Abstract
Background
This study aimed to evaluate the usefulness of the first-trimester crown-rump length (CRL) and nuchal translucency (NT) discordance in monochorionic diamniotic twins (MCDA) for the prediction of complications—twin–twin transfusion syndrome (TTTS), selective fetal growth restriction (sFGR) or intrauterine fetal demise (IUFD).
Methods
Intertwin discordance in the CRL and NT was calculated as a percentage of the larger CRL and NT, respectively. The performance of inter twin discordance (CRL ≥ 10% and NT≥ 20%) for predicting complications was analysed using standard statistical screening test methods.
Results
Fifty-eight MCDA twin pregnancies were studied. Out of them, 19 (32%) pregnancies resulted in one of the complications studied (4 TTTS, 10 sFGR, 5 IUFD). CRL and NT discordance showed an increased probability of developing complications positive likelihood ratio (LR+) {95% confidence interval}: 2.05 {0.46–9.23} and 1.88 {1.03–3.45}, respectively. NT discordance showed a sensitivity of 57%.
Conclusions
Although discordant first-trimester CRL and NT in monochorionic twins are poor screening tools for early prediction, if positive, they increase the risk of developing complications.
Keywords: MCDA twins, CRL discordance, NT discordance, First-trimester screening
Introduction
Monochorionic (MC) twin pregnancies are considered high-risk pregnancies because of the risk of developing unique complications like twin–twin transfusion syndrome (TTTS), selective fetal growth restriction (sFGR) or intrauterine fetal demise (IFUD) of one or both the twins, resulting in increased perinatal mortality and morbidity [1]. Mostly, these complications are either attributable to unidirectional large vascular anastomoses in the placenta connecting two fetal circulations (more specific for the development of TTTS) or due to unequal sharing of placenta between the twins (resulting in more in sFGR cases). TTTS complicates around 10–15% of monochorionic pregnancies [2], resulting in hemodynamic and fluid imbalances among the fetuses, and if left untreated, results in fetal demise in up to 90% cases [3]. sFGR (traditionally defined as estimated weight discordance of more than 25% among fetuses) occurs in up to 15% of monochorionic pregnancies, which aside from increasing the risk of preterm deliveries, is also a high-risk factor for resulting demise of the growth-restricted fetus [2, 3]. This can, in turn, because of the presence of vascular anastomoses, have neurological consequences in the surviving twin [2]. Many international guidelines recommend extensive monitoring of monochorionic pregnancies starting from 16 weeks [2–4], which entails fortnightly scanning to look for growth and amniotic fluid discordance and Dopplers. This approach allows the identification of complications early so that patients are offered fetal therapy.
Various predictive markers/screening tests are proposed to identify high-risk monochorionic pregnancies which may end up with these complications but more importantly to reassure couples at the start of the pregnancy that they potentially are in a low-risk group.
The objective of this study was to evaluate the usefulness of the first-trimester crown-rump length (CRL) and nuchal translucency (NT), both markers are performed routinely in all cases and to see whether discordance of CRL and NT between the two twins would predict complications such as—twin–twin transfusion syndrome (TTTS), selective fetal growth restriction (sFGR) or intrauterine demise (IUFD).
Methods
This was a retrospective study conducted at Apollo Centre for Fetal Medicine, between January 2010 and December 2018, a tertiary care referral centre in Delhi.
We retrieved data of all relevant parameters from an obstetric database Astraia software Gmbh (Version 1.25.2, Germany), of all viable monochorionic pregnancies who had their CRL and NT measured between 11 and 13 + 6 weeks’ gestation, as a part of first-trimester aneuploidy screening. The Fetal Medicine Foundation (FMF)-UK NT accredited specialists performed all the scans according to the defined criteria for CRL and NT measurements [5]. We ascertained the chorionicity by identifying the T-sign (monochorionic) or lambda sign (dichorionic), and the number of placental masses for all pregnancies [6].
Pregnancies referred to us at later gestations (≥ 14 weeks) with or without complications, even where the first-trimester CRL and NT measurements were available but not performed by us were excluded from the study as were those MCDA twins who did not follow up with us.
Following our protocol, all monochorionic pregnancies were followed by ultrasound at two-weekly intervals from 16 weeks onwards and more frequently if there were any ultrasound signs suggestive of complications.
