Abstract
An increasing number of studies have indicated that red blood cell distribution width (RDW) may be a novel biomarker for the diagnosis and prognosis of various malignancies. However, to date, data on the association of RDW with non-small cell lung cancer (NSCLC) are unclear. Our present study aimed to explore the value of RDW in NSCLC patients. A total of 338 NSCLC patients, 109 small cell lung cancer (SCLC) patients, and 302 healthy participants were retrospectively analyzed between January 2016 and December 2018. In the present study, we found that RDW was significantly increased in NSCLC patients. Receiver-operating characteristic (ROC) analysis showed that the area under the ROC curve (AUC) of RDW was 0.753 in discriminating NSCLC patients from healthy participants, the optimal cut-off value of RDW was 12.95, and the specificity and sensitivity were 76.33% and 76.16%, respectively. Further analysis found that RDW can enhance the diagnostic performance of Cyfra21-1 and NSE in discriminating NSCLC patients from healthy participants or SCLC patients. Among NSCLC patients, RDW was significantly correlated with TNM stage, T stage, N stage, M stage, and Cyfra21-1, indicating that RDW may be helpful for predicting the prognosis of NSCLC patients. Our findings suggest that RDW can be used as an auxiliary marker for the diagnosis and prognosis of NSCLC.
Subject terms: Cancer, Biomarkers
Introduction
Lung cancer, regarded as the 1st fatal cancer in the whole world, is also one of the most common cancers in China1. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and accounts for approximately 80% of all diagnosed lung cancer patients. Unfortunately, most of the NSCLC patients have metastasis at the time of diagnosis1,2. Although there are many treatments for NSCLC, including surgery, radiotherapy, and chemotherapy, these treatments bring limited improvements in the long-term survival of NSCLC patients due to the high incidence of recurrence and distant metastasis2. Mortality from NSCLC could be reduced by timely screening. Until recently, X-rays, computed tomography (CT) scans, and lung biopsies have been used to diagnose lung cancer, but various limitations restrict their clinical application, such as low sensitivity, high expense or invasive3,4. Therefore, to improve treatment strategies, the investigation of simple and readily available biomarkers is urgently needed for the clinic.
Recently, many studies have reported the relationships between various hematological parameters and the clinical diagnosis and treatment process of cancer patients, such as the neutrophil-to-lymphocyte ratio (NLR)5, platelet distribution width (PDW)6,7, and platelet-to-lymphocyte ratio (PLR)8. Red blood cell distribution width (RDW), one of the routine blood indexes, indicates the variability of red blood cell size within a blood sample. In the past, RDW was mainly used for the differential diagnosis of anemia. As an inexpensive and easy-to-measure marker, RDW is gradually attracting attention and being used more and more widely in the clinic9–12 . Emerging evidence suggests that RDW plays an important role in tumor diagnosis and prognosis13, such as renal cell cancer14, breast cancer15, ovarian cancer16, colorectal cancer17, esophageal cancer18, endometrial cancer19, and gastric cancer20.
However, to date, data about the association of RDW with NSCLC are still scarce. In 2013, Yasuko et al. first found that RDW is significantly associated with clinical cancer stage and higher RDW values suggested that lung cancer patients will have poor clinical outcomes21. In 2014, Richard et al. found that RDW plays a significant role in determining potentially curative resection in NSCLC patients22. In 2016, Mehmet et al. found that RDW levels are significantly and negatively correlated with survival rate23. Recently, two reports suggested that RDW is an independent predictor of worse prognosis in lung cancer24,25 . These limited reports suggest that RDW may be helpful in predicting prognosis in lung cancer, but there are few studies about the role of RDW in the diagnosis of NSCLC. Additionally, the relationships between histological types of lung cancer and RDW have not been clarified in some studies21. Thus, further investigations are needed to analyze the significance of RDW in NSCLC.
In this study, we assessed RDW values to analyze the significance of RDW in diagnosing NSCLC. Moreover, the ability of RDW to indicate prognosis in NSCLC patients was evaluated by analyzing the correlation between clinicopathological features and RDW levels. Our findings suggest that RDW can be used as an auxiliary marker for the diagnosis and prognosis of NSCLC.
