The study of human T cell development is hampered by the lack of genetic tools that have been successfully used in mice. In both mice and humans, T lymphocytes develop in the thymus from progenitors that originate in the bone marrow. In mice, targeted mutations (“knockout” mice) and transgenics have provided a better understanding of T cell development [1,2]. Mostly descriptive studies exist for human T cell development, although patients with rare genetic defects, such as SCID patients have been instrumental in obtaining insight into this intricate process.
A healthy human immune repertoire includes billions of T cells with different T cell receptors (TCRs) to help recognize and respond to virtually any pathogenic invasion. During T cell development, this diverse repertoire is generated by gene recombination of V, (D), and J TCR segments. Progenitors from hematopoietic stem cells (HSCs) in the bone marrow migrate to the thymus where they proliferate and differentiate into mature T cells. Surprisingly, only a subset of these progenitors is needed to reconstitute a diverse repertoire of human T cells in immune-deficient mice [3]. Partially due to data from mouse studies, it is generally assumed that an early thymocyte progenitor has lost the long-term self-renewal potential, but whether a self-renewing T cell progenitor exists in humans is not known.
In the last issue, Kury et al describe an intriguing X-linked SCID case [4], reporting a somatic reversion of the IL2RG mutation in all T cells but not in other immune cells. As such an event is extremely rare, the authors hypothesise that this reversion did not happen in more than one progenitor cell. The rescue of T cell development is illustrated by the presence of a functioning, albeit limited T cell repertoire, and thus far has kept the 18-year old patient healthy and without needing an allogeneic HSC transplantation.
How diverse is the T cell repertoire that stems from one progenitor? The authors discuss this but rightfully conclude that this is very hard to measure. Using CDR3β sequencing, they found 87562 unique sequences in 10 samples from the patient and compared this with a healthy donor. Because the sequencing never captures all clones in a given sample, the number is always an underrepresentation. For a healthy individual, the repertoire diversity is estimated to be in the range of 106 to 1012. Despite improvements in sequencing and associated bioinformatic analysis, it remains difficult to objectively determine the degree of limitation of this patient's T cell repertoire. Regardless, the repertoire is severely limited, yet sufficient, and impressive considering it is generated from one progenitor.
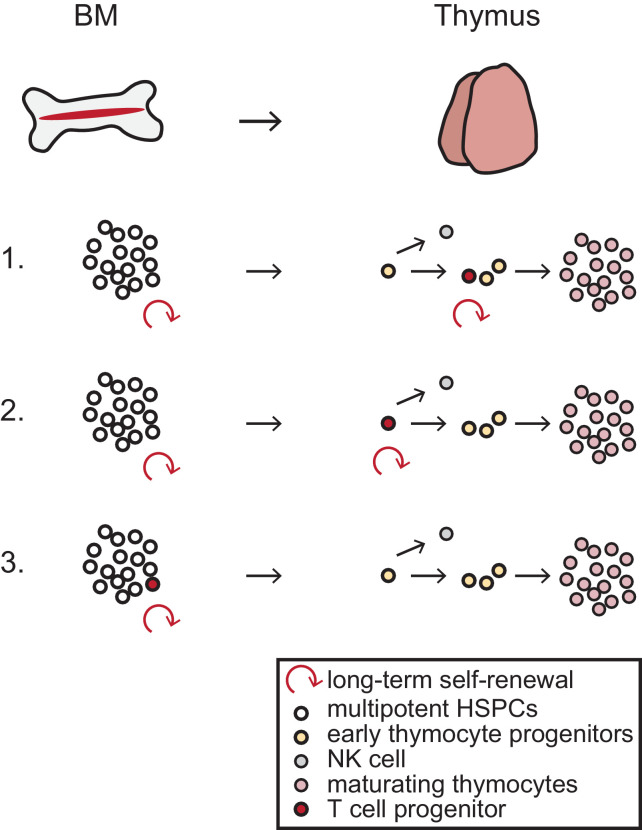
Conceptually, this case study generates some interesting biological questions regarding the nature and origin of this T cell progenitor with long-term self-renewal ability (Fig. 1). While the somatic reversion in a T cell progenitor resulted in T cell restoration, NK cells that also depend on IL2RG were absent, indicating the ‘reverted’ progenitor had already committed to the T cell lineage. On the other hand, IL2RG-SCID HSC xenografts in mice revealed that the T cell development arrest of this SCID is one of the earliest because it is almost immediately after reaching the thymus before the thymocytes acquire CD7 (and CD5) expression [5]. If NK cells can arise from a T cell progenitor in the thymus [6], why did NK cells not develop from this very early ‘reverted’ T cell progenitor? One explanation could be that NK cell development in the thymus branches off before the point of the IL2RG reversion, suggesting the presence of one or more even earlier, very rare, and yet unidentified cell populations in the thymus. Another could be that the ‘reverted’ progenitor is not solely T cell committed and in principle still has NK potential, but selective pressure in the thymus directs the generation of T cells over NK cells in an environment of severely reduced T cell output. Both options place the unidentified T cell progenitor in the thymus and assume a yet-to-be-proven long-term self-renewal capacity in the thymus. Data from gene therapy studies in X-linked SCID also indicate that despite a lack of gene marking in HSCs, long term T cell reconstitution with ongoing thymic output can occur [7]. Kury and colleagues [4] speculate that the T cell progenitor is a multipotent cell with T cell bias from the bone marrow. Indeed, functional studies at the single- cell level have revealed that HSPC subsets can be lineage-biased without losing their self-renewal or multipotency [8,9]. An argument against this is that despite the lineage bias, one would expect multipotent cells to occasionally yield B cells and NK cells with the reverted genotype. This was not observed, but B cells could have easily been missed in the sequencing of a pool of cells.