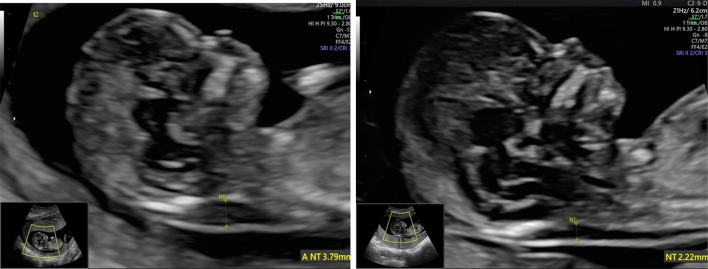
For each pregnancy, CRL and NT intertwin discordance percentage were calculated: (CRL or NT of the larger twin minus the CRL or NT of the smaller twin)/CRL or NT of the larger twin × 100. CRL discordance of ≥ 10% and NT discordance of ≥ 20%, criteria defined by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) guidelines on twins, was considered as significant discordance [3] (See Fig. 1).
Fig. 1.
Image showing NT discordance at 12 + 1 weeks
The pregnancies were followed for the development of any of the following complications:
TTTS was defined as the occurrence of oligohydramnios in one sac (donor twin, deepest vertical pocket {DVP} < 2 cm) and polyhydramnios in the other sac (recipient twin, DVP > 8 cm) [7].
sFGR was defined according to following criteria: estimated fetal weight (EFW) of one twin < 3rd centile or presence of at least 2 out of four contributory parameters (EFW of one twin < 10th centile, the abdominal circumference of one twin < 10th centile, EFW discordance of ≥ 25%, and umbilical artery pulsatility index of the smaller twin > 95th centile) [8].
The intrauterine fetal demise of one or both the twins after 16 weeks, not secondary to TTTS or sFGR.
We collected data of other outcomes, i.e. gestation at delivery, mode of delivery, birth weight of both the fetuses and any other specific pregnancy-related complications from the labour ward database, telephonically from the parents or their obstetricians.
The primary objective was to evaluate the predictive ability of the first-trimester CRL or NT intertwin discordance, expressed as a percentage of the larger twin measurement, for subsequent development of any adverse events (TTTS, sFGR or IUFD).
Data Analysis
All continuous variables were tested for normality assumptions using the Kolmogorov–Smirnov test. Descriptive statistics such as mean, standard deviations and range values were calculated for normally distributed data. Categorical variables were expressed as frequency and percentages. Diagnostic measures such as sensitivity, specificity, positive likelihood ratios and negative likelihood ratios (for assessing the value of performing a particular test) were calculated along with the 95% confidence intervals (CI) Receiver operating characteristic (ROC) curves were constructed for NT and CRL discordance, and area under the curve along with 95% CI was calculated for each.
SPSS statistics software IBM version 23.0 (Armonk, NY: IBM Corp) was used for calculations.
Results
A total of 58 monochorionic diamniotic pregnancies were recruited after satisfying the inclusion and exclusion criteria. The mean age of the participants was 30.4 years (SD 5.4), and mean BMI was 24.8 kg/m2 (SD 3.6). Nulliparous women formed 61.6% of the cohort and most conceived spontaneously (85%).
Out of 58 MCDA twin pairs, 19 (33%) pregnancies resulted in one or the other complications studied—4 had TTTS, 10 pregnancies were complicated by sFGR, and 5 pregnancies resulted in the intrauterine demise of one or both the twins (Fig. 2).
Fig. 2.
Flow diagram summarising pregnancies who developed adverse outcomes and explaining possible causes for IUFD
Table 1 shows the details of pregnancies which had any of these complications, along with NT and CRL discordance calculated in each pregnancy and subsequent outcomes of the pregnancies.
Table 1.
