components of the multiple complex bone microenvironments (FIG. 1). Bone metastases are established through complex mechanisms involving interactions with osteoblasts, osteocytes, and osteoclasts, as well as bone marrow stem cells and haematopoietic cells, as demonstrated in preclinical models and clinical studies49–51. Growth factors, such as bone morphogenetic proteins, transforming growth factor-β, insulin-like growth factor 1, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and endothelin-1 (REF.52), released by prostate cancer cells in the bone, disrupt the tightly regulated crosstalk between osteoblasts and osteoclasts and dysregulate their activity, leading to an abundance of new disorganized (woven) bone. In turn, osteoblasts express growth factors, including VEGF, monocyte chemoattractant protein 1, interleukin-6 and interleukin-8 (REF.53), that promote prostate cancer cell growth and survival. These bidirectional positive-feedback loops among tumour cells, osteoblasts and osteoclasts and the effects on the bone matrix constitute a vicious cycle of osteoblastic bone metastasis, characteristic of prostate cancer50,51 (FIG. 1). Prostate cancer cell proliferation within the bone is further promoted by osteoblast-mediated intratumoural steroidogenesis54 and bone-marrow-induced activation of PDGF receptor-α through a ligand-independent mechanism55, whereas physical contact between tum our cells and osteoblasts can disrupt osteoblast alignment and bone organization through heterotypic cell-cell adhesions and gap junctions56. Dysregulated bone metabolism in terms of resorption and deposition leads to pathological bone morphology and physiology, which account for considerable morbidity, bone fracture, pain and ultimately death57,58.
Fig. 1 |. 223Ra and prostate cancer bone metastases.
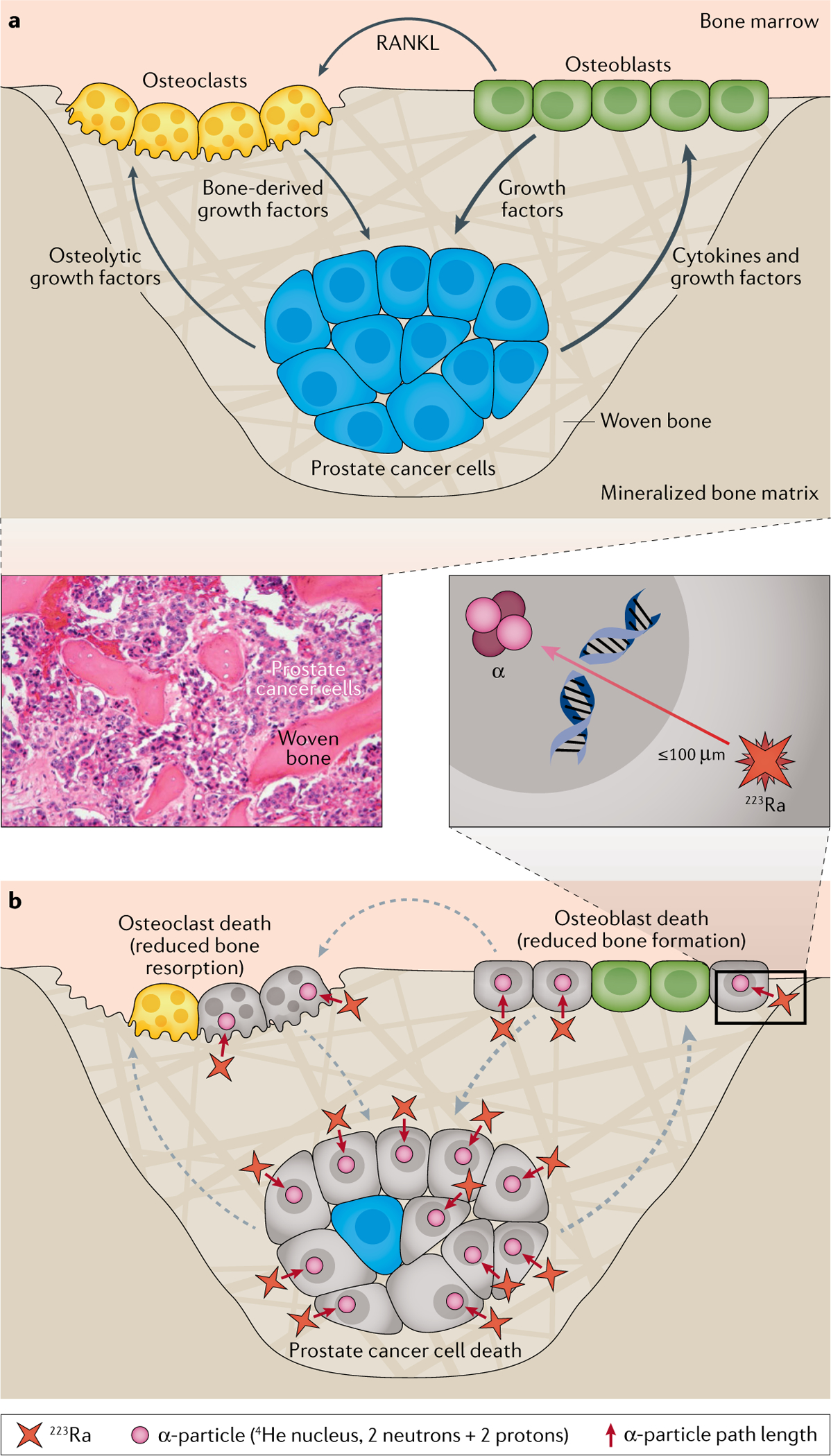
a | Vicious cycle of osteoblastic prostate cancer bone metastases. Growth factors released by prostate cancer cells in bone disrupt the tightly regulated osteoblast and osteoclast activity that is essential for normal bone integrity. Prostate cancer bone metastases mainly promote osteoblast activity, which stimulates osteoclast activity through the secretion of RANKL. In turn, osteoblasts express growth factors that promote prostate cancer cell growth and survival, resulting in a bidirectional positive-feedback loop between tumour cells and osteoblasts. In response to RANKL secreted by osteoblasts, activated osteoclasts release growth factors from the bone and further stimulate tumour growth, leading to the growth of new disorganized (woven) bone.
b | Mechanism of action of radium-223 (223Ra). 223Ra substitutes for calcium in hydroxyapatite complexes in woven bone surrounding prostate cancer metastatic lesions. 223Ra emits high-energy alpha particles over a range of ≤100μm. The alpha radiation leads to localized cytotoxicity through the induction of DNA double-strand breaks in adjacent tumour cells, osteoblasts and osteoclasts, which disrupts the positive-feedback loops between these cells, leading to inhibition of tumour growth and pathological bone reaction and a stabilization of the normal structure. Adapted from REF.24 by permission from the American Association for Cancer Research: Suominen, M. I. et al. Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone microenvironment in mouse models. Clin. Cancer Res. (2017) 23, 15, 4335–4346.
Evidence from preclinical models.
Animal models of metastatic prostate cancer to bone are notoriously difficult to develop owing to the complexity of the metastatic cascade, which requires recapitulation of each step of the metastatic development, tumour heterogeneity and immunological processes59. Thus, preclinical models aimed at investigating the mechanism of action and treatment effects of 223Ra were developed and published in 2017 (REF.24), well after the results of initial clinical trials of 223Ra became available, and even after the drug was approved by the FDA in 2013 following the pivotal randomized phase III ALSYMPCA trial18,21. Preclinical studies with bone metastasis models of cancers other than prostate cancer demonstrated that 223Ra substituted for calcium in hydroxyapatite complexes within areas of osteoblast-mediated new bone formation at sites of metastatic lesions23,25.
