Abstract
Background
Initial Glasgow Coma Score (iGCS) is a well-known predictor of adverse outcomes following chronic subdural hemorrhage (cSDH). Frailty, i.e. a reduced physiologic reserve, is associated with poorer outcomes across the surgical literature, however, there is no consensus on the best measure of frailty. To date, no study has compared frailty’s ability to predict cSDH outcomes versus iGCS. The goal of this study was to, therefore, examine the prognostic value of the 5- (mFI-5) and 11-factor (mFI-11) modified frailty index, and Charlson Comorbidity Index (CCI) versus iGCS following cSDH.
Methods
Between January, 2016 and June, 2018, patients who presented to the emergency department with cSDH were retrospectively identified using the International Classification of Diseases (ICD) codes. mFI-5, mFI-11, and CCI scores were calculated using patient baseline characteristics. Primary endpoints were death and discharge home and subgroup analyses were performed among operative cSDH. Univariate and multivariate logistic regressions were used to determine predictors of primary endpoints.
Results
Of the 109 patients identified, the average age was 72.6±1.6 years and the majority (69/109, 63.3%) were male. The average CCI, mFI-5, and mFI-11 were 4.5 ±0.2, 1.5 ±0.1, and 2.2 ±0.1, respectively. Fifty (45.9%) patients required surgical intervention, 11 (10.1%) died, and 48 (43.4%) were discharged home. In the overall cohort, while the only multivariate predictor of mortality was iGCS (OR=0.58; 95%CI:0.44-0.77; p=0.0001), the CCI (OR=0.73; 95%CI:0.58-0.92; p=0.0082) was a superior predictor of discharge home compared to iGCS (OR=1.46; 95%CI:1.13-1.90; p=0.0041). Conversely, among those who received an operative intervention, the CCI, but not iGCS, independently predicted both mortality (OR=4.24; 95%CI:1.01-17.86; p=0.0491) and discharge home (OR=0.55; 95%CI:0.33-0.90; p=0.0170). Neither mFI nor age predicted primary outcomes in multivariate analysis.
Conclusion
While frailty is associated with worse surgical outcomes, the clinical utility of the mFI-5, mFI-11, and CCI in cSDH is unclear. We show that the iGCS is an overall superior predictor of mortality following cSDH but is outperformed by the CCI after operative intervention. Similarly, the CCI is the superior predictor of discharge home in cSDH patients overall and following an operative intervention. These results indicate that while the iGCS best predicts mortality overall, the CCI may be considered when prognosticating post-operative course and hospital disposition.
Keywords: modified frailty index, subdural hemorrhage, charlson comorbidity index, mortality, age, gcs
Introduction
Chronic subdural hemorrhage (cSDH) is an increasingly common pathology encountered in modern neurosurgical practice given its high frequency among the elderly [1]. The outcomes following cSDH are generally positive, but one large, recent cohort study found that age is correlated with poorer functional outcomes and lower rates of discharge home, and the majority of patients with cSDH are in their 80s [2]. While both advanced chronological age and low initial Glasgow Coma Score (iGCS) are established predictors of poorer outcomes following cSDH, [2] there is a paucity of information regarding the effect of a patients’ underlying comorbidities on cSDH prognosis.
Frailty, i.e. a reduced physiologic reserve, is an emerging concept across the surgical literature that aims to elucidate the differential effects of age versus the cumulative effect of multiple comorbidities on surgical outcomes. While frailty, as most often measured by the modified frailty index (mFI-11), has been associated with poorer neurosurgical outcomes, its effect among those with cSDH is unclear [3-6]. To date, no study has compared the effect of frailty versus iGCS for predicting outcomes in both operative and non-operative cSDH. Complicating matters further, with over 215 different frailty indices in the literature [7], there remains no consensus regarding which frailty index best predicts outcomes; an effect that may be pathology and patient-population specific. Other common measures of frailty are the Charlson Comorbidity Index (CCI) and the new 5-factor modified frailty index (mFI-5). As such, the goal of this study is to perform a comparative analysis of multiple measures of frailty including the CCI, mFI-5, and mFI-11, versus iGCS for predicting mortality and discharge location among both operative and non-operative cSDH patients.
Materials and methods
Study design and setting
This retrospective study was performed between January 2016 and June 2018 at a quaternary academic referral center (Westchester Medical Center) with high neurosurgical volume. Institutional Review Board Approval with a waiver of informed consent was obtained from New York Medical College & Westchester Medical Center (IRB 12921). All data were retrospectively collected using the electronic medical record system by trained and monitored data abstractors.
Subject selection
cSDH patients were identified by reviewing the International Classification of Diseases (ICD) codes for subdural hemorrhage for all patients presenting to our emergency department in the study period. Inclusion criteria were patients 14 years or older who presented with a chronic subdural hemorrhage. Patients were excluded if they had a history of (non-subdural related) neurosurgical procedure or were found to not have a chronic SDH on in-house imaging. We defined cSDH as any subdural hemorrhage with a chronic component including mixed, acute on chronic, pure chronic, or subacute SDH.
Measures and endpoints
For each patient, demographics, smoking and alcohol abuse history, anti-coagulant or anti-platelet (AC/AP) drug use, GCS score, chief complaint, and cSDH characteristics (thickness, prior SDH), and presence of an isolated head injury were collected. The eleven-factor modified frailty index (mFI-11) was calculated by assigning one point for the presence of each of the following pre-hemorrhage characteristics for a maximum of 11 points: hypertension requiring medication, congestive heart failure, myocardial infarction, previous percutaneous coronary intervention or angina, transient ischemic attack or cerebrovascular accident without neurological deficit, cerebrovascular accident with neurological deficit, peripheral vascular disease or ischemic chest pain, chronic obstructive pulmonary disease or current pneumonia, diabetes mellitus, non-independent functional status, and impaired sensorium (Table 1). Non-independent functional status was defined as requiring assistance from another person for activities of daily living [8-11]. The five-factor modified frailty index (mFI-5) was defined similarly but with the presence of hypertension requiring medication, congestive heart failure, chronic obstructive pulmonary disease or current pneumonia, diabetes mellitus, or non-independent functional status [12,13]. The CCI was calculated as described extensively in prior literature (Table 2) [14,15].
