Abstract
After successful laboratory rearing of both males and females from a single clutch of eggs, the genus Nanophyllium Redtenbacher, 1906 (described only from males) and the frondosum species group within Phyllium (Pulchriphyllium) Griffini, 1898 (described only from females) are found to be the opposite sexes of the same genus. This rearing observation finally elucidates the relationship of these two small body sized leaf insect groups which, for more than a century, have never been linked before. This paper synonymizes the frondosum species group with Nanophyllium Redtenbacher, 1906 in order to create a singular and clearly defined taxonomic group. Five species are transferred from the Phyllium (Pulchriphyllium) frondosum species group and create the following new combinations: Nanophylliumasekiense (Größer, 2002), comb. nov.; Nanophylliumchitoniscoides (Größer, 1992), comb. nov.; Nanophylliumfrondosum (Redtenbacher, 1906), comb. nov.; Nanophylliumkeyicum (Karny, 1914), comb. nov.; Nanophylliumsuzukii (Größer, 2008), comb. nov. The only taxon from this species group not transferred from the frondosum species group to Nanophyllium is Phyllium (Pulchriphyllium) groesseri Zompro, 1998. Based on protibial exterior lobes, this species belongs in the schultzei species group as described in Hennemann et al. 2009 and is therefore excluded from further discussion here. The rearing of Nanophyllium also yielded the male Nanophylliumasekiense (Größer, 2002), comb. nov. thus, enabling comparison of this male to the other previously known Nanophyllium species. Two new species of nano-leaf insects are described within, Nanophylliummiyashitaisp. nov., from Morobe Province, Papua New Guinea, and Nanophylliumdaphnesp. nov., from Biak Island, Papua Province, Indonesia. With such distinct sexual dimorphism in Nanophyllium between sexes, which have only now been matched up via captive rearing, illustrated within are numerous specimens which might represent the unknown opposite sexes of the many currently known species of Nanophyllium. Due to pronounced sexual dimorphism in Nanophyllium, only future captive rearing or molecular analysis will match up the many unknown sexes. To conclude, with the description of two new Nanophyllium species, dichotomous keys to species for known males and females are presented.
Keywords: Nano-leaf insect, Phasmida, Phylliinae, Phyllium , sexual dimorphism, taxonomy, walking leaf, West Papua
Introduction
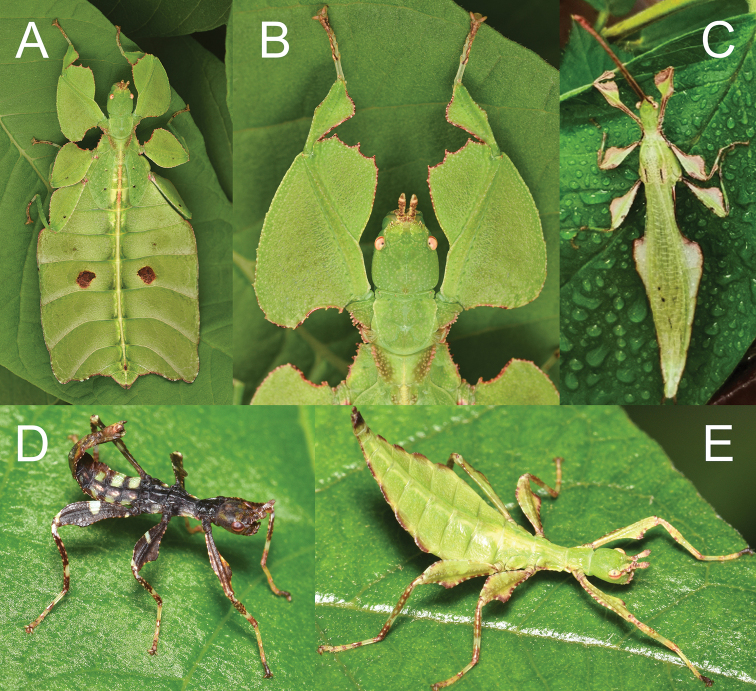
The leaf insects (Phylliidae) represent a lineage within the stick insects (Phasmatodea), which are popular as pets and within insect collections due to their impressive leaf-like camouflage. The Phylliidae have an impressive array of morphological adaptations to mimic different types of leaves. Present in many species is the ability for various color forms which allow them to look like living, dying, or dead leaves (Fig. 1). These masters of leaf-like camouflage can be found throughout Southeast Asia, with some of the biodiversity hotspots being Indonesia, Malaysia, and Papua New Guinea (Brock et al. 2020).
Figure 1.
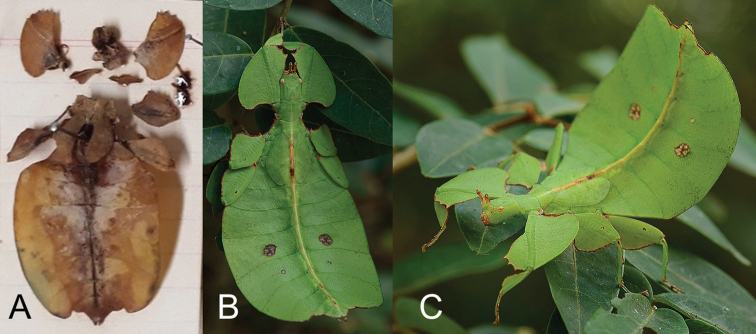
Live Nanophylliumasekiense (Größer, 2002), comb. nov. females, three primary color forms photographed in Morobe Province, Papua New Guinea.
Included within the Phylliidae are the Nanophyllium Redtenbacher, 1906 a group of small bodied species which for more than 100 years have only been known from rarely collected male specimens. Throughout a majority of those years only the single type species Nanophylliumpygmaeum Redtenbacher, 1906 was recognized. It was not until the last couple of decades that a majority of new Nanophyllium species began to be described (Brock et al. 2020). With numerous species described throughout the island of New Guinea solely from male specimens, the question begs to be asked, “where are the female Nanophyllium?”. Could the lack of females be that they are incredibly elusive, or already well-known and simply not recognized as female Nanophyllium?
Brock and Hasenpusch (2003: 203, fig. 6) were the first to suggest and illustrate the unknown Nanophyllium female, an individual from Nabire, Irian Jaya, collected along with a male which would later become the holotype of Nanophylliumhasenpuschi Brock & Größer, 2008. Unfortunately, however, the female specimen is missing both front legs (an important morphological identification feature frequently used for differentiation) but all other features place it morphologically close to Phyllium (Pulchriphyllium) frondosum Redtenbacher, 1909. Additionally, Brock and Hasenpusch (2003) discuss the subadult female found by G. B. Monteith in 1971 in the Iron Range, Queensland as additional evidence that a “frondosum-like species” is probably the unknown female for the Nanophyllium. As it turns out, the frondosum species group, as defined by Hennemann et al. (2009), are also somewhat of a quandary since they are only known from female specimens, except for Phyllium (Pulchriphyllium) groesseri Zompro, 1998. In light of the speculation by Brock and Hasenpusch (2003) the authors scoured major collections around the world for additional Nanophyllium male specimens and females of the frondosum species group looking for shared morphological features which might support the thoughts of Brock and Hasenpusch (2003).
As it so happens, the breakthrough was not due to the exhaustive review of museum specimens, but instead came as a surprise at the Montreal Insectarium, Quebec, Canada. In April 2018 the Insectarium received eggs of Phyllium (Pulchriphyllium) asekiense Größer, 2002 from Morobe Province, Papua New Guinea with the hopes of raising this beautifully variable species in captivity (Fig. 1). The surprise came in early 2019 when the three sole survivors of the very slow developing nymphs reached adulthood; as one female Phylliumasekiense and two male ‘Nanophyllium’. This breakthrough at long last was the evidence we had been searching for to remove the speculation of these two known, but never associated, taxa.
This pairing of the frondosum species group with the Nanophyllium morphologically was not surprising given numerous morphological similarities between the taxonomic groups (discussed below). From our extensive review of museum specimens, we found males ranging in length from ~ 27 to 40 mm and females ranging from ~ 46 to 71 mm, a difference which is not outside of the norm with the male to female ratio seen throughout the family.
Materials and methods
The holotype specimen of Nanophylliummiyashitai sp. nov. was loaned to the Montreal Insectarium (Stéphane Le Tirant, collection manager) from the extensive collection of Tetsuo Miyashita, Japan. See the abbreviations section below for a full list of collections (both institutional and large private) in which relevant material was recovered.
Photos of specimens that were loaned to/held within the Montreal Insectarium were taken by René Limoges of using a Nikon D810 DSLR camera with Nikon Micro-Nikkor 200 mm f/4 lens on Manfrotto 454 micrometric positioning sliding plate. Lighting was provided by two Nikon SB-25 flash units with a Cameron Digital diffusion photo box. Adobe Photoshop Elements 13 was used as post processing software. The Nanophylliummiyashitai sp. nov. holotype specimen is deposited in the Montreal Insectarium (Quebec, Canada) type collection.
Photographs of the female Nanophylliumchitoniscoides (Größer, 1992) comb. nov. (Fig. 16A) were taken by Frank Hennemann within his personal collection using a Nikon D7000 camera equipped with a Nikon DX AF-S Micro 40 mm lens and a wireless Nikon SU-800 dual speed light system. Background lightning was provided by a 18W 6000K LED panel light plate.
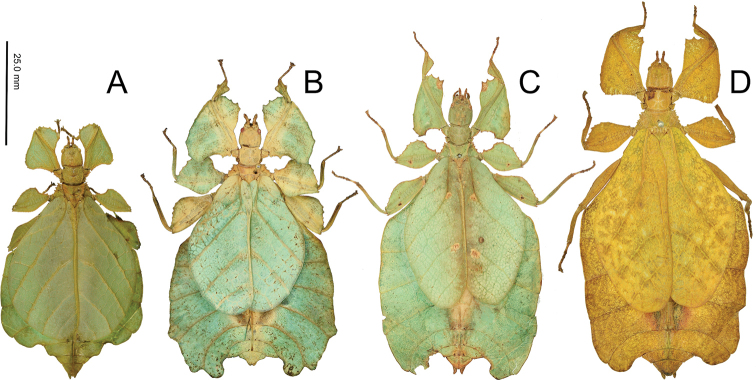
Figure 16.
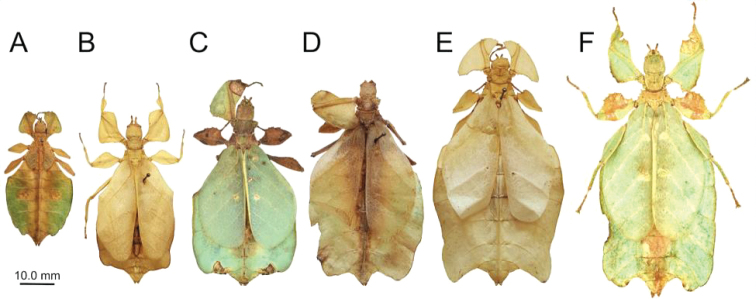
Notable known Nanophyllium females scaled to relative size ANanophylliumchitoniscoides comb. nov. Coll FHBNanophylliumsuzukii comb. nov. CNanophylliumfrondosum comb. nov. DNanophylliumkeyicum comb. nov. (RMNH).
The photograph of the female holotype Nanophylliumsuzukii (Größer, 2008) comb. nov. (Fig. 16B) was taken by Mandy Schröter under direction of Stephan Blanke at the Senckenberg German Entomological Institute Müncheberg using a Nikon D7200 digital camera and a Nikon Micro Nikkor 105 mm f/2.8 G ED objective. Lightning was from the Yongnuo Digital Speedlight YN 560 IV reflected by the inner surface of a Styrofoam box set up around the specimen. A grey card was used for white balance. Composite images with an extended depth of field were created using the software StackShot Macro Rail Package (Cognisys Inc., U.S.A.) and Zerene Stacker (release November 7, 2017; Zerene Systems LLC, U.S.A.).
Photographs of the types of Nanophylliumdaphne sp. nov. (Fig. 21C) and Nanophylliumkeyicum (=Phylliuminsulanicum) (Fig. 16D) were taken by Yvonne van Dam at Naturalis using a Nikon D600 with a 60 mm macro lens.
Figure 21.
Female holotype of Nanophylliumdaphne sp. nov. A front left leg showing lobes and serration B antennae, head, and thorax dorsal details C full body dorsal D thorax, lateral view E genitalia, ventral.
All other photographs were taken by unknown photography equipment/by simple camera phone images, and where photo credit/equipment is known it is stated within the figure caption. Egg orientation terminology follows Clark (1978). Species group organizations follow the classification presented in Hennemann et al. (2009) and Cumming (2017). Wing venation terminology follows Burt (1932) and Ragge (1955). Measurements of the holotype specimens were made to the nearest 0.1 mm using digital calipers.
The following institutional abbreviations are used:
AMNH American Museum of Natural History, New York, New York, U.S.A.
ANIC Australian National Insect Collection, Canberra, Australia
ANSP Academy of Natural Sciences, Philadelphia, Pennsylvania, U.S.A.
CAS California Academy of Sciences, San Francisco, California, U.S.A.
CFIA California Academy of Sciences, San Francisco, California, U.S.A.