223Ra has a half-life of 11.4 days and decays to produce predominantly alpha particles that have a high linear energy transfer (LET) over a short range (100 μM)60. This action leads to localized cytotoxicity, possibly through DNA double-strand breaks in adjacent cells25,26,61 (FIG. 1). The mode of action of 223Ra was investigated in an intratibial murine xenograft model of osteoblastic bone metastasis using patient-derived LuCaP-58 cells, in which mice bearing LuCaP-58 tumours were randomized according to the lesion grade and/or serum PSA level. 223Ra or vehicle was administered every 4 weeks twice, and soft-tissue tumours and tibiae were analysed using radiographic and micro-CT imaging24. Using this model, the vast majority of 223Ra deposits were observed within the surrounding bone matrix and especially in the vicinity of activated osteoblasts24. The researchers identified a dual mode of action consisting of the induction of potentially cytotoxic DNA double-strand breaks in adjacent tumour cells leading to tumour cell death and also in osteoblasts and osteoclasts, which would be expected to further disrupt the positive-feedback loops between these cell types and to suppress tumour-induced bone pathogenesis. This disruption led to an inhibition of tumour growth and of the pathological bone reaction, resulting in stabilization of normal bone structure. These findings were confirmed in a second intratibial bone metastasis model using human LNCaP prostate cancer cell line xenografts24.
In contrast to high-LET radiation, low-LET radiation, including beta and gamma radiation, induces a different pattern of DNA damage comprising single-strand breaks, base-pair deletions and substitutions, as well as DNA crosslinking26. The type of DNA damage from exposure to low LET is more easily repaired than that to high LET, and the likelihood of a single lethal event is much lower. For example, 1,000 DNA hits from beta particles are required for cell death, whereas only 1–4 DNA hits are required from high-LET alpha particles26,62. Furthermore, the mechanism of action of 223Ra is based on the direct non-reparable damage of tumour cell DNA, leading to tumour cell death and becoming less reliant on active cell proliferation, which might be advantageous for the killing of tumours with a low proliferation rate or dormant micrometastases25. In addition, TAT might be less susceptible to the development of tumour resistance mechanisms, including hypoxia, as DNA damage resulting from TAT is largely independent of oxygen levels26, in contrast to the oxygen-dependent DNA damage induced by external beam radiation therapy (EBRT), and use of alternate signalling pathways that become activated in response to other therapies, such as chemotherapy and cell signalling inhibitors24–26. Compared with beta particle emitters and EBRT, TAT approaches might also have the advantage of minimizing the damage to normal surrounding tissue owing to their comparatively short track length, which corresponds to 3–6 cell diameters63.
223Ra biodistribution and interaction with the bone and tumour compartments were investigated in prostate cancer bone metastases mouse models using LNCaP and PC3 cell lines64. 223Ra was found to be deposited at the bone surface, but not in the tumour mass, and skeletal accumulation was dependent on the local blood vessel density, indicating that vascular access is important for the effective delivery of 223Ra (REF.64).
223Ra might also enhance immune system activity, inhibit immunosuppression and alter the phenotype of cancer cells, rendering them susceptible to immune-mediated cell killing65,66. In vitro studies using human prostate, breast, and lung cancer cell lines demonstrated that sublethal doses of 223Ra enhanced T cell mediated lysis of tumour cells through antigen-specific CD8+ cytotoxic T lymphocytes. This effect seemed to be mediated through the induction of the endoplasmic reticulum stress response pathway that involves PERK transmembrane protein mediating the unfolded protein response, which translates into elevated expression of multiple antigen-processing machinery components67. 223Ra might also augment immune responses via the stimulator of interferon genes (STING) pathway68,69. STING, an endoplasmic reticulum translocon-associated transmembrane protein, detects DNA secreted from pathogens or damaged host DNA (from apoptosis or necrosis) in the cell cytoplasm, leading to the initiation of innate immune defence mechanisms69. The STING pathway is essential for radiation-induced adaptive immune responses, which are dependent on type I interferon signalling in dendritic cells70. However, whether the STING pathway is linked to antitumour T cell responses following TAT is currently unknown.
223Ra dosimetry.
Evaluation and knowledge of TAT distribution, absorbed radiation doses and radiation exposure in tumour and normal tissues have the potential to optimize treatment plans and to maximize the antitumour effects of TAT, while sparing normal tissues71. However, the short range of alpha-particle emitters makes the dosimetric calculations in organs difficult when the activity is localized to specific tissue compartments or cell types71.
In vivo dosimetry relies on 223Ra uptake quantification. Specifically, 223Ra decay produces a series of six products, before becoming a stable molecule. Of the total emitted energy (28.2 MeV), 95% comes from alpha emissions, 3.2% from beta particles and 2% from gamma emissions. Gamma emissions enable quantitative imaging of 223Ra72,73. However, microscopic energy deposition within the bone matrix presents challenges for internal dosimetry in the marrow and skeleton72. Currently, several small-scale modelling and microdosimetric approaches are being developed, with potential application in clinical imaging and dosimetry71,74–76. For example, for 223Ra, the trabecular marrow cavity model calculates the fraction of marrow volume receiving a cytotoxic absorbed dose. These methods might enable improved estimates of the dose of radiation delivered by radium and other alpha emitters in the future.
Some findings demonstrate a correlation between absorbed dose and local lesion response to 223Ra. In nine patients with mCRPC and bone metastases, a correlation between the uptakes of 223Ra in a metastatic lesion and the scintigraphy agent technetium-99m-methylene diphosphonate was observed73. Thus, conventional radiolabelled diphosphonate scintigraphy might help to define lesion extent73.
Rationale for treatment combinations in mCRPC
Based on the current understanding of the mechanism of action of 223Ra, and its efficacy and safety profile in the clinic, several treatment combination strategies have been proposed (TABLE 1). Their goal is to increase treatment efficacy by using agents with different mechanisms of action together with 223Ra, while keeping toxic effects to a minimum.
Table 1 |.
Summary of selected ongoing 223Ra combination trials in mCRPC
| Combination treatment with 223Ra | Mechanism of action | Trial phase and design | Primary outcomes | Patient characteristics (estimated enrolment) | Estimated completion date (primary) | Trial ID |
|---|---|---|---|---|---|---|
| Hormone therapies | ||||||
| Abiraterone149 | CYP17 inhibitor | Phase III (vs abiraterone only) | Symptomatic skeletal event-free survival | CRPC with asymptomatic or mildly symptomatic BM (n = 806) | December 2019 (February 2018) | NCT02043678a |
| Enzalutamide93 | AR inhibitor | Phase III (vs enzalutamide only) | Radiological progression-free survival | CRPC with asymptomatic or mildly symptomatic BM (n = 560) | April 2021 (November 2019) | NCT02194842 |
| Enzalutamide150 | AR inhibitor | Phase II | Safety (grade ≥3 AEs, by NCI CTCAE version 4) | Progressing mCRPC; BM and VD allowed (n = 44) | April 2021 (July 2017) | NCT02225704 |
| Chemotherapy | ||||||
| Docetaxel109 | Microtubule inhibitor | Randomized phase III (vs docetaxel only) | Overall survival | mCRPC (n = 738) | June 2023 (June 2022) | NCT03574571 |
| Immunotherapy | ||||||
| Atezolizumab151 | PD-L1 inhibitor | Phase I | DLT; adverse events; objective response (RECIST v1.1) | Progressing mCRPC; BM and VD allowed (n = 44) | March 2020 | NCT02814669 |
| Pembrolizumab121 | PD-1 inhibitor | Randomized phase II (vs 223Ra only) | Extent of immune cell infiltration | mCRPC with BM (n = 45) | June 2024 (June 2020) | NCT03093428 |
| Sipuleucel-T112 | ACT using PA2024-pretreated cells to stimulate tumour-specific immune responses | Randomized phase II (vs sipuleucel-T only) | Immune responses measured by peripheral PA2024 T cell proliferation | CRPC with asymptomatic or minimally symptomatic BM (n = 34) | December 2020 (December 2018) | NCT02463799 |
| DNA damage response | ||||||
| Niraparib131 | PARP inhibitor | Phase Ib | Maximum tolerated dose of niraparib to combine with standard dose of 223Ra | mCRPC; BM and VD allowed (n = 27) | November 2020 (November 2021) | NCT03076203 |
| Olaparib132 | PARP inhibitor | Randomized phase I/II (vs 223Ra only) | Maximum tolerated dose of olaparib and 223Ra; radiographic progression-free survival | CRPC with BM (n = 138) | April 2020 | NCT03317392 |
223Ra, radium-223; ACT, autologous cell therapy; AEs, adverse events; AR, androgen receptor; BM, bone metastases; CYP17, cytochrome P450 c17; DLT, dose-limiting toxicity; mCRPC, metastatic castration-resistant prostate cancer; PA2024, fusion protein of hum an prostatic antigen phosphatase and granulocyte-macrophage colony-stimulating factor; PARP, poly(ADP-ribose) polymerase; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; VD, visceral disease.
