Highlights
-
•
Achalasia is a rare motor disorder of the oesophagus.
-
•
Achalasia is thought to arise from organ-specific autoimmunity due to vitiligo that present in the patient.
-
•
Clinical awareness is imperative when approaching an achalasia due to its similar symptom to GERD and respiratory disease, and must always be considered in autoimmune patient.
-
•
We performed Laparoscopic Heller’s myotomy and repaired with Dor’s fundoplication.
Abbreviations: LOS, lower oesophagus sphincter; GERD, gastroesophageal reflux disease; MHC, major histocompatibility complex; IFN, interferon; HLA, human leucocyte antigen; PTPN22, protein of tyrosine phosphatase N22 gene; LYP, lymphoid-specific phosphatase
Keywords: Achalasia, Heller laparoscopy, Vitiligo, Autoimmune disease, Case report
Abstract
Background
Achalasia is a rare motor disorder of the oesophagus that typically characterized by the absence of oesophagus peristalsis and failure of swallow induced relaxation of oesophagus sphincter (LOS). The prevalence of achalasia is eight cases per million population.
Presentation of case
A 35-year-old woman presented with progressive dysphagia for 6 years. Her symptoms worsened in the last 14 days followed by vomiting undigested and retained food. She was previously diagnosed with a variant respiratory problem but her symptoms did not improve with medication. Clinical evaluation and investigation revealed features of multiple depigmented patches with sharply defined borders and leucotrichia on the neck, abdomen, hand, knee, and lateral malleolus. The patient had vitiligo for 18 years. The upper gastrointestinal endoscopy showed the dilatation from distal oesophagus (38 cm from incisors) with retained food. The diagnosis of achalasia was given. After laparoscopic Heller’s myotomy was performed and the opening of the oesophagus was repaired with Dor’s fundoplication, her symptoms were much improved.
Discussion and conclusion
We hereby report on a rare case of achalasia in a woman presenting with vitiligo which may suggest an autoimmune disorder in the onset of achalasia. Achalasia must be considered in vitiligo or any autoimmune disease presenting with the oesophagus-related problem.
1. Introduction
Achalasia is a rare motor disorder of the oesophagus that typically presents with slowly progressive dysphagia [1]. The prevalence of achalasia is eight cases per million population with an estimated incidence of approximately 0.3–1.63 per 100,000 habitants per year, and the morbidity rate is 1 per 100,000 population [2,3]. Achalasia affects equally in both men and women and has a peak incidence at age 30–60 years [4]. Etiology and pathogenesis of achalasia are still unknown, many hypotheses suggest a multifactorial etiopathogenic mechanism [2]. Genetic inheritance, infectious, neuronal degradation, and autoimmune mechanism have each been proposed with loss of ganglion cells in the oesophagus and in lower oesophagus sphincter (LOS) [5,6]. Achalasia is characterized by the absence of oesophagus peristalsis and failure of swallow induced relaxation of LOS, and increased in LOS resting tone [2,7]. The transit of the food into the stomach is impaired with resulting in food static, and the patient usually presents symptoms of dysphagia, regurgitation of saliva or undigested food, respiratory symptoms (nocturnal cough, recurrent aspiration, and pneumonia), heartburn, and retrosternal chest pain [8].
However, the low prevalance of achalasia can delay diagnosis and the similarity of its presenting symptom to gastrooesophagus reflux disease (GERD) and respiratory problem that can lead to misdiagnosis [6]. Recently, many researchers have turned their focus towards determining the pathophysiological of achalasia but to treat the target specific causes of the disease [6]. Here we report a patient with vitiligo who presented with achalasia and correlate our case with the suggested theory of an autoimmune etiology of achalasia.
This work has been reported in line with the SCARE criteria [9].
