Abstract
Purpose
To investigate the efficacy and safety of a newly developed thermo-responsive sol-gel, ABT13107, for reducing the formation of intrauterine adhesions (IUAs) after hysteroscopic surgery.
Materials and Methods
In this multicenter, prospective, randomized trial (Canadian Task Force classification I), 192 women scheduled to undergo a hysteroscopic surgery at one of the eight university hospitals in South Korea were randomized into the ABT13107 group or the comparator (Hyalobarrier®) group in a 1:1 ratio. During hysteroscopic surgery, ABT13107 or Hyalobarrier® was injected to sufficiently cover the entire intrauterine cavity.
Results
The patients returned to their respective sites for safety assessments at postoperative weeks 1 and 4 and for efficacy assessments at postoperative week 4. The post-surgery incidence of IUAs was 23.4% in the ABT13107 group and 25.8% in the comparator group; this difference met the criteria for ABT13107 to be considered as not inferior to the comparator. No differences were found in the extent of adhesions, types of adhesions, or the cumulative American Fertility Society score between the two treatment groups. Most adverse events were mild in severity, and no serious adverse events occurred.
Conclusion
ABT13107, a new anti-adhesive barrier containing hyaluronic acid, was not inferior to the highly viscous hyaluronic acid anti-adhesive barrier, Hyalurobarrier® in IUA formation after hysteroscopic surgery (Clinical trial registration No. NCT 04007211).
Keywords: Hysteroscopy, hyaluronic acid, infertility, tissue adhesions, uterus
INTRODUCTION
Intrauterine adhesions (IUAs) refer to the scar tissue formed in the uterine cavity in thin film-like or thick band-like structures. IUAs can result in serious complications such as amenorrhea, abnormal uterine bleeding, recurrent miscarriages, and infertility due to improper embryo implantation. Patients with IUAs can also experience abnormal uterine bleeding or amenorrhea. IUAs are usually caused by intrauterine trauma or infection, such as traumatic curettage, endometritis, prolonged retention of an intrauterine device, or operative hysteroscopy using electrocautery.1,2
Many attempts have been made to prevent the formation of IUAs after intrauterine surgery, hysteroscopic surgery, and curettages.3,4,5,6,7 To reduce the adhesion formation process occurring immediately after intrauterine surgery, hormonal treatments or anti-adhesion barriers have been applied, including mechanical devices or chemical agents with anti-inflammatory properties, antioxidants, anticoagulants, and fibrinolytics.8,9,10,11
Among chemical agents, hyaluronan, a glycosaminoglycan with repeated disaccharide units, is the most commonly used. As hyaluronic acid is an elastoviscous fluid that degrades rapidly, it does not remain in the cavity long enough to maintain the mechanical distension effect during the endometrium's healing process after surgery; this is in contrast to mechanical interference devices, such as intrauterine balloons.12
The uterine cavity may vary in size and shape and is surrounded by a muscular layer of interlacing smooth muscle fibers. Therefore, unlike the nasal or ear cavities that are surrounded by bony structures, the uterine cavity usually collapses during the postoperative healing process, unless it is mechanically distended. In addition to that, the uterine cavity covered with normal endometrium without IUAs is important for the basic functions of the uterus, such as menstruation, and conception. Therefore, we need an anti-adhesion barrier specifically designed for the uterine cavity. In an effort to modify hyaluronic acid by increasing its viscosity and decreasing its rate of degradation, a crosslinked modification has recently been adopted.1,6,13,14,15
ABT13107, a new thermo-responsive sol-gel, was developed to have both the versatility of a liquid and the adhesiveness of a viscous gel. ABT13107 was specifically created for use in body cavities such as the intrauterine or nasal cavities. It was made from non-animal derived hyaluronic acid and synthetic poloxamers, which are known to have an excellent biocompatibility and anti-adhesion effects. As far as we know, no study has compared the efficacy of ABT13107 in preventing IUAs after hysteroscopic surgery.
The aim of this randomized clinical trial was to compare the efficacy and safety of the newly developed thermo-responsive sol-gel ABT13107 (Medytox Inc., Ochang, South Korea) with those of the highly viscous hyaluronic acid anti-adhesive barrier, Hyalobarrier® Gel Endo (Hyalurobarrier®) (Anika Therapeutic Inc., Bedford, MA, USA), in reducing IUA formation after hysteroscopic surgery.