Unblinded owing to safety concerns in the combination arm.
Androgen receptor-axis inhibitors.
AR signalling regulates prostate cancer growth, and ADT is a standard treatment for patients with advanced or recurrent disease77,78. Despite initial responses to ADT, the disease invariably progresses to CRPC, which remains dependent on the AR-signalling axis in the majority of patients. Abiraterone acetate (abiraterone) is a selective steroidal inhibitor of cytochrome P450 c17 (CYP17), a key enzyme for testosterone and oestrogen biosynthesis78,79 (FIG. 2). Inhibition of CYP17 blocks steroid production in the testes, adrenal glands and tumour, limiting the amount of hormones available to stimulate AR signalling79. Enzalutamide, a non-steroidal antiandrogen, binds to the AR ligand-binding domain and inhibits the AR signalling pathway by preventing the binding of androgens to AR, by preventing nuclear translocation of activated AR and by impairing the binding of activated AR to DNA80 (FIG. 2).
Fig. 2 |. Mechanism of action of anticancer agents in prostate cancer.
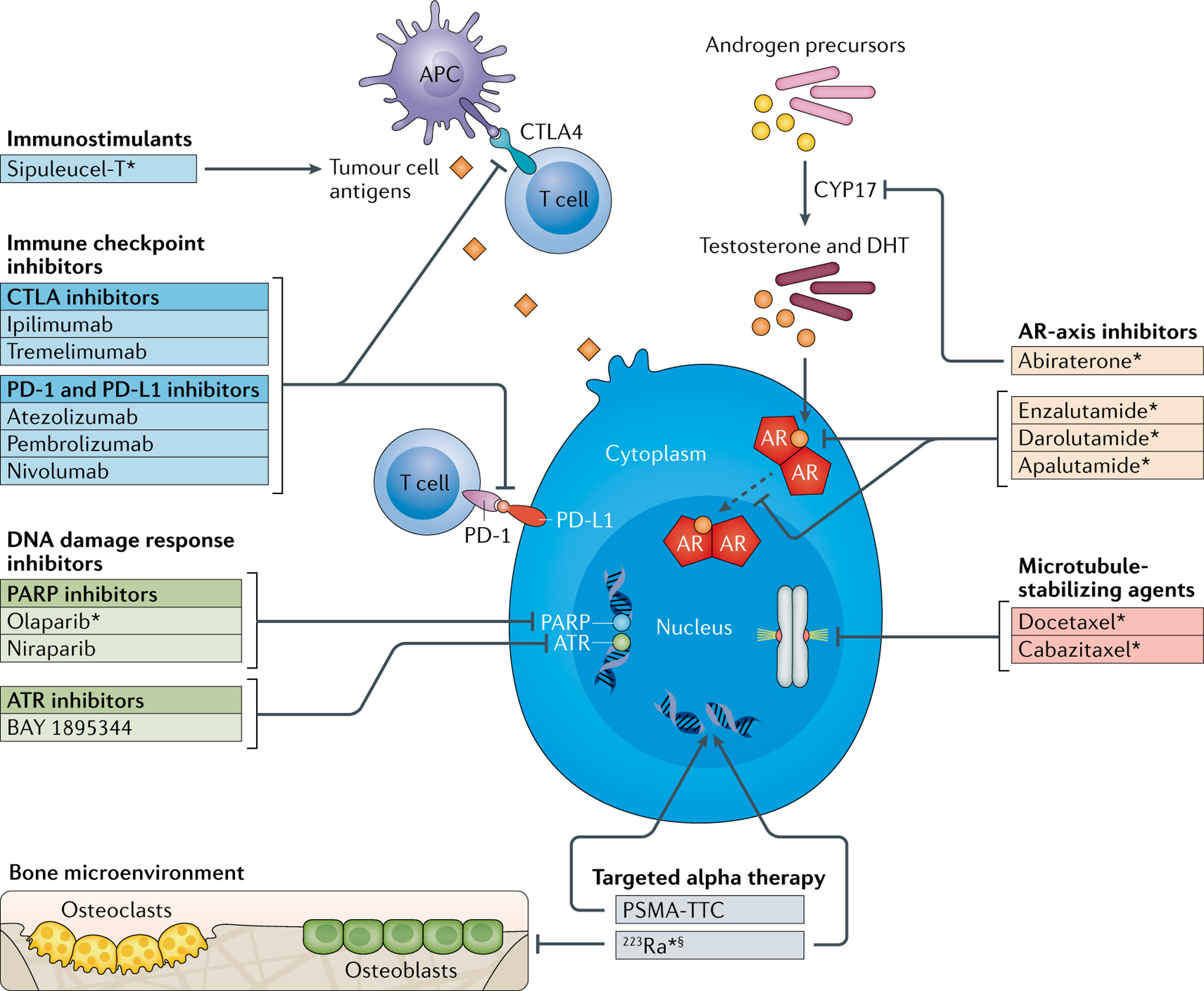
Several anticancer agents with differing mechanisms of action are in use in routine clinical practice or are under preclinical or clinical investigation in prostate cancer. Androgen receptor (AR)-axis inhibitors, microtubule-stabilizing agents, DNA damage response inhibitors, immune checkpoint inhibitors and immunostimulants, each with different but complementary mechanisms of action to radium-223 (223Ra), might have potential in combination treatments with 223Ra in metastatic castration-resistant prostate cancer. APC, antigen-presenting cell; ATR, ataxia telangiectasia and Rad3-related; CTLA, cytotoxic T lymphocyte antigen; DHT, dihydrotestosterone; PARP, poly(ADP-ribose) polymerase; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; PSMA-TTC, prostate-specific membrane antigen-targeted thorium conjugate. *Approved by the FDA. §223Ra also inhibits cellular activity within the adjacent bone microenvironment through induction of DNA double-strand breaks in osteoblasts and osteoclasts24.
Data from large randomized phase III clinical trials, COU-AA-301 and COU-AA-302 for abiraterone plus prednisone12,15, and AFFIRM and PREVAIL for enzalutamide10,81, led to the recommendation of these agents by the ESMO and NCCN guidelines for use in patients with mCRPC44,82. In phase III trials in mCRPC, abiraterone was generally well tolerated and adverse effects included increased incidence of mineralocorticoid-related events, hepatotoxicity and cardiac disorders12,15. The main adverse effects of enzalutamide included fatigue and hypertension10,81. Subsequently, the combination of abiraterone plus prednisone or prednisolone with primary ADT was shown to significantly extend the median overall survival (not reached versus 34.7 months; HR for death 0.62, 95% CI 0.51–0.76; P < 0.001) in patients with hormone-sensitive metastatic prostate cancer6,83, leading to guideline recommendation as a standard of care in the hormone-sensitive setting in suitable patients44,84.
The differing mechanisms of action of 223Ra and abiraterone and enzalutamide and their non-overlapping safety profiles provide a rationale for combining 223Ra with abiraterone or enzalutamide in patients with metastatic prostate cancer. Preliminary data for these combinations were provided by post hoc analyses of early access programmes85,86, patients treated in routine clinical practice87 and from a phase II study88, which suggested that 223Ra could be safely combined with abiraterone or enzalutamide.
The multicentre phase III ERA-223 trial evaluated the efficacy and safety of adding 223Ra treatment or placebo to abiraterone plus prednisone or prednisolone in patients with asymptomatic or minimally symptomatic mCRPC43 (TABLE 1). Primary analysis revealed no statistically significant difference in overall survival between the groups (33.3 versus 30.7 months; HR 1.195, 95% CI 0.950–1.505; P = 0.13)43. However, patients in the 223Ra combination arm had an unexpected increased incidence of fractures (29% versus 11%). Consequently, the combination of 223Ra with abiraterone and prednisolone or prednisone is currently contraindicated by the EMA42,89.