Results
RDW levels are increased in NSCLC patients
The RDW levels of 338 NSCLC patients, 109 small cell lung cancer (SCLC) patients, and 302 healthy participants were analyzed in this study. The results showed that the RDW levels were significantly higher in the NSCLC group [13.0 (IQR 13.0–14.0)] and the SCLC group [14.0 (IQR 12.85–15.0)] than in the control group [12.5 (IQR 12.18–12.90)] (P < 0.05). However, no difference was found in terms of RDW between NSCLC and SCLC patients (Fig. 1). Our data demonstrated that the levels of RDW are increased in NSCLC patients.
Figure 1.
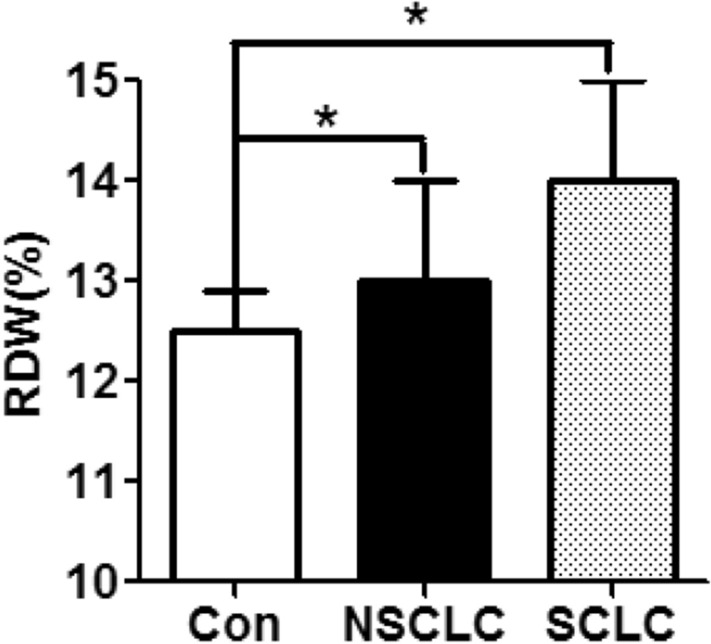
RDW levels in the NSCLC, SCLC, and control groups. RDW levels in NSCLC patients (n = 338), SCLC patients (n = 109), and healthy controls (n = 302) were tested by hematology analyzer. Data are expressed as median and interquartile range. *p < 0.05.
RDW is a useful blood marker to help diagnose NSCLC
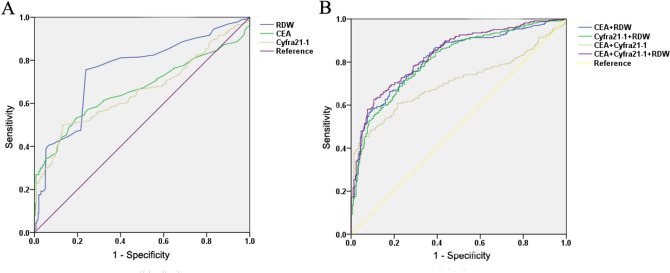
ROC curve analysis was used to analyze the power of RDW in the differential diagnosis of NSCLC patients and healthy participants. The results showed that the AUC of RDW was 0.753 (Fig. 2 and Table 1). When the cut-off value of RDW was 12.95, the sensitivity and specificity were 76.33% and 76.16%, respectively (Table 1).
Figure 2.
ROC analysis based on RDW for NSCLC diagnosis. (A) ROC analysis of value of RDW alone, CEA alone, and Cyfra21-1 alone for NSCLC diagnosis. (B) ROC analysis of value of combined detecting RDW, CEA and Cyfra21-1 for NSCLC diagnosis.
Table 1.