Table 1. 11- and 5-factor modified frailty index (mFI) characteristics and prevalence in patients with chronic subdural hemorrhage (cSDH).
| History of: | mFI-5 | mFI-11 |
| Hypertension on medications | 77 (70.6%) | |
| Congestive Heart Failure | 14 (12.8%) | |
| Diabetes mellitus | 24 (22.0%) | |
| Chronic Obstructive Pulmonary Disease, Pneumonia | 9 (8.3%) | |
| Non-independent functional status | 35 (32.1%) | |
| History of transient ischemic attack or cerebrovascular accident without neurological deficit | - | 17 (15.6%) |
| Myocardial Infarction | - | 9 (8.3%) |
| Peripheral Vascular Disease or ischemic rest pain | - | 5 (4.6%) |
| Cerebral vascular accident with deficit | - | 9 (8.3%) |
| Previous coronary intervention or angina | - | 24 (22.0%) |
| Impaired Sensorium | - | 21 (19.3%) |
Table 2. Charlson Comorbidity Index (CCI) characteristics and prevalence in patients with chronic subdural hemorrhage (cSDH).
| Characteristic | Points | Prevalence | ||
| Age (years) | ||||
| <50 | 0 | 6 (5.5%) | ||
| 50-59 | 1 | 18 (16.5%) | ||
| 60-69 | 2 | 20 (18.3%) | ||
| 70-79 | 3 | 17 (15.6%) | ||
 80 80 |
4 | 48 (44.0%) | ||
| Myocardial Infarction | 1 | 9 (8.3%) | ||
| Congestive Heart Failure | 1 | 14 (12.8%) | ||
| Peripheral Vascular Disease | 1 | 5 (4.6%) | ||
| Cerebrovascular Accident or Transient Ischemic Attack | 1 | 17 (15.6%) | ||
| Dementia | 1 | 19 (17.4%) | ||
| Chronic Obstructive Pulmonary Disease | 1 | 9 (8.3%) | ||
| Connective Tissue Disease | 1 | 3 (2.8%) | ||
| Peptic Ulcer Disease | 1 | 5 (4.6%) | ||
| Liver Disease | ||||
| Mild | 1 | 3 (2.8%) | ||
| Moderate to Severe | 3 | 5 (4.6%) | ||
| Diabetes Mellitus | ||||
| Uncomplicated | 1 | 22 (20.2%) | ||
| End organ damage | 2 | 2 (1.8%) | ||
| Hemiplegia | 2 | 2 (1.8%) | ||
| Moderate to Severe Chronic Kidney Disease | 2 | 14 (12.8%) | ||
| Solid tumor | ||||
| Localized | 2 | 13 (11.9%) | ||
| Metastatic | 6 | 1 (0.9%) | ||
| Leukemia | 2 | 3 (2.8%) | ||
| Lymphoma | 2 | 1 (0.9%) | ||
| AIDS | 6 | 0 (0.0%) | ||
Primary endpoints were discharge home and death. Secondary endpoints needed for surgical intervention (craniotomy, burr hole craniotomy, or subdural evacuating port system (SEPS) treatment), hospital length of stay (hLOS), ICU length of stay (ICU-LOS), deep vein thrombosis (DVT), pulmonary embolism (PE), pneumonia, tracheostomy, gastrostomy, and discharge GCS (excluding deaths).
Statistical analysis
Normal distributions were determined using an Anderson-Darling normality test. T-tests were used for normally distributed continuous samples and Mann-Whitney tests were used for non-normally distributed continuous samples. Continuous data were shown using mean ± standard error of the mean (SEM). Fisher’s exact tests were used for binary variables and odds ratios (OR) are shown with 95% confidence intervals (95% CI). Subgroup analyses were conducted among those who required an operation. Similar to prior studies, multivariate logistic regressions were performed via the enter method using known predictors of outcomes sex, prior subdural hemorrhage, craniotomy, AC/AP use, iGCS, and SDH thickness followed by forward conditional addition of either mFI-11, mFI-5, or CCI to the model. Age was not included due to collinearity with the CCI. No collinearity was detected in either model, as defined as a variance inflation factor of less than one or greater than 10. Statistical analysis was performed using Prism 8.3.0 (GraphPad Software Inc., La Jolla, CA) and SPSS version 25 (IBM Corp., Armonk, NY). Significance was defined as p<0.05.