Several reasons for the increase in skeletal fractures in the combination therapy group compared with the control group have been suggested90–92. Treatment with low-dose prednisone monotherapy has previously been shown to have a negative effect on bone metabolism through the inhibition of osteoblast function and the promotion of osteoclast activity, leading to decreased bone formation90. In addition to the potentially harmful effects of glucocorticoids and the potential hormonal changes within the bone microenvironment as a consequence of abiraterone, ionizing radiation can also contribute by augmenting osteoclastogenesis91,92. Overall, the additive effects of abiraterone and prednisone and continuous androgen depletion, glucocorticoids and 223Ra might result in compromised bone health, and, in turn, might lead to more frequent skeletal fractures.
In the ERA-223 trial, concurrent use of bone health agents (BHAs), including bisphosphonates (zoledronic acid) and denosumab from baseline, was associated with a lower incidence of fractures in both the 223Ra and placebo groups (15% and 7%, respectively) compared with patients who did not receive BHAs (37% and 15%, respectively)43. The hazard ratio for SSE-free survival for the 223Ra and placebo group was 0.932 (95% CI 0.666–1.306) when BHAs were used and 1.252 (95% CI 0.971–1.615) without BHAs43. These findings are underscored by data from the ALSYMPCA trial, in which patients treated with 223Ra in combination with BHAs seemed to have a superior outcome with respect to risk of SSEs compared with those who received 223Ra without BHAs18,38. In a univariate analysis of time to first SSE, current use of bisphosphonates at baseline (yes versus no) was an independent predictor of decreased risk of SSEs (HR 0.56, 95% CI 0.44–0.71). In multivariate analysis, the treatment effect of 223Ra compared with placebo was maintained (HR 0.65, 95% CI 0.51–0.82) and concurrent use of bisphosphonates at study entry was also independently associated with reduced risk of SSEs (HR 0.49, 95% CI 0.38–0.64)38. Notably, the treatment effect of 223Ra versus placebo on the time to first SSE seemed to be greater in patients who received current bisphosphonates at baseline (median 19.6 versus 10.2 months; HR 0.49, 95% CI 0.33–0.74) than those who did not receive bisphosphonates (median 11.8 versus 8.4 months; HR 0.77, 95% CI 0.58–1.02). These data suggest that bisphosphonates should be given with 223Ra to maximize treatment benefit, prevent fragility fractures and preserve bone health38.
Whether the increase in fractures will be seen in patients receiving a combination of 223Ra and AR-axis inhibitors other than abiraterone remains to be determined. The randomized phase III trial PEACE 3 (REF.93) is currently investigating 223Ra in combination with enzalutamide in patients with mCRPC who are asymptomatic or mildly symptomatic (TABLE 1).
Chemotherapy.
Taxanes are antimitotic chemotherapy agents that bind to and abnormally stabilize microtubules in dividing cells94,95 (FIG. 2). This effect impairs intracellular organelle trafficking, might impair AR signalling and leads to cell cycle arrest and apoptosis94–98.
Docetaxel was first recommended by the NCCN and ESMO guidelines for patients with mCRPC (particularly those who are symptomatic or have a high disease burden), and cabazitaxel was recommended in patients whose disease progresses on docetaxel treatment44,82. Results of two phase III trials5,7 support the recommendation for combining chemotherapy with primary ADT in newly diagnosed patients with metastatic castration-naive disease. The STAMPEDE study showed improved survival in patients treated with long-term hormone therapy combined with docetaxel chemotherapy compared with long-term hormone therapy alone (71 months versus 81 months; HR 0.78, 0.66–0.93; P = 0.006)5. The CHAARTED study also demonstrated an advantage of the combination therapy (ADT therapy plus docetaxel) in extending overall survival in patients with metastatic hormone-sensitive prostate cancer (58 months versus 44 months; HR for death 0.61, 95% CI 0.47–0.80; P < 0.001)7.
Several factors support the combination of 223Ra with docetaxel. First, chemotherapy has been shown to have a radiosensitizing effect through different mechanisms, including elevated radiation-induced damage, reduced DNA repair, increased vulnerability of hypoxic cells to cytotoxic agents, inhibition of prosurvival pathways and reduced ability of tumour cells to repopulate during radiotherapy, which lead to an additive cytotoxic effect when combined with radiotherapy in cancer patients99–101. However, no data currently exist on radiosensitizing effects when combining chemotherapy with alpha-particle emitters. In addition, the anti-proliferative effects of chemotherapy might reduce the cell repopulation effect associated with radiation therapy, in which rapid regrowth of tum our cells commonly occurs between doses of radiation99.
Additional rationale for combining bone-targeted alpha therapy with chemotherapy is provided by the concept of simultaneous targeting of the tum our and the bony compartment of the disease, each agent independently prolonging survival, and by the possibility that each agent might enhance the other by cross-sensitization102. The potential benefits of combining radiopharmaceuticals with chemotherapy in patients with mCRPC have previously been demonstrated in clinical studies of chemotherapy in combination with palliative beta particle-emitting agents103–105. For 223Ra, exploratory efficacy analysis of a randomized phase I/II clinical trial showed that 223Ra (55 kBq/kg every 6 weeks, n = 36) plus docetaxel (60 mg/m2 every 3 weeks) might enhance antitumour activity compared with docetaxel alone (75 mg/m2 every 3 weeks; n = 17)106. The combination arm had more durable suppression of PSA (median time to progression 6.6 versus 4.8 months, P = 0.02), alkaline phosphatase (ALP) (9 versus 7 months, P = 0.44) and osteoblastic bone deposition markers (bone ALP and procollagen type I N propeptide)106–108. Combination therapy was well tolerated and presented no greater safety concerns than treatment with docetaxel alone106. The most common adverse event was neutropenia, which occurred less frequently in the combination arm (30%) than in the treatment arm with docetaxel alone (38%), and no clinically significant thrombocytopenia was reported. Grade 3/4 treatment-emergent adverse events occurred in 48% and 62% of patients treated with the combination therapy or docetaxel alone, respectively. On the basis of these encouraging results, a phase III trial of this treatment combination is currently recruiting patients109 (TABLE 1).
Immunotherapy.
Combining 223Ra with immunotherapies, particularly those designed to generate and expand endogenous tumour-antigen-specific T cell populations, might be a beneficial approach, particularly when application of a lethal dose of radiation needed to reduce the tum our burden is constrained by toxic effects (FIG. 2). In a preclinical in vitro study, sublethal doses of 223Ra have been shown to sensitize the human prostate, breast and lung carcinoma cells to T cell-mediated lysis by CD8+ cytotoxic T lymphocytes specific for tumour antigens mucin-1, brachyury and carcinoembryonic antigen110. Sipuleucel-T is an autologous cell therapy, in which patient-enriched monocytic cells are activated with a recombinant fusion protein of hum an prostatic acid phosphatase and granulocyte-macrophage colony-stimulating factor (termed PA2024)111. Sipuleucel-T is currently approved by the FDA for the treatment of patients with mCRPC who have few or no symptoms, following data from a phase III clinical trial in which sipuleucel-T compared with placebo was shown to prolong overall survival in patients with mCRPC19,44. The treatment is generally well tolerated: the most common adverse events are fevers, chills, fatigue, nausea and headache19. A current randomized phase II study is investigating the combination of 223Ra with sipuleucel-T in patients with mCRPC and asymptomatic or mildly symptomatic bone metastases and is due to be completed in November 2019 (REF.112) (TABLE 1).
Therapeutic intervention with agents targeting programmed cell death 1 (PD-1) or the programmed cell death 1 ligand 1 (PD-L1) seem to be effective in a range of cancer types113–115. PD-L1 is selectively expressed on many cancer cells and on cells within the tumour microenvironment in response to inflammatory stimuli113. PD-L1 and PD-1 negatively regulate immune activation, mainly through the inhibition of T cell function. Atezolizumab, a monoclonal PD-L1 antibody, binds to PD-L1 on tumour cells and prevents its interaction with PD-1 and B7.1 receptors on immunosuppressed T cells, resulting in an activation of T cells and tumour cell death through specific immune responses116.