Diagnostic value of RDW alone, CEA alone and Cyfra21-1 alone and combined detecting for NSCLC diagnosis.
| Variables | AUC | Cut off | Sensitivity (%) | Specificity (%) | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Upper limit | Lower limit | |||||
| RDW | 0.753 | 12.95 | 76.33 | 76.16 | 0.721 | 0.797 |
| CEA | 0.665 | 5.00 | 34.32 | 94.04 | 0.620 | 0.705 |
| Cyfra21-1 | 0.657 | 3.30 | 50.31 | 86.42 | 0.614 | 0.700 |
| RDW + CEA | 0.816 | 82.10 | 66.20 | 0.783 | 0.850 | |
| RDW + Cyfra21-1 | 0.808 | 71.60 | 75.20 | 0.775 | 0.842 | |
| CEA + Cyfra21-1 | 0.705 | 60.80 | 78.50 | 0.663 | 0.746 | |
| RDW + CEA + Cyfra21-1 | 0.834 | 62.70 | 89.40 | 0.803 | 0.865 | |
Serum CEA and Cyfra21-1 are common tumor markers in the clinic, but they cannot be widely used in the diagnosis of NSCLC because they lack specificity26. In this study, we found that RDW can enhance the diagnostic sensitivity of CEA and Cyfra21-1(Table 1). Importantly, in the differential diagnosis of patients with NSCLC and healthy participants, the AUC values of CEA alone and Cyfra21-1 alone were 0.665 and 0.657, respectively. When CEA, Cyfra21-1, and RDW were combined for detection, the AUC values for CEA + RDW and Cyfra21-1 + RDW were 0.816 and 0.808, respectively, which were significantly higher than those for CEA + Cyfra21-1, CEA alone and Cyfra21-1 alone. When combined detection of lung cancer serum markers (CEA and Cyfra21-1) and blood markers (RDW) was applied, the AUC value was further increased, which was 0.834 (Fig. 2 and Table 1). In brief, these results indicated that RDW can enhance the diagnostic performance of CEA and Cyfra21-1 in the diagnosis of NSCLC.
NSCLC and SCLC are different in terms of their clinical course and response to treatment; thus, it is very important to differentially diagnose NSCLC and SCLC. NSE, a serum tumor marker, is widely used in SCLC patient diagnosis27. Next, the results of ROC curve analysis showed that the AUCs of RDW and NSE were 0.581 and 0.798, respectively. When the combined detection of RDW and NSE was applied, the AUC was further increased to 0.824 (Fig. 3 and Table 2), suggesting that RDW alone did not perform well, but can slightly enhance the diagnostic performance of NSE in the differential diagnosis of NSCLC and SCLC.
Figure 3.
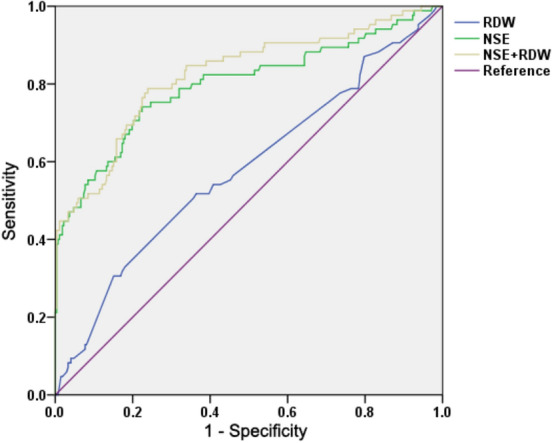
ROC analysis of value of RDW alone and NSE alone, and the combination in differential diagnosis between NSCLC and SCLC. RDW levels in 272 NSCLC patients and 85 SCLC patients were analyzed by ROC curve analysis (NSE levels of some patients is missing).
Table 2.
Diagnostic values of RDW alone and NSE alone, and combined detecting in differential diagnosis between NSCLC and SCLC.
| Variables | AUC | Cut off | Sensitivity (%) | Specificity (%) | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Upper limit | Lower limit | |||||
| RDW | 0.581 | 14.95 | 30.60 | 84.90 | 0.508 | 0.653 |
| NSE | 0.798 | 16.30 | 75.30 | 72.10 | 0.736 | 0.861 |
| RDW + NSE | 0.824 | 78.80 | 72.10 | 0.768 | 0.880 | |
Relationships between RDW levels and clinicopathological characteristics in NSCLC patients
The patients were categorized according to the RDW cut-off value, which was 12.95%. The correlations between RDW levels and clinicopatholotical characteristics are shown in Table 3. RDW levels were associated with TNM stage, T stage, M stage, N stage, and Cyfra21-1. However, no significant difference in age, sex, histological type, neutrophils, C-reactive protein (CRP), procalcitonin (PCT), or albumin (ALB) was shown in NSCLC patients (Table 3).
