Abstract
This study evaluated in vitro the potential prebiotic effects of a freeze-dried juice extracted from cladodes of Pilosocereus gounellei (A. Weber ex K. Schum.) Bly. Ex Rowl, an unconventional edible plant from Brazilian Caatinga biome and popularly known as xique-xique. Prebiotic effects of freeze-dried xique-xique cladode juice (XCJ, 20 g/L) were evaluated by measurements of prebiotic activity scores and stimulatory effects on growth and metabolic activities of probiotic Lactobacillus acidophilus LA-05, L. casei L-26 and L. paracasei L-10, which are beneficial species found as part of human gut microbiota. XCJ showed positive prebiotic activity scores on all examined probiotics, indicating a selective stimulatory effect on these microorganisms in detriment to enteric pathogens. Examined probiotics had high viable counts (> 8 log CFU/mL) after 48 h of cultivation in media with XCJ (20 g/L), representing an increase of > 2 log CFU/mL when compared to viable counts found on time zero. Cultivation of probiotics in media with XCJ resulted in decreased pH during the 48 h-incubation. Contents of fructose and glucose decreased in media with XCJ inoculated with L. acidophilus LA-05, L. casei L-26 or L. paracasei L-10 during the 48 h-cultivation, in parallel with an increase in contents of acetic and lactic acids. Measured effects of XCJ on probiotics were overall similar to those exerted by fructoligosaccharides (20 g/L), a proven prebiotic ingredient. These results showed that XCJ could exert selective stimulatory effects on different Lactobacillus species, which are indicative of potential prebiotic properties.
Keywords: Cactaceae, Prebiotic, Probiotic, Metabolic activity, Valorization
Introduction
Interest in rational explotaiton of plant biodiversity as a natural source of sustainable food products and health promoting bioactive compounds has been growing due to its abundance, safety and impacts in local economy (Maciel et al. 2019). Pilosocereus gounellei (A. Weber ex K. Schum.) Bly. Ex Rowl, Cactaceae, popularly known as “xique-xique”, is an endemic plant species from Brazilian Caatinga biome and considered an unconventional and sub-utilized edible plant (de Assis et al. 2019; Monteiro et al. 2015). In semi-arid region of northeast Brazil, xique-xique has been traditionally consumed by local population using uncommon processing methods to formulate a variety of products, such as juices, jellies, cake, flour and couscous, some of them commercialized in small scale (de Almeida et al. 2007; de Assis et al. 2019). Increased consumption of xique-xique has stimulated investigations on its nutritional composition, bioactive compounds and health-promoting properties (Monteiro et al. 2015; Sousa et al. 2018).
Early investigations on biological properties of xique-xique have shown gastroprotective effects of ethanol extracts from stem and roots (Sousa et al. 2018), antipyretic activity of cladode saline extract (de Oliveira et al. 2018) and intestinal anti-inflammatory and antioxidant effects of xique-xique cladode juice in in vivo animal models (de Assis et al. 2019). These biological effects have been primarily linked to presence of a variety of phenolics and/or high amounts of dietary fiber, especially of soluble fibers, in xique-xique edible parts (de Assis et al. 2019; Sousa et al. 2018).
Soluble fibers and phenolics have been associated with prebiotic effects of plant-derived products (Danneskiold-Samsøe et al. 2019; de Albuquerque et al. 2020). Prebiotics are substrates selectively utilized by microorganisms forming the gut microbiota, causing a range of benefits to host health primarily associated with their modulatory effects on intestinal microbiota (Chlebowska-Smigiel et al. 2017; Gibson et al. 2017; Massa et al. 2020). Criteria considered for selection of prebiotics include non-absorbability and fermentability by beneficial intestinal microorganisms, besides to ability to stimulate the growth and metabolism of probiotics in large intestine (Gibson et al. 2017; Mohanty et al. 2018). Lactobacillus has been the most common genera studied for probiotic use and found as part of human intestinal microbiota (Chlebowska-Śmigiel et al. 2017; Das et al. 2020), in addition to be ordinarily selected as test microorganisms in in vitro investigations to evaluate prebiotic properties of foods (de Albuquerque et al. 2020; Gómez et al. 2019; Massa et al. 2020).
This study aimed to evaluate the potential prebiotic effects of a juice extracted from xique-xique cladodes through measurements of stimulatory effects on growth and metabolic activities of different probiotic Lactobacillus isolates using in vitro experimental models.
Materials and methods
Xique-xique collection and freeze-dried juice preparation
Xique-xique (Pilosocerus gounellei) cladodes were collected in a private cultivation area located at the municipality of Boa Vista (coordinates 07° 15′ 32″ S 36° 14′ 24″ O, Paraíba, Brazil). The plant was identified by Prof. Dr. Leonardo Person Felix (Center of Agricultural Sciences, Federal University of Paraíba, Bananeiras, Paraíba, Brazil) and a certified voucher specimen (number 15437) was deposited in Herbarium Prof. Jaime Coelho Morais (Center of Agricultural Sciences, Federal University of Paraíba, Areia, Paraíba, Brazil).