IMQC Insectarium de Montréal, Montréal, Québec, Canada
MNHU Museum für Naturkunde der Humboldt-Universität, Berlin, Germany
MSNG Museo Civico di Storia Naturale, Genova, Italy
MZSF Strasbourg Zoological Museum, Strasbourg, France
NHMB Naturhistorisches Museum Basel, Basel, Switzerland
NHMUK Natural History Museum United Kingdom, London, United Kingdom
RBINS Royal Belgian Institute of Natural Sciences, Brussels, Belgium
RMNH Naturalis Biodiversity Center, Leiden, Netherlands
SDEI Senckenberg Deutsches Entomologisches Institut, Müncheberg, Germany
SDNHM San Diego Natural History Museum, San Diego, California, U.S.A.
SMTD Staatliches Museum für Tierkunde, Dresden, Germany
UMMZ University of Michigan, Museum of Zoology, Ann Arbor, Michigan, U.S.A.
Coll FH Private collection of Frank H. Hennemann, Bad Homburg, Germany
Coll RC Private collection of Royce T. Cumming, California, U.S.A.
Coll SLT Private collection of Stéphane Le Tirant, Montreal, Canada
Results
Captive rearing of Nanophyllium in the Montreal Insectarium
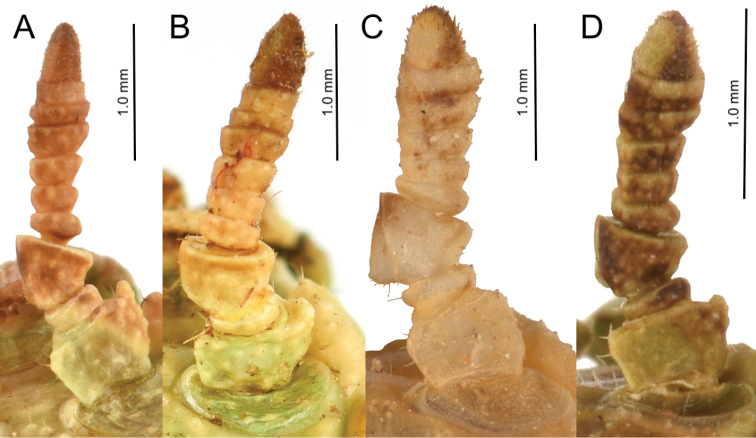
In January 2018, Stéphane Le Tirant of the Montreal Insectarium applied for a live insect import permit from the CFIA to allow the importation of live eggs to Canada from local insect suppliers in Papua New Guinea. Fortunately, one of those suppliers was able to export a small series of eggs freshly laid by a wild caught female Phylliumasekiense, collected in Morobe Province, Papua New Guinea. In April 2018, the Montreal Insectarium received 13 eggs, all black in color, and with the same morphology across the series. Eggs were incubated at room temperature on a plastic screen mesh within a plastic box with coco fiber at the base which was regularly sprayed with water to maintain humidity. Of these thirteen received eggs, during a period of seven to eleven months only five nymphs hatched (Fig. 2D). In an attempt to find at least one species of food which the fresh nymphs would accept, nymphs were offered Psidiumguajava (Guava), Rubus sp. (Bramble), and Gaultheriashallon (Salal) to eat. These three plants are commonly accepted host plants regularly used within the Montreal Insectarium.
Figure 2.
Live Nanophylliumasekiense (Größer, 2002), comb. nov. raised in the Montreal Insectarium A subadult female, full body dorsal B subadult female, head and front legs, dorsal C adult male, full body dorsal D freshly hatched nymph E nymph, later instar.
Of these five nymphs, two nymphs refuse to eat any of the host plants offered entirely and died within a few days. The other three nymphs thankfully accepted bramble and during the first few instars all three individuals looked very similar in morphology (Fig. 2E). All three nymphs were quite active both day and night and fed continuously. It was not until the last two molts that the sexual dimorphism became noticeable, and after a period of 90 to 130 days after first hatching all three reached adulthood as two males and one female. Unfortunately, the males became adult (Fig. 2C) whilst the female was still only a subadult (Fig. 2A). The males were active by day and night (readily flying) and lived only for about four months, dying before the female was adult.
The female lived as an adult for about nine months before dying, producing a total of 245 eggs of many different colors (Fig. 3). During the peak of her egg producing life she produced three to four eggs per day, and as many as 112 eggs in a single month. In adulthood, the female was no longer active during the day and only fed at night. In the months since the 245 eggs were laid, they have been incubating and during this period very few nymphs have successfully hatched, and all refused to feed on the three host plant species that were offered and accepted by the original culture. To try and ensure that the species was successfully brought into culture, the Montreal Insectarium shared many eggs with other experienced Phylliidae breeders but they also were unsuccessful with obtaining a subsequent generation.
Figure 3.
Nanophylliumasekiense (Größer, 2002), comb. nov. eggs laid by the single adult female which made it to adulthood in the Montreal Insectarium; note the variability of color within the eggs laid by this singular female.
Despite the failed attempt to bring this species into culture, the successful rearing of both sexes from a batch of eggs from a single female has allowed the much needed definitive evidence to back up the morphological observations that the females of the frondosum species group and the males of Nanophyllium are simply one and the same. Therefore, the frondosum species group of Phyllium (Pulchriphyllium) is here transferred to the genus Nanophyllium (except for Ph.groesseri, which instead belongs to the Phyllium (Pulchriphyllium) schultzei species group based in the protibial lobes and is therefore left within the Phyllium (Pulchriphyllium) subgenus). The genus Nanophyllium is here revised with most species illustrated and a general description for the male, female, and egg morphology after more than 100 years is now summarized together.
Taxonomic accounts
Nanophyllium Redtenbacher, 1906: 180.
Type species.Nanophylliumpygmaeum Redtenbacher, 1906, by monotypy.
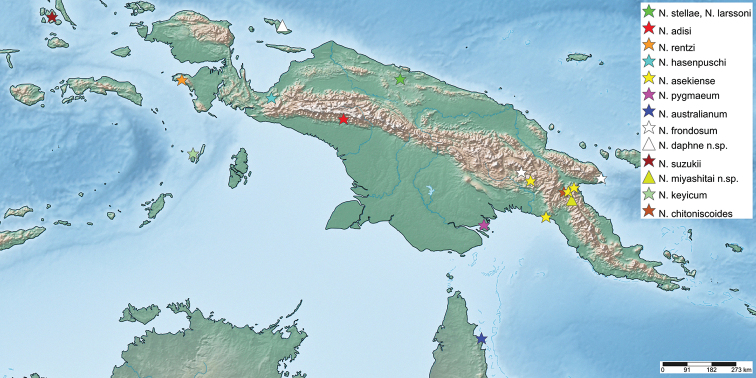
Distribution. East of Weber’s biogeographic line of faunal balance (Gressitt, 1982), primarily on the island of New Guinea and surrounding islands, as well as on the northern tip of Australia (Fig. 4).
Figure 4.
Distribution map showing localities for the known and herein described species.
Species group checklist and sex which is known
pygmaeum species group
Nanophylliumadisi Zompro & Größer, 2003 (Male)
Nanophylliumasekiense (Größer, 2002) comb. nov. (Female, Egg, Male)
Nanophylliumaustralianum Cumming, Le Tirant, & Teemsma, 2018 (Male)
Nanophylliumchitoniscoides (Größer, 1992) comb. nov. (Female, Egg)
Nanophylliumfrondosum (Redtenbacher, 1906) comb. nov. (Female)
Nanophylliumhasenpuschi Brock & Größer, 2008 (Male)
Nanophylliumkeyicum (Karny, 1914) comb. nov. (Female, Egg)
Nanophylliumpygmaeum Redtenbacher, 1906 (Male)
Nanophylliumrentzi Brock & Größer, 2008 (Male)
Nanophylliumsuzukii (Größer, 2008) comb. nov. (Female, Egg)
Nanophylliumdaphne Cumming, Willemse, Le Tirant, Teemsma, Hennemann and Büscher, sp. nov. (Female)
stellae species group
Nanophylliumstellae Cumming, 2016 (Male)
Nanophylliumlarssoni Cumming, 2017 (Male)
Nanophylliummiyashitai Cumming, Le Tirant, Teemsma, Hennemann, Willemse and Büscher, sp. nov. (Male)
Female Nanophyllium general morphology
1. Antennae: consisting of nine or ten segments (Fig. 5).
Figure 5.
Female Nanophyllium antennae, dorsal view ANanophylliumasekienseColl SLTBNanophyllium species, Indonesia, West Papua, Mapia, Coll SLTCNanophylliumdaphne sp. nov., RMNHDNanophyllium species NHMUK 012497230.
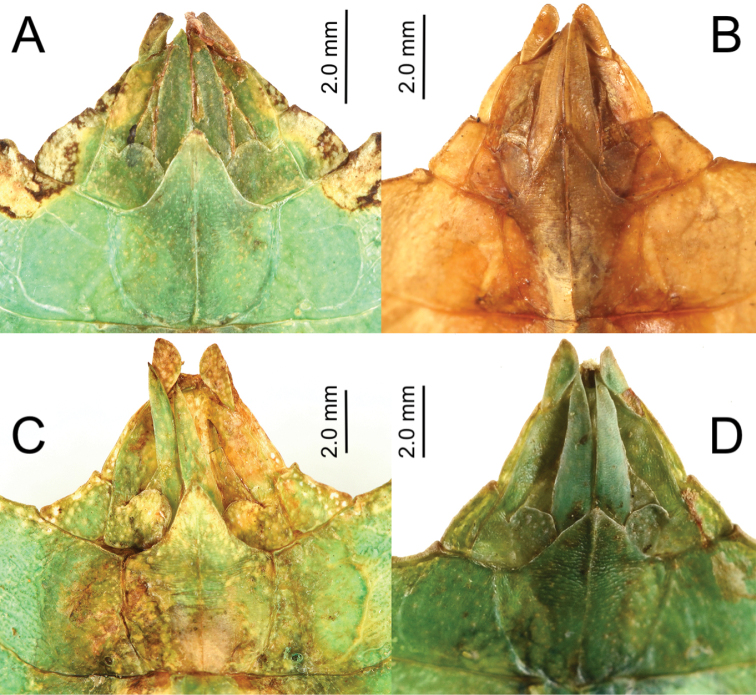
2. Posteriormedial tubercle of the head capsule, split into two points (Fig. 6A–C) not a single tubercle as is present in most of the remaining Phylliidae (Fig. 6D).
Figure 6.
Head capsule posteriomedial tubercles compared between select genera/species ANanophyllium species female NHMUK 012497230 BNanophylliumfrondosum female, Coll SLTCNanophylliummiyashitai sp. nov. holotype male DPhylliumletiranti Cumming & Teemsma, 2018 female, Coll SLT (note the singular tubercle in this Phyllium species versus the double tubercles of Nanophyllium).
3. Thorax: mesopleurae on their anterior end are notably wider than the prescutum anterior width. Mesopleurae always with prominent tubercles. Prescutum length to width ratio from 1 : 1.8 (more typical) to 1 : 3.4 (in the more extreme width like which is seen in N.suzukii) (Fig. 7).
Figure 7.
Thorax of various Nanophyllium females, dorsal ANanophyllium species female Coll RC 16-224 BNanophylliumfrondosum female Coll RC 16-049 CNanophylliumchitoniscoidesColl FHDNanophyllium species NHMUK 012497230.
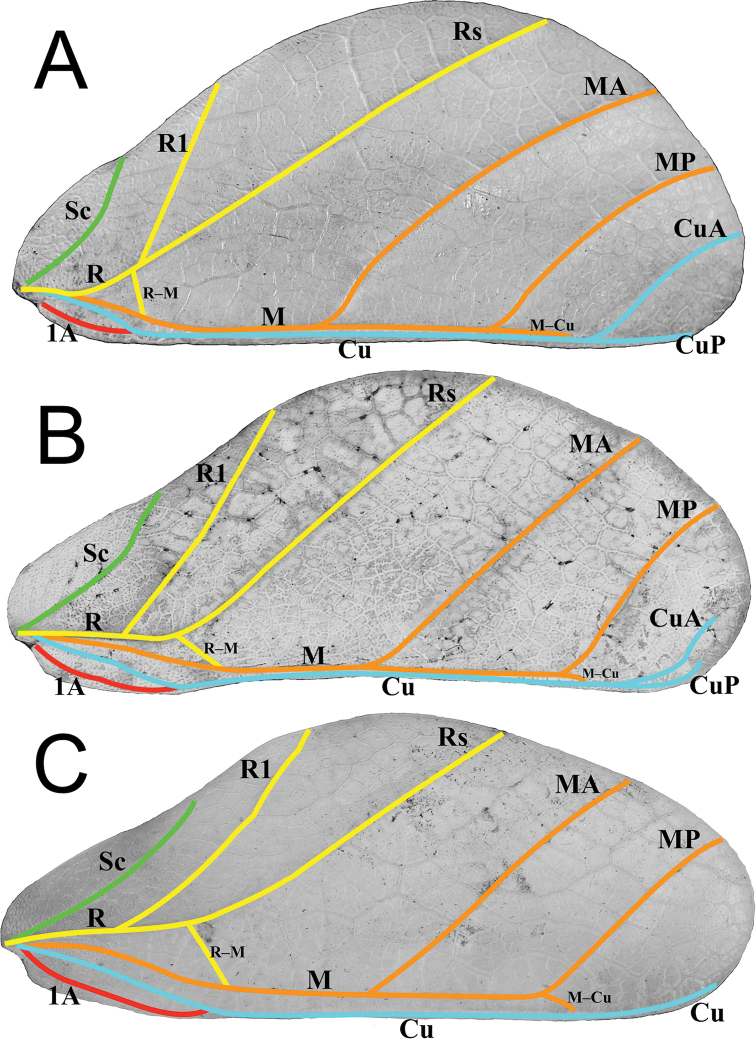
4. Tegmina: within the Nanophyllium there are three primary venation patterns (Fig. 8).
Figure 8.