Table 3.
Relationship between RDW and clinical characteristics of NSCLC patients.
| Variables | Total | RDW ≤ 12.95 | RDW > 12.95 | p |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Sample size | 338 | 80 (23.6) | 258 (76.4) | |
| Gender | 0.146 | |||
| Male | 196 | 52 (26.5) | 144 (73.5) | |
| Female | 142 | 28 (19.7) | 114 (80.3) | |
| Age | 0.151 | |||
| < 60 | 158 | 43 (27.2) | 115 (72.8) | |
| ≥ 60 | 180 | 37 (20.5) | 143 (79.5) | |
| Histological type | 0.138 | |||
| Adenocarcinoma | 263 | 60 (22.8) | 203 (77.2) | |
| Squamous cell carcinoma | 65 | 15 (23.1) | 50 (76.9) | |
| Others | 10 | 5 (50.0) | 5 (50.0) | |
| TNM stage | 0.000 | |||
| Early (I + II + IIIa) | 220 | 37 (16.8) | 183 (83.2) | |
| Advance (IIIb + IV) | 118 | 43 (36.4) | 75 (63.6) | |
| pT stage | 0.000 | |||
| T1 | 145 | 20 (13.8) | 125 (86.2) | |
| T2 | 75 | 17 (22.7) | 58 (77.3) | |
| T3 | 49 | 17 (34.7) | 32 (65.3) | |
| T4 | 69 | 26 (37.7) | 43 (62.3) | |
| pN stage | 0.004 | |||
| N0 | 181 | 29 (16.0) | 152 (84.0) | |
| N1 | 22 | 7 (31.8) | 15 (68.2) | |
| N2 | 70 | 25 (35.7) | 45 (64.3) | |
| N3 | 65 | 19 (29.2) | 46 (70.8) | |
| pM stage | 0.041 | |||
| M0 | 257 | 54 (21.0) | 203 (79.0) | |
| M1 | 81 | 26 (32.1) | 55 (67.9) | |
| CEA | 0.135 | |||
| <5 | 222 | 47 (21.2) | 175 (78.8) | |
| ≥5 | 116 | 33 (28.4) | 83 (71.6) | |
| Cyfra21-1* | 0.008 | |||
| < 3.3 | 161 | 29 (18.0) | 132 (82.0) | |
| ≥ 3.3 | 163 | 50 (30.7) | 113 (69.3) | |
| Neutrophile | 0.094 | |||
| ≤ 7.00 | 279 | 71 (25.4) | 208 (74.6) | |
| > 7.00 | 59 | 9 (15.3) | 50 (84.7) | |
| CRP* | 0.067 | |||
| ≤ 8 | 48 | 6 (12.5) | 42 (87.5) | |
| > 8.0 | 35 | 10 (28.6) | 25 (71.4) | |
| PCT* | 0.847 | |||
| ≤ 0.05 | 25 | 9 (36.0) | 16 (64.0) | |
| > 0.05 | 18 | 7 (38.9) | 11 (61.1) | |
| ALB | 0.961 | |||
| ≤ 35 | 64 | 15 (23.4) | 49 (76.6) | |
| > 35 | 274 | 65 (23.7) | 209 (76.3) |
*Data of some patients is missing. Bold indicates a statistically significant.
Discussion
Our findings showed that RDW levels were increased in NSCLC patients and that increased RDW levels can enhance the diagnostic performance of Cyfra21-1 in distinguishing NSCLC patients from healthy participants. Moreover, RDW is closely related to prognostic factors in NSCLC patients, such as tumor stage and tumor markers. Our results showed that RDW may be a useful biomarker in assisting diagnosis and predicting the outcome of NSCLC.