2. Presentation of case
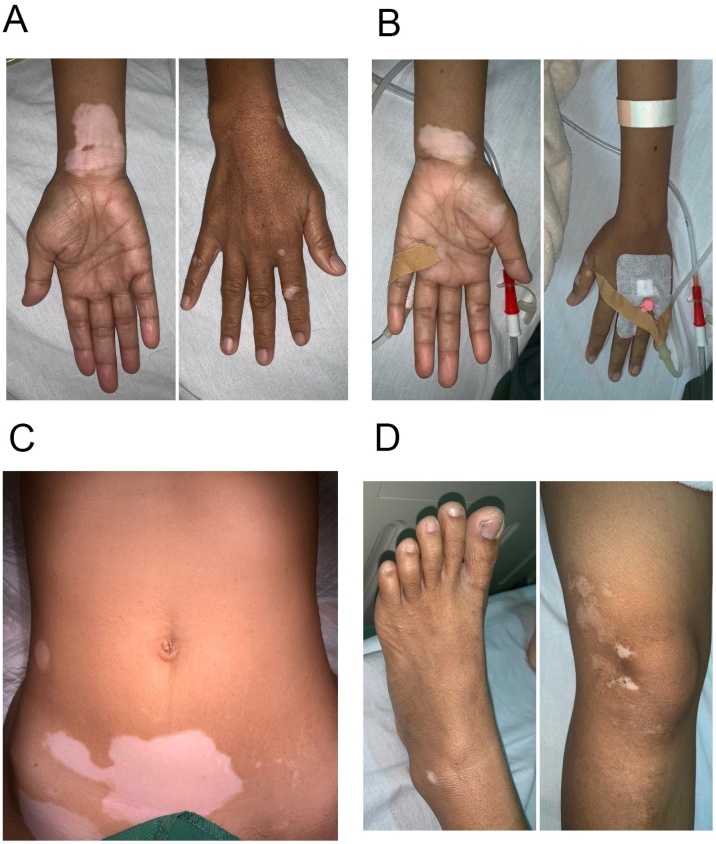
A 35-year-old woman was admitted to the emergency department with progressive dysphagia for 6 years. Her symptoms worsened in the last 14 days followed by vomiting undigested and retained food eaten. The patient also complained about coughing since 6 years ago and was previously diagnosed with a variant respiratory problem but her symptoms didn’t improve with medication. The patient had a heartburn and a history of weight loss. On physical examination she was found to have temperature 36.6 °C, a heart rate of 113 bpm, a blood pressure of 117/83 mm Hg, and respiratory rate 21 bpm. She had an appearance of multiple depigmented patches with sharply defined borders and leucotrichia on the neck, abdomen, hand, knee, and lateral malleolus (Fig. 1). The patient had vitiligo for 18 years. The lesion altered in size from small dot, were itchy, progressive, not painful, and no blister. The patient had a sister with the appearance of vitiligo.
Fig. 1.
Depigmented patch with leucotrichia on A, the left hand, B, right hand C, lower quadrant of the abdomen D, right knee, right lateral malleolus.
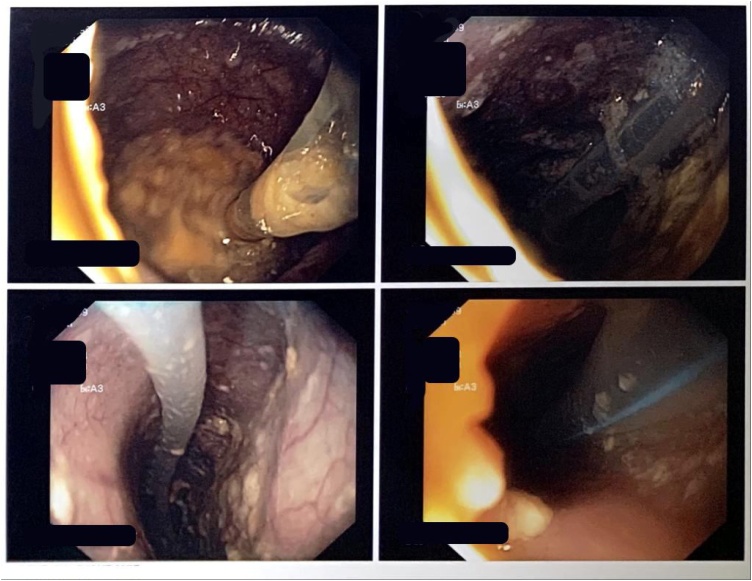