MATERIALS AND METHODS
Study subjects and study design
This study was designed as a multi-center, double-blind, randomized, parallel, active-controlled pivotal study to assess the anti-adhesive effect and safety of ABT13107 for the prevention of IUAs after hysteroscopic surgery. Women between 19 and 70 years of age who were scheduled to undergo a hysteroscopic surgery for submucosal myoma, endometrial polyp, IUA, uterine septa, or abnormal uterine bleeding at eight sites in South Korea between February 2017 and August 2018 were enrolled in this study. Participants were excluded if they had any of the following: gynecologic malignancies, uterine cervix stenosis, uterovaginal prolapse, pelvic inflammatory disease, or excessive uterine bleeding requiring hysterectomy. Subjects with a history of hypersensitivity or allergy to any component of the two study materials were also excluded.
This study was conducted according to the regulations of Korean Good Clinical Practice and the Declaration of Helsinki. This study protocol was approved by the Asan Medical Center Institutional Review Board (approval No. 2016-1326), and the clinical trial was registered (URL: https://www.clinicaltrials.gov. Unique identifier: NCT04007211). Informed consent was obtained from each patient.
Randomization and blinding
Subjects who satisfied the inclusion criteria and provided written informed consent were randomly assigned to either the study group (ABT13107) or the comparator group (Hyalobarrier®) in a 1:1 ratio using the dynamic allocation method with stratification by site and indication for hysteroscopic operation (cause of surgery). Allocation to each group was performed in the order of enrollment.
Surgical and study procedures
Subjects were treated in a sterilized environment after confirming complete hemostasis and removal of the distension media. During the procedure, a suction tool with a blunt tip was used to prevent additional endometrial injury. A narrow catheter was inserted through the cervix to locate the upper third of the uterine cavity. Up to 10 mL of either ABT13107 or Hyalobarrier® were injected to sufficiently cover the entire intrauterine cavity.
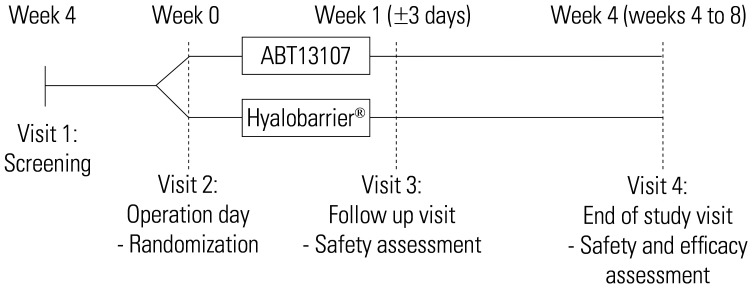
The subjects returned to their respective sites for safety assessments at postoperative weeks 1 and 4 and for efficacy assessments at postoperative week 4 (Fig. 1). The primary efficacy outcome was measured by the incidence of de novo IUAs at week 4. Secondary efficacy outcomes were measured by American Fertility Society (AFS) scores for adhesion extent(area), adhesion type, and their cumulative score at week 4 based on the independent evaluators' and investigators' assessments. The incidence of de novo IUAs based on the judgment of the investigator was also included as a secondary efficacy outcome. Any side-effects associated with the application of ABT13107 or Hyalobarrier® were the safety outcomes of this study.
Fig. 1. CONSORT flow diagram of participants.
The laboratory tests, including hematology, coagulation testing, electrolyte testing, liver function tests, lipid testing, and urinalysis were performed at week 0 and week 4 for safety assessment. Adverse events (AEs) were classified as mild, moderate, and severe. The criteria were divided based on the severity of symptoms and the need of intervention.
Three gynecology professors capable of assessing adhesion formation, extent, and type of adhesion were selected as independent evaluators. The independent evaluators assessed the adhesions using photographs of the intrauterine cavity taken via hysteroscopy at postoperative week 4 according to the AFS classification of IUAs (Supplementary Table 1, only online).16 To maintain blinding, the evaluators were not allowed to discuss assessment results with the other investigators or the study sponsor until completion of all assessments.