Details of pregnancies which developed complications, along with NT and CRL discordance and subsequent outcomes
| Patient | Gestational age at scan (weeks) | NT1 (mm) | NT2 (mm) | NT discordance (%) | CRL1 (mm) | CRL2 (mm) | CRL discordance (%) | Complications TTTS-1, sFGR-2, IUD-3 |
Outcome (weeks) {Live birth-LB} |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 + 1 | 1.2 | 0.9 | 25 | 52.5 | 48.5 | 8 | 2 | LB (35 + 2) |
| 2 | 12 | 1.0 | 4.1 | 75 | 50.0 | 51.5 | 3 | 2 | LB (35 + 1) |
| 3 | 12 + 1 | 1.4 | 1.1 | 21 | 50.0 | 55.4 | 10 | 2 | LB (35 + 2) |
| 4 | 12 + 2 | 1.6 | 1.7 | 5 | 60.3 | 63.8 | 6 | 3 | IUD (16 + 6) |
| 5 | 12 | 1.5 | 1.2 | 20 | 51.5 | 51.4 | 0.2 | 2 | LB (35 + 1) |
| 6 | 13 + 4 | 1.5 | 1.5 | 0 | 83.1 | 83.6 | 0.6 | 3 | 1LB (34 + 3) |
| 7 | 13 | 1.9 | 1.7 | 11 | 67.4 | 63.8 | 5.3 | 1 | LB (28) |
| 8 | 13 + 5 | 2.4 | 2.3 | 4 | 77.0 | 77.0 | 0 | 3 | IUD (18 + 6) |
| 9 | 12 + 1 | 1.7 | 10 | 83 | 55.8 | 55 | 0.1 | 3 | IUD (17) |
| 10 | 12 + 4 | 1.2 | 2.1 | 42 | 57.3 | 63.6 | 10 | 2 | LB (35 + 1) |
| 11 | 12 + 4 | 1.6 | 1.5 | 6 | 67.4 | 69.7 | 3 | 2 | LB (32 + 5) |
| 12 | 12 + 2 | 1.6 | 1.4 | 13 | 63.9 | 64.8 | 1.4 | 1 | 1LB (28) |
| 13 | 12 + 4 | 4.4 | 1.6 | 63 | 61.5 | 62 | 0.8 | 3 | IUD (20) |
| 14 | 13 + 3 | 2.6 | 2.0 | 23 | 72.7 | 72 | 0.9 | 1 | Termination (17 + 6) |
| 15 | 12 + 4 | 1.1 | 1.7 | 35 | 66.1 | 68.8 | 4 | 1 | LB (28 + 5) |
| 16 | 13 + 6 | 1.1 | 1.2 | 8 | 87.0 | 84 | 3 | 2 | LB (35 + 1) |
| 17 | 13 + 3 | 1.2 | 1.6 | 25 | 54.0 | 74 | 27 | 2 | LB (28 + 2) |
| 18 | 12 + 3 | 1.6 | 1.4 | 13 | 58.0 | 58 | 0 | 2 | LB (34) |
| 19 | 11 + 4 | 1.6 | 5.7 | 72 | 53.3 | 56.4 | 5 | 2 | LB (29 + 4) |
Based on combined first-trimester screening (ultrasound markers plus dual marker), 7 women screened positive for Down syndrome. As a protocol of our unit, invasive testing was offered to them; 5 out of 7 opted for chorionic villous sampling, and 2 declined further testing. In those tested, no chromosomal aneuploidies were detected.
NT discordance (≥ 20%) as a predictor of adverse outcomes had a low sensitivity [57.9%; 95% CI 33.5–79.8%] but a relatively good specificity [69.2%; 95% CI 52.4–82.9%]. The accuracy of CRL discordance (≥ 10%) as a predictor of complications was very low, the sensitivity of CRL discordance was 15.8% [95% CI 3.4–39.6%]; however, it showed high specificity for predicting low adverse outcomes [92.3%; 95% CI 79.1–98.4%].
Table 2 summarises the performance of NT and CRL discordance for prediction of adverse pregnancy outcomes.
Table 2.
Performance of NT and CRL discordance for prediction of adverse pregnancy outcomes
| Sensitivity (%) | Specificity (%) | Positive likelihood ratio [95% CI] | Negative likelihood ratio [95% CI] | |
|---|---|---|---|---|
| NT discordance (≥ 20%) | 57.9 | 69.2 | 1.88 [1.03–3.45] | 0.61 [0.34–1.07] |
| CRL discordance (≥ 10%) | 15.8 | 92.3 | 2.05 [0.46–9.23] | 0.91 [0.74–1.13] |
CRL and NT discordance showed a moderate increased probability of developing adverse fetal outcomes, positive likelihood ratio (LR+) {95% CI}: 2.05 {0.46–9.23} and 1.88 {1.03–3.45%}, respectively.
The prediction of the subsequent development of complications provided by NT discordance (≥ 20%) [Fig. 3], expressed as the area under the ROC curve was 0.63 (95% CI 0.47–0.78), while it was 0.60 (95% CI 0.45–0.77) for ≥ 10% CRL discordance [Fig. 4].