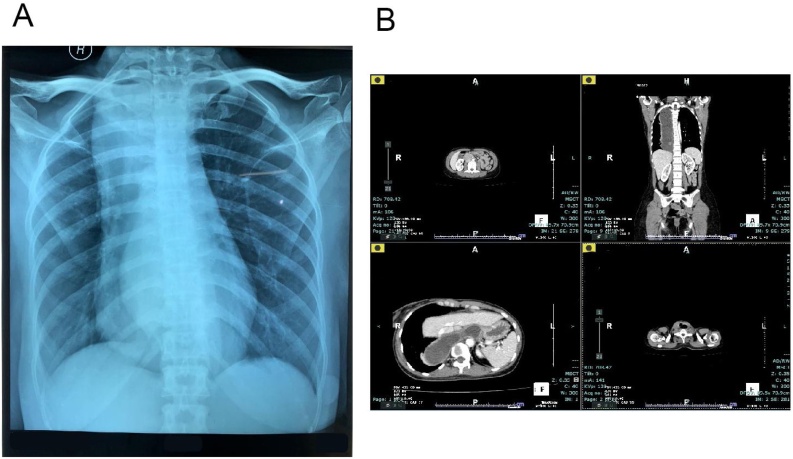
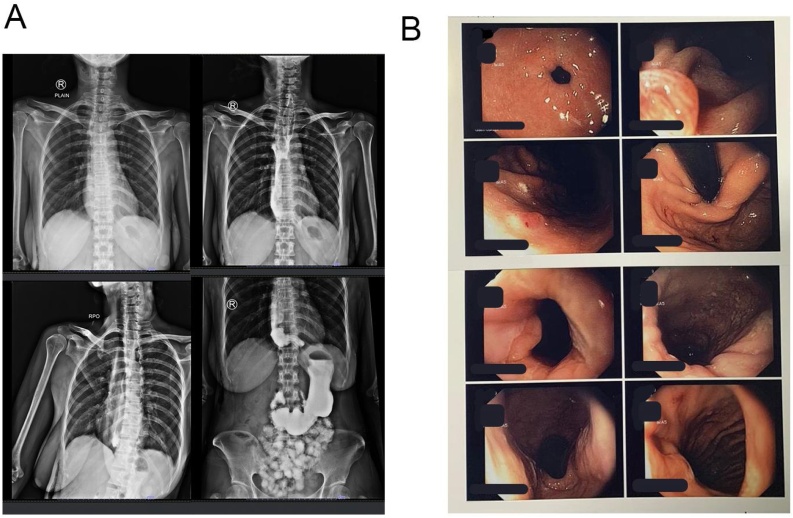
The patient underwent an upper gastrointestinal endoscopy and showed dilatation from distal oesophagus (38 cm from incisors) with retained food caused by narrowing gastro oesophagus junction (Fig. 2). The biopsy was not performed. Computed Tomography Scan Thorax revealed severe dilatation of oesophagus (diameter 4–7 cm) with narrowing in the distal level of gastrooesophagus sphincter, intraluminal fluid, shifting trachea to the right, shifting thyroid lobes to anterior and shifting left carotid artery to posterolateral left (Fig. 3).
Fig. 2.
Upper gastrointestinal endoscopy findings showed dilatation of oesophagus with retained food caused by narrowing gastro oesophagus junction.
Fig. 3.
A, Chest X-Ray showed convex opacity overlapping the right mediastinum and absent of gastric bubble. B, Computed Tomography Scan Thorax showed severe dilatation of oesophagus (diameter 4–7 cm) with narrowing in the distal level of gastrooesophagus sphincter.
After examining the symptoms and investigations, we conclude the diagnosis as achalasia. Considering the clinical history and physical exam findings, the diagnosis of vitiligo was given.