Statistical analysis
The optimal sample size for an adequate power to detect statistical significance was determined by reviewing the available literature.13,14,17,18,19,20 The average incidence of IUAs was 10% among patients who had received anti-adhesion treatments and 44% among non-treated patients. Based on this information, the non-inferiority margin was set to 17% from half the difference of the efficacy results. A total of 192 subjects, taking into account a 15% drop-out rate, was determined to be the minimum number required to determine the non-inferiority of ABT13107 when compared to Hyalobarrier®.
All statistical analyses were performed using the StatView System® (SAS Institute, Cary, NC, USA). A p-value less than 0.05 was considered statistically significant. For the primary efficacy outcome, a one-sided 97.5% confidence interval (CI) was presented for the difference between the study and comparator group. If the upper limit of the confidence interval was lower than the non-inferiority margin, it was determined that the study group was non-inferior to the comparator group. For continuous variables, the number, mean, standard deviation, median, maximum, and minimum were presented; for categorical variables, frequency and percentage were presented. Values were not adjusted for analysis in this study.
RESULTS
Subject disposition and baseline characteristics
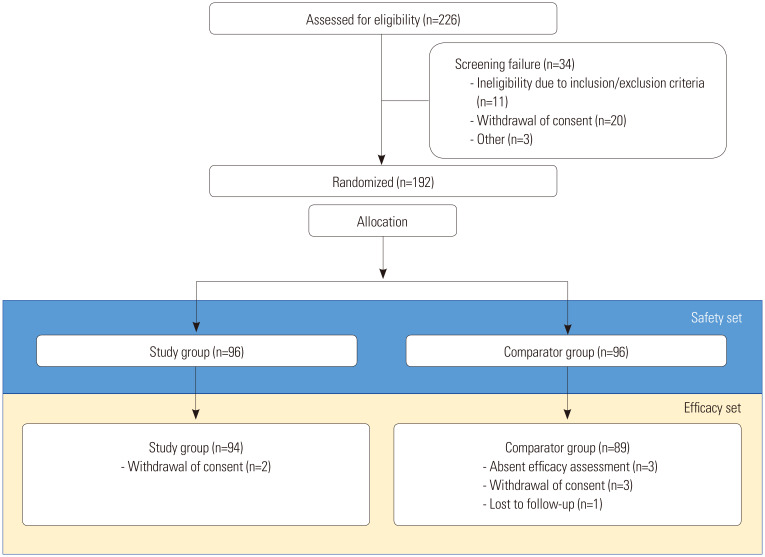
Of the 226 screened patients, 192 (study group: 96; comparator group: 96) were included and randomized in this study. After randomized allocation, six patients withdrew from the study (study group: 2; comparator group: 4) and three did not complete the efficacy assessment (comparator group: 3). In all, 192 patients who were administered the study material and who had received at least one safety assessment were included in the set for safety analysis; 183 patients who completed the study and received at least one efficacy assessment were included in the set for efficacy analysis (Fig. 2). The demographics and baseline characteristics obtained at screening, including age, height, weight, body mass index, and indication for operation, did not differ significantly between the treatment groups (Table 1).
Fig. 2. Number of patients assessed and enrolled in the clinical trial.
Table 1. Demographics and Baseline Characteristics of the Study Subjects.
| Variable | Study group (n=96) | Comparator group (n=96) | p value |
|---|---|---|---|
| Age (yr) | 40.8±9.1 | 41.2±9.0 | 0.750* |
| Height (cm) | 160.32±6.02 | 160.63±5.37 | 0.711* |
| Weight (kg) | 59.56±9.26 | 59.28±8.88 | 0.830* |
| BMI (kg/m2) | 23.25±3.94 | 23.00±3.43 | 0.637* |
| Indication for operation | 1.000† | ||
| Submucosal myoma | 28 (29.2) | 28 (29.2) | |
| Endometrial polyp | 57 (59.4) | 57 (59.4) | |
| Others | 11 (11.4) | 11 (11.4) | |
| Intra-uterine adhesions | 6 (6.25) | 7 (7.29) | - |
| Uterine septae | 0 | 3 (3.13) | - |
| Dysfunctional uterine bleeding | 5 (5.21) | 1 (1.04) | - |
BMI, body mass index.