For juice extraction, xique-xique cladodes were washed with running tap water and sanitized by immersion in 150 ppm chlorinated water for 15 min. Peel and central stem were removed and remaining flesh was mashed in a pulping machine (MecVal®, Gambolo, Italy), sieved (20 mesh), portioned in sterile screw-capped glass flasks and frozen (− 18 ± 1 °C). Prior to use in assays to evaluate the potential prebiotic effects, frozen juice was lyophilized using a bench top liophylizer (L-101 model, Liotop, São Carlos, São Paulo, Brazil) for approximately 48 h (average condenser temperature: -49 ± 2 °C, vacuum pressure: < 138 µHg, freeze-drying speed: 1 mm/h). Freeze-dried xique-xique cladode juice (XCJ) was packaged in polypropylene bags and stored at -20 °C.
Phyisicochemical characteristics of XCJ
Physicochemical characteristics of XCJ were determined using standard procedures (AOAC 2016), to cite: molar acidity measured by titration; moisture, ash and total dry extract measured by drying up to reaching a constant weight; and protein content measured by Kjeldahl method. Lipid content was measured with a cold extraction method (Folch et al. 1957). An enzymatic–gravimetric method was used to measure soluble and insoluble fiber contents (Horwitz et al. 2005; Prosky et al. 1992). Sugar and organic acid contents were measured using procedures described in "Measurements of pH, organic acids and sugars in cultivation media".
Prebiotic activity assays
Microorganisms and inoculum preparation
Three isolates of different Lactobacillus species with well-known probiotic properties were used, to cite: L. acidophilus LA-05, L. casei L-26 and L. paracasei L-10 (Menezes et al. 2017; Sánchez-Zapata et al. 2013; Sousa et al. 2015). These Lactobacillus species have been commonly reported as part of human intestinal microbiota (David et al., 2014; de Albuquerque et al. 2020; Sun et al., 2018). Stocks were kept in de Mann, Rogosa and Sharpe (MRS) broth (HiMedia, Mumbai, India) with glycerol (Sigma Aldrich, St. Louis, MA, USA; 15 mL/100 mL) at—20 °C. Before use in assays, each isolate was grown in MRS broth at 37 °C during 20–24 h, collected by centrifugation (4500×g, 15 min, 4 °C), washed twice and re-suspended in sterile saline (0.85 g/100 mL) to obtain a cell suspension with an optical density reading at 625 nm (OD625) of approximately 0.8. Each isolate was tested separately in experiments (Duarte et al. 2017; Massa et al. 2020).
Escherichia coli ATCC 11,303 and E. coli ATCC 25,922 were used to prepare an enteric inoculum to determine the prebiotic activity scores. Stocks were kept on Muller-Hinton (MH) agar (MHA; HiMedia) with glycerol (15 mL/100 mL, Sigma Aldrich) at -20 °C. Before use in assays, each isolate was cultivated in Brain Heart Infusion (BHI) broth (HiMedia) at 37 °C for 18–20 h. Each culture was collected by centrifugation (4500 × g, 15 min, 4 °C), washed twice and re-suspended in sterile saline to obtain a cell suspension with OD625 of approximately 0.1. The enteric inoculum was obtained by mixing the two different E. coli suspensions in a rate of 1:1 (Duarte et al. 2017; Massa et al. 2020).
Cultivation media
A MRS broth modified in relation to the sole carbon source was used as a basal medium to evaluate the growth and metabolic activity of examined probiotic isolates (Sánchez-Zapata et al. 2013; Sousa et al. 2015). Composition of different cultivation media used in experiments was: tryptone 10 g/L, meat extract 8 g/L, yeast extract 4 g/L, hydrogen di-potassium phosphate 2 g/L, Tween 80 g/L, sodium acetate 5 g/L, tribasic ammonium citrate 2 g/L, magnesium sulphate 0.2 g/L, manganese sulphate 0.04 g/L and respective carbon source 20 g/L. To measure the probiotic growth and parameters indicative of metabolic activities, three different media were formulated: MRS broth with 20 g/L glucose (standard MRS broth), MRS broth with 20 g/L fructooligosaccharides (FOS, a proven prebiotic ingredient) (de Albuquerque et al. 2020; Gibson et al. 2017) and MRS broth with 20 g/L XCJ. All ingredients used to formulate the cultivation media were obtained from Sigma-Aldrich, with the exception of FOS obtained from Galena Industrial (Campinas, São Paulo, Brazil).
Measurements of prebiotic activity scores
A 0.2 mL-aliquot of examined probiotic suspension was homogenized with 10 mL of MRS broth with glucose, FOS or XCJ (20 g/L). In parallel, a 0.2 mL-aliquot of the mixed enteric inoculum was homogenized with 10 mL of M9 broth (Sigma-Aldrich) with glucose, FOS or XCJ (20 g/L). On time zero (just after homogenization) and after 48 h of incubation (37 °C), a 1 mL-aliquot of each cultivation medium was diluted (1:9) in sterile saline solution and a 100-µL aliquot of each dilution was plated on MRS agar (HiMedia) or eosin methylene blue agar (HiMedia) for probiotic and enteric mixture enumeration, respectively, and incubated at 37 °C for 24 h. The viable counts (Log CFU/mL) were enumerated at the end of the incubation period and prebiotic activity score was determined with the formula:
| 1 |
A positive prebiotic activity score indicates that examined cultivation media ingredient is metabolized as well as or better than glucose by inoculated probiotic, besides to the occurrence of selective stimulatory effects on examined probiotic growth in detriment to the enteric mixture, which is characteristic of potential prebiotic activity (Duarte et al. 2017; Zhang et al. 2018).