The FDA has approved the PD-1 inhibitory antibody pembrolizumab for MSI-high and DNA mismatch repair-deficient cancers, including mCRPC117. Early analyses showed that responses to PD-1 or PD-L1 therapy might occur in ~50% of patients with defects in DNA mismatch repair genes118. Studies in men with mCRPC not selected for MSI-high disease or DNA mismatch repair deficiencies are ongoing119.
Preclinical studies suggest that the PD-L1-PD-1 axis might dampen the immune response to ionizing radiation120. In a mouse model, PD-L1 in the tumour microenvironment was upregulated following treatment with ionizing radiation, leading to the suppression of radiation-induced immune responses120. Concurrent administration of inhibitory PD-L1 antibodies enhanced the efficacy of the ionizing radiation through a mechanism that was dependent on cytotoxic T cells. Clinical trials are investigating 223Ra in combination with the PD-L1 inhibitory antibody atezolizumab in patients with mCRPC who have progressed on treatment with an androgen pathway inhibitor, and 223Ra in combination with pembrolizumab in patients with mCRPC and bone metastases121 (TABLE 1).
DNA damage response.
The DNA damage response maintains cellular integrity and homeostasis. Cellular recognition of DNA damage activates DNA damage response pathways, resulting in cell cycle arrest and induction of DNA repair or cell death122. Genomic defects in DNA repair have been identified in 12–30% of men with advanced CRPC123–125. In an integrative clinical sequencing analysis of 150 patients with mCRPC, 23% of tumours had aberrations in DNA repair pathways and 8% of patients had germline variants124. The incidence of germline mutations in DNA repair genes associated with autosomal dominant cancer predisposition syndromes in a cohort of 692 patients with metastatic prostate cancer was 12%125.
Some of these genetic alterations are associated with sensitivity to DNA-damaging anticancer agents, including platinum-based chemotherapy, and sensitivity to inhibitors of DNA damage response proteins, including poly(ADP-ribose) polymerase (PARP)126 (FIG. 2). This finding suggests that using PARP inhibitors in patients whose tumours harbour a DNA repair defect (mutation in DNA repair genes) might exploit a synthetic lethal interaction127.
Olaparib, a PARP inhibitor, is used in the treatment of patients with advanced ovarian cancer who have germline BRCA1 or BRCA2 gene m utations128. In a phase II trial in patients heavily pretreated with mCRPC, olaparib treatment resulted in a response rate of 33%127. Next-generation sequencing identified homozygous deletions and deleterious mutations in DNA repair genes in 33% of patients, and 88% of these patients had a response to olaparib. Deleterious BRCA2 mutations or other deleterious alterations and ATM gene mutations were found in the majority of patients showing a response to olaparib127. The FDA granted breakthrough therapy designation for olaparib monotherapy for the treatment of BRCA1 or BRCA2 or ATM-mutated mCRPC in patients who have received prior taxane-based chemotherapy and at least one next-generation antihormonal agent (abiraterone or enzalutamide)129. A small study in patients with mCRPC treated with 223Ra (n = 49) found that 4 of 10 (40%) responders who had undergone DNA sequencing had an alteration to a DNA repair gene130. As 223Ra induces double-strand DNA breaks, defects in DNA damage repair pathways might confer sensitivity to 223Ra, but this hypothesis requires confirmation in a prospective study130. Combination of 223Ra with PARP inhibitors might be useful, as a PARP inhibitor might further inhibit double-strand break repair capacity in 223Ra-irradiated cells, even in those patients whose tumours do not harbour alterations in DNA repair genes. An ongoing phase Ib study is investigating the PARP inhibitor niraparib in combination with 223Ra in patients with mCRPC who do not have alterations in DNA repair genes131, and a randomized phase I/II trial is planned to investigate the combination of olaparib and 223Ra (REF.132) (TABLE 1).
The combination of 223Ra with other inhibitors of DNA damage response proteins might also be considered. BAY 1895344, an inhibitor of the ataxia telangiectasia and Rad3-related (ATR) kinase (FIG. 2), which is involved in the repair of DNA double-strand breaks, is currently in phase I clinical testing133. A preclinical study demonstrated synergistic antitumour activity in a mouse xenograft model of mCRPC with bone metastases treated with BAY 1895344 in combination with 223Ra (REF.134). BAY 1895344 doses ranging from 3% to 13% of the single-agent maximum tolerated dose in the combination treatment with 223Ra showed antitumour activity, with the highest activity achieved 24 h after administration of the first 223Ra dose.
A study published in 2018 evaluated whether pathogenic mutations in homologous recombination DNA repair genes correlate with 223Ra efficacy in patients with mCRPC and bone metastases135. The study found that patients with homologous recombination deficiency (HRD) who were treated with 223Ra showed improved ALP responses (80% versus 39%; P = 0.04) and time to ALP progression (median 10.4 versus 5.8 months, HR 6.4; P = 0.005), and longer overall survival (median 36.9 versus 19.0 months, HR 3.3; P = 0.11) compared with patients without the deficiency135. Unfortunately, the patient population was small (n = 28)135 and the correlation between the HRD status and response to 223Ra needs further validation.
Future perspectives on alpha therapy
223Ra specifically targets hydroxyapatite and bone metastases in men with prostate cancer, yielding a clinical benefit in prolonging overall survival and delaying time to first SSE. However, by using a bone-targeting agent such as 223Ra, soft-tissue disease is not targeted. Further, because the depth of penetration of an alpha particle is so shallow, disease that is more than a few cell lengths away from newly deposited bone will not be directly affected by the treatment. Future approaches will be focused on delivering alpha-emitting therapy, not so much to newly deposited bone but to the cancer cell itself. Such an approach has the potential to directly kill more tumour tissue, regardless of location. The conjugation of alpha particle-emitting radionuclides with tumour-targeted carrier molecules, including antibodies, peptides and small molecules, has the potential to deliver tumour-specific targeting136.
Antibodies targeted against tumour antigens and conjugated to the alpha emitter thorium-227 (227Th) have been evaluated in preclinical studies and include 227Th-CD-70 conjugates in renal cell carcinoma models137; 227Th-lintuzumab (CD33 antibody) in acute myeloid leukaemia xenografts138; 227Th-rituximab (CD20 antibody) in lymphoma xenografts139; and 227Th-trastuzumab (HER2 antibody) in breast and ovarian cancer xenografts140. In addition, a phase I clinical trial of 227Th-epratuzumab (CD22 antibody) in non-Hodgkin lymphoma is in progress141. Prostate-specific membrane antigen (PSMA) is expressed on both normal and cancerous prostate tissues and PSMA targeting is successfully used in imaging techniques142. A PSMA-targeted thorium conjugate (227Th-labeled anti-PSMA IgG1 (PSMA-TTC)) showed statistically significant prevention of tumour growth at a dose of 100 kBq/kg in an orthotopic bone xenograft model (LNCaP-Luc), which supports its clinical development for the treatment of patients with metastatic prostate cancer143,144. Importantly, applying the principle underlying PSMA-TTC could extend the use of 223Ra to patients with mCRPC and visceral disease. Actinium-225 (225Ac) is also under clinical investigation in mCRPC. In an analysis of patients who received an 225Ac radiolabelled form of the PSMA-targeting small molecule PSMA-617, a dose of 100 kBq/kg per cycle every 8 weeks was well tolerated145 and demonstrated a median duration of tumour control of 9 months in 40 patients146. Similarly, a bismuth-213 (213Bi)-labelled small-molecule PSMA inhibitor (213Bi-PSMA I&T) rapidly targeted PSMA-expressing LNCaP tumour xenografts leading to accumulation of DNA double-strand breaks in the tumour tissue147. In a patient with mCRPC that progressed under conventional therapy, two cycles of treatment with another 213Bi-labelled small-molecule PSMA inhibitor (213Bi-PSMA-617) achieved a remarkable molecular imaging response and reduction of PSA levels148.