Data are presented as mean±standard deviation or number (%).
*p-value by independent t-test, †p-value by chi-square test.
In terms of indications for operation, endometrial polyp was the most common indication (57/92 patients, 59.4%), and submucosal myoma (28/92 patients, 29.2%), and others (11/92 patients, 11.4%) were followed. Others were intra-uterine adhesions (6 patients, 6.25% for study group; 7 patients, 7.29% for comparator group), dysfunctional uterine bleeding (5 patients, 5.21% for study group; 1 patient, 1.04% for comparator group), and uterine septae (3 patients, 3.13% for comparator group).
If there was a medical history or an operation history, as of the time of written informed consent (present illness), then the diagnosis name was coded according to the System Organ Class (SOC) and Preferred Term (PT) of Medical Dictionary for Regulatory Activitie (MedDRA) 20.0. As a result of classification according to the SOC, “reproductive system and breast disorders” was investigated the most for both study and comparator groups. The most common PT terms were, “adenomyosis” in 6.25% (6/96 subjects), “endometrial hyperplasia” in 4.17% (4/96 subjects), and “uterine polyp” in 4.17% (4/2.96 subjects) of the study group, and “ovarian cyst” in 8.33% (8/96 subjects), “cervical polyp” in 5.21% (5/96 subjects), and “uterine polyp” in 5.21% (5/96 subjects) of the comparator group.
Efficacy evaluation
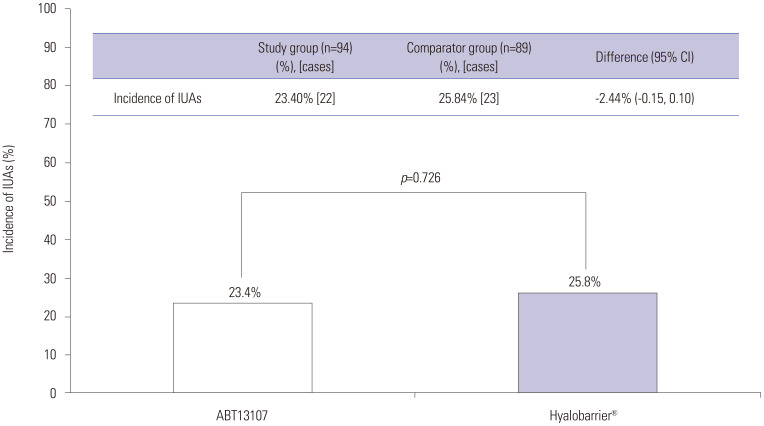
The primary outcome–incidence of de novo IUAs post-surgery according to the independent evaluators' assessments–revealed the following; from the efficacy set of 183 patients, 24.6% (45 patients) experienced IUA formation: 23.40% (22/94 patients) in the study group and 25.84% (23/89 patients) in the comparator group. The difference in the incidence of adhesions between the two groups (study group-comparator group) was −2.44% (95% CI: −0.15, 0.10), and the upper limit of the 95% CI was 10, which satisfied the non-inferiority margin of <17 (Table 2, Fig. 3).
Table 2. Primary Efficacy Outcome: IUAs at Postoperative Week 4.
| Variable | Study group (n=94) | Comparator group (n=89) | Difference (95% CI) | p value |
|---|---|---|---|---|
| Independent evaluator-assessed | ||||
| Incidence of IUAs | 23.40 (22) | 25.84 (23) | −2.44 (−0.15, 1.10) | 0.726† |
| Extent of cavity involved | 1.16±0.34 | 1.18±0.40 | −0.02 (−0.20, 0.16) | 0.824* |
| Type of adhesion | 1.36±0.45 | 1.40±0.52 | −0.05 (−0.28, 0.19) | 0.701* |
| Cumulative score | 2.49±0.62 | 2.58±0.87 | −0.09 (−0.46, 0.27) | 0.609* |
| Investigator-assessed | ||||
| Incidence of IUAs | 19.15 (18) | 13.48 (12) | 5.67 (−0.05, 0.16) | 0.271† |
| Extent of cavity involved | 1.06±0.24 | 1.58±1.16 | −0.53 (−1.27, 0.22) | 0.148* |
| Type of adhesion | 1.39±0.50 | 1.25±0.45 | 0.14 (−0.23, 0.51) | 0.447* |
| Cumulative score | 2.44±0.62 | 2.83±1.59 | −0.39 (−1.42, 0.65) | 0.432* |
IUAs, intrauterine adhesions.