For decades, RDW has been routinely used as a useful index for the differential diagnosis of anemia. In recent years, RDW has attracted notable attention7. Various studies have found that RDW is a simple, robust, and convenient biomarker in various malignancies13. However, reports on the relationship between RDW and NSCLC are still scarce21–23, and further studies are still needed to investigate the role of RDW in NSCLC.
In the current study, the results showed that the levels of RDW were increased in NSCLC patients, which is consistent with the finding of previous report21–23. ROC curve analysis showed that RDW was an convenient marker for differentiating NSCLC patients and healthy participants with a cut-off value of 12.95%, sensitivity of 76.33%, and specificity of 76.16%. Cyfra21-1 and NSE, two important serum tumor markers, are often used for the differential diagnosis of lung cancer26. However, the low specificity of Cyfra21-1 and NSE limit their clinical application in lung cancer screening. Our results showed that RDW can improve the diagnostic ability of Cyfra21-1 and NSE, suggesting that combining the detection of RDW and Cyfra21-1 or NSE may be a better marker for discriminating NSCLC patients from healthy participants or SCLC patients. To our knowledge, our study is the first to report the clinical utility of the combination of RDW and Cyfra21-1 or NSE for the differential diagnosis of patients with NSCLC.
Next, we investigated whether increased RDW levels might be a potential biomarker for predicting the prognosis of NSCLC patients, and the results showed that reduced RDW was associated with TNM stage, T stage, M stage, N stage, and Cyfra21-1 in NSCLC patients. In contrast, Yasuko et al. reported that RDW is positively correlated with the clinical cancer stage of lung cancer21. NSCLC and SCLC have different clinical courses and responses to treatment. However, Yasuko et al. did not analyze the relationships between RDW and NSCLC or SCLC21. In addition, the inconsistent findings may be due to the selected populations, small sample sizes, and different RDW cut-off values21. Therefore, we need to provide more data to make clarify the associations between RDW and NSCLC. Considering that TNM stage, T stage, M stage, N stage, and Cyfra21-1 are strong prognostic factors for NSCLC, we hypothesize that RDW can be used as a potential prognostic factor in NSCLC patients.
The mechanisms explaining our findings are unclear, but there are some possible explanations. First, some reports have suggested that chronic inflammation plays a significant role in the development and progression of NSCLC, and inflammatory cells release various signaling molecules, which may affect the synthesis or activity of erythropoietin, thus impairing red blood cell maturation and causing immature red blood cells to enter the blood flow28. At the same time, inflammation can also lower red blood cell survival, thus resulting in the mixing of red blood cell volumes in peripheral circulation29. Previous studies have shown that there is a positive relationship between RDW and inflammatory markers30. Second, cancer growth leads to nutritional deficiency, which weakens red blood cell maturation and causes a increased RDW. In this study, we analyzed the relationship between RDW and inflammatory markers (neutrophils, CRP, and PCT) and malnutrition markers (ALB) and found that RDW levels were increased in NSCLC patients with high levels of inflammation markers (neutrophils, CRP, and PCT), but these result were not statistically significant. In addition, RDW levels were not associated with serum ALB levels in NSCLC patients. Therefore, we speculate that RDW is increased and that the relationship between the pathological features and RDW of NSCLC patients may be only partly related to the inflammatory response in NSCLC patients, and the main reasons that may affect RDW levels in NSCLC patients need further study.
Several limitations of the present study remain to be resolved. First, our study was a single-institution, retrospective, relatively small sample size study, which may lead to bias in sample selection and analysis. Second, our study did not assess the association between RDW values and overall survival in NSCLC patients, which limits the generalization of our findings. Therefore, the clinical role of RDW in NSCLC needs to be further verified by other research centers.
Overall, our study indicated that RDW might be used as an auxiliary marker for the diagnosis and prognosis of NSCLC.
Subjects and methods
Study subjects
We retrospectively reviewed patients newly diagnosed with lung cancer from January 2016 to December 2018. The exclusion criteria of patients were as follows: anemia, hematologic diseases, and active inflammation. Finally, 338 NSCLC patients [196 male and 142 female, median age (interquartile range age): 61 (53–67) years], 109 SCLC patients [95 male and 14 female, median age (interquartile range age): 64 (57–68) years], and 302 healthy participants [171 male and 131 female, median age (interquartile range age): 60 (55–65) years] were included. The clinicopathological characteristics and laboratory data of the patients were obtained by screening the hospital medical records system. This research was approved by the ethics committee of the Fujian Medical University Union Hospital. Informed consent was obtained from all participants included in the study. All methods were carried out in accordance with relevant guidelines and regulations.