Measurements of probiotic viable counts
The inoculum of examined probiotic isolate was dispensed (2 mL/100 mL) in sterile flasks with the respective cultivation media (100 mL; final viable counts of approximately 6.5 log CFU/mL). The mixtures were gently hand-shaken for 30 s and incubated at 37 ºC. At different incubation times (0—just after homogenization and after 6, 12, 18, 24 and 48 h), a 100 μL-aliquot of each mixture was diluted (1:9) in sterile saline solution, and, subsequently, a 20 μL-aliquot of each dilution was plated on MRS agar. Plates were incubated at 37 °C for 24 h and results were expressed as log CFU/mL (de Albuquerque et al. 2020).
Measurements of pH, organic acids and sugars in cultivation media
Metabolic activity of probiotics was evaluated by measuring the pH values and contents of sugars and organic acids in cultivation media at different incubation time intervals. pH values were measured (time zero—immediately after homogenization and after 6, 12, 18, 24 and 48 h) with a digital potentiometer following standard procedures (AOAC 2016). Contents of sugars (glucose and fructose) and organic acids (acetic and lactic acids) were simultaneously measured (after 12, 24 and 48 h) with HPLC—diode array detector (DAD)—refractive index detector (RID) using an Agilent chromatograph (model 1260 Infinity LC, Agilent Technologies, St. Clara, CA, USA) equipped with a quaternary solvent pump (G1311C model), degasser, thermostatic column compartment (G1316A model) and automatic auto-sampler (G1329B model), coupled with a DAD (G1315D model) and RID (G1362A model) according to a previously described method for simultaneous sugar and organic acid detection (Coelho et al. 2018). The other analytical conditions were: an Agilent Hi-Plex H column (8 μm, 7.7 × 300 mm); mobile phase H2SO4 4 mM/L in ultrapure water; flow rate 0.7 mL/min; separation temperature 70 °C; and sample injection volume 10 μL. Organic acids were detected by DAD at 210 nm and sugars were detected by RID. Data were processed with OpenLAB CDS ChemStation EditionTM software (Agilent Technologies). HPLC sample peaks were identified by comparing their retention times with those of organic acid and sugar standards. Samples were filtered in 0.45 micron membranes and double injected. Mean peak areas were used for quantification. Glucose and fructose standards were obtained from Sigma-Aldrich; acetic and lactic acid standards were obtained from Vetec Química Fina (Rio de Janeiro, Rio de Janeiro, Brazil).
Statistical analysis
Experiments were done in triplicate in three different occasions. Results were expressed as average ± standard deviation. Data were submitted to Student’s t test or analysis of variance (ANOVA) followed by Tukey's test, using p ≤ 0.05. Statistical analyses were done with Graphpad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
Results
Results of physicochemical parameters of XCJ showed a low acidic pH and high contents of ash (30.10 ± 0.03 g/100 g) and soluble fibers (14.4 ± 0.02 g/100 g). XCJ had low contents of proteins (7.59 ± 0.06 g/100 g) and fat (2.35 ± 0.01 g/100 g). Glucose (0.28 ± 0.05 g/100 g) and fructose (0.21 ± 0.06 g/100 g) were found in XCJ (Table 1).
Table 1.
Physicochemical parameters (mean ± standard deviation, n = 3) of juices extracted from xique-xique (Pilosocereus gounellei) cladodes. Results are expressed under a dry matter basis
| Parameters | Values |
|---|---|
| Glucose (g/100 g) | 0.28 ± 0.05 |
| Fructose (g/100 g) | 0.21 ± 0.06 |
| Maltose (g/100 g) | 0.05 ± 0.02 |
| Rhamnose (g/100 g) | < LOD |
| Total dietary fiber (g/100 g) | 16.23 ± 0.03 |
| Soluble fiber (g/100 g) | 14.40 ± 0.02 |
| Insoluble fiber (g/100 g) | 1.83 ± 0.02 |
| Ash (g/100 g) | 30.10 ± 0.03 |
| Protein (g/100 g) | 7.59 ± 0.06 |
| Lipid (g/100 g) | 2.35 ± 0.01 |
| pH | 5.02 ± 0.02 |
< LOD: below the limit of detection
XCJ showed positive prebiotic activity scores (0.9 ± 0.2–1.1 ± 0.3) on L. acidophilus LA-05, L. casei L-26 and L. paracasei L-10. Prebiotic activity scores found for XCJ on examined probiotics were similar (p > 0.05) to those found for FOS (0.9 ± 0.3–1.3 ± 0.2). XCJ showed similar prebiotic activity scores (p > 0.05) on three examined probiotics (Table 2).
Table 2.