Data are presented as mean±standard deviation. Incidence of IUAs is presented as the frequency and (number of cases).
*p-value by independent t-test, †p-value by Cochran-Mantel-Haenszel test (adjusted to underlying disease).
Fig. 3. The primary efficacy outcomes: intrauterine adhesions (IUAs) between ABT13107 and Hyalobarrier® assessed by independent evaluators at week 4. CI, confidence interval.
As to the secondary efficacy outcomes assessed by the independent evaluators and the investigator at postoperative week 4, we found no significant difference between the treatment groups in the extent of adhesion, type of adhesion, or the cumulative score, verifying the non-inferiority of ABT13107 to Hyalobarrier® (Table 2).
Safety evaluation
AEs after treatment occurred in 25.0% (24/96 patients, 37 events) of the study group and 25.0% (24/96 patients, 29 events) of the comparator group. Most of the AEs were mild in severity, and no severe AEs occurred in either group. Mild AEs were 83.78% (31 cases) for the study group and 86.21% (25 cases) for the comparator group. Moderate AEs were 16.22% (6 cases) and 13.79% (4 cases) for the study and comparator group, respectively. There was no significant difference between the two groups for the severity of AEs. Adverse device effects (ADEs) occurred in 6.3% (6/96 subjects, 8 events) of the study group and 3.1% (3/96 subjects, 3 events) of the comparator group, and there was no significant difference between the two groups (p=0.497) (Table 3). In the study group, increase in serum low density lipoprotein (LDL) concentration was the most frequent occurring in 2.08% (2/96 subjects, 2 cases) of subjects, followed by ‘dysmenorrhea,’ ‘pelvic pain,’ ‘palpitation,’ ‘abdominal pain,’ ‘chest pain,’ and ‘tendonitis,’ each occurring in 1.04% (1/96 subjects, 1 case) of subjects. In the comparator group, increase in serum LDL concentration, pyrexia, and vaginal infection was reported in each 1.04% (1/96 subjects, 1 case) of subjects. Overall, the ADEs reported in this clinical trial were classified as either expected or not likely to be relevant to the study device, and there were no significant findings in either group related to the laboratory tests, vital signs, or on physical examination.
Table 3. Overall Summary of AEs (Safety Set).
| Study group (n=96) n (%) [events] | Comparator group (n=96) n (%) [events] | p value* | |
|---|---|---|---|
| Treatment emergent Aes | 24 (25.0) [37] | 24 (25.0) [29] | 0.999 |
| Adverse device effects | 6 (6.3) [8]† | 3 (3.1) [3]‡ | 0.497 |
| Severity of AEs | 0.785 | ||
| Total | 37 (100.0) | 29 (100.0) | |
| Mild | 31 (83.78) | 25 (86.21) | |
| Moderate | 6 (16.22) | 4 (13.79) | |
| Severe | 0 | 0 |
AE, adverse event; LDL, low density lipoprotein.
Data are presented as the number, (frequency), and [number of cases].
*p-value by chi-square test, †Increase in serum LDL concentration (2 cases), dysmenorrhoea (1 case), pelvic pain (1 case), palpitation (1 case), abdominal pain (1 case), chest pain (1 case), and tendonitis (1 case), ‡Increase in serum LDL concentration (1 case), pyrexia (1 case), and vaginal infection (1 case).
DISCUSSION
This randomized clinical trial revealed no difference in efficacy or safety for the prevention of IUAs after hysteroscopic surgeries between ABT13107, the newly developed thermo-responsive sol-gel, and Hyalobarrier®, a composition of highly purified auto-cross-linked hyaluronic acid gel.