Three primary venation patterns seen in female Nanophyllium tegmina, dorsal view ANanophylliumchitoniscoidesColl FHBNanophylliumsuzukii holotype, SDEICNanophylliumkeyicumColl FH. Abbreviations used: Sc (subcosta); R (radius); R1 (radius 1); Rs (radial sector); R–M (radius and medial crossvein); M (media); MA (media anterior); MP (media posterior); Cu (cubitus); CuA (cubitus anterior); CuP (cubitus posterior); 1A (first anal).
a. In the smallest species (individuals ~ 56.0 mm or less), the radial bend occurs before the splitting of the first radial and the radial sector, therefore the radial sector is straight (for example see N.chitoniscoides comb. nov.; Fig. 8A). These females also have a radius and medial crossvein present on the radial bend at or before the splitting of the first radial (Fig. 8A). The cubitus at its terminus is clearly split into the anterior cubitus and posterior cubitus veins with a clearly defined gap between them (Fig. 8A). Example species are N.chitoniscoides comb. nov., N.daphne sp. nov.
b. For the larger species, the bend in the radial vein happens on the radial sector after the splitting of the first radial from the radius (Fig. 8B). Also, the radius and medial crossvein occurs after the splitting of the first radial, instead originating on the radial sector (Fig. 8B). The cubitus at its terminus can be weakly split into the anterior cubitus and posterior cubitus, but in many specimens this vein is simple and unbranched (it is never clearly split with a large gap between the anterior cubitus and posterior cubitus like is seen in the smaller species; Fig. 8A). Example species are N.asekiense comb. nov., N.frondosum comb. nov., or N.suzukii comb. nov.
c. The final venation pattern is found in Nanophylliumkeyicum comb. nov., the only species in this genus which has a wide gap between the media and cubitus veins which persists throughout the entire length of the media, this gap is several times wider than a single vein width. This feature is only seen on this species from Kei Island, Indonesia (Fig. 8C). All other examined Nanophyllium species have the media and cubitus veins running side by side throughout the entire length, no farther than a single vein width apart (for example in the larger species like N.asekiense comb. nov. or N.suzukii comb. nov.; Fig. 8B), or with veins moderately wide for the anterior portion (at most two or three vein widths apart) but as they reach the splitting of the media posterior the width between the media and cubitus veins is reduced and the veins are side by side (this example can be seen in the smaller species like N.daphne sp. nov.). Also, in N.keyicum comb. nov., the radius and medial crossvein occurs after the splitting of the first radial, instead originating on the radial sector (Fig. 8C). Similar to the larger species, the cubitus at its terminus can be weakly split into the anterior cubitus and posterior cubitus (like those seen in Fig. 8B), but in most specimens of N.keyicum comb. nov. this vein is simple and unbranched (as illustrated in Fig. 8C).
5. Alae: absent.
6. Genitalia: gonapophyses protruding from abdominal segment VIII as long as the terminal abdominal segment, gonapophyses protruding from abdominal segment IX thinner and shorter, not exceeding the terminal abdominal segment, and subgenital plate short and moderately broad with the point just reaching the anterior margin of the terminal abdominal segment (Fig. 9).
Figure 9.
Female Nanophyllium genitalia, ventral view ANanophyllium species NHMUK 012497230 BNanophyllium species, Papua New Guinea, Central Province, Coll RC 16-224 CNanophyllium species, Indonesia, West Papua, Mapia, Coll SLTDNanophylliumchitoniscoides, Coll FH.
Male Nanophyllium general morphology
The Nanophyllium based on male morphology can be separated into two distinct species groups, the pygmaeum species group (Fig. 10A) and the stellae species group (Fig. 10B). Males of several species for the two species groups are known, but females and eggs are only known for the pygmaeum species group, the female and eggs are not yet known for the stellae species group.
Figure 10.

Side by side Nanophyllium males of the two species groups Apygmaeum species group, Nanophylliumasekiense male from Morobe Province, Papua New Guinea Bstellae species group, Nanophylliumstellae HT from Jayapura, Indonesia.
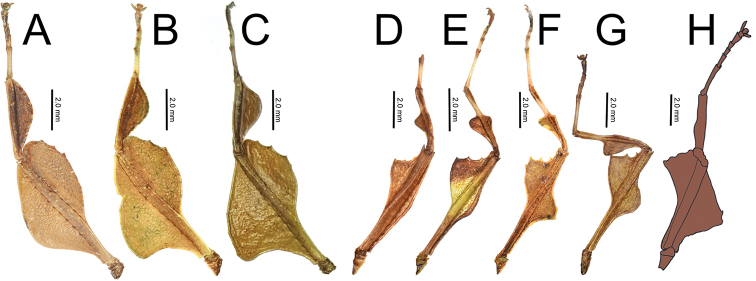
Easily observed morphological features which differentiate the species groups are the femoral lobes. The pygmaeum species group has profemoral interior lobes which are angular (Fig. 11D–H) and mesofemoral interior lobes which do not reach from end to end of the shaft and have distinct serrate teeth. The stellae species group has profemoral interior lobes which are rounded without a sharp angle (Fig. 11A–C) and mesofemoral interior lobes which are a large rounded triangle, reaching from end to end without prominent spination.
Figure 11.
Front legs and lobes of males of the stellae species group (A–C) and the pygmaeum species group (D–H) ANanophylliumstellaeBNanophylliummiyashitai sp. nov. CNanophylliumlarssoniDNanophylliumaustralianumENanophylliumasekiense comb. nov. FNanophylliumrentziColl SLTGNanophylliumhasenpuschiHNanophylliumadisi line drawing based on holotype.
1. Profemoral interior lobe, in both species groups with most often three small teeth, but occasionally four teeth (rarely), never more than four teeth (Fig. 11A–H).
2. Posteromedial tubercle of the head capsule split into two points (Fig. 7A–C) not a single tubercle as is present in most of the remaining Phylliidae (Fig. 7D).
3. Thorax: mesopleurae on their anterior end are moderately wider than the prescutum anterior width (Fig. 12). Prescutum length to width ratio ranges from 1 : 2.6 to 1 : 3.3 (Fig. 12).
Figure 12.
Pronotum and thorax of males for the two species groups, dorsal ANanophylliumstellae (stellae species group) BNanophylliumasekiense comb. nov. (pygmaeum species group).
4. Tegmina short, length never exceeding the posterior of the metathorax. The venation of the tegmina appears to be rather simple/too sclerotized to identify details of venation. From a review of several different species it appears as though the subcostal vein is lost within an area that is highly sclerotized. The radial vein is moderately present and runs along the edge of the highly sclerotized patch. The medial vein is the most prominent and runs through the center of the tegmina and occasionally has a weak vein splitting from it, but for most observed specimens the medial vein was not prominently branched. The cubitus and first anal are only moderately formed and give stability to the other half of the tegmina and are not notably branched.
5. A unique feature of the alae which appears to be a symplesiomorphy for the Nanophyllium is that the radius splits into the first radial and the radial sector on the distal half of the wing and these two veins run separately to the wing margin without fusing to others (the only other group which also has the radial split on the distal half is the Pulchriphyllium, but their radial sector fuses with the media anterior, media posterior, and the cubitus instead of running by itself to the wing margin; all other leaf insect genera have the radial split happening on the proximal half of the wing). Within the Nanophyllium the alae venation differs between the two species groups.
a. In the pygmaeum species group the key differences are that the media posterior fuses back to the media anterior before reaching the wing margin, and then the fused media runs on its own to the wing margin without fusing with the radial sector (Fig. 13A).
Figure 13.
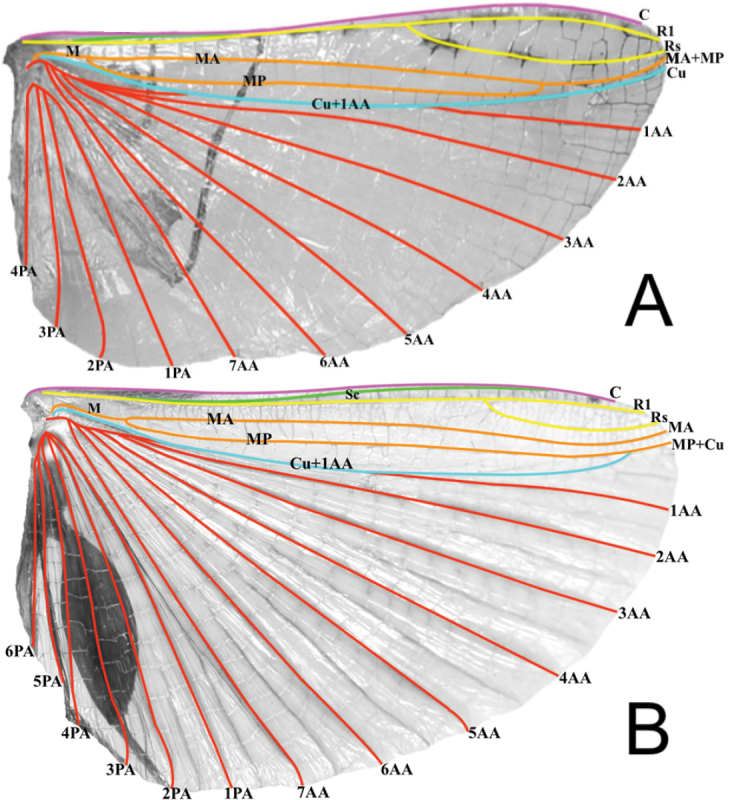
Alae wing venation of males for the two species groups, dorsal ANanophylliumrentzi (pygmaeum species group) BNanophylliumstellae (stellae species group). Abbreviations used: C (costa); Sc (subcosta); R (radius); R1 (radius 1); Rs (radial sector); M (media); MA (media anterior); MP (media posterior); Cu (cubitus); Cu+1AA (cubitus and first anterior anal); 1AA–7AA (first through seventh anterior anal); 1PA–6PA (first through fifth posterior anal).
b. In the stellae species group the media anterior and the media posterior do not fuse, instead they both run to the wing margin, and the cubitus after splitting from the first anterior anal fuses with the media posterior near the wing margin and then they run fused to the margin as one (Fig. 13B).
6. Vomer long and slender with a single apical hook (Fig. 19C).
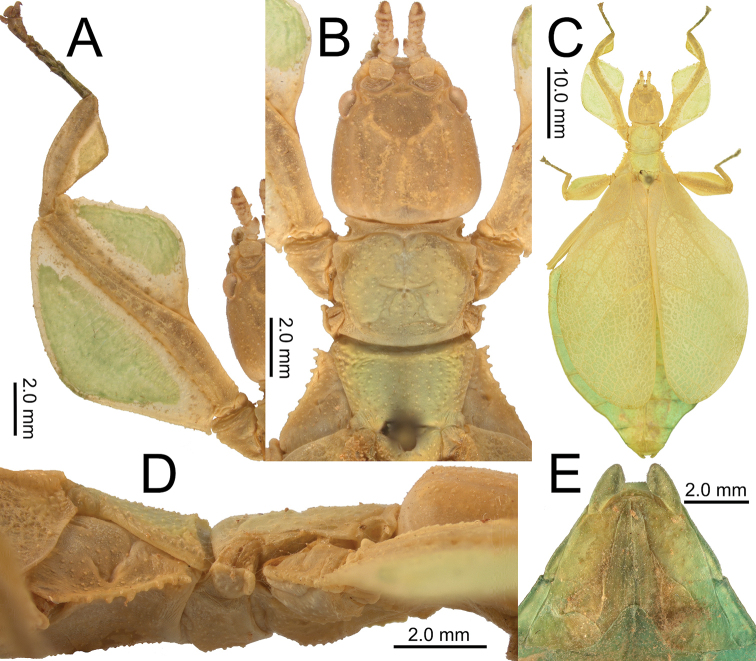
Figure 19.
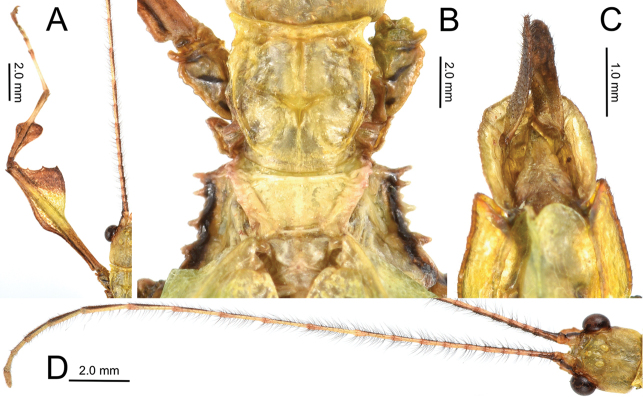
Male Nanophylliumasekiense (Größer, 2002), comb. nov. morphological details A profemoral and protibial lobes B dorsal thorax spination C ventral view of the genitalia D dorsal view of the head and antennae.