Results
Demographics and baseline characteristics
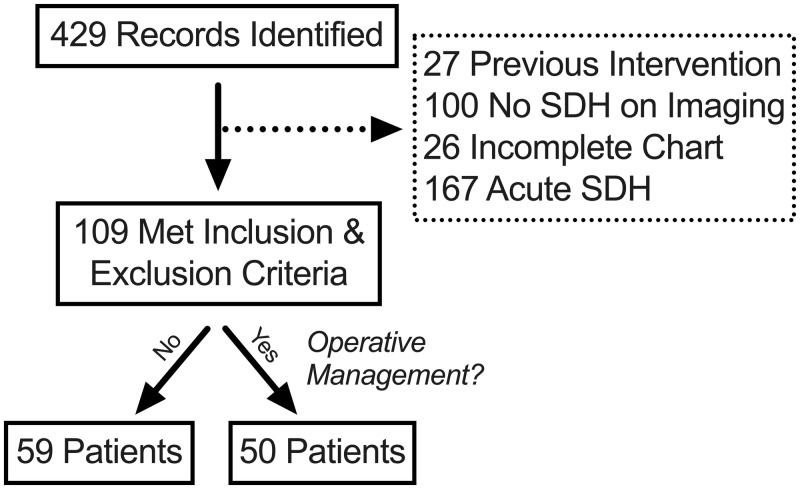
Of the 429 records identified, 109 met inclusion and exclusion criteria (Figure 1). The average age (range: 14-98 years) of the cohort was 72.6 ±1.6 years, the majority of patients were men (69/109, 63.3%), and 25 (63.3%) had a history of prior subdural hemorrhage (Table 3). As expected, the most common chief complaint was fall (74/109, 67.9%) and half of the patients had taken AC/AP medication prior to hemorrhage (55/109, 50.5%). The average iGCS was 13.4 ±0.3 (range: 3T-15) and the majority of patients had an isolated head injury (100/109, 91.7%). Most hemorrhages were of mixed density (93/109, 85.3%) and the average thickness was 16.0 ±0.7mm. For other baseline characteristics, see Table 3.
Figure 1. Patient identification and selection.
SDH: subdural hemorrhage
Table 3. Baseline characteristics and comparison between operative and non-operative patients.
^indicates non-normally distributed sample. AC/AP: Anticoagulation/Antiplatelet medication, MVC: Motor Vehicle Collision, iGCS: initial Glasgow Coma Score, cSDH: chronic subdural hemorrhage, CCI: Charlson Comorbidity Index, mFI: modified frailty index.
| Overall (n=109) | Non-operative (n=59) | Operative (n=50) | P-value | ||
| Age (years)^ | 72.6 ±1.6 | 76.5 ±2.1 | 67.9 ±2.2 | 0.0020 | |
| Men | 69 (63.3%) | 39 (57.4%) | 30 (60.0%) | 0.8509 | |
| Prior subdural hemorrhage | 25 (22.9%) | 14 (23.7%) | 11 (22.0%) | >0.9999 | |
| Smoking history | 39 (35.8%) | 24 (40.7%) | 15 (30.0%) | 0.3167 | |
| Alcohol abuse | 18 (16.5%) | 9 (15.3%) | 9 (18.0%) | 0.7978 | |
| AC/AP | 55 (50.5%) | 27 (45.8%) | 28 (56.0%) | 0.3384 | |
| Chief Complaint | |||||
| Fall | 74 (67.9%) | 43 (72.9%) | 31 (62.0%) | 0.3034 | |
| Altered Mental Status | 12 (11.0%) | 7 (11.9%) | 5 (10.0%) | >0.9999 | |
| MVC | 3 (2.8%) | 0 (0.0%) | 3 (6.0%) | 0.0934 | |
| Assault | 2 (1.8%) | 1 (1.7%) | 1 (2.0%) | >0.9999 | |
| Other/unknown | 20 (18.3%) | 10 (17.0%) | 10 (20.0%) | 0.8050 | |
| iGCS^ | 13.4 ±0.3 | 12.9 ±0.5 | 13.9 ±0.3 | 0.0423 | |
| Isolated head Injury | 100 (91.7%) | 51 (86.4%) | 49 (98.0%) | 0.0370 | |
| cSDH thickness (mm)^ | 16.0 ±0.7 | 13.6 ±0.9 | 18.8 ±1.0 | 0.0003 | |
| Type of cSDH | |||||
| Mixed Density | 93 (85.3%) | 49 (83.1%) | 44 (88.0%) | 0.5900 | |
| Subacute | 5 (4.6%) | 4 (6.8%) | 1 (2.0%) | 0.3721 | |
| Pure Chronic | 11 (10.1%) | 6 (10.2%) | 5 (10.0%) | >0.9999 | |
| CCI | 4.5 ±0.2 | 5.1 ±0.3 | 3.8 ±0.3 | 0.0033 | |
| mFI-5^ | 1.5 ±0.1 | 1.5 ±0.1 | 1.4 ±0.1 | 0.2580 | |
| mFI-11^ | 2.2 ±0.1 | 2.5 ±0.2 | 1.9 ±0.2 | 0.0471 | |
The average CCI was 4.5 ±0.2 (range: 0-11) and 42 (38.5%) had a CCI³6. The most commonly identified CCI characteristic, beyond age, was a history of uncomplicated diabetes (22/109, 20.2%) followed by a history of dementia (19/109, 17.4%) (Table 2). The average mFI-11 (range: 0-7) and mFI-5 (range: 0-4) was 2.2 ±0.1 and 1.5 ±0.1, respectively, with 73 (67.0%) having a mFI-11 ³2 and 55 (50.5%) having a mFI-5 ³2. The most commonly identified characteristic in both the mFI-5 and mFI-11 systems was hypertension on medications (77/109, 70.6%) followed by non-independent functional status (35/109, 32.1%) (Table 1).
Clinical course and outcomes
One hundred and five (96.3%) patients were admitted and 50 (45.9%) required a surgical evacuation of SDH. Five (4.6%) patients developed a DVT, 1 (0.9%) a PE, and 5 (4.6%) pneumonia. Six (5.5%) patients required a tracheostomy tube and 9 (8.3%) a gastrostomy tube. The average ICU-LOS was 5.0 ±0.5 days (range: 0-22) while the hLOS was 9.0 ±0.9 days (range: 1-62). Ultimately, the average discharge GCS was 14.3 ±0.2, a plurality of patients were discharged home (48/109, 44.0%), and 11 (10.1%) patients died (Table 4).
Table 4. Patient Outcomes and comparison between operative and non-operative patients.