Laboratory analysis
Peripheral blood samples were collected prior to the start of treatment. Routine blood tests were performed with a Beckman Coulter LH 780 automated hematology analyzer (Beckman Coulter, Brea, CA, USA), the normal range of neutrophils was less than or equal to 7.0× 109/L. The serum carcinoembryonic antigen (CEA), cytokeratin 19 fragments (Cyfra21-1), neuron-specific enolase (NSE), C-reactive protein (CRP), and procalcitonin (PCT) levels were determined by a Cobas 6000 Analyzer (Roche Diagnostics, Basel, Switzerland). According to the manufacturer’s instructions, the normal range of CEA is less than 5 ng/mL, the normal range of CYFRA21-1 is less than 3.3 ng/mL, the normal range of NSE is less than 16.3 ng/mL, the normal range of CRP is less than or equal to 8 mg/L, and that for the normal range of PCT is less than or equal to 0.05 ng/mL. Serum albumin (ALB) was detected with a Beckman DXC800 Biochemical analyzer (Beckman Coulter, Brea, CA, USA), and the normal range of the ALB is more than or equal to 35 g/L.
Statistical analysis
Statistical analyses were performed using SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA). RDW values are summarized as the median (interquartile range, IQR), and the Mann–Whitney U-test was used to determine the differences between the two groups. ROC curve analysis was performed to assess the diagnostic value of RDW in NSCLC patients. The optimal cut-off value for RDW was determined by the Youden index (Youden index = specificity + sensitivity − 1). The categorical variables are presented as the numbers of patients and percentages, and the Chi-square test was used to compare the differences in incidence of abnormal RDW data between the different groups. All statistical tests were considered statistically significant at a p-value < 0.05.
Acknowledgements
The Authors would like to thank all the participants who took part in this study. The Authors acknowledge funding for this study from Joint Funds for the innovation of science and Technology, Fujian province (2017Y9051, 2019Y9060), Natural Science Foundation of Fujian Province (2019J01151), and the Training Project for Young and Middle-aged Core Talents in Health System of Fujian Province (2018-ZQN-69).
Author contributions
X.Z. and Y.C. conceived the research and took overall supervision in the study. B.S., P.S., J.X., Y.S., M.Z. collected data. X.Z., Y.C. and B.S. performed data analysis. X.Z. and Y.C. wrote the manuscript. X.Z., Y.C., B.S. and P.S. contributed to the discussion of results and to the review of the manuscript. All authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bin Song and Pengchong Shi.
Contributor Information
Yingping Cao, Email: caoyingping@aliyun.com.
Xianjin Zhu, Email: zxj5027667@163.com.