Conclusions
223Ra seems to act through multiple mechanisms, including direct effects via DNA damage to tumour and effector cells of pathological bone remodelling that are in close proximity to newly formed, hydroxyapatite-rich bone matrix, and possibly also through activation of cellular immune defences within the bone microenvironment via the endoplasmic reticulum stress response and STING pathways. Understanding the mechanisms of action of 223Ra should be useful for designing combination strategies that include agents that target tumour growth by inhibiting DNA repair, modulating the immune system or directly targeting cell proliferation. Clinical trials to investigate novel 223Ra combinations are underway (TABLE 1).
Mechanistically, combining 223Ra with other anticancer agents seems feasible and data from preclinical studies are encouraging; however, the results of randomized controlled trials are required before 223Ra combinations can be used in clinical practice, as emphasized by the unexpected findings of the ERA-223 trial, despite a strong rationale for combining 223Ra with abiraterone in mCRPC.
Our understanding of how 223Ra improves patient outcomes might also pave the way for new developments in TAT. Combining the cytotoxic properties of alpha-particle emitters with agents that enable tumour-specific delivery has the potential to expand the spectrum of cancers in which TAT might prove to be an effective treatment option.
Key points.
Radium-223 (223Ra) deposited in the intralesional bone matrix emits high-energy alpha-particles that induce potentially cytotoxic DNA double-strand breaks in cells within a 100-μm distance while sparing surrounding normal tissue.
223Ra acts via a multi-modal mechanism, killing both tumour cells and the effector cells of pathological bone metabolism, osteoblasts and osteoclasts; 223Ra might also promote local antitumour immune responses.
Understanding the mode of action of 223Ra and its interactions with those of other anticancer agents offers potential for new treatment combinations for bone-metastatic cancers.
Clinical trials are investigating 223Ra in combination with agents targeting the androgen receptor signalling axis, cell microtubules, the immune response and the DNA damage response.
Current combination studies are preliminary and further clinical testing will be required to demonstrate safety, efficacy and clinical benefit.
223Ra treatment in patients with metastatic castration-resistant prostate cancer has led to the development of other targeted alpha therapies for the treatment of patients with different cancers that also have bone-predominant metastases.
Acknowledgements
The authors thank P. Hoban (Cancer Communications and Consultancy Ltd, Knutsford, UK) for providing writing assistance funded by Bayer.
Footnotes
Competing interests
M.J.M. declares participation as a compensated member of advisory boards for Advanced Accelerated Applications, an uncompensated member of advisory boards for Bayer, Endocyte and Progenics and research funding (for institute) from Bayer, Endocyte, Progenics and Corcept. E.C. and T.A.G. declare participation as compensated members of a round-table meeting from Bayer and research funding (for institute) from Bayer. W.K.K. declares clinical trial support from Bayer. D.I.Q. declares participation as a compensated member of advisory boards from Astellas, Bayer, Janssen, Genzyme, Dendreon and AstraZeneca. A.S. is a full-time employee of Bayer. G.S. is a consultant for Bayer and holds ownership in a start-up company that Bayer has used for imaging and dosimetry analyses. J.L.G declares no competing interests.
Peer review information
Nature Reviews Urology thanks S. Nilsson, J. O’Sullivan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.American Institute for Cancer Research. Prostate cancer statistics World Cancer Research Fund; https://www.wcrf.org/dietandcancer/cancer-trends/prostatecancer-statistics (2018). [Google Scholar]
- 2.Pernar CH, Ebot EM, Wilson KM & Mucci LA The epidemiology of prostate cancer. Cold Spring Harb. Perspect. Med 8, a030361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute. SEER cancer statistics review 1975–2014 https://seer.cancer.gov/archive/csr/1975_2014/.
- 4.Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016. JAMA Oncol 4, 1553–1568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James ND et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fizazi K et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med 377, 352–360 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Sweeney CJ et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med 373, 737–746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono JS et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Tannock IF et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med 351, 1502–1512 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Beer TM et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med 4 371, 24–433 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Fizazi K et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol 15, 1147–1156 (2014). [DOI] [PubMed] [Google Scholar]
- 12.de Bono JS et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med 364, 1995–2005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logothetis CJ et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol 13, 1210–1217 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Fizazi K et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13, 983–992 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Ryan CJ et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med 368, 138–148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan CJ et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16, 152–160 (2015). [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication FDA; https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm (2017). [Google Scholar]
- 18.Parker C et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med 369, 213–223 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Kantoff PW et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med 363, 411–422 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Beer TM et al. Quality of life after sipuleucel-T therapy: results from a randomized, double-blind study in patients with androgen-dependent prostate cancer. Urology 82, 410–415 (2013). [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Adminstration. Xofigo (radium Ra 223 dichloride). Prescribing Information FDA; https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203971lbl.pdf (2013). [Google Scholar]
- 22.European Medicines Agency. Xofigo: summary of product characteristics EMA; https://www.ema.europa.eu/documents/referral/xofigo-article-20-procedureannex-i-ii-iii_en.pdf (2018). [Google Scholar]
- 23.Henriksen G, Breistol K, Bruland OS, Fodstad O & Larsen RH Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res 62, 3120–3125 (2002). [PubMed] [Google Scholar]
- 24.Suominen MI et al. Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone microenvironment in mouse models. Clin. Cancer Res 23, 4335–4346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruland OS, Nilsson S, Fisher DR & Larsen RH High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin. Cancer Res 12, 6250s–6257s (2006). [DOI] [PubMed] [Google Scholar]
- 26.Sgouros G et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med 51, 311–328 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suominen MI et al. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J. Natl. Cancer Inst 105, 908–916 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Coleman R et al. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res. Treat 145, 411–418 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueno NT et al. Phase II study of Ra-223 combined with hormonal therapy and denosumab for treatment of hormone receptor-positive breast cancer with bone-dominant metastasis. J. Clin. Oncol 36, 1065 (2018). [Google Scholar]
- 30.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02366130?term=radium-223&cond=Breast+Cancer&rank=4 (2019). [DOI] [PubMed]
- 31.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02258451 (2019). [DOI] [PubMed]
- 32.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02258464 (2019). [DOI] [PubMed]
- 33.Suominen MI et al. Abstract 5202: additive benefits of radium-223 dichloride and bortezomib combination in a syngeneic 5TGM1 multiple myeloma mouse model. Cancer Res 77, 5202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02928029 (2019). [DOI] [PubMed]
- 35.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02406521 (2019). [DOI] [PubMed]
- 36.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02283749 (2019). [DOI] [PubMed]
- 37.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02390934 (2019). [DOI] [PubMed]
- 38.Sartor O et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 15, 738–746 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Vogelzang NJ et al. Hematologic safety of radium-223 dichloride: baseline prognostic factors associated with myelosuppression in the ALSYMPCA trial. Clin. Genitourin. Cancer 15, 42–58 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Nilsson S et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann. Oncol 27, 868–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sternberg CN et al. A randomized phase 2 study investigating 3 dosing regimens of radium-223 dichloride (Ra-223) in bone metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol 36, 5008 (2018). [Google Scholar]