^indicates non-normally distributed sample. hLOS: hospital length of stay, ICU-LOS: Intensive care unit length of stay.
| Overall (n=109) | Non-operative (n=59) | Operative (n=50) | P-value | ||
| hLOS (days)^ | 9.0 ±0.9 | 8.3 ±1.4 | 9.7 ±0.9 | 0.0139 | |
| ICU LOS (days) | 5.0 ±0.5 | 4.2 ±0.9 | 5.7 ±0.7 | 0.0011 | |
| Deep Vein Thrombosis | 5 (4.6%) | 2 (3.4%) | 3 (6.0%) | 0.6591 | |
| Pulmonary Embolism | 1 (0.9%) | 0 (0.0%) | 1 (2.0%) | 0.4587 | |
| Pneumonia | 5 (4.6%) | 2 (3.4%) | 3 (6.0%) | 0.6591 | |
| Tracheostomy | 6 (5.5%) | 3 (5.1%) | 3 (6.0%) | >0.9999 | |
| Gastrostomy | 9 (8.3%) | 5 (8.5%) | 4 (8.0%) | >0.9999 | |
| Discharge GCS (without death) ^ | 14.3 ±0.2 | 14.2 ±0.2 | 14.5 ±0.2 | 0.0793 | |
| Discharge location | |||||
| Home | 48 (44.0%) | 26 (44.1%) | 22 (44.0%) | >0.9999 | |
| Acute Rehab | 30 (27.5%) | 9 (15.3%) | 21 (42.0%) | 0.0025 | |
| Subacute Rehab | 6 (5.5%) | 4 (6.8%) | 2 (4.0%) | 0.6853 | |
| Nursing Home | 13 (11.9%) | 12 (20.3%) | 1 (2.0%) | 0.0029 | |
| Death | 11 (10.1%) | 7 (11.9%) | 4 (8.0%) | 0.5439 | |
Clinical course and outcomes: the effect of operative management
Patients who received operative evacuation of SDH were significantly younger (67.9 ±2.2 versus 76.5 ±2.1; p=0.0020), had a higher iGCS (13.9 ±0.3 versus 12.9 ±0.5; p=0.0423), larger SDH thickness (18.8 ±1.0 versus 13.6 ±0.9; p=0.0003), and were more likely to have an isolated head injury (OR=7.7; 95%CI: 1.1-86.9; p=0.0370), indicating careful selection of surgical candidates. Likewise, those that received an operation had decreased CCI (3.8 ±0.3 versus 5.1 ±0.3; p=0.0033) and mFI-11 (1.9 ±0.2 versus 2.5 ±0.2; p=0.0370) but not mFI-5 (p=0.2580) scores compared to those who did not have an operation. There were no significant baseline differences in history of SDH, smoking or alcohol abuse history, AC/AP use, or type of SDH between those who did or did not receive an intervention (Table 3).
As expected, those that received an operation had longer hospital (9.7 ±0.9 versus 8.3 ±1.4 days; p=0.0139) and ICU (5.7±0.7 versus 4.2±0.9days; p=0.0011) length of stay but no differences in complications (p>0.05) (Table 4). Interestingly, there were no differences in rates of discharge home or death between those who did or did not receive surgical drainage. However, patients in the operative group were more likely to be discharged to acute rehabilitation (OR=4.0; 95%CI: 1.7-9.2; p=0.0025), less likely to be discharged to a nursing home (OR=0.08; 95%CI: 0.007-0.48; p=0.0029), and trended to have a higher discharge GCS scores (14.5 ±0.2 versus 14.2±0.2; p=0.0793). Of the patients who received operative evacuation of SDH, 33 (66%) had a craniotomy while 17 (34%) had either a burr hole craniotomy or a subdural evacuation port system device placement. There were no significant differences in any primary or secondary endpoints between the two strategies of operative management (Table 4).
Clinical course and outcomes: the effect of patient frailty
As expected, patients in the CCI 6, mFI-11
6, mFI-11 2, and mFI-5
2, and mFI-5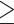 2 groups were each older than their non-frail counterparts (Table 5). Those in the CCI
2 groups were each older than their non-frail counterparts (Table 5). Those in the CCI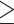 6 (p=0.0080) and mFI-11
6 (p=0.0080) and mFI-11 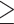 2 (p=0.0093) groups also presented with significantly lower iGCS scores. Those in the mFI-5
2 (p=0.0093) groups also presented with significantly lower iGCS scores. Those in the mFI-5 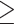 2 group also trended to have decreased iGCS scores compared to those in the mFI-5
2 group also trended to have decreased iGCS scores compared to those in the mFI-5  1 group. As noted above, those that received an operation had significantly lower CCI and mFI-11 scores compared to those that did not receive an intervention (Table 4). Despite this, there were no significant differences in SDH thickness, hospital or ICU LOS, complications, tracheostomy, or gastrostomy tube need. Patients in the mFI-11
1 group. As noted above, those that received an operation had significantly lower CCI and mFI-11 scores compared to those that did not receive an intervention (Table 4). Despite this, there were no significant differences in SDH thickness, hospital or ICU LOS, complications, tracheostomy, or gastrostomy tube need. Patients in the mFI-11  2 (p=0.0108) and mFI-5
2 (p=0.0108) and mFI-5  2 (0.0186) groups, but not CCI
2 (0.0186) groups, but not CCI 6, were discharged with significantly lower GCS scores. There were also significant reductions in rates of discharge home and corresponding increases in discharge to nursing home or hospice in each frailty group. Finally, while there were no significant differences in mortality, there were more deaths in each of the frail groups.
6, were discharged with significantly lower GCS scores. There were also significant reductions in rates of discharge home and corresponding increases in discharge to nursing home or hospice in each frailty group. Finally, while there were no significant differences in mortality, there were more deaths in each of the frail groups.