Nanophyllium egg general morphology
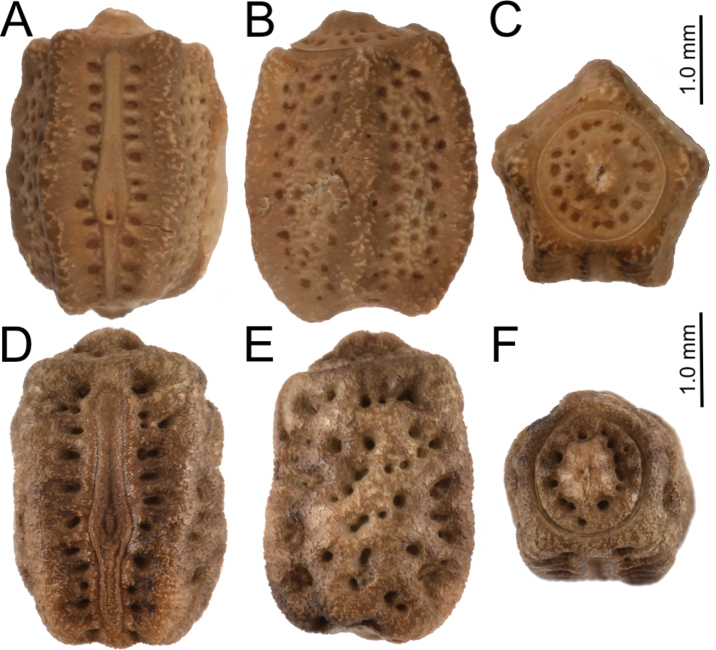
Figure 14.
A–CNanophylliumasekienseColl RC 18-046 A dorsal view B lateral view C Opercular (anterior) view D–F egg from Nanophyllium sp. NHMUK 012497230 D dorsal view E lateral view F opercular (anterior) view.
Fortunately, there are several Nanophyllium species with the egg morphology known.
These species include: Nanophylliumasekiense (Figs 3, 14A–C) comb. nov.; Nanophylliumchitoniscoides (Größer, 1992: 165, fig. 3), comb. nov.; Nanophylliumkeyicum (Karny, 1914: see Größer, 2008: 123, fig. 146), comb. nov.; Nanophylliumsuzukii (Größer, 2008: 137, fig. 171), comb. nov.; and the unidentified Nanophyllium female from the NHMUK 012497230, has a single egg mounted to a card below the specimen (Fig. 14D–F). From these known eggs a generalized list of morphological features can be compiled.
1. Cross-section is roundly pentagonal (Fig. 14C, F).
2. Surface is roughly textured, with pitting of various sizes throughout the capsule surface. Surface lacks pinnae. Pits on the capsule surface depending on the species can either have significant depth to them or in other species can have shallow pits.
3. Operculum has distinct pitting surrounding the central gently raised apex (Fig. 14C, F).
4. Micropylar plate is elongate, nearly reaching from end to end of the capsule and with an approximately uniform width throughout except for around the micropylar cup where it is slightly wider. Running parallel along the micropylar plate margin are pits, which vary in number from species to species (Fig. 14A, D).
5. Lateral surface with irregular pitting in no detectable pattern, with some pits very near each other or touching to form wider irregular shapes (Fig. 14B, E).
Nanophyllium Distribution
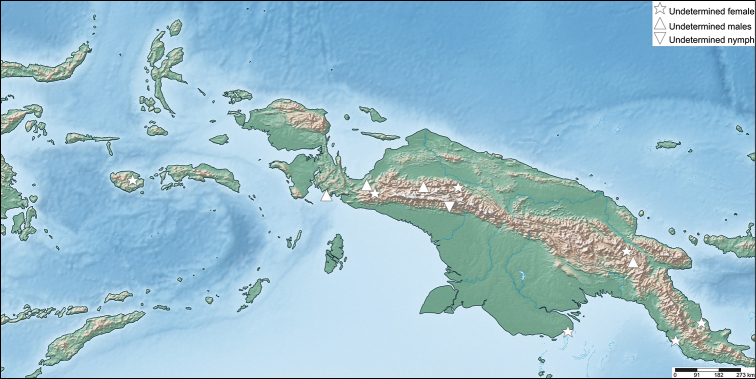
Figure 15.
Distribution map showing the undetermined Nanophyllium specimens with mappable localities.
INDONESIA
North Maluku Province:
Batjan Island (N.suzukii: SDEI, Fig. 16B)
Maluku Province:
Buru Island (Nanophyllium sp. Undetermined female)
Kei Island (Nanophylliumkeyicum: NHMUK; RMNH; SDEI; FH Coll, Fig. 16D)
West Papua Province:
Fak Fak (Nanophylliumrentzi: NHMUK, Fig. 18A; Coll SLT, Fig. 18B)
Figure 18.
Nanophylliumrentzi males dorsal, note the variation in abdominal shape A holotype in the NHMUK (copyright NHMUK 2020 online Data Portal; https://data.nhm.ac.uk/object/b03f481f-1a1c-4ccd-8558-505668fc78f3/1591228800000) B male from Fak Fak, Indonesia, Coll SLT.
Aiduma Island (Nanophyllium sp. Undetermined male, Fig. 22)
Figure 22.
Live observations of unidentifiable male NanophylliumA individual observed by Mike Wild in Mokndoma, Indonesia B individual observed by Achmad Rian Dietra, May, 2017 on Aiduma Island, Indonesia.
Papua Province:
Biak Numfor Regency, Biak Island (N.daphne sp. nov., RMNH, Fig. 21C)
Nabire Regency (N.hasenpuschi: NHMUK)
Dogiyai Regency, Mapia (Nanophyllium sp. Undetermined female: Coll SLT, Fig. 24F)
Figure 24.
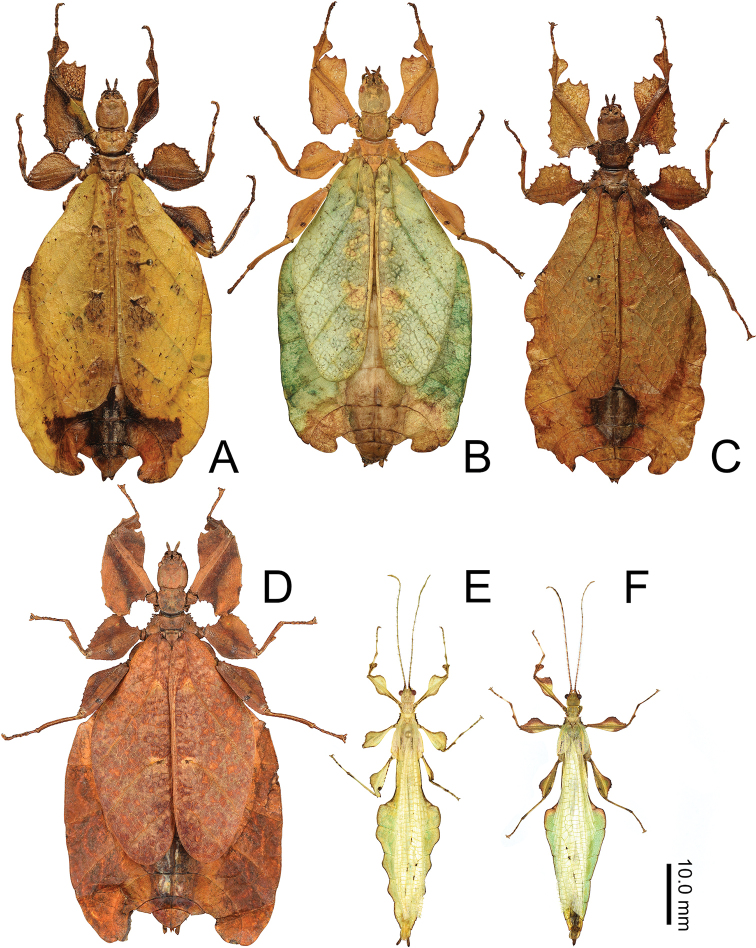
Nanophyllium females and their collection data which likely represent the unknown female Nanophylliumrentzi, Nanophylliumhasenpuschi, Nanophylliumpygmaeum, Nanophylliumadisi, or possibly undescribed species, scaled to relative size A Eastern Highlands District LJBrass, Coll., Sixth Archbold Exped. To Papua New Guinea, No. 7, Kotuni, south slopes Mt. Otto, 2200m. Aug.4–20.1959, AMNHB Papua New Guinea, Central Province, Along Hiritano HWY., E. of Vanama River Crossing: June 1989, Coll RC 16-224 C Papua New Guinea, Central, 20 km SE Port Moresby, I.1985, NHMUK 012497230 D Daru, Papua (New Guinea) Mouth of Fly R., VII-1941, Collector RG Wind, CASE New Guinea, West Irian, Kobakma, North Slope of Central Range N. of Baliem Valley 3500’ October 1971, Robert Mitton Coll, CASF Indonesia, West Papua, Mapia, May 1999, Coll SLT.
Mimika Regency, Utakwa River (Nanophyllium sp. Undetermined male, Wollaston Expedition, NHMUK)
Nduga Regency “Hoofdbivak” (N.adisi, Stirling Expedition, SMTD)
Nduga Regency “Kloofbivak” (Third South New Guinea Expedition, Nanophyllium sp. Undetermined nymph, ANSP)
Central Mamberamo Regency, Kobakma (Nanophyllium sp. Undetermined female, CAS, Fig. 24E)
Jayapura Regency, Cyclops Mts. (N.stellae (Fig. 10B and N.larssoni, SDNHM)
PAPUA NEW GUINEA
Western Province:
Katau (N.pygmaeum: MSNG)
Daru Island (Nanophyllium sp. Undetermined female, CAS, Fig. 24D)
Chimbu Province:
Kerowagi District (N.frondosum, Coll RC, Fig. 16C)
Eastern Highlands Province:
Mt. Otto (Nanophyllium sp. Undetermined female nymph, Sixth Archbold Expedition, AMNH, Fig. 24A)
Buntibasa dist., N. Guinea, Kratke Mts, 4,000–5,000’, February 1933. (F. Shaw Mayer), (Nanophyllium sp. Undetermined male nymph, NHMUK)
Herowana Village (observational record for N.asekiense by Daniel Levitis, USA)
Gulf Province:
Kerema (N.asekiense, Coll RC)
Morobe Province:
Wau (N.miyashitai sp. nov., IMQC, Fig. 20)
Figure 20.
Holotype male Nanophylliummiyashitai sp. nov. A full body dorsal B full body ventral C right front leg D abdominal segment X and dorsal view of cerci E genitalia, ventral F head through thorax, dorsal.
Lae (N.asekiense, Coll RC)
Sattelberg (N.frondosum, UMMZ)
Menyama District, Aseki (N.frondosum and N.asekiense, Coll RC; N.chitoniscoides, Miyashita Private collection, and Coll FH Fig. 16A)
Watut (N.chitoniscoides, paratype in the Detlef Größer private collection)
Central Province:
Vanama River (Nanophyllium sp. Undetermined female, Coll RC, Fig. 24B)
Port Moresby (Nanophyllium sp. Undetermined female, NHMUK, Fig. 24C)
Northern Province:
Popondetta (Nanophyllium sp. Undetermined female, NHMUK)
Milne Bay Province:
Normanby Island (Nanophyllium sp. Undetermined female)
AUSTRALIA
Queensland:
Iron Range National Park (N.australianum, ANIC)
Lockhart (N.australianum, Fig. 23)
Figure 23.
Likely N.australianum subadult females A preserved specimen which was collected by G. B. Monteith in June 1971 on the edge of Iron Range, Queensland (photograph by Susan Wright, collections manager, Queensland Museum) B dorsal, live individual photographed by Chien C. Lee (Malaysia) in July 2014; Lockhart, Queensland C same individual as in B but viewed from the lateral aspect.
Nanophyllium asekiense
(Größer, 2002) comb. nov.