- 42.European Medicines Agency. EMA restricts use of prostate cancer medicine Xofigo EMA; http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Xofigo/human_referral_prac_000071.jsp&mid=WC0b01ac05805c516f (2018). [Google Scholar]
- 43.Smith M et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20, 408–419 (2019). [DOI] [PubMed] [Google Scholar]
- 44.National Comprehensive Cancer Network. NCCN guidelines. Prostate cancer version 1 NCCN; https://www.nccn.org (2018). [Google Scholar]
- 45.American Urological Association. Castration-resistant prostate cancer AUA; https://www.auanet.org/guidelines/prostate-cancer-castration-resistant-guideline (2018). [Google Scholar]
- 46.European Association of Urology. Prostate cancer guidelines EAU; https://uroweb.org/guideline/prostate-cancer/#6 (2019). [Google Scholar]
- 47.Parker CC et al. Three-year safety of radium-223 dichloride in patients prostate with castration-resistant cancer and symptomatic bone metastases from phase 3 randomized alpharadin in symptomatic prostate cancer trial. Eur. Urol 10.1016/j.eururo.2017.06.021 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Dizdarevic S et al. Interim analysis of the REASSURE (Radium-223 alpha Emitter Agent in non-intervention Safety Study in mCRPC popUlation for long-teRm Evaluation) study: patient characteristics and safety according to prior use of chemotherapy in routine clinical practice. Eur. J. Nucl. Med. Mol. Imaging 46, 1102–1110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Body JJ, Casimiro S & Costa L Targeting bone metastases in prostate cancer: improving clinical outcome. Nat. Rev. Urol 12, 340–356 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Casimiro S, Guise TA & Chirgwin J The critical role of the bone microenvironment in cancer metastases. Mo!. Cell Endocrinol 310, 71–81 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Gdowski A, Ranjan A & Vishwanatha J Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Cancer Res 36, 108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Logothetis CJ & Lin SH Osteoblasts in prostate cancer metastasis to bone. Nat. Rev. Cancer 5, 21–28 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Ottewell PD The role of osteoblasts in bone metastasis. J. Bone Oncol 5, 124–127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagberg Thulin M et al. Osteoblasts promote castration-resistant prostate cancer by altering intratumoral steroidogenesis. Mol. Cell Endocrinol 422, 182–191 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Liu Q, Jernigan D, Zhang Y & Fatatis A Implication of platelet-derived growth factor receptor alpha in prostate cancer skeletal metastasis. Chin. J. Cancer 30, 612–619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimura Y, Matsugaki A, Sekita A & Nakano T Alteration of osteoblast arrangement via direct attack by cancer cells: new insights into bone metastasis. Sci. Rep 7, 44824 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagiwara M, Delea TE, Saville MW & Chung K Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis 16, 23–27 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Oefelein MG, Ricchiuti V, Conrad W & Resnick MI Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J. Urol 168, 1005–1007 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Berish RB, Ali AN, Telmer PG, Ronald JA & Leong HS Translational models of prostate cancer bone metastasis. Nat. Rev. Urol 15, 403–421 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Deshayes E et al. Radium 223 dichloride for prostate cancer treatment. Drug Des. Dev. Ther 11, 2643–2651 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ritter MA, Cleaver JE & Tobias CA High-LET radiations induce a large proportion of non-rejoining DNA breaks. Nature 266, 653–655 (1977). [DOI] [PubMed] [Google Scholar]
- 62.Mantel E MIRD monograph: radiobiology and dosimetry for radiopharmaceutical therapy with alpha-particle emitters (ed. Sgouros G). J. Nucl. Med. Technol 44, 216 (2016). [Google Scholar]
- 63.Ceder J & Elgqvist J Targeting prostate cancer stem cells with alpha-particle therapy. Front. Oncol 6, 273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abou DS et al. Whole-body and microenvironmental localization of radium-223 in naive and mouse models of prostate cancer metastasis. J. Natl. Cancer Inst 108, djv380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodge JW, Guha C, Neefjes J & Gulley JL Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology 22, 1064–1070; discussion 1075, 1080–1, 1084 (2008). [PMC free article] [PubMed] [Google Scholar]
- 66.Gameiro SR, Ardiani A, Kwilas A & Hodge JW Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. Oncoimmunology 3, e28643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiner LM & Lotze MT Tumor-cell death, autophagy, and immunity. N. Engl. J. Med 366, 1156–1158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abe T et al. STING recognition of cytoplasmic DNA instigates cellular defense. Mol. Cell 50, 5–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barber GN STING: infection, inflammation and cancer. Nat. Rev. Immunol 15, 760–770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng L et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sgouros G & Hobbs RF Dosimetry for radiopharmaceutical therapy. Semin. Nucl. Med 44, 172–178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flux GD Imaging and dosimetry for radium-223: the potential for personalized treatment. Br. J. Radiol 90, 20160748 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pacilio M et al. Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting (223)Ra-dichloride. Eur. J. Nucl. Med. Mol. imaging 43, 21–33 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Akabani G & Zalutsky MR Microdosimetry of astatine-211 using histological images: application to bone marrow. Radiat. Res 148, 599–607 (1997). [PubMed] [Google Scholar]
- 75.Akabani G, Kennel SJ & Zalutsky MR Microdosimetric analysis of alpha-particle-emitting targeted radiotherapeutics using histological images. J. Nucl. Med 44, 792–805 (2003). [PubMed] [Google Scholar]
- 76.Chouin N et al. Evidence of extranuclear cell sensitivity to alpha-particle radiation using a microdosimetric model. II. Application of the microdosimetric model to experimental results. Radiat. Res 171, 664–673 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Coutinho I, Day TK, Tilley WD & Selth LA Androgen receptor signaling in castration-resistant prostate cancer: a lesson in persistence. Endocr. Relat. Cancer 23, T179–T197 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Crona DJ, Milowsky MI & Whang YE Androgen receptor targeting drugs in castration-resistant prostate cancer and mechanisms of resistance. Clin. Pharmacol. Ther 98, 582–589 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Attard G, Belldegrun AS & de Bono JS Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int 96, 1241–1246 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Schalken J & Fitzpatrick JM Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int 117, 215–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scher HI et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med 367, 1187–1197 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Parker C, Gillessen S, Heidenreich A, Horwich A & Committee EG Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol 26, v69–v77 (2015). [DOI] [PubMed] [Google Scholar]
- 83.James ND et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med 377, 338–351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morris MJ et al. Optimizing anticancer therapy in metastatic non-castrate prostate cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol 36, 1521–1539 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Saad F et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol 17, 1306–1316 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Sartor O et al. Radium-223 safety, efficacy, and concurrent use with abiraterone or enzalutamide: first U.S. experience from an expanded access program. Oncologist 23, 193–202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harshman LC et al. First interim results of the radium-223 (Ra-223) REASSURE observational study: analysis of patient (Pt) characteristics and safety by use of abiraterone and/or enzalutamide (Abi/Enza). Ann. Oncol 28, 870P (2017). [Google Scholar]
- 88.Shore ND et al. eRADicAte: a prospective evaluation combining radium-223 dichloride and abiraterone acetate plus prednisone in patients with castration-resistant prostate cancer. Clin. Genitourin. Cancer 16, 149–154 (2018). [DOI] [PubMed] [Google Scholar]
- 89.O’Sullivan JM et al. The case against the European Medicines Agency’s change to the label for radium-223 for the treatment of metastatic castration-resistant prostate cancer. Eur. Urol 75, e51–e52 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Ton FN, Gunawardene SC, Lee H & Neer RM Effects of low-dose prednisone on bone metabolism. J. Bone Miner. Res 20, 464–470 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Faruqi S et al. Vertebral compression fracture after spine stereotactic body radiation therapy: a review of the pathophysiology and risk factors. Neurosurgery 83, 314–322 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Zhang J et al. Differences in responses to X-ray exposure between osteoclast and osteoblast cells. J. Radiat. Res 58, 791–802 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02194842 (2018). [DOI] [PubMed]
- 94.Martin SK, Kamelgarn M & Kyprianou N Cytoskeleton targeting value in prostate cancer treatment. Am. J. Clin. Exp. Urol 2, 15–26 (2014). [PMC free article] [PubMed] [Google Scholar]