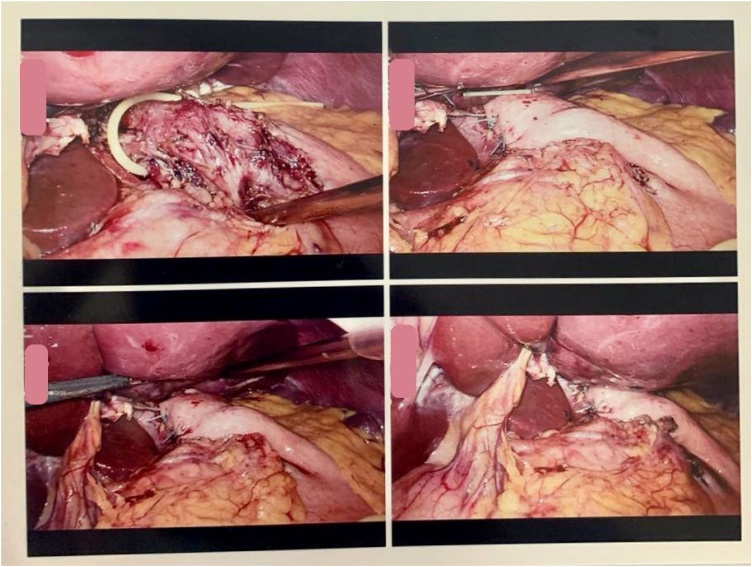
She was prescribed ranitidine and metoclopramide injection as a plan for initial management prior to surgery. Regarding the procedure, Laparoscopic Heller’s myotomy was performed. The patient was placed in the French position (supine with the legs abducted on straight leg boards) with the operator stand between the legs and the surgical assistants stand one on each side of the patient. A 3-port procedure was taken, with a 10 mm port was used for camera port. The left liver lobe was retracted to the abdominal wall. We released oesophagus from crus anterior and posterior, and retracted with sling catheter modification. The myotomy of oesophagus musculature was performed with scissor and ultrasonic scissor (harmonic device) with the active blade in the upper side about 5 cm to proximal. The myotomy of the gastric side with monopolar cautery and splitting with grasper 2 cm distal to LOS. After the myotomy was made and leak testing was done with blowing the nasogastric tube, the opening of the esophagus was repaired with Dor’s fundoplication (Fig. 4).
Fig. 4.
Laparoscopic view. Heller myotomy was performed with oesophagus side 5 cm to proximal and gastric side 2 cm to distal, Dor fundoplication was performed for anti-reflux and to cover the exposed submucosa.
A post-operative study with a barium swallow of upper gastrointestinal radiograph was carried out 5 days following surgery, in which passage from oesophagus to jejunum without leakage, and no narrowing in oesophagus gastric junction at the distal oesophagus (Fig. 5A). Patient compliance was good throughout the treatment, and we also assess patient tolerance by monitoring blood routine and vital signs. The patient was discharged from the hospital on postoperative day 8 and is undergoing follow-up as an outpatient, with no dysphagia. Five months later the patient presented back with improvement were observed in upper gastrointestinal endoscopy, with a reduction in the sigmoid shape of oesophagus, good condition of LOS, and polyp 2 mm presented in the gastric corpus (Fig. 5B).
Fig. 5.
Post-operative A, Barium swallow on postoperative day 5 barium showed no narrowing in oesophagus gastric junction at the distal oesophagus, no leakage from oesophagus to jejunum. B, Upper gastrointestinal endoscopy findings showed a reduction in sigmoid shape, no esophagitis, good condition of LOS, and polyp 2 mm presented in the gastric corpus.
3. Discussion
Achalasia is a disorder affecting the oesophagus motility [7]. Patients suffering from this disorder have an imbalance of excitatory (acetylcholine, substance P) and inhibitory (nitric oxide, vasoactive intestinal peptide) neurotransmission which results in hypertensive non-relaxing LOS [3]. It is considered a rare disease with the prevalence is eight cases per million population [2]. Achalasia has a peak incidence most frequently in the age group of 30–60 years [4]. In our case, the patient is a 35-year-old female diagnosed with achalasia and present with vitiligo.
The pathology examination of affected LOS tissue has revealed that the mechanism of neuronal destruction is an inflammatory response with T-cell infiltrates [5]. Meanwhile, the cause of neuronal degeneration in primary achalasia is not known, but increasing evidence suggests that autoimmunity, particularly anti-myenteric antibodies may play a role [10,11]. The autoimmune mechanism is highly associated with class II antigens of the major histocompatibility complex (MHC), the expression of MHC class II molecules in oesophagus ganglion cells induced by IFN- release initiates an autoimmune process, the autoimmune mechanism could resulting in oesophagus ganglion cell destruction in achalasia [12]. There is a correlation between the disease and particular HLA class II antigens, evidence of myenteric antiplexus antibodies was detected in serum samples from patients diagnosed with achalasia, especially in HLA DQA1*0103 and DQBI*0603 alleles [6]. The protein of tyrosine phosphatase N22 gene (PTPN22) is believed to encodes a lymphoid-specific phosphatase (LYP) that down-regulates T-cell activation, the PTPN22-allele 1858T promotes an autoimmune response that results in chronic inflammation and the association with some autoimmune conditions [13].