Prebiotic activity scores (mean ± standard deviation, n = 3) of freeze-dried xique-xique (Pilosocereus gounellei) cladode juice (XCJ, 20 g/L) and fructooligossaccharides (FOS, 20 g/L) on different probiotic Lactobacillus isolates
| Probiotics | XCJ | FOS |
|---|---|---|
| L. acidophilus LA-05 | 1.0 ± 0.3 | 1.0 ± 0.2 |
| L. casei L-26 | 0.9 ± 0.2 | 0.9 ± 0.3 |
| L. paracasei L-10 | 1.1 ± 0.3 | 1.3 ± 0.2 |
No significant difference (p > 0.05) among values found for XCJ or FOS on different probiotic isolates, as well as for XCJ and FOS on a same probiotic isolate, based on Tukey’s test and Students’ t test, respectively
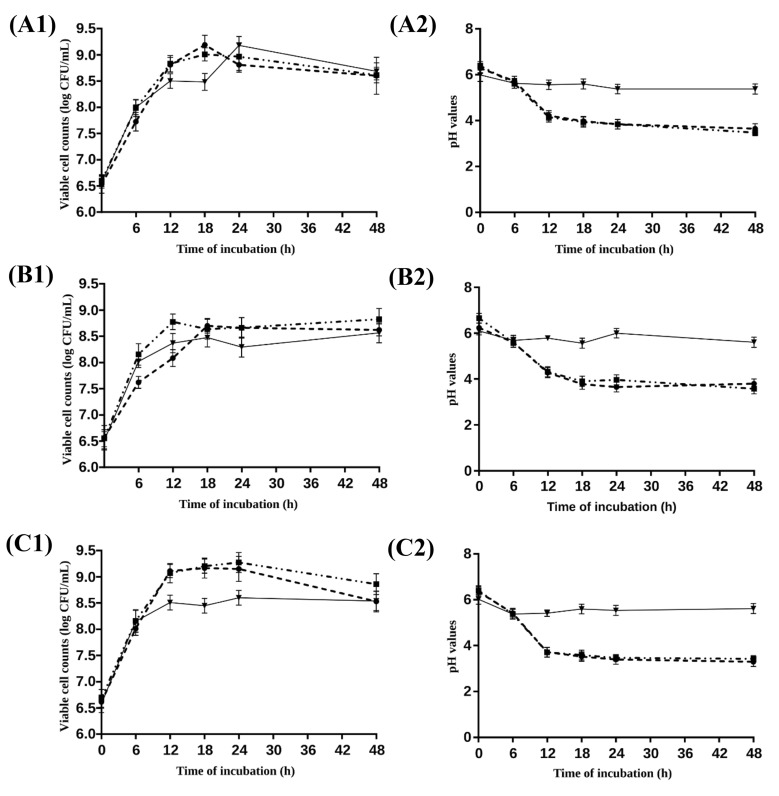
L. acidophilus LA-05, L. casei L-26 and L. paracasei L-10 had high viable counts (> 8 log CFU/mL) after 48 h of cultivation in media with XCJ, glucose or FOS. Viable counts of examined probiotics had an increase of > 2 log CFU/mL in media with XCJ, glucose or FOS after 48 h of cultivation when compared to viable counts found on time zero. Examined probiotics had overall increased viable counts up to 12 or 18 h of cultivation, followed for steady viable counts up to 48 h of cultivation regardless of the carbon source in media. L. acidophilus LA-05 and L. casei L-26 had similar viable counts (p > 0.05) after 48 h of cultivation in media with XCJ, FOS or glucose, while L. paracasei L-10 had higher viable counts (p ≤ 0.05) in media with FOS, followed by media with glucose or XCJ. L. acidophilus LA-05, L. casei L-26 and L. paracasei L-10 had similar viable counts (p > 0.05) after 48 h of cultivation in media with a same carbon source (Fig. 1a1–c1).
Fig. 1.
Viable counts (1) of L. acidophillus LA-05 (a), L. casei L-26 (b) and L. paracasei L-10 (c) and pH values (2) in media with glucose (20 g/L,  ), fructooligosaccharides (20 g/L,
), fructooligosaccharides (20 g/L,  ) and freeze-dried xique-xique (Pilosocereus gounellei) cladode juice (XCJ, 20 g/L,
) and freeze-dried xique-xique (Pilosocereus gounellei) cladode juice (XCJ, 20 g/L,  ) during a 48 h-cultivation (37 °C) (n = 3)
) during a 48 h-cultivation (37 °C) (n = 3)
Cultivation of probiotics in media with XCJ, glucose or FOS resulted in lower pH after 48 h when compared to time zero (p ≤ 0.05). Lowest pH values of cultivation media were reached after 18 or 24 h of incubation, which did not change (p > 0.05) up to 48 h. Cultivation media with glucose or FOS had lower pH after 48 h (pH of approximately 4) when compared to cultivation medium with XCJ (pH of approximately 5) regardless of the inoculated probiotic. No difference (p > 0.05) in pH was found after 48 h of cultivation of a same probiotic in media with XCJ, glucose or FOS (Fig. 1a2–c2).
Contents of glucose and/or fructose decreased (p ≤ 0.05) in media with XCJ, glucose or FOS inoculated with L. acidophilus LA-05, L. casei L-26 or L. paracasei L-10 during the 48 h-cultivation. Glucose was the sugar found at the highest contents after 12 h of cultivation in media with XJ regardless of the inoculated probiotic, being rapidly depleted already after 24 h of cultivation. Contents of acetic and lactic acid increased (p ≤ 0.05) during the 48 h-cultivation in media with XCJ regardless of the inoculated probiotic. Acetic acid was found at higher contents than lactic acid during the 48 h-cultivation in media with XCJ regardless of the inoculated probiotic. In most cases, contents of acetic and lactic acid increased (p ≤ 0.05) during the 48 h-cultivation in media with glucose or FOS inoculated with examined probiotics. Contents of lactic acid were higher in media with glucose or FOS when compared to media with XXJ during the measured cultivation period regardless of the inoculated probiotic. Contents of acetic acid were higher in media with XXJ after 24 and 48 h of cultivation when compared to media with glucose or FOS (Table 3).
Table 3.