Table 5. The effect of frailty on primary and secondary outcomes.
CCI: Charlson Comorbidity Index, mFI: modified frailty index, iGCS: initial Glasgow Coma Score, hLOS: hospital length of stay, ICU-LOS: Intensive Care Unit length of stay.
| CCI | mFI-11 | mFI-5 | ||||||||
CCI 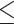 5 (n=67) 5 (n=67) |
CCI 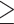 6 (n=42) 6 (n=42) |
P-value | mFI 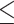 1 (n=36) 1 (n=36) |
mFI 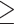 2 (n=73) 2 (n=73) |
P-value | mFI-5 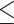 1 (n=54) 1 (n=54) |
mFI-5 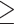 2 (n=55) 2 (n=55) |
p-value | ||
| Age (years) ^ | 66.8 ±2.1 | 81.8 ±1.6 | <0.0001 | 66.1 ±3.3 | 75.7 ±1.6 | 0.0035 | 69.1 ±2.4 | 75.9± 1.9 | 0.0349 | |
| iGCS^ | 13.6 ±0.4 | 13.0 ±0.4 | 0.0080 | 14.2 ±0.4 | 13.0 ±0.4 | 0.0093 | 13.7 ±0.4 | 13.1 ±0.4 | 0.0850 | |
| SDH thickness | 15.6 ±0.9 | 16.5 ±1.2 | 0.5550 | 16.4 ±1.3 | 15.8 ±0.9 | 0.6953 | 16.4 ±1.0 | 15.5 ±1.0 | 0.5363 | |
| hLOS (days)^ | 9.7 ±1.2 | 7.8 ±1.0 | 0.2720 | 8.8 ±1.3 | 9.0 ±1.1 | 0.7781 | 7.9 ±1.0 | 9.9 ±1.4 | 0.4039 | |
| ICU LOS (days) | 5.0 ±0.7 | 4.9 ±0.9 | 0.3948 | 4.8 ±0.8 | 5.0 ±0.7 | 0.4745 | 4.7 ±0.7 | 5.2 ±0.9 | 0.6792 | |
| Deep Vein Thrombosis | 4 (6.0%) | 1 (2.4%) | 0.6470 | 1 (2.8%) | 4 (5.5%) | >0.9999 | 1 (1.9%) | 4 (7.3%) | 0.3634 | |
| Pulmonary Embolism | 1 (1.5%) | 0 (0.0%) | >0.9999 | 0 (0.0%) | 1 (1.4%) | >0.9999 | 0 (0.0%) | 1 (1.8%) | >0.9999 | |
| Pneumonia | 4 (6.0%) | 1 (2.4%) | 0.6470 | 1 (2.8%) | 4 (5.5%) | >0.9999 | 1 (1.9%) | 4 (7.3%) | 0.3634 | |
| Tracheostomy | 5 (7.5%) | 1 (2.4%) | 0.4025 | 1 (2.8%) | 5 (6.8%) | 0.6616 | 2 (3.7%) | 4 (7.3%) | 0.6787 | |
| Gastrostomy | 6 (9.0%) | 3 (7.1%) | >0.9999 | 3 (8.3%) | 6 (8.2%) | >0.9999 | 6 (11.1%) | 3 (5.5%) | 0.3203 | |
| Discharge GCS (without death)^ | 14.3 ±0.2 | 14.5 ±0.2 | 0.5264 | 14.7 ±0.2 | 14.1 ±0.2 | 0.0108 | 14.7 ±0.1 | 14.0 ±0.3 | 0.0186 | |
| Discharge Location | Home | 35 (51.5%) | 13 (31.0%) | 0.0477 | 22 (61.1%) | 26 (35.6%) | 0.0144 | 31 (57.4%) | 17 (30.9%) | 0.0070 |
| Acute Rehab | 22 (32.8%) | 8 (19.1%) | 0.1295 | 9 (25.0%) | 21 (28.8%) | 0.8204 | 13 (24.1%) | 17 (30.9%) | 0.5211 | |
| Subacute Rehab | 2 (3.0%) | 4 (9.5%) | 0.2022 | 1 (2.8%) | 5 (7.9%) | 0.4121 | 3 (5.6%) | 3 (5.5%) | >0.9999 | |
| Nursing Home/ hospice | 3 (4.5%) | 10 (23.8%) | 0.0045 | 1 (2.8%) | 12 (16.4%) | 0.0566 | 2 (3.7%) | 11 (20.0%) | 0.0153 | |
| Death | 5 (7.4%) | 6 (14.3%) | 0.3281 | 2 (5.6%) | 9 (12.3%) | 0.3325 | 4 (7.4%) | 7 (12.7%) | 0.5269 | |
Multivariate logistic regressions
To examine the influence of each frailty measure as predictors of mortality and discharge home, stepwise multivariate logistic regressions were performed while including variables known to be associated with poorer outcomes following cSDH. Age was not included for this analysis due to co-linearity with frailty measures. In multivariate analysis, the single most significant predictor of mortality was the iGCS (OR=0.58; 95%CI: 0.44-0.77; p<0.0001) and was not independently predicted by any frailty measure (Table 6). Discharge home, however, was independently predicted by both iGCS (OR=1.46; 95%CI: 1.13-1.90; p=0.0041) and CCI (OR=0.73; 95%CI: 0.58-0.92; p=0.0082) score but not either mFI.
Table 6. Multivariate logistic regressions for primary endpoints among all patients in the cohort.