5779D91D-4D07-5D0E-A363-A566F894971C
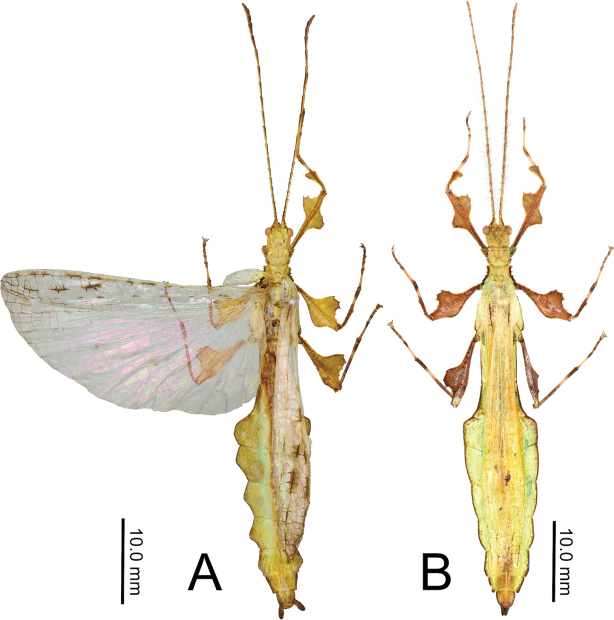
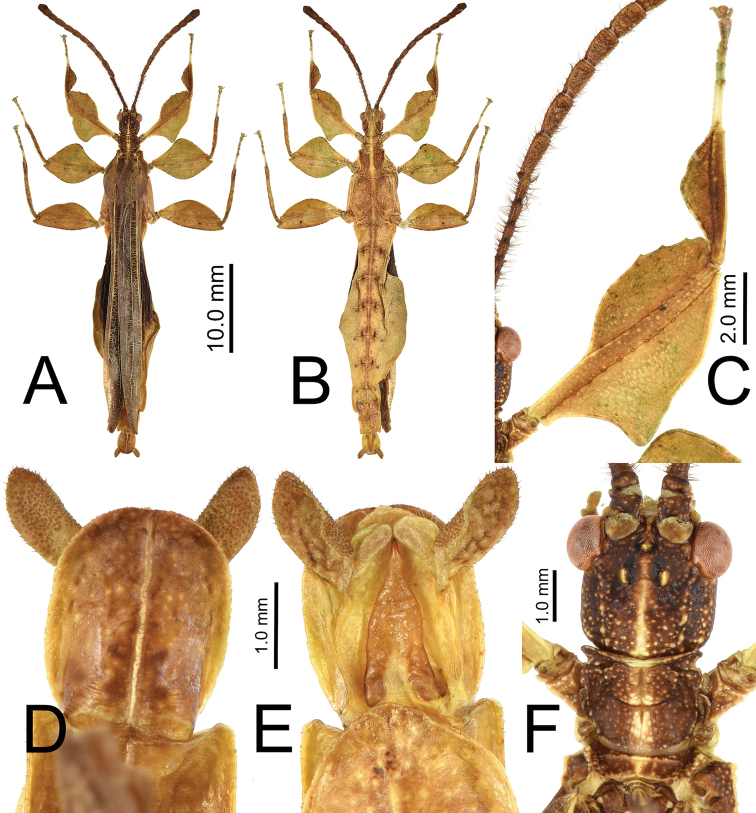
Figures 1 , 2 , 3 , 6A , 10A , 11E , 12B , 14A–C , 17 , 19
Figure 17.
Nanophylliumasekiense (Größer, 2002), comb. nov. males and females, all originating from Papua New Guinea, Morobe Province. Note the variation in the male abdominal shape and the female abdominal, color, and femoral lobe variability A yellow form female, Morobe Province, Aseki, Oiwa Village, July, 2016, Coll RC 16-268 B green form female, Papua New Guinea, Morobe, Aseki (Oiwa), Nov. 2000, Coll SLTC brown form female, Morobe Province, Aseki, Oiwa Village, July, 2016, Coll RC 16-264 D red/brown form female, Papua New Guinea, Morobe, Aseki (Oiwa), Nov. 2000, Coll SLTE serrate abdominal male bred by the Montreal Insectarium, IMQCF spade shaped male bred by the Montreal Insectarium, Coll RC 19-055.
Discussion.
Female N.asekiense comb. nov. is most often confused with the sympatric species N.frondosum comb. nov., but N.asekiense comb. nov. can be differentiated by the presence of small exterior pro- and meso-tibial spurs, which N.frondosum comb. nov. lacks.
Only two male N.asekiense comb. nov. are known at present, but their morphology is consistent between them for all features except for the abdominal shape (see Fig. 17E, F for side by side comparison of these two males). An additional specimen of Nanophylliumrentzi also shows how variable male abdominal morphology can be within the same species while the other morphological features remain stable (Fig. 18A, B). Nanophylliumasekiense comb. nov. males can be differentiated from the other known Nanophyllium males based on the profemoral lobe morphology. In N.asekiense comb. nov. the interior profemoral lobe is distinctly right-angled (a feature present in all species of the pygmaeum species group (Fig. 11E–H) except N.australianum which has a thinner, obtuse angle; Fig. 11D), but the exterior profemoral lobe of N.asekiense comb. nov. is narrow, only about the same width as the profemoral shaft; Fig. 11E (more like N.australianum than any other species, as the other species instead have an exterior profemoral lobe which is broader than the width of the profemoral shaft; Fig. 11F–H).
Description.
Male. Coloration. Each antennal segment with dark brown and tan coloration. The rest of the body and legs are of a yellow-green based color, with variable brown margins. In the two males bred by the Montreal Insectarium the individual with the undulating abdomen has minimal brown markings, with only brown along the leg margins and the abdomen margins (Fig. 17E). The male with the straight abdominal margins has more prominent brown markings throughout, with around half of the leg lobes marked with brown and a wider brown abdominal margin (Fig. 17F). The alae and tegmina on both males are a translucent pale green, with small flecks of dark brown along the prominent veins.
Morphology.Head. Head capsule about as long as wide, with a vertex that is lumpy without notable granulation, two posteromedial tubercles are not notably large but are present with a slight furrow between them (Fig. 19D). Compound eyes are notably protruding from the head capsule and there are three well-developed ocelli between and slightly posterior to them (Fig. 19D). Antennae. The antennae are longer than the outstretched forelegs and consist of 23 antennomeres (Fig. 19D). The scapus and pedicellus are nearly bare, with only a few short clear setae. All segments beyond the scapus and pedicellus except for the terminal four are covered in stiff dark setae which are each longer than the segment they are on is wide. The terminal four antennal segments also have dark setae, but these setae are shorter than the segments are wide (Fig. 19D). Thorax. Pronotum as wide as it is long with moderately formed rims on the lateral margins that are mostly parallel and only gently converging near the posterior, the anterior margin is slightly curved with a prominent rim, and the posterior margin is weakly formed (Fig. 19B). Surface of the pronotum has a distinct sagittal furrow and central lateral furrow, and the surface is irregularly lumpy, but not granular (Fig. 19B). Prescutum significantly wider than long, at its widest on the anterior it is 2.5 times wider than long. The prescutum margins evenly converge toward the posterior and have two or three notable nodes on the anterior portion, and the remainder of the margin is irregularly lumpy. Surface of prescutum is nearly smooth without significant features, and the anterior prescutum margin is simple, also lacking nodes or spines (Fig. 19B). Mesopleurae gradually diverging wider from anterior to the posterior, and marked with irregularly shaped tubercles throughout, with around four notably larger ones and smaller nodes intermixed (Fig. 19B). Mesopleurae surface irregularly lumpy with a single distinct pit in the center and no notable nodes. Pro-, meso-, and meta-sternum with irregularly spaced granules mostly along the sagittal plane but with the margins occasionally with sparse granules. Wings. Tegmina short, not exceeding the posterior of the metathorax. The subcostal vein and any splitting of the radius is obscured within an area of the wing that is highly sclerotized. The radius is the most prominent vein and runs through the center of the tegmina to the posterior margin (along the edge of the highly sclerotized patch). The medial vein is also prominent and runs through the center of the tegmina parallel with the radius and does not appear as though the media has any notable splits. The cubitus and first anal are moderately formed and give stability to the other half of the tegmina, are not notably branched, and this half of the tegmina is not as heavily sclerotized as the other half. The first anal fuses with the cubitus around two thirds of the length, and the cubitus runs nearly to the wing margin. Alae developed, with the exposed section of folded alae slightly sclerotized, but not as sclerotized as the alae. The costa runs along the wing margin with a weak subcosta running along and eventually fusing with it. The radius splits into the first radial and the radial sector just distal to the wing midline and these two veins run separately to the wing margin without fusing to others. The media splits into the media anterior and the media posterior almost immediately near the base of the wing. The media posterior fuses back to the media anterior near the distal one fifth of the wing, and then the fused medial veins run to the wing margin (Fig. 13A). The cubitus runs with the first anterior anal for most of the length and then near the distal one fifth of the wing they split and the cubius runs unbranched and unfused to the wing margin. There are seven anterior anals which run simply to the wing margin and four or five well-formed posterior anals which run simply to the wing margin. Abdomen. Abdominal segment II with parallel sides, segment III widening in a smooth arc, segment IV widening slightly for the first half, then gently curving in for the second half, segments V through VIII variable with margins that are either straight and converging uniformly to the apex which gives the abdomen a straight spade-shaped appearance or with margins which each expand and then contract which gives the abdomen a lobed appearance. Abdominal segment IX with margins which slightly converge to the abdominal segment X which is longer than wide and ends in a rounded apex. Genitalia. Poculum broad and ending in a slightly cleft apex that reaches the anterior margin of segment X (Fig. 19C). Cerci densely covered in nodes throughout the surface and short setae mostly on the distal half (Fig. 19C). Vomer long, and slightly bending to the side, not perfectly straight; with sides gradually converging to the upward hooked apex (Fig. 19C). Legs. Profemoral interior lobe angular (approximately 90 degrees) with three small evenly spaced teeth with a nearly straight gap between each tooth (Fig. 19A). Exterior profemoral lobe significantly thinner than the interior lobe (about as wide as the profemoral shaft), only present as a smoothly curved lobe just distal to the midline, not spanning the entire length (Fig. 19A). Protibiae with lobes only present on the proximal half, the distal half is bare. The protibial exterior lobe is a scalene triangle only as wide as the protibial shaft. The interior lobe is also a scalene triangle and about two times as wide as the exterior lobe (Fig. 19A). Mesofemoral exterior lobe smoothly arcing the full length, with the widest portion on the distal half and only about one and a half times as wide as the mesofemoral shaft, with fine serration on the widest portion only. Mesofemoral interior lobe with the majority of the lobe on the distal half, the proximal half with only a sliver of the lobe and lacks teeth versus the wide proximal expanse (about three times as wide as the mesofemoral shaft) has four to five notable serrate teeth. Interior metafemoral lobe with the majority of the lobe on the distal half, with only a thin sliver on the proximal half. The distal half of the metafemoral lobe is two and a half times as wide as the shaft and has five to six serrated teeth. Exterior metafemoral lobe slightly thinner than the metafemoral shaft, spanning the full length, and lacking serration. Mesotibial exterior has a small roundly triangular lobe near the midline which is only about as wide as the mesotibial shaft. Metatibiae lacking exterior and interior lobes.
Nanophyllium miyashitai sp. nov.
D7798876-1147-5C8F-98AE-1232313315AD
http://zoobank.org/2C3C5029-9E78-4687-A680-BDC9645DD3CB
Type material.
Holotype: ♂, Papua New Guinea: Morobe Province, Wau: IX, 2000. From the collection of Tetsuo Miyashita, Japan. Deposited in the Montreal Insectarium (Quebec, Canada) type collection.
Differentiation.
With the interior lobe of the profemora rounded, not angular, and the mesofemoral interior lobe broad and reaching fully end to end in a rounded triangle, this new species falls within the stellae species group. This is the first species from the stellae species group recorded from Papua New Guinea. The other two species are known from Jayapura, Irian Jaya, Indonesia (very near the border with Papua New Guinea so it is likely they also occur there but to date, we have not confirmed any specimens). This new species can be differentiated from the other two species in the stellae species group by the mesopleurae which have a prominent anterior tubercle followed by four additional small tubercles (only a single anterior tubercle in the other two species) and tegmina that are shorter, only about half the length of the metathorax (almost the length of the metathorax in the other two species).
Like the other members of the Nanophylliumstellae species group, the holotype is a male specimen and the female is unknown. It is expected that the female is larger than other known female Nanophyllium as the stellae species group members are larger than the pygmaeum species group members.
Description.
Male. Coloration. Antennae dark brown, a similar brown to that found throughout the head and thorax. The majority of the dorsal aspect throughout the remainder of the body and legs is of a similar lighter brown, but not a light as the stripe of light brown running along the sagittal plane along the head and thorax. Alae and tegmina have a similar dark brown to that found on the antennae. Throughout the ventral surface the coloration is the same as that found on the legs. Granulation on the body is mostly of a lighter brown than the surface it is found on.
Morphology.Head. Head capsule slightly longer than wide, with a vertex that is heavily granulose, which includes the two posteromedian tubercles which are no larger than the surrounding granulation around them (Fig. 20F). Three well-developed ocelli are slightly posterior to the compound eyes which are ovular and slightly protrude from the head capsule (Fig. 20F). Antennae. Antennae in the holotype are both damaged and repaired so the original number of antennomeres is unknown. The antennae are longer than the outstretched forelegs and the left antennae consist of 21 antennomeres and the right of 19 (including the scapus and pedicellus). Scapus and pedicellus with short clear setae and the scapus has a notable spur on the anterior rim lateral side. All segments beyond the scapus and pedicellus covered in stiff dark setae each longer than the segment is wide until the terminal four segments where the setae begin to steadily decrease in size until the terminal segment which has dense short setae. Thorax. Pronotum wider than long (width to length, 1 : 0.75) with parallel lateral margins, and all margins slightly granulose. Surface of the pronotum heavily granulose like the vertex of the head capsule. Prescutum significantly wider than long (width to length, 3.3 : 1), with converging lateral margins with a granular surface of at least five nodes (Fig. 20F). Surface of prescutum slightly granular but lacking significant features. Mesopleurae gently diverging, anterior edge armed with a single tubercle, remainder of the rim with four small tubercles with a single seta protruding from the tip of each. Mesopleurae surface irregularly granular with a single distinct pit in the center. Pro-, meso-, and metasternum covered in irregularly spaced granules. Wings. Tegmina short, only reaching about halfway through the metathorax. Alae developed; exposed section of folded alae moderately sclerotized. Abdomen. Abdominal segments with folding in the holotype so shape description is only approximate. Abdominal segment II slightly tapering, III gradually widening, IV widening for the first quarter, then parallel, V through the first half of VI parallel, VII converging, VIII–IX parallel to subparallel. Anal abdominal segment X longer than wide with a broad rounded apex (Fig. 20D). Poculum broad, about as broad as segment IX, ending in a broad rounded apex that reaches the anterior margin of segment X (Fig. 20E). Cercus about as wide as the vomer but slightly shorter, margins marked with a row of thin tan setae and a dorsal surface that is heavily granular. Vomer long, reaching the majority of the length to the apex, with sides gradually converging to the hooked apex (Fig. 20E). Legs. Profemora, interior lobe rounded with three small nubby, evenly spaced teeth (Fig. 20C). Exterior lobe wider than the interior lobe and with a slight recurve and an edge that is smooth with a row of single stout setae along the entire length. Protibiae lacking an exterior lobe, interior lobe a rounded scalene triangle spanning the entire length of the protibia (Fig. 20C). Mesofemora, exterior lobe smoothly arcing the length of the mesofemora, interior lobe smoothly triangular with five to six small nubby teeth on the distal half and about one and a half times as wide as exterior lobe. Interior and exterior lobe of metafemora smoothly arcing with interior lobe about twice as wide as the exterior lobe and the interior with a few small nubby teeth near the distal end. Meso- and metatibiae lacking exterior and interior lobes.