Contents of sugars and organic acids (g/L, n = 3) in media with glucose (20 g/L), fructooligosaccharides (FOS, 20 g/L) or freeze-dried xique-xique (Pilosocereus gounellei) cladode juice (XCJ, 20 g/L) inoculated with Lactobacillus acidophilus LA-05, L. casei L-26 or L. paracasei L-10 during a 48 h-cultivation (37 °C)
| Parameters | Time (h) | L. acidophilus LA-05 | L. casei L-26 | L. paracasei L-10 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | FOS | XXJ | Glucose | FOS | XXJ | Glucose | FOS | XXJ | ||
| Sugars | ||||||||||
| Glucose | 12 | 7.87 ± 0.07Aa | 0.68 ± 0.05Ab | 0.29 ± 0.03c | 7.81 ± 0.07Aa | 0.54 ± 0.05Ab | 0.19 ± 0.02c | 4.46 ± 0.07Aa | 0.30 ± 0.05Ab | 0.24 ± 0.03b |
| 24 | 3.21 ± 0.06Ba | 0.57 ± 0.05Ab | < LOD | 3.50 ± 0.06Ba | 0.42 ± 0.05Ab | < LOD | 0.89 ± 0.06Ba | 0.29 ± 0.01Ab | < LOD | |
| 48 | 0.03 ± 0.01Ca | 0.03 ± 0.01Ba | < LOD | 0.06 ± 0.01Cb | 0.39 ± 0.01Aa | < LOD | 0.50 ± 0.01Ca | 0.25 ± 0.05Ab | < LOD | |
| Fructose | 12 | Nd | 5.52 ± 0.07Aa | 0.04 ± 0.03Ab | Nd | 3.61 ± 0.06Aa | 0.02 ± 0.01Ab | Nd | 6.45 ± 0.07Aa | 0.05 ± 0.01Ab |
| 24 | Nd | 2.28 ± 0.06Ba | 0.03 ± 0.01Ab | Nd | 2.18 ± 0.05Ba | 0.03 ± 0.02Ab | Nd | 0.60 ± 0.03Ba | 0.03 ± 0.02Ab | |
| 48 | Nd | 0.08 ± 0.01C | < LOD | Nd | 0.45 ± 0.02Ca | 0.04 ± 0.03Ab | Nd | 0.24 ± 0.02C | < LOD | |
| Organic acids | ||||||||||
| Lactic acid | 12 | 11.43 ± 0.07Ca | 8.43 ± 0.05Cb | 2.19 ± 0.03Bc | 9.75 ± 0.07Ca | 6.06 ± 0.05Cb | 2.01 ± 0.03Ac | 10.04 ± 0.06Ca | 9.99 ± 0.05Ca | 1.55 ± 0.03Cb |
| 24 | 12.76 ± 0.06Bb | 14.40 ± 0.05Ba | 2.83 ± 0.03Ac | 14.75 ± 0.06Ba | 14.11 ± 0.05Bb | 1.96 ± 0.03Bc | 18.35 ± 0.07Ba | 11.04 ± 0.05Bb | 2.50 ± 0.03Bc | |
| 48 | 18.34 ± 0.01Aa | 16.52 ± 0.07Ab | 2.46 ± 0.03Cc | 22.17 ± 0.01Aa | 14.80 ± 0.05Ab | 1.86 ± 0.03Cc | 22.22 ± 0.07Aa | 21.58 ± 0.07Ab | 3.40 ± 0.03Ac | |
| Acetic acid | 12 | 2.44 ± 0.06Bb | 1.92 ± 0.05Bc | 3.30 ± 0.03Ba | 1.98 ± 0.03Bb | 1.42 ± 0.07Cc | 2.46 ± 0.03Ca | 3.02 ± 0.04Bc | 3.31 ± 0.04Aa | 3.20 ± 0.03Bb |
| 24 | 1.82 ± 0.03Cb | 1.89 ± 0.06Bb | 3.46 ± 0.02Ca | 1.93 ± 0.03Bb | 1.92 ± 0.06Bb | 2.68 ± 0.03Ba | 3.24 ± 0.04Aa | 2.01 ± 0.06Cb | 3.28 ± 0.03Ba | |
| 48 | 3.20 ± 0.03Aa | 3.21 ± 0.03Aa | 3.95 ± 0.04Ab | 2.37 ± 0.03Ab | 2.23 ± 0.01Ac | 3.18 ± 0.04Aa | 2.58 ± 0.04Cb | 2.57 ± 0.01Bb | 3.63 ± 0.03Aa | |
A–C: different superscript capital letters in the same column for the same sugar/organic acid at different cultivation time for each tested isolated denote differences (p ≤ 0.05), based on Tukey’s test; a–c: different superscript small letters in the same row and different measured sugars/organic acids for each isolate among media with glucose, FOS and XCJ denote difference, based on Tukey’s test (p ≤ 0.05)
Nd not determined
Discussion
Positive prebiotic activity scores found for XCJ indicate its capability of stimulating selectively Lactobacillus species in detriment to enteric pathogens (de Albuquerque et al. 2020; Duarte et al. 2017; Zhang et al. 2018). This selective stimulatory effect is an important characteristic for a prebiotic food because it could impose a competitive advantage of Lactobacillus species on enteric pathogens co-existing in intestinal environment (de Albuquerque et al. 2020; Massa et al. 2020). Examined Lactobacillus isolates had overall similar ability to increase their populations and reduce the pH over time in cultivation media with XCJ or FOS, the main prebiotic ingredient in use by food industry (de Paulo Farias et al. 2019; Gibson et al. 2017), which are indicative of intense stimulatory effects on Lactobacillus growth and metabolism (Duarte et al. 2017; Massa et al. 2020).