CCI: Charlson Comorbidity Index, mFI: modified frailty index, iGCS: initial Glasgow Coma Score, AC/AP: anticoagulation/antiplatelet medication.
| Characteristic | Multivariate OR (95% CI) | P-value | |
| Mortality | Sex | 2.03 (0.26-15.60) | 0.4961 |
| Prior SDH | 0 (0-infinity) | 0.9980 | |
| Craniotomy for SDH | 1.46 (0.17-12.62) | 0.7310 | |
| AC/AP | 1.31 (0.19-8.89) | 0.7815 | |
| iGCS | 0.58 (0.44-0.77) | 0.0001 | |
| SDH Thickness | 1.07 (0.92-1.24) | 0.3605 | |
| mFI-11 | Not Included | 0.6560 | |
| mFI-5 | Not Included | 0.6387 | |
| CCI | Not Included | 0.3296 | |
| Discharge Home | Sex | 0.65 (0.26-1.67) | 0.3716 |
| Prior SDH | 0.50 (0.16-1.52) | 0.2218 | |
| Craniotomy for SDH | 0.46 (0.15-1.38) | 0.1647 | |
| AC/AP | 1.55 (0.58-4.15) | 0.3808 | |
| iGCS | 1.46 (1.13-1.90) | 0.0041 | |
| SDH Thickness | 1.00 (0.93-1.06) | 0.8932 | |
| mFI-11 | Not Included | 0.6812 | |
| mFI-5 | Not Included | 0.1833 | |
| CCI | 0.73 (0.58-0.92) | 0.0082 |
Subgroup analysis of operative patients
Stepwise multivariate logistic regressions, in the same manner as above, were performed among those who received an operative intervention. Among this group, the only independent predictor of mortality was the CCI (OR=4.24; 95%CI: 1.01-17.86; p=0.0491) (Table 7). Similarly, the only independent predictor of discharge home among this group was also the CCI (OR=0.55; 95%CI: 0.33-0.90; p=0.0170). Unlike the cohort overall, the iGCS was not independently associated with either endpoint among those that received an operative intervention.
Table 7. Multivariate logistic regressions for primary endpoints among operative cSDH patients.
CCI: Charlson Comorbidity Index, mFI: modified frailty index, iGCS: initial Glasgow Coma Score, AC/AP: anticoagulation/antiplatelet medication; cSDH: chronic subdural hemorrhage.
| Characteristic | Multivariate OR (95% CI) | P-value | |
| Mortality | Sex | 0.93 (0.05-17.53) | 0.9615 |
| Prior SDH | 0 (0-infinity) | 0.9980 | |
| Craniotomy for SDH | 0.34 (0.01-16.75) | 0.5840 | |
| AC/AP | infinity (0-infinity) | 0.9973 | |
| iGCS | 1.17 (0.67-2.02) | 0.5831 | |
| SDH Thickness | 0.69 (0.41-1.16) | 0.1606 | |
| mFI-11 | Not Included | 0.3296 | |
| mFI-5 | Not Included | 0.4914 | |
| CCI | 4.24 (1.01-17.86) | 0.0491 | |
| Discharge Home | Sex | 2.08 (0.45-9.69) | 0.3499 |
| Prior SDH | 0.20 (0.03-1.57) | 0.1246 | |
| Craniotomy for SDH | 0.58 (0.12-2.81) | 0.4943 | |
| AC/AP | 0.90 (0.17-4.90) | 0.9039 | |
| iGCS | 1.02 (0.68-1.54) | 0.9128 | |
| SDH Thickness | 1.05 (0.94-1.17) | 0.3841 | |
| mFI-11 | Not Included | 0.7254 | |
| mFI-5 | Not Included | 0.5783 | |
| CCI | 0.55 (0.33-0.90) | 0.0170 |
Discussion
Frailty predicts outcomes in a variety of surgical and non-surgical patients. Despite cSDH being a common neurosurgical condition associated with cerebral atrophy and advanced age, the utility of frailty has not been explored in this population. In this study, we show that frail patients, regardless of scoring system, are less likely to be discharged home compared to their non-frail counterparts. Along with iGCS, the CCI was independently associated with discharge home in multivariate analysis in the cohort overall and was the only independent predictor of this endpoint in patients who received an operation. Similarly, while mortality overall was best predicted by iGCS, the CCI was the only independent predictor of death in subgroup analysis of operative cases. These results indicate that the CCI may have clinical utility in predicting functional outcome in all cSDH patients and mortality among those who receive an operation.
It is well known that patient comorbidities contribute to poorer prognosis following a neurologic insult. Frailty is an emerging concept that aims to quantify the cumulative effect of these comorbidities in order to more accurately prognosticate outcomes. In neurosurgery specifically, frailty has been associated with poorer outcomes following subarachnoid hemorrhage [5,11], spine surgery [16], intracranial hemorrhage [17], and intra-cranial tumor surgery [18]. Despite this, there are over 215 established frailty indices [7] and no consensus on the optimal scoring system or appropriate cutoffs [19]; something that may be disease and patient-population specific. For example, in our own work we have shown in the same time period and setting as the study herein, the superiority of the mFI-5 and mFI-11 over the CCI in predicting outcomes following angiogram-negative subarachnoid hemorrhage (under review, BJN). This is in contrast to the study herein showing the opposite finding for cSDH. This indicates that despite frailty being associated with poorer outcomes across all of neurosurgery [20], individual scoring systems must be validated for each disease type.