In the present study, the overall incidence of IUAs was 23.40% in the ABT13107 group and 25.84% in the comparator group. The mean cumulative AFS score for IUAs found during the follow-up hysteroscopy was 2.49 in the ABT13107 group and 2.58 in the comparator group at week 4 (as assessed by independent evaluators). These AFS scores show that the adhesion types were between “filmy” and “filmy and dense”, and the extent of the cavity involved was between “<1/3” and “1/3 to 2/3” for both groups. Although lower than the incidence with no use of prophylaxis,21 the incidence of IUAs seemed to be higher, whereas the adhesion score was similar to those of previous studies using other cross-linked hyaluronic acid treatments.1,14,22 However, since the indications for the surgeries (dilation and curettage for termination, adhesiolysis, or mass excision), as well as the patient characteristics differed across studies, the efficacy of IUA prevention should be compared only with controls within the same study. For example, the mean age in the current study (about 41 years old) was higher than that of other studies (mainly early 30s); therefore, there were more participants who did not have plans for future pregnancies. Consequently, the surgeons may not have been as concerned with avoiding damage to the endometrium during these patients' hysteroscopic surgeries.
In recent years, anti-adhesive gels derived from hyaluronic acid have been widely used to prevent IUAs, as they reduce inflammation and improve re-epithelialization and wound healing.23 A hyaluronic acid-based adhesion barrier has been widely used for preventing IUA after operative hysteroscopy. Although there are controversies, there is a lack of reproducible evidence that hyaluronic acid may decrease the incidence and severity of postoperative IUAs. A recent meta-analysis revealed that the use of cross-linked hyaluronic acid gel after hysteroscopic operations effectively reduced IUAs24; now, application of a cross-linked hyaluronic acid after operation is recommended to reduce the development of IUAs.25
ABT13107 is also a hyaluronic-acid-based anti-adhesive barrier with a sol-gel transition property in response to a rise in temperature, called the thermo-responsive sol-gel transition, and has the unique characteristic of exerting the anti-adhesion effect in the uterine cavity. In a liquid state, it can easily be applied into the cavity and can spread evenly inside uterine cavities varying in size and shape. Then, the solution becomes a gel in response to the body's temperature. Unlike liquid-type barriers, the gel-state of ABT13107 can remain for longer in the uterine cavity without an immediate push back. That is, the thermo-responsive sol-gel transition provides both the ease of use and extension of exposure to the targeted body parts. Although the anti-adhesive efficacy of ABT13107 was comparable with Hyalobarrier® in the present study, the thermo-responsive solgel could theoretically be a better option for preventing IUAs in clinical practice.
This study had several strengths. First, it was a multicenter, double-blind, randomized controlled trial investigating the efficacy and safety of the newly developed adhesion barrier. Second, almost all of the participants completed the study, and only a small number were lost to follow-up. Third, we stratified the subjects according to the indication for the operation, as the risks for developing IUAs after surgery are known to differ between myomectomy and polypectomy (3.6% after polypectomy and 31.3% after myomectomy).18 This stratification can provide a clearer result regarding the efficacy of the two antiadhesives. Fourth, blinded, experienced clinicians evaluated the existence and severity of the IUAs found during the second hysteroscopy. Because visual measurement of an adhesion is inevitably subjective, blinding of this assessment was helpful to avoid ascertainment bias.
However, this study also had some limitations. Firstly, the follow-up duration for determining the degree of IUAs was relatively short (4 weeks). However, as entire re-epithelialization of the uterine cavity and stromal growth usually begins by day 5–6 of the menstrual cycle, we considered 4 weeks of follow-up to be sufficient.26 Secondly, there was a lack of data regarding pregnancy outcomes, changes in menstruation, and other symptoms. Finally, clinical heterogenicity may have existed regarding operating conditions. The distension media (normal saline) was the same at all study sites, but the power of electrocautery varied. Formation of adhesions may be associated with the location or size of the mass, the surgical procedure, including cautery use, the surgeon's skill, or the type of surgery performed. However, these factors were not considered together in previous studies investigating the anti-adhesive effects of various methods.