These stimulatory effects could be related to a range of nutrients available in XCJ for use as fermentable substrates by Lactobacillus, particularly the contents of soluble fibers. Specifically, soluble fibers are resistant to digestion and absorption in human small intestine, reaching the colon where they are selectively fermented, being characterized as important energy sources for beneficial bacteria part of intestinal microbiota (Franco-Robles and López 2015; Wang et al. 2019). Sugars (e.g., glucose and fructose) found in XCJ could be also fermentable substrates for examined Lactobacillus (Massa et al. 2020).
An early study identified different phenolics in XCJ (e.g., catechin, epicatechin, quercetin 3-glucoside, rutin, kaempferol 3-glucoside, gallic acid, caffeic acid, chlorogenic acid and hesperidin) (de Assis et al. 2019), many of which have been reported to exert stimulatory effects on probiotic Lactobacillus (de Souza et al. 2019). Phenolics found in XCJ have been reported to be metabolized in human colon by some Lactobacillus species with production of secondary metabolites with high bioactivity and bioavailability (de Sousa et al. 2018; Filannino et al. 2016; Shen et al., 2018). A previous study found that fermentation of a cactus (Opuntia ficus-indica L., Cactaceae) cladode pulp with Lactobacillus species caused alteration in phenolic profile, which was directly linked to enhanced in vitro bioactive properties found in fermented pulp (Di Cagno et al. 2016).
Our results indicated that XCJ was metabolized by examined Lactobacillus isolates with fast consumption of glucose and fructose to promote high viable counts and production of acetic and lactic acids during the measured cultivation period, which are positive results in investigations with candidates to be used as prebiotics (Gibson et al. 2017; Sanders et al. 2019). Acetic and lactic acids were produced in cultivation media with XCJ regardless of the inoculated Lactobacillus isolate. Acetic acid is an important metabolite produced by lactic acid bacteria during fermentation of carbohydrates in the colon, being a natural energy source for host organism. Lactic acid is one of the most important end-products of glucose and fructose fermentation by lactic acid bacteria, being the majority organic acid produced by metabolism of carbohydrates by homofermentative Lactobacillus species (Chlebowska-Śmigiel et al. 2017; Gibson et al. 2017). The intense metabolic activity of examined probiotics found in media with XCJ are important results because the beneficial effects of prebiotics on host health (e.g., increased nutrient bioavailability and improved immunological and inflammatory responses) have been linked to increased population of beneficial microorganisms, decreased pH and increased production of organic acids in colon (Gibson et al. 2017; Saarela 2019). An early investigation found that consumption of dehydrated cactus cladodes (Opuntia ficus-indica L.) induced beneficial alterations in gut microbiota (including an increase in population Lactobacillus species populations) of rats fed a high fat and sucrose diet, which could support the use of cactus cladodes as a prebiotic food (Sánchez-Tapia et al. 2017).
It is noteworthy to cite that results obtained with in vitro experiments revealing the potential prebiotic effects of XJC, as indicated by its selective stimulatory effects on probiotic Lactobacillus isolates, should have limitations to be extrapolated to achievement of prebiotic effects under in vivo conditions and outcomes on host health (de Albuquerque et al. 2020). Nevertheless, results of in vitro experiments, as those found in this study, have been considered valuable evidence of prebiotic properties in conventional and non-conventional foods (Duarte et al. 2017; Massa et al. 2020; Gómez et al. 2019; Huang et al. 2019; Martinez-Gutierrez et al. 2017; Zhang et al. 2018). Additionally, although few reports on available literature have focused on bioactive compounds and biological activities (e.g., anti-inflammatory, antioxidant, antimicrobial and anti-hypertensive) of underutilized edible plants from Brazilian biomes (de Almeida et al. 2007; de Assis et al. 2019; Gadioli et al. 2018; Maciel et al. 2019; Vieira et al. 2019), investigations on their potential prebiotic properties are still scarce.
In conclusion, the results of this study showed that XCJ could induce stimulatory effects on growth and metabolism of different Lactobacillus isolates, which were indicated by high viable counts, decreased pH values and increased sugar consumption and organic acid production in media during cultivation. Furthermore, these effects were similar to those induced by FOS, a well-known prebiotic ingredient. These results show that XCJ has potential prebiotic properties and, consequently, capability of exerting benefits to consumers. Finally, these results should help to promote the valorization of an unconventional and still few exploited edible plant from Caatinga biome, which could be rationally exploited as source of health-related bioactive compounds by food and pharmaceutical industry.