References
- 1.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25:iii27–iii39. doi: 10.1093/annonc/mdu199. [DOI] [PubMed] [Google Scholar]
- 3.Tanoue LT, Tanner NT, Gould MK, Silvestri GA. Lung cancer screening. Am J Respir Crit Care Med. 2015;191:19–33. doi: 10.1164/rccm.201410-1777CI. [DOI] [PubMed] [Google Scholar]
- 4.Usman Ali M, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev. Med. 2016;89:301–314. doi: 10.1016/j.ypmed.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Mei Z, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat. Rev. 2017;58:1–13. doi: 10.1016/j.ctrv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Guo F, Zhu X, Qin X. Platelet distribution width in hepatocellular carcinoma. Med. Sci. Monit. 2018;24:2518–2523. doi: 10.12659/MSM.909474. [DOI] [PubMed] [Google Scholar]
- 7.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 8.Li B, et al. Platelet-to-lymphocyte ratio in advanced cancer: review and meta-analysis. Clin. Chim. Acta. 2018;483:48–56. doi: 10.1016/j.cca.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Goyal H, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J. Gastroenterol. 2017;23:4879–4891. doi: 10.3748/wjg.v23.i27.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, et al. The important role of circulating CYFRA21-1 in metastasis diagnosis and prognostic value compared with carcinoembryonic antigen and neuron-specific enolase in lung cancer patients. BMC Cancer. 2017;17:96. doi: 10.1186/s12885-017-3070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazici P, Demir U, Bozkurt E, Isil GR, Mihmanli M. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark. 2017;18:19–25. doi: 10.3233/CBM-160668. [DOI] [PubMed] [Google Scholar]
- 12.Parizadeh SM, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. BioFactors. 2019 doi: 10.1002/biof.1518. [DOI] [PubMed] [Google Scholar]
- 13.Montagnana M, Danese E. Red cell distribution width and cancer. Ann. Transl. Med. 2016;4:399. doi: 10.21037/atm.2016.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zyczkowski M, et al. The Relationship between red cell distribution width and cancer-specific survival in patients with renal cell carcinoma treated with partial and radical nephrectomy. Clin. Genitourin. Cancer. 2017 doi: 10.1016/j.clgc.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Huang DP, Ma RM, Xiang YQ. Utility of red cell distribution width as a prognostic factor in young breast cancer patients. Medicine. 2016;95:e3430. doi: 10.1097/MD.0000000000003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin Y, et al. The value of red cell distribution width in patients with ovarian cancer. Medicine. 2017;96:e6752. doi: 10.1097/MD.0000000000006752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, et al. Clinical usefulness and prognostic value of red cell distribution width in colorectal cancer. Biomed. Res. Int. 2018;2018:9858943. doi: 10.1155/2018/9858943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen GP, Huang Y, Yang X, Feng JF. A nomogram to predict prognostic value of red cell distribution width in patients with esophageal cancer. Mediators Inflamm. 2015;2015:854670. doi: 10.1155/2015/854670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemal Y, et al. The value of red blood cell distribution width in endometrial cancer. Clin. Chem. Lab. Med. 2015;53:823–827. doi: 10.1515/cclm-2014-0699. [DOI] [PubMed] [Google Scholar]
- 20.Wei TT, et al. Relationship between red blood cell distribution width, bilirubin, and clinical characteristics of patients with gastric cancer. Int. J. Lab. Hematol. 2017;39:497–501. doi: 10.1111/ijlh.12675. [DOI] [PubMed] [Google Scholar]
- 21.Koma Y, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS ONE. 2013;8:e80240. doi: 10.1371/journal.pone.0080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warwick R, et al. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014;45:108–113. doi: 10.1093/ejcts/ezt275. [DOI] [PubMed] [Google Scholar]
- 23.Kos M, et al. Evaluation of the effects of red blood cell distribution width on survival in lung cancer patients. Contemp. Oncol. (Pozn) 2016;20:153–157. doi: 10.5114/wo.2016.60072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C, Wang X, Zhao R. Associations of lymphocyte percentage and red blood cell distribution width with risk of lung cancer. J. Int. Med. Res. 2019 doi: 10.1177/0300060519850417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyokawa G, Shoji F, Yamazaki K, Shimokawa M, Takeo S. significance of the red blood cell distribution width in resected pathologic stage I nonsmall cell lung cancer. Semin. Thorac. Cardiovasc. Surg. 2019 doi: 10.1053/j.semtcvs.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Sone K, et al. Predictive role of CYFRA21-1 and CEA for subsequent docetaxel in non-small cell lung cancer patients. Anticancer Res. 2017;37:5125–5131. doi: 10.21873/anticanres.11932. [DOI] [PubMed] [Google Scholar]
- 27.Harmsma M, Schutte B, Ramaekers FC. Serum markers in small cell lung cancer: opportunities for improvement. Biochim. Biophys. Acta. 2013;1836:255–272. doi: 10.1016/j.bbcan.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J. Interferon Cytokine Res. 1998;18:555–559. doi: 10.1089/jir.1998.18.555. [DOI] [PubMed] [Google Scholar]
- 29.Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr. Opin. Hematol. 2000;7:113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Lippi G, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009;133:628–632. doi: 10.1043/1543-2165-133.4.628. [DOI] [PubMed] [Google Scholar]