Careful selection of surgical candidates for cSDH evacuation incorporates a variety of factors including hemorrhage size, MLS, age, overall clinical history and exam, and the iGCS. In particular, the iGCS following cSDH is thought to be the factor with the highest prognostic utility [21,22]. In line with this, patients in our study who had an operation were younger, were more likely to have an isolated head injury, a better iGCS, larger cSDH thickness, and lower CCI and mFI-11 scores (Table 3). Likewise, while patients who had an evacuation had longer lengths of stay, they were more likely to be discharged to acute rehabilitation, equally as likely to be discharged home, less likely to be discharged to a nursing home, and trended to have lower mortality compared to their non-operative counterparts. Moreover, among the subgroup of operative patients, the CCI was the only independent predictor of both mortality and discharge location. Together, this reflects careful patient selection for those who are likely to do well after surgery, but given the retrospective nature of this study, we cannot comment on the clinical utility of frailty in patient selection for surgery. Prospective studies with larger samples of those who received a craniotomy or burr hole only may provide more conclusive evidence that the CCI, in addition to better-documented criteria, may be considered for operative candidate selection.
While the mFI-11 is the most commonly used frailty measure in the literature, to date, no study has examined the mFI-11 or mFI-5 among patients with cSDH; an effect that may reflect a publication bias. Furthermore, while there have been previous studies investigating the relationship between the CCI and cSDH prognosis, there are conflicting results with some finding that the CCI is independently associated with cSDH recurrence [23], increased complications [24,25] and long-term mortality [26], while others have shown no such effects [2,27]. Differences in these studies may be attributed to inclusion criteria, with some groups, including our own, examining all cSDH patients, while others only examined certain types of operative patients. For example, Shimizu et al. retrospectively examined 211 patients ≥ 65 years of age who underwent burr hole craniotomy to determine predictors of three month modified Rankin scores, non-home discharge, and unfavorable prognosis. This group showed, in multivariate analysis, that age, nutritional status, and recurrence better predicted endpoints than the clinical frailty score, an established functional measure of frailty, and the CCI. Of note, this group did not include iGCS in multivariate regressions and retrospectively calculated functional frailty scores based on documentation thus limiting the relative clinical utility of their findings [28]. In a similar limitation, we did not include age in multivariate analyses as this variable was included in the CCI score and to do so would incur co-linearity between these variables. Along with this, our goal was to compare frailty with the iGCS and we showed that the iGCS was the most potent predictor of both mortality and discharge home in cSDH overall but was inferior to the CCI among operative patients.
Limitations
This study was chiefly limited by its retrospective design as outlined elsewhere [29] and by a limited sample size. Larger samples may have elucidated more significant effects of frailty on secondary endpoints and perhaps on mortality. In the latter endpoint, there was a trend toward increased mortality with all examined frailty indices. Second, we included all patients who presented with SDH that had a chronic component including mixed, acute-on-chronic, and pure chronic SDH. Larger prospectively obtained cohorts would allow for subgroup analysis of each cSDH type and for stronger comparisons of craniotomy versus burr hole craniotomy.
Conclusions
While frailty is associated with worse surgical outcomes, the clinical utility of the mFI-5, mFI-11, and CCI versus the iGCS following cSDH is unclear. We show that the iGCS is an overall superior predictor of mortality following cSDH but is outperformed by the CCI after operative intervention. Similarly, the CCI is the superior predictor of discharge home in cSDH patients overall and following an operative intervention. These results indicate that while the iGCS best predicts mortality overall, the CCI may be considered when prognosticating post-operative course and hospital disposition.
Acknowledgments
We would like to thank Christine Loscri, Monique Carrero-Tangle, and Lizbel Aquino for their support of neurosurgical research.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
This work was supported, in part, by a grant from New York Medical College for medical student research.
Human Ethics
Consent was obtained by all participants in this study. New York Medical College issued approval 12921. IRB approval for this retrospective study was obtained from New York Medical College with a waiver of informed consent.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Öhman J, Helén P. J Neurosurg. 2019;132:1147–1157. doi: 10.3171/2018.12.JNS183035. [DOI] [PubMed] [Google Scholar]
- 2.Morbidity and mortality after burrhole craniostomy versus craniotomy for chronic subdural hematoma evacuation: a single-center experience. Raghavan A, Smith G, Onyewadume L, et al. World Neurosurg. 2020;134:0. doi: 10.1016/j.wneu.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Association of patient frailty with increased morbidity after common ambulatory general surgery operations. Seib CD, Rochefort H, Chomsky-Higgins K, Gosnell JE, Suh I, Shen WT, Duh QY. JAMA Surg. 2018;153:160–168. doi: 10.1001/jamasurg.2017.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The modified frailty index and 30-day adverse events in oncologic neurosurgery. Youngerman BE, Neugut AI, Yang J, Hershman DL, Wright JD, Bruce JN. J Neurooncol. 2018;136:197–206. doi: 10.1007/s11060-017-2644-0. [DOI] [PubMed] [Google Scholar]