References
- AOAC—Official Methods of Analysis of the Association of Official Analytical Chemists (2016), 20th edn. Association of Official Analytical Chemist, Rockville
- Chlebowska-Smigiel A, Gniewosz M, Kieliszek M, Bzducha-Wrobel A. The effect of pullulan on the growth and acidifying activity of selected stool microflora of human. Curr Pharm Biotechnol. 2017;18:121–126. doi: 10.2174/1389201017666161229154324. [DOI] [PubMed] [Google Scholar]
- Chlebowska-Śmigiel A, Kycia K, Neffe-Skocińska K, Kieliszek M, Gniewosz M, Kołożyn-Krajewska D. Effect of pullulan on physicochemical, microbiological, and sensory quality of yogurts. Curr Pharm Biotechnol. 2017;20:489–496. doi: 10.2174/1389201020666190416151129. [DOI] [PubMed] [Google Scholar]
- Coelho EM, Padilha CVS, Miskinis GA, Sá AGB, Pereira GE, Azevêdo LC, Lima MS. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: method validation and characterization of products from northeast Brazil. J Food Compos Anal. 2018;66:160–167. doi: 10.1016/j.jfca.2017.12.017. [DOI] [Google Scholar]
- Danneskiold-Samsøe NB, Barros HDFQ, Santos R, Bicas JL, Cazarin CBB, Madsen L, Kristiansen K, Pastore GM, Brix S, Maróstica Júnior MR. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23–31. doi: 10.1016/j.foodres.2018.07.043. [DOI] [PubMed] [Google Scholar]
- Das DJ, Shankar A, Johnson JB, Thomas S. Critical insights into antibiotic resistance transferability in probiotic Lactobacillus. Nutrition. 2020;69:110567. doi: 10.1016/j.nut.2019.110567. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Button JE, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Albuquerque TMR, Borges CWP, Cavalcanti MT, dos Santos LM, Magnani M, de Souza EL. Potential prebiotic properties of flours from different varieties of sweet potato (Ipomoea batatas L.) roots cultivated in Northeastern Brazil. Food Biosci. 2020;36:100614. doi: 10.1016/j.fbio.2020.100614. [DOI] [Google Scholar]
- de Almeida CA, de Figueiredo RM, Queiroz AJM, de Oliveira FM. Physical and chemical characteristics of xiquexique pulps. Rev Ciênc Agron. 2007;38:440. [Google Scholar]
- de Assis POA, Guerra GCB, de Souza Araújo DF, de Araújo AA, de Araújo RF, de Carvalho TG, de Souza MFV, Borges GSC, Lima MS, Rolim FRL, Rodrigues RAV, Queiroga RCRE. Intestinal anti-inflammatory activity of xique–xique (Pilosocereus gounellei A. Weber ex K. Schum. Bly. Ex Rowl) juice on acetic acid-induced colitis in rats. Food Funct. 2019;10:7275–7290. doi: 10.1039/C9FO00920E. [DOI] [PubMed] [Google Scholar]
- de Oliveira AM, de Luna Freire MO, da Silva WAV, Ferreira MRA, Paiva PMG, Soares LAL, Medeiros PL, Carvalho BM, Napoleão TH. Saline extract of Pilosocereus gounellei stem has antinociceptive effect in mice without showing acute toxicity and altering motor coordination. Regul Toxicol Pharm. 2018;95:289–297. doi: 10.1016/j.yrtph.2018.04.004. [DOI] [PubMed] [Google Scholar]
- de Paulo Farias D, de Araújo FF, Neri-Numa IA, Pastore GM. Prebiotics: trends in food, health and technological applications. Trends Food Sci Technol. 2019;93:23–35. doi: 10.1016/j.tifs.2019.09.004. [DOI] [Google Scholar]
- de Souza EL, de Albuquerque TMR, dos Santos AS, Massa NML, de Brito Alves JL. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—a review. Crit Rev Food Sci Nutr. 2019;59:1645–1659. doi: 10.1080/10408398.2018.1425285. [DOI] [PubMed] [Google Scholar]
- Duarte FND, Rodrigues JB, da Costa LM, Lima MDS, Pacheco MTB, Pintado MME, Aquino JS, de Souza EL. Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro-industrial byproduct on Lactobacillus species. J Sci Food Agric. 2017;97:3712–3719. doi: 10.1002/jsfa.8232. [DOI] [PubMed] [Google Scholar]
- Filannino P, Cavoski I, Thlien N, Vincentini O, De Angelis M, Silano M, Gobetti M, Di Cagno R. Lactic acid fermentation of cactus cladodes (Opuntia ficus-indica L.) generates flavonoid derivatives with antioxidant and anti-inflammatory properties. PLoS ONE. 2016;29:e0152575. doi: 10.1371/journal.pone.0152575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Franco-Robles E, López MG. Implication of fructans in health: immunomodulatory and antioxidant mechanisms. Sci World J. 2015;2015:289267. doi: 10.1155/2015/289267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadioli IL, da Cunha MSB, de Carvalho MVO, Costa AM, Pineli LLO. A systematic review on phenolic compounds in Passiflora plants: exploring biodiversity for food, nutrition, and popular medicine. Crit Rev Food Sci Nutr. 2018;24:785–807. doi: 10.1080/10408398.2016.1224805. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Hutkins RW, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. The international Scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;1:1–12. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Gómez B, Peláez C, Martínez-Cuesta MC, Parajó JC, Alonso JL, Requena T. Emerging prebiotics obtained from lemon and sugar beet byproducts: evaluation of their in vitro fermentability by probiotic bacteria. LWT Food Sci Technol. 2019;109:17–25. doi: 10.1016/j.lwt.2019.04.008. [DOI] [Google Scholar]