Fig. 3.

The ROC curves of NT discordance as a predictor of adverse fetal events
Fig. 4.

The ROC curves of CRL discordance as a predictor of adverse fetal events
Discussion
Main Findings Out of 58 MCDA twin pregnancies analysed in our study, complications specific to monochorionic placentation (TTTS, sFGR or IUFD) occurred in 33% of the cases, which is slightly higher than quoted but that may be because we are a referral unit for MCDA pregnancies [1]. Our data showed that first-trimester CRL discordance (≥ 10%) and NT discordance (≥ 20%) were not useful predictors of an adverse outcome [sensitivity (58% and 15%). However they had a reasonable specificity (69% and 92%). These parameters can at least be used for initial triaging of patients, the high risk ones to be referred for a second opinion to a fetal therapy centre for counselling and the low risk ones followed up locally.
Mackie et al. [9], in their study of 177 monochorionic diamniotic twins, demonstrated a statistically significant increase in the odds of developing complications specific to monochorionic placentation for each 1 percentage increase in NT and CRL discordance.
Systematic reviews and meta-analysis [10, 11] done to evaluate the role of ultrasound markers (NT or CRL discrepancy) as early predictors of outcomes in monochorionic pregnancies have shown nearly similar results as ours, that is, a moderate increase in the likelihood of developing adverse outcomes with intertwin CRL discrepancy (≥ 10%) and NT discrepancy (≥ 20%), LR + 2.63 [95% CI, 1.51–4.58] and 1.92 [95% CI 1.25–2.96], respectively [11] with overall low sensitivity (52% and 15–20%, respectively) and moderate specificity (73% and 90%, respectively) for detecting complications in these studies [10, 11]. Thus, their clinical utility as a robust screening test remains to be established. This was similar to our findings.
A recently published study by Mogra et al. [12] showed first-trimester NT discordance (> 20%) as a reliable predictor for TTTS (n = 9) with a reported sensitivity of 88% and specificity of 70% unlike ours where subgroup analysis using NT discordance as a screening method for TTTS (n = 4) showed a sensitivity of 50% with a specificity of 59%. Even they found a poor correlation of CRL discordance with subsequent TTTS development. None of the patient in our group who had the first-trimester CRL discordance developed TTTS and 4 who did were concordant for CRL. Theoretically, CRL discordance should be more specific for sFGR prediction, but it failed to achieve the statistically significant values on subgroup analysis [12]. However, the numbers in each group of complications were perhaps too few to draw any meaningful conclusion if analysed individually.
According to the results of the present study absence of ultrasound features of discordance in the first trimester, CRL and NT cannot be considered as a benign feature as these screening methods do not have a consistently high negative predictive value. The presence of these features, however, also cannot be regarded as an ominous sign as we have assumed till now due to its low predictive value.
In conclusion, it is not possible to identify which pregnancies will develop the complications specific to MCDA pregnancies; therefore, as recommended by most of the guidelines [2–4, 6, 13], we need to continue with extensive ultrasound monitoring, every 2 weekly from 16 weeks onwards, irrespective of the first-trimester ultrasound signs of CRL/NT discordance, for timely identification, with appropriate referral to higher centres for management of these severe complications.
Those screened negative could be triaged as potentially low risk yet followed up locally but with the same rigorous fortnightly fetal surveillance protocol.
Strengths and Limitations Main advantage of our study is that all monochorionic pregnancies were followed up with the currently recommended standard screening protocol for adverse outcomes (TTTS and sFGR were defined according to the latest internationally accepted definitions [7, 8] in a centre, where early recourse to fetal therapy is available).
The relatively small cohort size and retrospective nature of the study are its main limitations. Also, CRL/NT discordance and their predictive values for TTTS or sFGR could not be studied separately, as done in most of the previously reported studies because of the small number of complications in each group.
Conclusion
Discordance in the first-trimester CRL (≥ 10%) and in NT (≥ 20%) in monochorionic twins, are poor screening tools for early prediction of adverse pregnancy outcomes (TTTS, sFGR or IUFD), but if positive, they increase the subsequent chances of developing these complications. Nevertheless, large cohort studies are required for evaluating the role of various markers (ultrasound and serum biomarkers) in monochorionic pregnancies as early predictor of adverse outcomes and to develop their role as screening methods.