Measurements of holotype
[mm]. Length of body (including cerci and head, excluding antennae) 40.0, length/width of head 2.6/2.5, antennae (repaired) 16.4, pronotum 1.8, mesonotum 1.8, length of tegmina 5.9, length of alae 30.5, greatest width of abdomen 8.0, profemora 7.4, mesofemora 6.8, metafemora 7.3, protibiae 3.9, mesotibiae 5.0, metatibiae 6.7.
Distribution.
Currently only known from the type locality of Wau, Morobe Province, Papua New Guinea (Fig. 4).
Etymology.
Patronym. This species is dedicated to Mr. Tetsuo Miyashita (Japan). Miyashita is a major private collector who has amassed one of the largest insect collections in the world. Miyashita and the specimens from his collection have allowed the description of several new beetle taxa over the years with this being the first phasmid described from his collection.
Nanophyllium daphne sp. nov.
BFB9DCC4-8E59-5CEC-B91D-50D00C3056F5
http://zoobank.org/A5CB1FE9-AA0A-4141-A291-C20654161E50
Type material.
Holotype ♀: Indonesia: Biak. 16/9.54; NNM-Leiden, ex collectie A. Veldhuyzen. In the collection RMNH, Leiden, Netherlands.
Discussion and differentiation.
This small species (body length of the holotype only 54.0 mm) has several interesting morphological features which differentiate it from other known Nanophyllium females. The tegmina venation places this species most closely aligned to N.chitoniscoides due to the venation pattern having the radial bend occurring before the splitting of the first radial and the radial sector, therefore the radial sector is straight (Fig. 9A). Additionally, a radial and medial crossvein is present on the radial bend at or before the splitting of the first radial (Fig. 9A).
This new species can be differentiated from all other Nanophyllium by several morphological features. First, it is the only species which has exterior profemoral lobes which are obtuse (Fig. 21A), not right angles like in N.keyicum (Fig. 16D) or recurved acute angles like in all other known Nanophyllium species (for example Fig. 16C). Additionally, this is the only species known where the female has the abdomen tapering towards the posterior, giving the abdomen a spade-shaped appearance (Fig. 21C), all other known species have females with abdominal segments VI and VII either parallel sided (like in N.frondosum and N.keyicum, Fig. 16C, D respectively) or as the broadest segments (like in N.chitoniscoides and N.suzukii, Fig. 16A, B, respectively).
These unique morphological features coupled with the geographic isolation from the mainland makes it unlikely that this female represents the unknown female sex of one of the many species which are only known from males from the mainland (Fig. 4). Instead, we here describe this species as Nanophylliumdaphne sp. nov. as the first recorded Phylliidae species from Biak Island, Indonesia.
Description.
Female. Coloration. Presently, only the dried holotype specimen is known, which is fairly well-preserved with only minimal discoloration along the midline due to a lack of gutting. The majority of the body is of a pale light green coloration, with the areas of discoloration (such as the head, thorax, and shafts of the legs) being a pale brown/tan in coloration. Leaf insects are more vibrantly colored in life and it can be assumed that this specimen was a brighter green in life.
Morphology.Head. Head capsule slightly longer than wide, vertex with small granulation throughout the surface and unevenly spaced in no detectable pattern (some right next to each other some with more spacing). The posteromedial tubercle is small, only slightly noticeable and split into two lobes. Frontal convexity stout, not prominently protruding, with a lumpy surface which is marked by numerous pale setae. Antennae. Antennae consisting of nine segments. The terminal segment has a narrower base than segment VIII, instead with a width only about as wide as segments IV or V, and it is about as long as the previous two segments combined length. All segments have setae present; segments I through III have sparse but long pale setae; segments IV through VIII have sparse, stout, tan setae; and the terminal segment IX has dense, stout, dark setae. Compound eyes slender and tightly formed to the head, only reaching across one quarter of the head capsule length. Ocelli absent. Antennal fields approximately the same dimensions as the compound eyes, wider than the base of the first antennomere, and not protruding back farther than the frontal suture. Thorax. Pronotum with anterior margin that is slightly concave and lateral margins that are straight that slightly converge to a broad, slightly convex posterior margin that is about the same width as the anterior rim (Fig. 21B). The pronotum surface has moderate granulation throughout that is evenly spaced, and the pronotum surface has a moderate pit in the center and furrows along the sagittal and lateral planes (Fig. 21B). Pronotum lacks prominent rims, with only the anterior rim moderately formed and with a rough texture (but no features as prominent as actual granulation present). Pro-, meso-, and metasternum with granulation throughout, with all granules evenly spaced and of even size. Prescutum wider than long, with an anterior margin 1.3 times wider than the posterior margin (Fig. 21B). Prescutum lateral rims and surface of the prescutum with granulation throughout, but no prominent spination. No prescutum crest present, the surface is only slightly raised so it is not perfectly flat, but it is not prominent. Prescutum anterior rim slightly raised in the center but not prominent, and lacks a sagittal spine, instead there is only weak granulation throughout the rim which is similar to the granulation found on the prescutum surface. Mesopleurae start near the anterior margin but not flush with it, instead they begin notably wider than the prescutum anterior margin. Mesopleurae are nearly straight and diverge evenly along their length (Fig. 21B). Mesopleurae margins on their anterior margin are marked by a prominent tubercle immediately adjacent to two more which are medium sized and followed by three small tubercles that are nearly evenly spaced throughout the remainder of the length of the mesopleurae with slight granulation interspersed (Fig. 21B). Face of the mesopleurae has a granular surface similar to the texture of the prescutum disk and marked with a distinct pit near the middle of the surface. Wings. Tegmina long, reaching past the anterior margin of abdominal segment VIII. The subcosta (Sc) is the first vein in the forewing and arcs smoothly unbranched towards the wing margin. The radius (R) gently bends towards the wing margin almost immediately and along this bend (first on the medial side) there is a notable radius to media crossvein (R-M), then following this first branching, the radius branches (on the distal side) into the first radius (R1) which runs unbranched to the wing margin, and the remainder of the radius as the radial sector (Rs) runs unbent to the wing margin, terminating slightly past the wings mid-length. The media (M) runs nearly parallel with the cubitus along the wing margin (there is a slightly wider than side by side gap near the anterior, but the veins are almost touching throughout a majority of their length). The media anterior (MA) diverges near the wing mid-length and arcs smoothly towards the wing margin where it terminates approximately three-quarters of the way through the length of the wing; this is followed by a splitting of the media posterior (MP) which runs parallel with the media anterior as it smoothly arcs towards the wing posterior margin. Following the media posterior split there is a small media to cubitus crossvein (M-Cu) which runs briefly parallel side by side with and then fuses to the cubitus. The cubitus (Cu) is bifurcate, branching into the cubitus anterior (CuA) and cubitus posterior (CuP) which diverge evenly, and both terminate at or near the wing posterior apex. The first anal vein (1A) is simple and fuses with the cubitus near the wing anterior margin. Alae rudimentary. Abdomen. Abdominal segments II through the anterior one third of IV uniformly diverging, posterior two thirds of IV through the anterior half of V parallel, the remainder of the abdominal segments are roundly converging to the broad rounded apex giving the abdomen an overall rounded appearance. Genitalia. Subgenital plate short and rounded, starting at the anterior margin of segment VIII and extending only about halfway onto segment IX, with straight, uniformly converging margins. Subgenital plate is only about a third the length of the gonapophyses, leaving a significant amount of the gonapophyses exposed. Gonapophyses are long and slender, not quite reaching the apex of the terminal abdominal segment (Fig. 21E). Cerci broad and slightly cupped, with a surface throughout that is rough in texture, and margins with only a few short setae, none prominent. Legs. Profemoral exterior lobe broad with a rounded obtuse angle, and slightly wider than the interior lobe. Edge of the profemoral exterior lobe without notable teeth but with a margin that is granular throughout the length (Fig. 21A). Profemoral interior lobe narrower than the exterior and shaped as a slightly obtuse angle marked with four small teeth (Fig. 21A). The proximal most tooth is very small, not much more than a bump along the margin, this is followed by a narrow gap, the first prominent tooth, then a larger gap twice as wide as the first, another prominent tooth the same size as the previous, a gap the same size as the first small gap, and then one more prominent tooth at the distal end which is about the same size as the previous two teeth. The gaps between teeth are not deep and looping, instead they are straight and shallow between each tooth (Fig. 21A). Mesofemoral exterior lobe arcs smoothly from end to end and lacks dentition. The interior and exterior mesofemoral lobes are of a similar width. Mesofemoral interior lobe arcs end to end with three serrate teeth only on the distal quarter of the lobe, which is slightly wider than the proximal portion of the lobe. Metafemoral interior lobe arcs end to end with the distal end wider than the proximal, and seven to eight irregularly shaped teeth on the distal third of the lobe only. Metafemoral exterior lobe is thin, smooth, and hugs the metafemoral shaft without teeth. Protibiae lacking an exterior lobe. Protibiae interior lobe spans the entire length of the protibiae and is not particularly wide, only about the same width as the protibial shaft itself. The lobe is smoothly triangular and is slightly wider towards the distal half. Mesotibiae and metatibiae lacking exterior and interior lobes.
Measurements of holotype
[mm]. Length of body (including cerci and head, excluding antennae) 54.0, length/width of head 5.7/5.1, antennae 2.9, pronotum 4.0, mesonotum 2.7, length of tegmina 36.0, greatest width of abdomen 28.0, profemora 10.0, mesofemora 8.3, metafemora 9.9, protibiae 5.7, mesotibiae 6.4, metatibiae 8.2.
Etymology.
Noun. Named for the nymph Daphne of Greek mythology who was pursued tirelessly by the god Apollo and was eventually after pleading with her father for a way to escape the relentlessness of Apollo, was turned into a laurel tree. Derived from Greek, Δάφνη.
Distribution.
Currently only known from Biak Island, Papua Province, Indonesia.
Possible unconfirmed sexes of known Nanophyllium species/ notable specimens which cannot be identified to species
Nanophyllium
species (male)
87E7EBAB-8BFC-505D-ABED-45375D2C0707
Collection data.
One male, observed and collected by Mike Wild (USA/Indonesia) in 2015. Indonesia: Papua Province, Puncak Jaya Regency, Mokndoma, around 2,180 meters elevation.
Discussion.
This individual was observed and photographed by Mike Wild, who notes that despite living in the area for more than 14 years, and actively observing and collecting insects there the entire time, this is the only leaf insect he has ever seen. This species has highly reduced exterior profemoral lobes, which places it morphologically most similar to N.australianum (Fig. 11D) from Australia. This particular feature is not observed in other New Guinea known males to such a slender degree. This unknown species can be differentiated from N.australianum by the orange head, pronotum, and mesonotum (Fig. 22A) a unique feature in and of itself as all other known Nanophyllium males have the head and thorax the same color as the rest of the body. It is possible that this male may represent the unknown sex of one of the known female Nanophyllium or represent an undescribed species, but at this time it cannot be determined with so many species only known from a single sex.
Originally proposed by Rentz (1988) we agree that these darker and slightly metallic Nanophyllium males appear to not mimic foliage, but to instead be mimicking a wasp. Easily observed in this individual from Mokndoma and noted by Rentz (1988) the dark coloration is “shining black with a bluish overcast”. This coloration is common within Scoliidae and Pompilidae, both of which are large and intimidating wasps within the correct size range of a Nanophyllium male. Additionally, this particular specimen from Mokndoma has a bright orange head, pronotum, and mesonotum, and many species of these large wasps also have yellow, orange, or red segments of their bodies. We hope that examination of wasp species from this region and additional Nanophyllium males will help to identify possible species models.
Nanophyllium
species (male)
473C8348-1BC3-5007-B1C7-D76F588DB75E
Observational collection data.
One male, observed by Achmad Rian Dietra (Indonesia) in May of 2017. Indonesia: West Papua Province, Kaimana Regency, Aiduma Island.