- Horwitz W, Latimer J, George W (2005) Official methods of analysis of the Association of Official Analytical Chemists, 18th edn. Current Through Revision 3, 2010. Gaithersburg, Maryland, pp 101–102. https://doi.org/10.2174/1389201020666190416151129
- Huang F, Liu Y, Zhang R, Bai Y, Dong L, Liu L, Jia X, Wang G, Zhang M. Structural characterization and in vitro gastrointestinal digestion and fermentation of litchi polysaccharide. Int J Biol Macromol. 2019;140:965–972. doi: 10.1016/j.ijbiomac.2019.08.170. [DOI] [PubMed] [Google Scholar]
- Maciel VBV, Yoshida CMP, Goycoolea FM. Agronomic cultivation, chemical composition, functional activities and applications of Pereskia species—a mini review. Curr Med Chem. 2019;26:4573–4584. doi: 10.2174/0929867325666180926151615. [DOI] [PubMed] [Google Scholar]
- Martinez-Gutierrez F, Ratering S, Juárez-Flores B, Godinez-Hernandez C, Geissler-Plaum R, Prell F, Zorn H, Czermak P, Schnell S. Potential use of Agave salmiana as a prebiotic that stimulates the growth of probiotic bacteria. LWT Food Sci Technol. 2017;84:151–159. doi: 10.1016/j.lwt.2017.05.044. [DOI] [Google Scholar]
- Massa NLM, Duarte de Menezes FND, Albuquerque TMR, de Oliveira SPA, Lima MS, Magnani M, de Souza EL. Effects of digested jabuticaba (Myrciaria jaboticaba (Vell) Berg.) by-product on growth and metabolism of Lactobacillus and Bifidobacterium indicate prebiotic properties. LWT Food Sci Technol. 2020;131:109766. doi: 10.1016/j.lwt.2020.109766. [DOI] [Google Scholar]
- Mohanty D, Misra S, Mohapatra S, Sahu PS. Prebiotics and synbiotics: recent concepts in nutrition. Food Biosci. 2018;26:152–160. doi: 10.1016/j.fbio.2018.10.008. [DOI] [Google Scholar]
- Monteiro ER, Mangolin CA, Neves AF, Orasmo GR, Silva JGM, Machado MFPS. Genetic structure of Pilosocereus gounellei (Cactaceae) as revealed by AFLP marker to guide proposals for improvement and restoration of degraded areas in Caatinga biome. Genet Mol Res. 2015;14:16966–16974. doi: 10.4238/2015. [DOI] [PubMed] [Google Scholar]
- Prosky L, Asp NG, Schweizer TF, Devries JW, Furda I. Determination of insoluble and soluble dietary fibers in foods and food products. J Assoc Anal Chem. 1992;2:360–367. doi: 10.1093/jaoac/75.2.360. [DOI] [PubMed] [Google Scholar]
- Saarela MH. Safety aspects of next generation probiotics. Curr Opin Food Sci. 2019;30:8–13. doi: 10.1016/j.cofs.2018.09.001. [DOI] [Google Scholar]
- Sánchez-Tapia M, Aguilar-López P-C, Pichardo-Ontiveros E, Wang M, Donovan SM, Tovar AR, Torres N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci Rep. 2017;5:4716. doi: 10.1038/s41598-017-05096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Zapata E, Fernández-López J, Pérez-Alvarez JA, Soares J, Sousa S, Gomes AM, Pintado MM. In vitro evaluation of “horchata” co-products as carbon source for probiotic bacteria growth. Food Bioprod Process. 2013;91:279–286. doi: 10.1016/j.fbp.2012.11.003. [DOI] [Google Scholar]
- Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- Shen Y, Sun H, Zeng H, Prinyawiwatkul W, Xu W, Xu Z. Increases of phenolics, fatty acids, phytosterols, and anticancer activity of sweet potato after fermented by Lactobacillus acidophilus. J Agric Food Chem. 2018;66:2735–2741. doi: 10.1021/acs.jafc.7b05414. [DOI] [PubMed] [Google Scholar]
- Sousa GA, Oliveira IS, Silva-Freitas FV, Viana AFS, Neto BP, Cunha FVM, Fernandes PD, Maciel JKS, da Silva TMS, Souza MFV, Oliveira FA. Gastroprotective effect of ethanol extracts of cladodes and roots of Pilosocereus gounellei (A. Weber ex K. Schum.) Bly. Ex Rowl (Cactaceae) on experimental ulcer models. J Ethnopharmacol. 2018;218:100–108. doi: 10.1016/j.jep.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Sousa S, Pinto J, Pereira C, Malcata FX, Pacheco MTB, Gomes AM, Pintado M. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food Bioprod Process. 2015;95:96–105. doi: 10.1016/j.fbp.2015.04.003. [DOI] [Google Scholar]
- Sun H, Chen Y, Cheng M, Zhang X, Zheng X, Zhang Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J Food Sci Technol. 2018;55:399–407. doi: 10.1007/s13197-017-2951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira CR, da Silva BP, da Carmo MAV, Azevedo L, Nogueira DA, Martino HSD, Silva RR. Effect of Pereskia aculeata Mill. in vitro and in overweight humans: a randomized controlled trial. J Food Biochem. 2019;43:e-12903. doi: 10.1111/jfbc.12903. [DOI] [PubMed] [Google Scholar]
- Wang M, Wichienchot S, He X, Fu X, Huang Q, Zhang B. In vitro colonic fermentation of dietary fibers: fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci Technol. 2019;88:1–9. doi: 10.1016/j.tifs.2019.03.005. [DOI] [Google Scholar]
- Zhang S, Hu H, Wang L, Liu F, Pan S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018;244:232–237. doi: 10.1016/j.foodchem.2017.10.071. [DOI] [PubMed] [Google Scholar]