This was not a placebo-controlled trial, we did not demonstrate that the incidence of IUAs in the study group was reduced compared to that of patients who received only a hysteroscopic operation without an anti-adhesive barrier. Therefore, to determine the best method to reduce IUAs, further well-designed, large-scale, randomized controlled trials are needed.
In conclusion, our findings showed that ABT13107, a new anti-adhesive barrier containing hyaluronic acid, was non-inferior to the highly viscous hyaluronic acid anti-adhesive barrier, Hyalurobarrier®, in IUA formation after hysteroscopic surgery. To determine the best method to reduce IUAs, further well-designed, large-scale, adequately powered randomized controlled trials are needed.
ACKNOWLEDGEMENTS
This study (NCT04007211) was supported by Medytox Inc., Seoul, Republic of Korea. The content of this publication reflects the opinions and experience of experts.
This research was supported by Medytox Inc. and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare of the Republic of Korea (grant number: HI17C0565).
Footnotes
Woo Shun Lee is an employee of Medytox Inc. The other authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Data curation: Dong-Yun Lee, Sa Ra Lee, and Woo Shun Lee.
- Formal analysis: Seul Ki Kim, Jong Kil Joo, and Woo Shun Lee.
- Funding acquisition: Woo Shun Lee.
- Investigation: Dong-Yun Lee, Sa Ra Lee, Seul Ki Kim, Jong Kil Joo, Jung-Ho Shin, SiHyun Cho, Joon Cheol Park, and Sung Hoon Kim.
- Methodology: all authors.
- Supervision: Joon Cheol Park and Sung Hoon Kim.
- Validation: Jung-Ho Shin and SiHyun Cho.
- Visualization: Dong-Yun Lee and Sa Ra Lee.
- Writing—original draft: Dong-Yun Lee and Sa Ra Lee.
- Writing—review and editing: Joon Cheol Park and Sung Hoon Kim.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
American Fertility Society Score for Intrauterine Adhesions
References
- 1.Guida M, Acunzo G, Di Spiezio Sardo A, Bifulco G, Piccoli R, Pellicano M, et al. Effectiveness of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a prospective, randomized, controlled study. Hum Reprod. 2004;19:1461–1464. doi: 10.1093/humrep/deh238. [DOI] [PubMed] [Google Scholar]
- 2.Al-Inany H. Intrauterine adhesions. An update. Acta Obstet Gynecol Scand. 2001;80:986–993. [PubMed] [Google Scholar]
- 3.Risberg B. Adhesions: preventive strategies. Eur J Surg Suppl. 1997;(577):32–39. [PubMed] [Google Scholar]
- 4.Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD000475. doi: 10.1002/14651858.CD000475. [DOI] [PubMed] [Google Scholar]
- 5.Watson A, Vandekerckhove P, Lilford R. Liquid and fluid agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD001298. doi: 10.1002/14651858.CD001298. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Wu L, Zhou Y, Fan X, Huang J, Wu J, et al. New crosslinked hyaluronan gel for the prevention of intrauterine adhesions after dilation and curettage in patients with delayed miscarriage: a prospective, multicenter, randomized, controlled trial. J Minim Invasive Gynecol. 2019;26:94–99. doi: 10.1016/j.jmig.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Bosteels J, Weyers S, Kasius J, Broekmans FJ, Mol BW, D'Hooghe TM. Anti-adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane Database Syst Rev. 2015;(11):CD011110. doi: 10.1002/14651858.CD011110.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Hellebrekers BW, Trimbos-Kemper TC, Trimbos JB, Emeis JJ, Kooistra T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000;74:203–212. doi: 10.1016/s0015-0282(00)00656-7. [DOI] [PubMed] [Google Scholar]
- 9.Ferland R, Mulani D, Campbell PK. Evaluation of a sprayable polyethylene glycol adhesion barrier in a porcine efficacy model. Hum Reprod. 2001;16:2718–2723. doi: 10.1093/humrep/16.12.2718. [DOI] [PubMed] [Google Scholar]
- 10.diZerega GS, Verco SJ, Young P, Kettel M, Kobak W, Martin D, et al. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum Reprod. 2002;17:1031–1038. doi: 10.1093/humrep/17.4.1031. [DOI] [PubMed] [Google Scholar]