Discussion. This is only known from photographs of a live individual taken by Achmad Rian Dietra (Indonesia). Based on pro- and mesofemoral lobes being strongly angular and not smoothly arcing from end to end, this individual belongs to the pygmaeum species group. This species group only has males known for six species: N.pygmaeum Redtenbacher, 1906, N.asekiense (Größer, 2002), comb. nov., N.adisi Zompro & Größer, 2003, N.rentzi Brock & Größer, 2008, N.hasenpuschi Brock & Größer, 2008, and N.australianum Cumming, Le Tirant & Teemsma, 2018.
Based on the profemoral exterior lobe that is wider than the shaft width and not larger than the interior lobe, that rules out N.australianum (exterior lobe of profemora same width as shaft width; Fig. 11D) and N.adisi (exterior lobe of profemora larger than interior lobe; Fig. 11H). Nanophylliumrentzi (Fig. 18) and N.asekiense (Größer, 2002), comb. nov. (Fig. 17E, F), can also be ruled out as possible identifications, as their entire body coloration is green and the alae are completely transparent, in contrast this specimen from Aiduma Island has a brown body and dark tegmina and alae. The two remaining identification possibilities are N.pygmaeum and N.hasenpuschi which can easily be morphologically separated by the coloration of the alae, solid brown in N.pygmaeum or alae with a large transparent patch in N.hasenpuschi. Unfortunately, this individual has its wings closed so the interior color is impossible to see. A definitive identification is unfortunately not possible at this time. Geographically this individual is located near collection sites of both N.hasenpuschi and N.pygmaeum so no inference can be drawn from locality (Fig. 4). This is however a unique opportunity to share photos of a live individual and to add a new distribution checkpoint to the map of Nanophyllium collection/observation localities (Fig. 15). It is also possible that this individual represents an undescribed species on its own, or the male for an undescribed species based on one of the below females illustrated.
Presumed records for female Nanophylliumaustralianum Cumming, Le Tirant, & Teemsma, 2018
Nanophylliumaustralianum specimens are exceedingly rare (likely due to a lack of extensive collecting in the area they are found in), with only four collections/observations known to the authors to date and all known from in/near Iron Range National Park of Northern Queensland, Australia (Fig. 4).
The first known record is a subadult female discovered by G. B. Monteith in June 1971 while he was collecting along the edge of the rainforest of Iron Range. Monteith recognized this individual as a second species for Australia and likened the species to a specimen from Popondetta, Papua New Guinea (Fig. 23A; Monteith 1971). This species was again referenced in Key (1974) but this time as “P.frondosum” based on the subadult nymph collected by Monteith (Monteith 1978).
The second collection record we are aware of is an early instar nymph collected by G. B. Monteith in February 1976, near Gordon’s Mine Area, Iron Range (see Cumming et al. 2018: fig. 2 for an image of this nymph, note the shape of the femoral lobes which are angled with distinct teeth like which can be seen in the adults).
The third record was the holotype male which was collected as a nymph by D. C. F. Rentz near Mt. Tozer within the Iron Range in December 1986 and which matured to adult in January 1987 (see Rentz (1988) for notes and photographs of the nymph when it was caught, and see Cumming et al. (2018) for images of the resulting adult and species description).
The only other individual we are aware of is a subadult female which was photographed by Chien C. Lee in July 2014 in Lockhart, Queensland (Fig. 23B, C). This female matches the morphology of the first nymph which was collected as a subadult by Monteith in 1971, and with it observed near the same general collecting locality as the holotype male N.australianum, coupled with the similarity in femoral shapes to the male, we expect these females represent the female Nanophylliumaustralianum. The authors hope that future collection efforts in the Iron Range area will yield additional specimens so we can better review the intraspecific variation of this rarely collected species and allow the morphological description of the female adult morphology.
Unidentified Nanophyllium species (females) (listed from the smallest to the largest specimens)
Nanophyllium sp. Female NYMPH (35 mm): Papua New Guinea: Eastern Highlands District LJBrass, Coll. Sixth Archbold Exped. To Papua New Guinea. No.7, Kotuni, south slopes Mt.Otto, 2200m. Aug. 4–20, 1959. (AMNH). (Fig. 24A).
This subadult could be N.asekiense or N.frondosum based on the geographic proximity to N.asekiense and N.frondosum known localities and how large it might be if it had reached adulthood. It is likely too large to have been one of the smaller species like N.pygmaeum.
Nanophyllium sp. Female (46.7 mm): Papua New Guinea: Central Province, Along Hiritano Highway, East of Vanapa River crossing. June 21st, 1989. Collected by L. M. Munsey, previously from the collection of Jerri Larsson (California), (Coll RC 16-224). (Fig. 24B).
Habitat. From the notes of L. M. Munsey the collector of the two specimens: “Daytime beating in a 1 to 3 acre area of cuttings with few small and large downed trees remaining”.
Nanophyllium sp. Female (54.0 mm): Papua New Guinea: Central Province, 20Km SE Port Moresby “bushes” 26.i.1985 J.W.Ismay. Ex Papua New Guinea DPI-CRIC Konedobu. C.I.E. COLL. A. 17440. NHMUK 012497230. (Fig. 24C).
Nanophyllium sp. Female (59.6 mm): Papua New Guinea: Daru, Papua (New Guinea) Mouth of Fly R. VII-1941. Collector RG Wind. Van Dyke Collection. (CAS). (Fig. 24D).
These three small adult females possibly represent the unknown female for Nanophylliumpygmaeum as they are the correct size and geographically are from southern Papua New Guinea, with the female from CAS from nearby the Fly River, which is the N.pygmaeum type locality.
Nanophyllium sp. Female (70.5 mm): Indonesia: New Guinea: West Irian, Kobakma, North Slope of Central Range N. of Baliem Valley 3500’ October 1971, Robert Mitton, Coll., Presented by H. Vannoy Davis, C.A.S. Accession. (CAS). (Fig. 24E).
Based on the size and morphology this female from Kobakma is similar to N.frondosum females which are known from other distant localities in Papua New Guinea. Unfortunately, the holotype N.frondosum has no other locality information other than “Neu-Guinea” (Redtenbacher, 1906) and therefore we cannot determine if N.frondosum is a wide-ranging species or if there is a complex of N.frondosum-like species. Hopefully future molecular comparison of freshly collected material can reveal the extent of N.frondosum.
An additional possibility is that this female could be the opposite sex of Nanophylliumadisi, which is known from nearby this collection location from “Hoofdbivak, 250 m Datum IX” from the Stirling Expedition of 1926 (present day Indonesia: Papua Province, Nduga Regency). This possibility is in our opinion unlikely as these localities are separated by the expansive Maoke Mountains which are most likely a geographic barrier between these populations.
Nanophyllium sp. Female (75.0 mm): Indonesia, West Papua, Mapia, V.1999 (Coll SLT) (Fig. 24F).
This female is from very near the type locality of N.hasenpuschi and could possibly represent the unknown female. Hopefully the holotype N.hasenpuschi can be sequenced one day to be compared and possibly matched with this female. Morphologically this female is similar to N.asekiense as it has slight exterior tibial lobes and similar prominent serration of the femoral lobes.
Biogeography
Phylliidae arose according to Bradler et al. (2015) in the Eocene approximately 55–65 mya ago. However, other phylogenetic approaches (Goldberg et al. 2015; Büscher et al. 2018a, b; Robertson et al. 2018) discuss monophyletic Phylliidae in various different relationships to other groups, which would suggest a later origin of the lineage. The early radiation of the extant Phyllium and Chitoniscus species is dated ~30–35 mya ago (Bradler et al. 2015; Robertson et al. 2018). The report of the fossil Eophylliummesselense Wedmann, Bradler & Rust, 2007 gives further indication that Phylliidae arose more than 47 mya ago and estimates the splitting of Nanophyllium from the remaining Phylliidae ~ 20 mya ago (Wedmann et al. 2007). Assuming an approximate splitting of Nanophyllium 20–35 mya from the remaining Phylliidae some conclusions on the biogeographical history of Nanophyllium can be drawn. Ancestors of Nanophyllium probably settled New Guinea close to that timeframe, after New Guinea emerged in the Eocene (~ 40 mya) and was separated from Australia during the Oligocene (~25 mya) after the New Guinea Passive Margin collided with the leading edge of the Eastern Philippines-Halmahera-New Guinea Arc System (Hall 2001). As extant phylliids inhabit most parts of the Oriental and Australian region, but Nanophyllium, so far known, is restricted to New Guinea, including the surrounding islands, and Australia (Fig. 4), this lineage probably evolved on New Guinea and later migrated to northern Australia. Nanophyllium likely diversified on New Guinea due to geographic isolation by the Central Cordillera (Chisholm 1911) and the Foja Mountain Ranges in the north (Richards et al. 2009; Oliver et al. 2011). During the Pleistocene (2.6 mya–11.7 ka) Australia and New Guinea were interconnected many times, due to climatic oscillation resulting in sea level fluctuations (Pillans et al. 1998), but the remaining parts of Indonesia remained separated from New Guinea (Ludt and Rocha 2015). The southernmost population of Nanophyllium possibly distributed to Australia and became separated, resulting in the speciation of N.pygmaeum and N.australianum after the Last Glacial Maximum ~ 17–19 ka ago (Ludt and Rocha 2015). The distributional records of the known Nanophyllium specimens suggest a well-established isolation of the distributions of N.miyashitai sp. nov. from other Nanophyllium by the Central Cordillera. As this formation arose quite early (probably during the Oligocene (Hall 2001), this population potentially has been isolated for a comparatively long time, that likely led to allopatric speciation. Likewise, most of the other Nanophyllium species are probably a result of allopatric isolation caused by this mountain range. Nanophylliumkeyicum and N.daphne sp. nov. in contrast, are probably a result of the isolation on the islands they are found, which became separated from mainland New Guinea.
Key to known Nanophyllium males*
| 1 | Profemoral interior lobes which are rounded without a sharp angle; mesofemoral interior lobes which are a large rounded triangle, reaching from end to end without prominent spination; (alae) the media anterior and the media posterior do no fuse, instead they both run to the wing margin, and the cubitus after splitting from the first anterior anal fuses with the media posterior near the wing margin and then they run fused to the margin as one; (stellae species group) | 1 |
| – | Profemoral interior lobe angular; mesofemoral interior lobes which do not reach from end to end of the shaft and have distinct serrate teeth; (alae) the media posterior fuses back to the media anterior before reaching the wing margin, and then the fused media runs on its own to the wing margin without fusing with the radial sector; (pygmaeum species group) | 4 |
| 2 | Mesopleurae with a single anterior tubercle, remainder lacking tubercles; tegmina longer (almost the length of the metathorax) | 3 |
| – | Mesopleurae with a prominent anterior tubercle followed by four additional small tubercles; tegmina shorter (only about half the length of the metathorax) | N.miyashitai sp. nov. |
| 3 | Exterior profemoral lobe smoothly rounded with an obtuse angle; abdominal segments with smooth edges creating a clean, spade-shaped abdomen | N.stellae |
| – | Exterior profemoral lobe slightly recurved creating an overall acute angle; abdominal segment V with two large clear spots; segments V–VII each with margins that extend and then contract creating a scalloped edge | N.larssoni |
| 4 | Alae completely transparent, not fully brown in color or with a brown band along the margin; tegmina transparent | 5 |
| – | Alae color either completely brown or with a transparent center and brown margin; tegmina completely brown | 6 |
| 5 | Profemoral interior lobe notably larger than the exterior lobe | N.asekiense |
| – | Profemoral interior lobe equal width to the exterior lobe | N.rentzi |
| 6 | Exterior profemoral lobe distinct, wider than the width of the profemoral shaft | 7 |
| – | Exterior lobe of profemora greatly reduced, not wider than the width of the profemoral shaft | N.australianum |
| 7 | Exterior profemoral lobe notably tapered on the distal and proximal ends; the interior profemoral lobe can be of the same size as the exterior lobe or larger than the exterior lobe | 8 |
| – | Exterior profemoral lobe only notably tapered on the proximal end, with the distal nearly reaching the end of the profemoral shaft; profemoral interior lobe always smaller than the exterior lobe | N.adisi |
| 8 | Alae almost completely brown, or completely brown in color | N.pygmaeum |
| – | Only the alae margin and sclerotized section brown, interior half of the alae transparent | N.hasenpuschi |
Key to known Nanophyllium females
| 1 | Small species (~ 56.0 mm or less); (tegmina) the radial bend occurs before the splitting of the first radial and the radial sector, therefore the radial sector is straight; the radius and medial crossvein is present on the radial bend at or before the splitting of the first radial | 2 |
| – | Larger species (> 56.0 mm); (tegmina) the bend in the radial vein happens on the radial sector after the splitting of the first radial from the radius; the radius and medial crossvein occurs after the splitting of the first radial, instead originating on the radial sector | 3 |
| 2 | Profemoral exterior lobe broad, with a slight recurve, giving the exterior angle an acute or right angle; mesofemoral interior lobe with the widest portion on the proximal half | N.chitoniscoides comb. nov. |
| – | Profemoral exterior lobe narrow, smoothly arcing from end to end with the exterior angle distinctly obtuse; mesofemoral interior lobe with the widest portion on the distal half | N.daphne sp. nov. |
| 3 | (Tegmina) there is a wide gap between the media and cubitus veins which persists throughout their entire length, this gap is several times wider than a single vein width; profemoral exterior lobe proximal margin is straight, not recurved | N.keyicum comb. nov. |
| – | (Tegmina) the media and cubitus veins run side by side throughout the entire length either touching or no farther than a single vein width apart; profemoral exterior lobe proximal margin is recurved, not straight | 4 |
| 4 | Prescutum width more than two times the length; mesofemoral exterior lobe broad, notably wider than the mesofemoral shaft | N.suzukii comb. nov. |
| – | Prescutum width less than two times the length; mesofemoral exterior lobe as wide as or thinner than the mesofemoral shaft | 5 |
| 5 | No protibial exterior lobes and no mesotibial exterior lobes, exteriors simple | N.frondosum comb. nov. |
| – | Distinct protibial exterior lobes and mesotibial exterior lobes, present as small spurs | N.asekiense comb. nov. |
Conclusions
Review of a wide number of institution and private collections as well as the successful rearing by the Montreal Insectarium has revealed the identity of a previously unconfirmed female Nanophyllium. This has allowed us to synonymize the frondosum species group (only known from females) with the Nanophyllium (only known from males) into a single genus.