- 5.Increasing frailty predicts worse outcomes and increased complications after angiogram-negative subarachnoid hemorrhages. McIntyre M, Gandhi C, Dragonette J, et al. World Neurosurg. 2020;134:0. doi: 10.1016/j.wneu.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Accumulating deficits model of frailty and postoperative mortality and morbidity: Its application to a national database. Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. J Surg Res. 2013;183:104–110. doi: 10.1016/j.jss.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 7.A comprehensive overview of activities of daily living in existing frailty instruments: a systematic literature search. Costenoble A, Knoop V, Vermeiren S, et al. Gerontologist. 2019:0. doi: 10.1093/geront/gnz147. [DOI] [PubMed] [Google Scholar]
- 8.Preoperative frailty and quality of life as predictors of postoperative complications. Saxton A, Velanovich V. Ann Surg. 2011;253:1223–1229. doi: 10.1097/SLA.0b013e318214bce7. [DOI] [PubMed] [Google Scholar]
- 9.A modified frailty index to assess morbidity and mortality after lobectomy. Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. J Surg Res. 2013;183:40–46. doi: 10.1016/j.jss.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 10.Frailty index as a predictor of adverse postoperative outcomes in patients undergoing cervical spinal fusion. Shin JI, Kothari P, Phan K, Kim JS, Leven D, Lee NJ, Cho SK. Spine. 2017;42:304–310. doi: 10.1097/BRS.0000000000001755. [DOI] [PubMed] [Google Scholar]
- 11.Age predicts outcomes better than frailty following aneurysmal subarachnoid hemorrhage: a retrospective cohort analysis. McIntyre MK, Gandhi C, Long A, et al. Clin Neurol Neurosurg. 2019;187:105558. doi: 10.1016/j.clineuro.2019.105558. [DOI] [PubMed] [Google Scholar]
- 12.New 5-factor modified frailty index using American College of Surgeons NSQIP data. Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. J Am Coll Surg. 2018;226:173–181. doi: 10.1016/j.jamcollsurg.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 13.The 5-Item modified frailty index is predictive of severe adverse events in patients undergoing surgery for adult spinal deformity. Yagi M, Michikawa T, Hosogane N, et al. Spine. 2019;44:0. doi: 10.1097/BRS.0000000000003063. [DOI] [PubMed] [Google Scholar]
- 14.A comparison of scoring systems for predicting short- and long-term survival after trauma in older adults. Meagher AD, Lin A, Mandell SP, Bulger E, Newgard C. Acad Emerg Med. 2019;26:621–630. doi: 10.1111/acem.13727. [DOI] [PubMed] [Google Scholar]
- 15.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Charlson M, Pompei P, Ales K, MacKenzie C. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Use of the modified frailty index to predict 30-day morbidity and mortality from spine surgery. Ali R, Schwalb JM, Nerenz DR, Antoine HJ, Rubinfeld I. J Neurosurg Spine. 2016;25:537–541. doi: 10.3171/2015.10.SPINE14582. [DOI] [PubMed] [Google Scholar]
- 17.Modified frailty index predicts postoperative outcomes of spontaneous intracerebral hemorrhage. Imaoka Y, Kawano T, Hashiguchi A, et al. Clin Neurol Neurosurg. 2018;175:137–143. doi: 10.1016/j.clineuro.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Factors related to frailty associated with clinical deterioration after meningioma surgery in the elderly. Isobe N, Ikawa F, Tominaga A, et al. World Neurosurg. 2018;119:0. doi: 10.1016/j.wneu.2018.07.080. [DOI] [PubMed] [Google Scholar]
- 19.The immense heterogeneity of frailty in neurosurgery: a comprehensive literature review [Epub ahead of print] Pazniokas J, Gandhi C, Theriault B, et al. Neurosurg Rev. 2020 doi: 10.1007/s10143-020-01241-2. [DOI] [PubMed] [Google Scholar]
- 20.Preoperative frailty score for 30-day morbidity and mortality after cranial neurosurgery. Tomlinson SB, Piper K, Kimmell KT, Vates GE. World Neurosurg. 2017;107:959–965. doi: 10.1016/j.wneu.2017.07.081. [DOI] [PubMed] [Google Scholar]
- 21.Development of the Subdural Hematoma in the Elderly (SHE) score to predict mortality. Alford EN, Rotman LE, Erwood MS, et al. J Neurosurg. 2019;132:1616–1622. doi: 10.3171/2019.1.JNS182895. [DOI] [PubMed] [Google Scholar]
- 22.Outcomes of subdural hematoma in the elderly with a history of minor or no previous trauma. Kuhn EN, Erwood MS, Oster RA, Davis MC, Zeiger HE, Pittman BC, Fisher III WS. World Neurosurg. 2018;119:0. doi: 10.1016/j.wneu.2018.07.168. [DOI] [PubMed] [Google Scholar]
- 23.Role of the patient comorbidity in the recurrence of chronic subdural hematomas [Epub ahead of print] Martinez-Perez R, Tsimpas A, Rayo N, Cepeda S, Lagares A. Neurosurg Rev. 2020 doi: 10.1007/s10143-020-01274-7. [DOI] [PubMed] [Google Scholar]
- 24.Outcomes of chronic subdural hematoma drainage in nonagenarians and centenarians: a multicenter study. Lee L, Ker J, Ng H, Munusamy T, King NKK, Kumar D, Ng WH. J Neurosurg. 2016;124:546–551. doi: 10.3171/2014.12.JNS142053. [DOI] [PubMed] [Google Scholar]
- 25.Predictors of recurrence and complications after chronic subdural hematoma surgery: a population-based study. Bartek J, Sjåvik K, Kristiansson H, Ståhl F, Fornebo I, Förander P, Jakola AS. World Neurosurg. 2017;106:609–614. doi: 10.1016/j.wneu.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 26.Chronic subdural hematoma in patients aged 80 years and older: a two-centre study. De Bonis P, Olei S, Mongardi L, et al. Clin Neurol Neurosurg. 2018;170:88–92. doi: 10.1016/j.clineuro.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 27.A propensity score analysis of the impact of surgical intervention on unexpected 30-day readmission following admission for subdural hematoma. Franko LR, Sheehan KM, Roark CD, Joseph JR, Burke JF, Rajajee V, Williamson CA. J Neurosurg. 2018;129:1008–1016. doi: 10.3171/2017.6.JNS17188. [DOI] [PubMed] [Google Scholar]
- 28.Importance of frailty evaluation in the prediction of the prognosis of patients with chronic subdural hematoma. Shimizu K, Sadatomo T, Hara T, Onishi S, Yuki K, Kurisu K. Geriatr Gerontol Int. 2018;18:1173–1176. doi: 10.1111/ggi.13436. [DOI] [PubMed] [Google Scholar]
- 29.Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Kaji AH, Schriger D, Green S. Ann Emerg Med. 2014;64:292–298. doi: 10.1016/j.annemergmed.2014.03.025. [DOI] [PubMed] [Google Scholar]