- 11.Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomed Mater Res. 2002;63:10–14. doi: 10.1002/jbm.10040. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Wei M, Li TC, Huang Q, Huang D, Zhou F, et al. A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome: a cohort study. Eur J Obstet Gynecol Reprod Biol. 2013;170:512–516. doi: 10.1016/j.ejogrb.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Lu Q, Zhang Z, Xue M, Zhang Y, Zhang Y, et al. A randomized controlled trial on the efficacy and safety of a new crosslinked hyaluronan gel in reducing adhesions after gynecologic laparoscopic surgeries. J Minim Invasive Gynecol. 2015;22:853–863. doi: 10.1016/j.jmig.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Acunzo G, Guida M, Pellicano M, Tommaselli GA, Di Spiezio, Bifulco G, et al. Effectiveness of auto-cross-linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective, randomized, controlled study. Hum Reprod. 2003;18:1918–1921. doi: 10.1093/humrep/deg368. [DOI] [PubMed] [Google Scholar]
- 15.Huberlant S, Fernandez H, Vieille P, Khrouf M, Ulrich D, deTayrac R, et al. Application of a hyaluronic acid gel after intrauterine surgery may improve spontaneous fertility: a randomized controlled trial in New Zealand White rabbits. PLoS One. 2015;10:e0125610. doi: 10.1371/journal.pone.0125610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The American Fertility Society. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49:944–955. doi: 10.1016/s0015-0282(16)59942-7. [DOI] [PubMed] [Google Scholar]
- 17.Di Spiezio Sardo A, Spinelli M, Bramante S, Scognamiglio M, Greco E, Guida M, et al. Efficacy of a polyethylene oxide-sodium carboxymethylcellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery. J Minim Invasive Gynecol. 2011;18:462–469. doi: 10.1016/j.jmig.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Taskin O, Sadik S, Onoglu A, Gokdeniz R, Erturan E, Burak F, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7:351–354. doi: 10.1016/s1074-3804(05)60478-1. [DOI] [PubMed] [Google Scholar]
- 19.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–273. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 20.Pabuccu R, Onalan G, Kaya C, Selam B, Ceyhan T, Ornek T, et al. Efficiency and pregnancy outcome of serial intrauterine deviceguided hysteroscopic adhesiolysis of intrauterine synechiae. Fertil Steril. 2008;90:1973–1977. doi: 10.1016/j.fertnstert.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 21.Yang JH, Chen MJ, Chen CD, Chen SU, Ho HN, Yang YS. Optimal waiting period for subsequent fertility treatment after various hysteroscopic surgeries. Fertil Steril. 2013;99:2092–2096.e3. doi: 10.1016/j.fertnstert.2013.01.137. [DOI] [PubMed] [Google Scholar]
- 22.Hooker AB, de Leeuw R, van de Ven PM, Bakkum EA, Thurkow AL, Vogel NEA, et al. Prevalence of intrauterine adhesions after the application of hyaluronic acid gel after dilatation and curettage in women with at least one previous curettage: short-term outcomes of a multicenter, prospective randomized controlled trial. Fertil Steril. 2017;107:1223–1231.e3. doi: 10.1016/j.fertnstert.2017.02.113. [DOI] [PubMed] [Google Scholar]
- 23.Valachová K, Volpi N, Stern R, Soltes L. Hyaluronan in medical practice. Curr Med Chem. 2016;23:3607–3617. doi: 10.2174/0929867323666160824162133. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Xu Y, Yi N, Yi W. Efficacy and safety of hyaluronic acid gel for the prevention of intrauterine adhesion: a meta-analysis of randomized clinical trials. Gynecol Obstet Invest. 2018;83:227–233. doi: 10.1159/000486674. [DOI] [PubMed] [Google Scholar]
- 25.AAGL Elevating Gynecologic Surgery. AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynaecological Endoscopy (ESGE) Gynecol Surg. 2017;14:6. doi: 10.1186/s10397-017-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig H, Spornitz UM. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann N Y Acad Sci. 1991;622:28–46. doi: 10.1111/j.1749-6632.1991.tb37848.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
American Fertility Society Score for Intrauterine Adhesions