- 95.Cheetham P & Petrylak DP Tubulin-targeted agents including docetaxel and cabazitaxel. Cancer J 19, 59–65 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Kraus LA et al. The mechanism of action of docetaxel (Taxotere) in xenograft models is not limited to bcl-2 phosphorylation. Invest. New Drugs 21, 259–268 (2003). [DOI] [PubMed] [Google Scholar]
- 97.Zhu ML et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res 70, 7992–8002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Darshan MS et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res 71, 6019–6029 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seiwert TY, Salama JK & Vokes EE The concurrent chemoradiation paradigm—general principles. Nat. Clin. Pract. Oncol 4, 86–100 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Perrotti M et al. Phase I/II trial of docetaxel and concurrent radiation therapy in localized high risk prostate cancer (AGUSG 03–10). Urol. Oncol 26, 276–280 (2008). [DOI] [PubMed] [Google Scholar]
- 101.Balcer-Kubiczek EK, Attarpour M, Jiang J, Kennedy AS & Suntharalingam M Cytotoxicity of docetaxel (Taxotere) used as a single agent and in combination with radiation in human gastric, cervical and pancreatic cancer cells. Chemotherapy 52, 231–240 (2006). [DOI] [PubMed] [Google Scholar]
- 102.Bentzen SM, Harari PM & Bernier J Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat. Clin. Pract. Oncol 4, 172–180 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Fizazi K et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J. Clin. Oncol 27, 2429–2435 (2009). [DOI] [PubMed] [Google Scholar]
- 104.Morris MJ et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J. Clin. Oncol 27, 2436–2442 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tu SM et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 357, 336–341 (2001). [DOI] [PubMed] [Google Scholar]
- 106.Morris MJ et al. Radium-223 in combination with docetaxel in patients with castration-resistant prostate cancer and bone metastases: a phase 1 dose escalation/randomised phase 2a trial. Eur. J. Cancer 114, 107–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morris MJ et al. Updated results: a phase I/IIa randomized trial of radium-223 + docetaxel versus docetaxel in patients with castrationresistant prostate cancer and bone metastases. J. Clin. Oncol 34(15S), 5075 (2016). [Google Scholar]
- 108.Morris MJ et al. Effects of radium-223 (Ra-223) with docetaxel versus docetaxel alone on bone biomarkers in patients with bone-metastatic castration-resistant prostate cancer (CRPC): a phase I/IIa clinical trial. J. Clin. Oncol 35, 154 (2017). [Google Scholar]
- 109.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03574571 (2019). [DOI] [PubMed]
- 110.Malamas AS, Gameiro SR, Knudson KM & Hodge JW Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget 7, 86937–86947 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geary SM, Lemke CD, Lubaroff DM & Salem AK Proposed mechanisms of action for prostate cancer vaccines. Nat. Rev. Urol 10, 149–160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02463799 (2019). [DOI] [PubMed]
- 113.Brahmer JR et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med 366, 2455–2465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.US Food and Drug Administration. TECENTRIQ (atezolizumab) prescribing information FDA; https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761034s000lbl.pdf (2016). [Google Scholar]
- 115.US Food and Drug Administration. KEYTRUDA (pembrolizumab) prescribing information FDA; https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s012lbl.pdf (2014). [Google Scholar]
- 116.Herbst RS et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.US Food and Drug Administration. FDA approves first cancer treatment for any solid tumor with a specific genetic feature FDA; https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm (2017). [Google Scholar]
- 118.Abida W et al. Microsatellite instability in prostate cancer and response to immune checkpoint blockade. J. Clin. Oncol 36, 5020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Bono J et al. KEYNOTE-199: pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol 36, 5007 (2018). [Google Scholar]
- 120.Deng L et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest 124, 687–695 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03093428 (2019). [DOI] [PubMed]
- 122.O’Connor MJ Targeting the DNA damage response in cancer. Mol. Cell 60, 547–560 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Mateo J et al. DNA repair in prostate cancer: biology and clinical implications. Eur. Urol 71, 417–425 (2017). [DOI] [PubMed] [Google Scholar]
- 124.Robinson D et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pritchard CC et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med 375, 443–453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fong PC et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med 361, 123–134 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Mateo J et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med 373, 1697–1708 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lorusso D et al. Spotlight on olaparib in the treatment of BRCA-mutated ovarian cancer: design, development and place in therapy. Drug Des. Dev. Ther 12, 1501–1509 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.AstraZeneca. Lynparza™ (olaparib) granted breakthrough therapy designation by US FDA for treatment of BRCA1/2 or ATM gene mutated metastatic castration resistant prostate cancer AstraZeneca; https://www.astrazeneca.com/media-centre/press-releases/2016/Lynparza-Olaparib-granted-Breakthrough-Therapy-Designation-by-USFDA-for-treatment-of-BRCA1-2-or-ATM-gene-mutated-metastatic-Castration-Resistant-Prostate-Cancer-28012016.html# (2016). [Google Scholar]
- 130.Ramos JD, Mostaghel EA, Pritchard CC & Yu EY DNA repair pathway alterations in metastatic castration-resistant prostate cancer responders to radium-223. Clin. Genitourin. Cancer 16, 106–110 (2018). [DOI] [PubMed] [Google Scholar]
- 131.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03076203 (2017). [DOI] [PubMed]
- 132.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03317392 (2019). [DOI] [PubMed]
- 133.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03188965 (2019). [DOI] [PubMed]
- 134.Wengner AM et al. ATR inhibitor BAY 1895344 shows potent anti-tumor efficacy in monotherapy and strong combination potential with the targeted alpha therapy radium-223 dichloride in preclinical tumor models. Cancer Res 77, 836 (2017). [Google Scholar]
- 135.Isaacsson Velho P et al. Efficacy of radium-223 in bone-metastatic castration-resistant prostate cancer with and without homologous repair gene defects. Eur. Urol 76, 170–176 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oyen WJ & de Bono JS Targeted alpha-based treatment of metastatic castration-resistant prostate cancer patients: revolutionizing systemic radiotherapy? J. Nucl. Med 57, 1838–1839 (2016). [DOI] [PubMed] [Google Scholar]
- 137.Hagemann UB et al. Targeted alpha therapy using a novel CD70 targeted thorium-227 conjugate in in vitro and in vivo models of renal cell carcinoma. Oncotarget 8, 56311–56326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hagemann UB et al. In vitro and in vivo efficacy of a novel CD33-targeted thorium-227 conjugate for the treatment of acute myeloid leukemia. Mol. Cancer Ther 15, 2422–2431 (2016). [DOI] [PubMed] [Google Scholar]
- 139.Dahle J et al. Assessment of long-term radiotoxicity after treatment with the low-dose-rate alpha-particle-emitting radioimmunoconjugate (227)Th-rituximab. Eur. J. Nucl. Med. Mol. Imaging 37, 93–102 (2010). [DOI] [PubMed] [Google Scholar]
- 140.Heyerdahl H, Abbas N, Brevik EM, Mollatt C & Dahle J Fractionated therapy of HER2-expressing breast and ovarian cancer xenografts in mice with targeted alpha emitting 227Th-DOTA-p-benzyltrastuzumab. PLOS ONE 7, e42345 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02581878 (2019). [DOI] [PubMed]
- 142.Chang SS Overview of prostate-specific membrane antigen Rev. Urol 6 (S10), 13–18 (2004). [PMC free article] [PubMed] [Google Scholar]
- 143.Hammer S et al. Preclinical pharmacology of the PSMA-targeted thorium-227 conjugate PSMA-TTC: a novel targeted alpha therapeutic for the treatment of prostate cancer. Proc. Am. Assoc. Cancer Res 58, 5202 (2017). [Google Scholar]
- 144.US National Library of Medicine. ClinicalTriais.gov https://clinicaltrials.gov/ct2/show/NCT03724747 (2019). [DOI] [PubMed]
- 145.Kratochwil C et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: dosimetry estimate and empiric dose finding. J. Nucl. Med 58, 1624–1631 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Kratochwil C et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J. Nucl. Med 59, 795–802 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Nonnekens J et al. (213)Bi-labeled prostate-specific membrane antigen-targeting agents induce DNA double-strand breaks in prostate cancer xenografts. Cancer Biother. Radiopharm 32, 67–73 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Sathekge M et al. (213)Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 44, 1099–1100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.US National Library of Medicine. ClinicalTriais.gov https://clinicaltrials.gov/ct2/show/NCT02043678 (2019). [DOI] [PubMed]
- 150.US National Library of Medicine. ClinicalTriais.gov https://clinicaltrials.gov/ct2/show/NCT02225704 (2018). [DOI] [PubMed]
- 151.US National Library of Medicine. ClinicalTriais.gov https://clinicaltrials.gov/ct2/show/NCT02814669 (2019). [DOI] [PubMed]