Fig. 1.
Potential origin of the T cell progenitor (in red) with long-term self-renewal ability.
Thus far, the origin and identity of the T cell progenitor remain unknown. Identifying this progenitor is clinically important as it would help future research to boost T cell immunity in immunocompromised patients or patients who are recovering after a stem cell transplantation. The T cell progenitor will likely not be identified solely by the existing surface markers since they have been used extensively and not led to early novel progenitor subsets. Instead, epigenetic studies or single-cell sequencing in combination with single-cell functional studies may provide a better chance at identifying the subtle differences between various lineage-biased multipotent progenitors that appear similar on the surface. A potential alternative is the in vitro generation of T cell progenitors using the Notch ligand DLL4, as has been proposed [10] and is now tried in clinical studies.
Collectively, the careful analysis of unique patients such as the one reported by Kury and colleagues remains invaluable for a better understanding of human lymphopoiesis.
Declaration of Interests
Authors have nothing to disclose.
Acknowledgements
KCB and FJTS are supported in part by the European Union's Horizon 2020 research and innovation programme under grant agreement No. 755170 (RECOMB).
References
- 1.Garcia-Perez L, Famili F, Cordes M, Brugman M, van Eggermond M, Wu H. Functional definition of a transcription factor hierarchy regulating T cell lineage commitment. Sci Adv. 2020;6(31):eaaw7313. doi: 10.1126/sciadv.aaw7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothenberg EV. Programming for T-lymphocyte fates: modularity and mechanisms. Genes Dev. 2019;33(17-18):1117–1135. doi: 10.1101/gad.327163.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brugman MH, Wiekmeijer AS, van Eggermond M, Wolvers-Tettero I, Langerak AW, de Haas EF. Development of a diverse human T-cell repertoire despite stringent restriction of hematopoietic clonality in the thymus. Proc Natl Acad Sci U S A. 2015;112(44):E6020–E6027. doi: 10.1073/pnas.1519118112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kury P, Führer M, Fuchs S, Lorenz MR, Giorgetti OB, Bakhtiar S. Long-term robustness of a T-cell system emerging from somatic rescue of a genetic block in T-cell development. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiekmeijer AS, Pike-Overzet K, H IJ, Brugman MH, Wolvers-Tettero IL, Lankester AC. Identification of checkpoints in human T-cell development using severe combined immunodeficiency stem cells. J Allergy Clin Immunol. 2016;137(2) doi: 10.1016/j.jaci.2015.08.022. 517-26.e3. [DOI] [PubMed] [Google Scholar]
- 6.Spits H, Blom B, Jaleco AC, Weijer K, Verschuren MC, van Dongen JJ. Early stages in the development of human T, natural killer and thymic dendritic cells. Immunol Rev. 1998;165:75–86. doi: 10.1111/j.1600-065x.1998.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 7.Cavazzana M, Six E, Lagresle-Peyrou C, Andre-Schmutz I, Hacein-Bey-Abina S. Gene therapy for X-linked severe combined immunodeficiency: where do we stand? Hum Gene Ther. 2016;27(2):108–116. doi: 10.1089/hum.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrelha J, Meng Y, Kettyle LM, Luis TC, Norfo R, Alcolea V. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature. 2018;554(7690):106–111. doi: 10.1038/nature25455. [DOI] [PubMed] [Google Scholar]
- 9.Velten L, Haas SF, Raffel S, Blaszkiewicz S, Islam S, Hennig BP. Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol. 2017;19(4):271–281. doi: 10.1038/ncb3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reimann C, Six E, Dal-Cortivo L, Schiavo A, Appourchaux K, Lagresle-Peyrou C. Human T-lymphoid progenitors generated in a feeder-cell-free Delta-like-4 culture system promote T-cell reconstitution in NOD/SCID/γc(-/-) mice. Stem Cells. 2012;30(8):1771–1780. doi: 10.1002/stem.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]