Saloni Arora
has done post-graduation in obstetrics and gynecology from Govt. Medical College and Hospital, Chandigarh in 2015. Following residency she joined Apollo Centre for Fetal Medicine to pursue her passion for fetal medicine and completed her fellowship in 2019. Presently, she is working as a consultant fetal medicine at Apollo Centre for Fetal Medicine, New Delhi.
Funding
No funding sources.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Written informed consent is taken from all the patients, visiting our centre, about the use of their personal data for future studies. Also for doing reanalysis of de-identified data permission was taken from the Institutional ethics committee.
Footnotes
Dr. Saloni Arora, MD, MRCOG, Fellowship Fetal Medicine, Consultant, Apollo Centre for Fetal Medicine, New Delhi, India. Dr. Smriti Prasad, MD, MRCOG, Fellowship Fetal Medicine, Consultant, Apollo Centre for Fetal Medicine, New Delhi, India. Dr. Akshatha Sharma, MD, MRCOG, Fellowship Fetal Medicine, Consultant, Apollo Centre for Fetal Medicine, New Delhi, India. Dr. Anita Kaul, MD, FRCOG, Diploma in Fetal Medicine, Clinical Coordinator and Head of Department, Apollo Centre for Fetal Medicine, New Delhi, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fichera A, Prefumo F, Stagnati V, et al. Outcome of monochorionic diamniotic twin pregnancies followed at a single center. Prenat Diagn. 2015;35(11):1057–1064. doi: 10.1002/pd.4643. [DOI] [PubMed] [Google Scholar]
- 2.Kilby MD, Bricker L, On behalf of the Royal College of Obstetricians and Gynaecologists Management of monochorionic twin pregnancy. BJOG. 2016;124:e1–e45. [Google Scholar]
- 3.Khalil A, Rodgers M, Baschat A, et al. ISUOG practice guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. 2016;47:247–263. doi: 10.1002/uog.15821. [DOI] [PubMed] [Google Scholar]
- 4.ACOG Multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Practice Bulletin no. 169. Obstet Gynecol. 2016;128:e131–e146. doi: 10.1097/AOG.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 5.FASP. Fetal anomaly screening programme. London: Crown. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/443865/FASP_ultrasound_handbook_July_2015_090715.pdf (2015). Accessed 14 May 2020.
- 6.National Collaborating Center for Women’s and Children’s Health (UK). Twin and triplet pregnancy. Commissioned by the National Institute for Clinical Excellence. 2019.
- 7.Kontopoulos E, Chmait RH, Quintero RA. Twin-to-twin transfusion syndrome: definition, staging, and ultrasound assessment. Twin Res Hum Genet. 2016;19(3):175–183. doi: 10.1017/thg.2016.34. [DOI] [PubMed] [Google Scholar]
- 8.Khalil A, Beune I, Hecher K, Reed K, et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: a Delphi procedure. Ultrasound Obstet Gynecol. 2019;53(1):47–54. doi: 10.1002/uog.19013. [DOI] [PubMed] [Google Scholar]
- 9.Mackie FL, Whittle R, Morris RK, et al. First-trimester ultrasound measurements and maternal serum biomarkers as prognostic factors in monochorionic twins: a cohort study. Diagn Progn Res. 2019;3:9. doi: 10.1186/s41512-019-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackie FL, Hall M, Morris R, et al. Early prognostic factors of outcomes in monochorionic twin pregnancy: systematic review and meta-analysis. Am J Obstet Gynecol. 2018;219(5):436–446. doi: 10.1016/j.ajog.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Stagnati V, Zanardini C, Fichera A, et al. Early prediction of twin-to-twin transfusion syndrome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49:573–582. doi: 10.1002/uog.15989. [DOI] [PubMed] [Google Scholar]
- 12.Mogra R, Saaid R, Tooher J, et al. Prospective validation of first-trimester ultrasound characteristics as predictive tools for twin-twin transfusion syndrome and selective intrauterine growth restriction in monochorionic diamniotic twin pregnancies. Fetal Diagn Ther. 2020;47:321–327. doi: 10.1159/000504049. [DOI] [PubMed] [Google Scholar]
- 13.Management of monochorionic twin pregnancy. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s Health/Statement and guidelines/Clinical-Obstetrics/Management-of-Monochorionic-Twins-(C-Obs-42)-review-July-2017.pdf?ext = .pdf (2017). Accessed 14 May 2020.