Furthermore, Booy, Takata, Tomlinson, Urbach (2011) [5] has been reported that patients with achalasia was 3.6 times more likely to suffer from any autoimmune condition. There are many different types of autoimmune diseases has been linked to achalasia: myasthenia gravis, polymyositis, autoimmune thyroiditis disease, anemia, Addison’s disease, inflammatory bowel disease, vitiligo, and idiopathic thrombocytopenia, contributing to the argument that there is an autoimmune component to achalasia’s etiology [13,11]. There are five autoimmune diseases that more prevalent present in the patient with achalasia: type I diabetes mellitus, hypothyroidism, Sjogren’s syndrome, systemic lupus erythematosus, and uveitis, but a coincidence of achalasia and vitiligo is rare [5].
This case reports and studies give rise to thought about the association of achalasia and vitiligo. Our patient herein presented with vitiligo characterized by an area of white macules and patches for 12 years before the dysphagia symptom was developed. We don’t know whether a unique genetic predisposition or any environmental or infection factor caused this, but interestingly, the patient’s sister also developed the symptoms of vitiligo.
We suggest autoimmunity as a probable contributing factor for the association of these two disorders. Vitiligo is an acquired depigmentation disorder, related to the destruction of melanocytes in the epidermis [14]. In order to explain this, several pathogenetic models have attempted to explain vitiligo onset and progression, but clinical and experimental findings point mainly to the autoimmune hypothesis as the most contributing factor [15]. The immune hypothesis theory stated there is an alteration of immune surveillance that has been proposed as the primary response resulting in impaired function and destruction of melanocytes, it then releases the antigenic components that lead to the development of the autoantibodies [16]. The dysfunction and destruction of melanocytes in vitiligo are mediated by an autoimmune attack of CD8+ T cells [14]. The IFN- plays an important role in vitiligo in addition to its release of chemokines such as CXCL9, 10, and 11, and the role of IFN- derived from cytotoxic T cells leading to induced apoptosis in melanocytes [15].
This might explain the increased organ-specific autoimmunity observed in ganglion cell destruction as the mechanism of achalasia, leading to their destruction by T cells. Saleem & Azim (2016) [17] reported that the frequency of associated autoimmune disorders significantly higher in patients with vitiligo than in the control group study. There are some reports of patients with achalasia responding to immunosuppressive drugs, lending further to the suggestion that this disease has an autoimmune component [18]. Additionally, patients presenting vitiligo or any autoimmune disease should be further investigated for the oesophagus-related problem, ranging from characteristic oesophagus dysmotility to more complex manifestation as occurred in our case.
4. Conclusion
The presentation of achalasia is usually nonspecific and often leads to diagnostic and treatment delays. Therefore, the pathophysiological of achalasia need to be understood to target the specific cause of the disease. We hereby report on a rare case of achalasia present with vitiligo which may suggest an autoimmune disorder in the onset of achalasia. Achalasia must be considered in vitiligo or any autoimmune disease presenting with the esophagus-related problem.
Declaration of Competing Interest
The authors report no declarations of interest.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The ethical approval has been exempted by our institution RSUD Abdul Wahab Sjahranie Samarinda No:168/KEPK-AWS/XI/2019.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Bambang Suprapto: Perform the surgery, Data collection, Data analysis, review.
Amalia Aswin: Data analysis, literature search, writing-review & editing, preparation of manuscript for publication.
Registration of research studies
NA.
Guarantor
Bambang Suprapto.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgment
The authors are grateful to our institute.