Unfortunately, due to the striking sexual dimorphism in Nanophyllium, this leaves many females and males with unknown opposite sexes and the possibility that some of the presently described species of either group might simply be the opposite sex of an already known species. Hopefully future collections of fresh material from throughout the region will either allow successful rearing of species to elucidate the unknown sex or allow pairing of sexes on a molecular basis. Additionally, we eagerly await extensive molecular analysis for the Phylliidae as a whole to elucidate the higher taxonomy within the family and the placement of the Nanophyllium.
Supplementary Material
Acknowledgments
Mr. Tetsuo Miyashita who recognized the importance of describing this new Nanophyllium species from his personal collection. Alexandre Banko for his collaboration and transportation of the holotype Nanophylliummiyashitai specimen. René Limoges, entomological technician at the Montreal Insectarium for taking many of the photos for this work, as well as for many professional courtesies. Paul Harrison, Mario Bonneau, Thierry Boislard, Thomas Théry, Jennifer De Almeida, and Dominic Ouellette, entomological technicians at the Montreal Insectarium for caring for the live Phylliidae cultures and for taking photos of the live Nanophyllium. Lorraine Bluteau, horticulturist at the Montreal Insectarium for growing the host plants for the breeding laboratory for many years. Maxim Larrivée (director of the Montreal Insectarium) and Michel Saint-Germain (head of research and entomological collections at the Montreal Insectarium) for their support to “Team Phyllies”. Stephan Blanke and Mandy Schröter at the Senckenberg German Entomological Institute Müncheberg (SDEI) for supplying photos of the holotype female Phylliumsuzukii. Achmad Rian Dietra (Indonesia) for allowing us to use his photograph of a live Nanophyllium male he observed on Aiduma Island. Mike Wild (USA/Indonesia) for allowing us to use his photo of a live Nanophyllium male he found in Mokndoma. Jim Berrian (SDNHM) for assistance with the museum’s camera equipment and much support over the years. Judith Marshall and Benjamin Price (NHMUK) for processing the loan of the Phylliidae specimens despite the lack of an Orthopteroid collection curator. Paul Brock for reading the first working draft and offering suggestions. Jason Weintraub (ANSP) for searching the collection for relevant specimens for this study and for information about the “Third South New Guinea Expedition”. Susan Wright, collection manager at the Queensland Museum for photos of their collection. Yvonne van Dam (RMNH) for preparing photographs of type material of Nanophylliumdaphne sp. nov. and Phylliuminsulanicum (newly accepted name: Nanophylliumkeyicum). Chien C. Lee (Malaysia) for sharing images of a wild female Nanophylliumaustralianum. Thank you to our peer reviewers for valuable feedback on this article.
Citation
Cumming RT, Le Tirant S, Teemsma SN, Hennemann FH, Willemse L, Büscher TH (2020) Lost lovers linked at long last: elusive female Nanophyllium mystery solved after a century of being placed in a different genus (Phasmatodea, Phylliidae). ZooKeys 969: 43–84. https://doi.org/10.3897/zookeys.969.56214
Footnotes
Adapted from Cumming et al. 2018.
References
- Bradler S, Cliquennois N, Buckley TR. (2015) Single origin of Mascarene stick insects: ancient radiation on sunken islands? BMC Evolutionary Biology 15: 1–196. 10.1186/s12862-015-0478-y [DOI] [PMC free article] [PubMed]
- Brock PD. (1999) The Amazing World of Stick and Leaf-Insects. The Amateur Entomologists’ Society, Orpington, Kent, England. The Amateur Entomologist 26: 1–165. [Google Scholar]
- Brock PD, Hasenpusch J. (2003) Studies on the leaf insects (Phasmida: Phylliidae) of Australia. Journal of Orthoptera Research 11: 199–205. 10.1665/1082-6467(2002)011[0199:SOTLIP]2.0.CO;2 [DOI]
- Brock PD, Büscher TH, Baker E. (2020) Phasmida SF: Phasmida Species File Version 5.0/5.0. In: Roskov Y (Eds) Species 2000 & ITIS Catalogue of Life. Species 2000: Naturalis, Leiden. https://www.catalogueoflife.org/col
- Buckley TR, Attanayake D, Bradler S. (2009) Extreme convergence in stick insect evolution: phylogenetic placement of the Lord Howe Island tree lobster. Proceedings of the Royal Society B 276: 1055–1062. 10.1098/rspb.2008.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher TH, Buckley TR, Grohmann C, Gorb SN, Bradler S. (2018a) The evolution of tarsal adhesive microstructures in stick and leaf insects (Phasmatodea). Frontiers in Ecology and Evolution 6: 1–11. 10.3389/fevo.2018.00069 [DOI] [Google Scholar]
- Büscher TH, Kryuchkov M, Katanaev VL, Gorb SN. (2018b) Versatility of Turing patterns potentiate rapid evolution in the attachment microstructures of stick and leaf insects (Phasmatodea). Journal of the Royal Society Interface 15: 1–7. 10.1098/rsif.2018.0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm H. (1911) New Guinea. In: Chisholm H. (Ed.) Encyclopædia Britannica 19 (11th edn.). Cambridge University Press, Cambridge, 486–490.
- Clark JT. (1978) The eggs of leaf insects (Insecta: Phasmida). Zoological Journal of the Linnean Society 63: 249–258. 10.1111/j.1096-3642.1978.tb02562.x [DOI] [Google Scholar]
- Cumming RT, Leong JV, Lohman DJ. (2017) Leaf insects from Luzon, Philippines, with descriptions of four new species, the new genus Pseudomicrophyllium, and redescription of Phyllium (Phyllium) geryon Gray, 1843, (Phasmida: Phylliidae). Zootaxa 4365: 101–131. 10.11646/zootaxa.4365.2.1 [DOI] [PubMed] [Google Scholar]
- Cumming RT, Le Tirant S, Teemsma SN. (2018) Northeastern Australia record of Nanophylliumpygmaeum Redtenbacher, 1906, now recognized as a new species, Nanophylliumaustralianum n. sp. (Phasmida, Phylliidae). Faunitaxys 6: 1–5. [Google Scholar]
- Cumming RT, Teemsma SN. (2018) Phyllium (Phyllium) letiranti sp. nov. (Phasmida, Phylliidae) a new leaf insect from Peleng Island, Indonesia. Insecta Mundi 0618: 1–16. [Google Scholar]
- Cumming RT. (2016) A new species of Nanophyllium Redtenbacher, 1906 from the northern coast of New Guinea (Phasmida, Phylliidae). Zootaxa 4147: 089–091. 10.11646/zootaxa.4147.1.7 [DOI] [PubMed] [Google Scholar]
- Cumming RT. (2017) A second new species of Nanophyllium Redtenbacher, 1906 from the northern coast of New Guinea (Phasmida, Phylliidae). Zootaxa 4238: 246–248. 10.11646/zootaxa.4238.2.3 [DOI] [PubMed] [Google Scholar]
- Goldberg J, Bresseel J, Constant J, Kneubühler B, Leubner F, Michalik P, Bradler S. (2015) Extreme convergence in egg-laying strategy across insect orders. Scientific Reports 5(7825): 1–7. 10.1038/srep07825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressitt JL. (1982) Ecology and biogeography of New Guinean Coleoptera (beetles). In: Gressitt JL. (Eds) Monographiae Biologicae 42, Biogeography and Ecology in New Guinea.Dr W. Junk Publishers, The Hague, 709–734. 10.1007/978-94-009-8632-9_36 [DOI]
- Größer D. (1992) Zwei neue Arten der Gattung Phyllium aus Neugenia (Phasmatodea: Phylliidae). Entomologische Zeitschrift 102: 162–167. [Google Scholar]
- Größer D. (2008) Wandelnde Blätter. Ein Katalog aller bisher beschriebenen Phylliinae-Arten und deren Eier mit drei Neubeschreibungen (2nd edn.). Chimaira, Frankfurt am Main, 175 pp. [Google Scholar]
- Hall R. (2001) Cenozoic reconstructions of SE Asia and the SW Pacific: changing patterns of land and sea. In: Metcalfe I, Smith JMB, Morwood M, Davidson ID (Eds) Faunal and Floral Migrations and Evolution in SE Asia-Australasia, Swets & Zeitlinger, Lisse, 35–56.
- Hennemann FH, Conle OV, Gottardo M, Bresseel J. (2009) On certain species of the genus Phyllium Illiger, 1798, with proposals for an intra-generic systematization and the descriptions of five new species from the Philippines and Palawan (Phasmida: Phylliidae: Phylliinae: Phylliini). Zootaxa 2322: 1–83. 10.11646/zootaxa.2322.1.1 [DOI] [Google Scholar]
- Herwaarden HCM. (1998) A guide to the genera of stick- and leaf-insects (Insecta: Phasmida) of New Guinea and the surrounding islands. Science in New Guinea 24: 55–117. [Google Scholar]
- Illiger JKW. (1798) Verzeichnis der Käfer Preussens. Johann Jacob Gebauer, Halle, 510 pp. https://biodiversitylibrary.org/page/52579286 [Google Scholar]
- Key KHL. (1974) Phasmatodea (Stick-insects). In: CSIRO (Ed.) The Insects of Australia.Supplement 1974. Melbourne University Press, Australia, 48–49.
- Ludt WB, Rocha LA. (2015) Shifting seas: the impacts of Pleistocene sea‐level fluctuations on the evolution of tropical marine taxa. Journal of Biogeography 42: 25–38. 10.1111/jbi.12416 [DOI] [Google Scholar]
- Monteith GB. (1971) Leaf Insects from Australia. The Entomological Society of Queensland 78: 14–15. [Google Scholar]
- Monteith GB. (1978) The First Male Leaf Insect from Australia. The Entomological Society of Queensland 5: 138–139. [Google Scholar]
- Oliver P, Krey K, Richards S. (2011) A new species of bent-toed gecko (Cyrtodactylus, Gekkonidae) from the North Papuan Mountains. Zootaxa 2930: 22–32. 10.11646/zootaxa.4671.1.9 [DOI] [Google Scholar]
- Pillans B, Chappell J, Naish TR. (1998) A review of the Milankovitch climatic beat: template for Plio-Pleistocene sea-level changes and sequence stratigraphy. Sedimentary Geology 122: 5–21. 10.1016/S0037-0738(98)00095-5 [DOI] [Google Scholar]
- Redtenbacher J. (1906) Die Insektenfamilie der Phasmiden. I. Phasmidae, Areolatae. Verlag W. Engelmann, Leipzig, 180 pp. [Google Scholar]
- Rentz DCF. (1988) Nanophylliumpygmaeum Redtenbacher (Phasmatodea: Phylliidae: Phylliinae), a leaf insect recently recognized in Australia. Australian Entomological Magazine 15: 1–3. http://hdl.handle.net/102.100.100/266346?index=1 [Google Scholar]
- Robertson JA, Bradler S, Whiting MF. (2018) Evolution of Oviposition Techniques in Stick and Leaf Insects (Phasmatodea). Frontiers in Ecology and Evolution 6: 1–216. 10.3389/fevo.2018.00216 [DOI] [Google Scholar]
- Richards SJ, Oliver PM, Krey K, Tjaturadi B. (2009) A new species of Litoria (Amphibia: Anura: Hylidae) from the foothills of the Foja Mountains, Papua Province, Indonesia. Zootaxa 2277: 1–13. 10.11646/zootaxa.2277.1.1 [DOI] [Google Scholar]
- Stål C. (1875) Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar. Recensio Orthopterorum. Revue critique des Orthopteres, Decrits Par, Linne De Geer et Thunberg. P. A. Norstedt & Söner, Stockholm, Sweden, 32(III), 105 pp. [Google Scholar]
- Wedmann S, Bradler S, Rust J. (2007) The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proceedings of the National Academy of Sciences of the United States of America 104: 565–569. 10.1073/pnas.0606937104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zompro O, Größer D. (2003) A generic revision of the insect order Phasmatodea: The genera of the areolate stick insect family Phylliidae (Walking Leaves). Spixiana 26: 129–141. https://www.zobodat.at/pdf/Spixiana_026_0129-0141.pdf [Google Scholar]
- Zompro O. (2001) Philippine phasmids from the collection of the Staatliches Museums fur Tierkunde, Dresden (Insecta: Phasmatodea). Reichenbachia 34: 49–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.