References
- 1.Herzig M., Tutuian R. Focal achalasia - case report and review of the literature. Clujul Med. 2018;91(1):120–128. doi: 10.15386/cjmed-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuzawa-Carballeda J., Aguilar-Leon D., Gamboa-Dominguez A., Valdovinos M., Nunez-Alvarez C., Martin-del-Campo L. Achalasia—an autoimmune inflammatory disease: a cross-sectional study. J. Immunol. Res. 2015:1–4. doi: 10.1155/2015/729217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarumpaet F., Dairi L. IOP Conf. Series: Earth and Environmental Science. IOP Publishing; Medan: 2018. A case report of achalasia; pp. 1–4. [DOI] [Google Scholar]
- 4.Amr B., Mamillapalli C. Achalasia in a patient with polyglandular autoimmune syndrome type II. Case Rep. Gastroenterol. 2015;9(2):160–164. doi: 10.1159/000430493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booy J., Takata J., Tomlinson G., Urbach D. The prevalence of autoimmune disease in patients with esophageal achalasia. Dis. Esophagus. 2012;25(3):209–213. doi: 10.1111/j.1442-2050.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 6.Furuzawa-Carballeda J., Torres-Landa S., Valdovinos M., Coss-Adame E., Martin-del-Campo L., Torres-Villalobos G. New insights into the pathophysiology of achalasia and implications for future treatment. World J. Gastroenterol. 2016;22(35):7892–7907. doi: 10.3748/wjg.v22.i35.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlottmann F., Neto R., Herbella F., Patti M. The Southeastern Surgical Congress. The American Surgeon; California: 2018. Esophageal achalasia: pathophysiology, clinical presentation, and diagnostic evaluation; pp. 67–70. [PubMed] [Google Scholar]
- 8.Zaninotto G., Bennett C., Boeckxstaens G., Costantini M., Ferguson M., Pandolfino J. 2018. Disease of the Esophagus. The 2018 ISDE Achalasia Guidelines. [DOI] [PubMed] [Google Scholar]
- 9.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A.J., Orgill D.P. The SCARE 2018 statement: Updating consensus Surgical Case Report (SCARE) guidelines. Int. J. Surg. Case Rep. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Kraichely R., Farrugia G., Pittock S., Castell D., Lennon V. Neural autoantibody profile of primary achalasia. Dig. Dis. Sci. 2010;55(2):307. doi: 10.1007/s10620-009-0838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avelar-Rodriguez D., Toro-Monjaraz E., Velez R., Yamazaki-Nakashi-mada M., Ramirez-Mayans J. A patient with esophageal achalasia and common variable immunodeficiency: a case report and review of the literature. Acta Pediatr. Mex. 2019;40(3):143–147. doi: 10.18233/apm40no3pp143-1471810. [DOI] [Google Scholar]
- 12.Zarate N., Mearin F., Gill-Vernet J., Camarasa F., Malagelada J. Achalasia and Down’s syndrome: coincidential association or something else? Am. J. Gastroenterol. 1999;94(6):1674–1677. doi: 10.1016/s0002-9270(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 13.Quidute R., Freitas E.V.d., Lima T.G.d., Feitosa A.M., Santos J.P.d., Correia J.W. Achalasia and thyroid disease: possible autoimmune connection? Arch. Endocrinol. Metab. 2012;56(9):677–682. doi: 10.1590/s0004-27302012000900013. [DOI] [PubMed] [Google Scholar]
- 14.Lukinovic-Skudar V. Autoimmune factors in the ethiopathogenesis of vitiligo. MOJ Anat. Physiol. 2015;1(3):60–62. doi: 10.15406/mojap.2015.01.00012. [DOI] [Google Scholar]
- 15.Baldini E., Odorisio T., Sorrenti S., Catania A., Tartaglia F., Carbotta G. Vitiligo and autoimmune thyroid disorders. Front. Endocrinol. 2017;8:1–6. doi: 10.3389/fendo.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborty D., Haneef N., Razvi F., Kumar B., Fatima N., Koganti N. A case report showing coexistence of two autoimmune disease-psoriasis and vitiligo. J. Med. Allied. Sci. 2017;7(2):114–117. doi: 10.5455/jmas.260021. [DOI] [Google Scholar]
- 17.Saleem K., Azim W. Association of vitiligo with other autoimmune disorders. Diabetes Case Rep. 2016;1(3):1–3. doi: 10.4172/2572-5629.1000114. [DOI] [Google Scholar]
- 18.Al-Jafar H., Laffan M., Al-Sabah S., Elmorsi M., Habeeb M., Alnajar F. Severe recurrent achalasia cardia responding to treatment of severe autoimmune acquired haemophilia. Case Rep. Gastroenterol. 2012;6(3):618–623. doi: 10.1159/000343435. [DOI] [PMC free article] [PubMed] [Google Scholar]