Abstract
Objective
The aim was to assess the safety and efficacy of up to 156 weeks of ixekizumab (an IL-17A antagonist) treatment in PsA patients.
Methods
In a phase III study, patients naïve to biologic treatment were randomized to placebo, adalimumab 40 mg every 2 weeks (ADA; active reference) or ixekizumab 80 mg every 2 weeks (IXEQ2W) or every 4 weeks (IXEQ4W) after an initial dose of 160 mg. At week 24 (week 16 for inadequate responders), ADA (after 8-week washout) and placebo patients were re-randomized to IXEQ2W or IXEQ4W. Outcomes were evaluated using a modified non-responder imputation [linear extrapolation for radiographic progression (modified total Sharp score = 0)] during extended treatment until week 156.
Results
Of 417 patients, 381 entered the extension, and 243 of 381 (63.8%) completed the 156-week study. Incidence rates of treatment-emergent and serious adverse events, respectively, were 38.0 and 5.2 with IXEQ2W (n = 189) and 38.1 and 8.0 with IXEQ4W (n = 197). One death occurred (IXEQ4W). With IXEQ2W and IXEQ4W, respectively, the response rates persisted to week 156 as measured by the ACR response ≥20% (62.5 and 69.8%), ≥50% (56.1 and 51.8%) and ≥70% (43.8 and 33.4%), psoriasis area and severity index (PASI) 75 (69.1 and 63.5%), PASI 90 (64.5 and 51.2%) and PASI 100 (60.5 and 43.6%). Inhibition of radiographic progression also persisted to week 156 in 61% of IXEQ2W and 71% of IXEQ4W patients.
Conclusion
In this 156-week study of ixekizumab, the safety profile remained consistent with previous reports, and improvements in signs and symptoms of PsA were observed, including persistent low rates of radiographic progression.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT01695239, EudraCT 2011-002326-49.
Keywords: DMARDs (biologic), psoriatic arthritis, psoriasis, ixekizumab, spondyloarthritis
Rheumatology key messages
Ixekizumab, a high-affinity monoclonal antibody with specificity for IL-17A, is an approved PsA/psoriasis treatment.
Ixekizumab treatment for ≤3 years demonstrated continued PsA improvements and a consistent safety profile.
Ixekizumab is a suitable long-term treatment for PsA.
Introduction
PsA is chronic and immune mediated, with manifestations including peripheral arthritis, axial arthritis, enthesitis, dactylitis and skin and nail psoriasis [1]. The disease can be progressive, leading to structural joint damage and significant impairment of patients’ health-related quality of life (HRQoL) [2].
Ixekizumab is a high-affinity monoclonal antibody that has specificity for IL-17A [3], a pro-inflammatory cytokine that promotes the pathogenesis of PsA and psoriasis [4], and is approved for the treatment of PsA and psoriasis. In a phase III study of patients naïve to biologic PsA treatments (SPIRIT-P1), ixekizumab was superior to placebo in improving signs and symptoms of PsA, slowing the progression of structural joint damage and improving patient HRQoL [5–7].
Here we report the safety, efficacy and HRQoL findings for ixekizumab in SPIRIT-P1 up to 156 weeks.
Methods
Study design and patient population
SPIRIT-P1 (NCT01695239, EudraCT 2011–002326-49) is a phase III, multicentre, randomized, active and placebo-controlled study in active PsA patients. The study consisted of three periods: double-blind (weeks 0–24), extension (weeks 24–52) and long-term extension (weeks 52–156). Week 156 was the last visit of the long-term extension. Detailed methodology has been reported previously (in brief, see Supplementary material, section Study Design, available at Rheumatology online) [5, 7]. Patients who participated in the extension periods received 80 mg ixekizumab every 2 weeks (IXEQ2W) or every 4 weeks (IXEQ4W) until study completion or treatment discontinuation.
Participants were naïve to biologic DMARDs (bDMARDs) for PsA, could not have been taking more than one conventional synthetic DMARD (csDMARD) and could not have taken more than four csDMARDs before enrolment. Patients were also ≥18 years of age, had a documented PsA diagnosis for ≥6 months, met Classification Criteria for Psoriatic Arthritis (CASPAR), had a tender joint count of at least three and a swollen joint count of at least three and had at least one PsA-related hand or foot joint erosion on centrally read X-rays or CRP >6 mg/l.
Patient and public involvement
Patients were not invited to comment on the study design, develop patient-relevant outcomes, interpret results or contribute to writing or editing this document. All patients gave written informed consent. This study was conducted in accordance with Good Clinical Practice, the principles of the Declaration of Helsinki and local laws and regulations and was approved by institutional review boards or independent ethics committees for each study site.
Outcomes
Safety was assessed by adverse events (AEs), laboratory monitoring, physical examinations and ECGs. For immunogenicity testing, venous blood samples were collected at regular intervals, and a validated acid capture and elution ELISA assay was used to determine treatment-emergent antidrug antibodies (TE-ADAs). AEs of special interest included, but were not limited to, infections (including serious Candida and tuberculosis), allergic reactions/hypersensitivity events (including, but not limited to, hypersensitivity reactions, and clinical manifestations of these reactions may include, but are not limited to, skin rash, pruritus, urticaria, angioedema and anaphylactic reaction) and injection-site reactions (ISRs). Patients were administered a purified protein derivative skin/QuantiFERON®-TB Gold test at week 52 and yearly thereafter; a purified protein derivative ≥5 mm induration or positive QuantiFERON-TB Gold test at week 52 or later required patient discontinuation from the study.
Efficacy was assessed by the percentage of patients achieving and/or change from baseline on the following measures: ACR response ≥20, ≥50 or ≥70% (ACR20/50/70) [8]; psoriasis area and severity index ≥75, ≥90 or 100% (PASI 75/90/100) among patients with baseline body surface area ≥3% [9, 10]; minimal disease activity [meeting at least five of seven criteria: tender joint count ≤1, swollen joint count ≤1, total PASI ≤1 or body surface area ≤3, patient pain visual analogue scale (VAS) ≤15, patient global disease VAS ≤20, HAQ-disability index (DI) ≤0.5 and tender entheseal points ≤1] [11, 12]; very low disease activity (all seven minimal disease activity criteria met) [11, 12]; remission-to-low disease activity [>4 and ≤14 disease activity index for psoriatic arthritis (DAPSA)] and remission (≤4 DAPSA) [13]; low disease activity [≤3.2 psoriatic arthritis disease activity (PASDAS)] and near remission (≤1.9 PASDAS) [14]; Leeds enthesitis index (LEI) in patients with baseline enthesitis [15]; Leeds dactylitis index-basic (LDI-B) in patients with baseline dactylitis [16]; nail psoriasis severity index (NAPSI) in patients with baseline fingernail psoriasis; HAQ-DI [including percentage improving by the minimal clinically important difference (MCID; ≥0.35) in patients with baseline HAQ-DI ≥0.35] [17]; 36-item short form health survey (SF-36) [18]; EuroQoL 5 dimension questionnaire VAS (EQ-5D VAS) [19]; work productivity and activity impairment questionnaire (WPAI) [20]; and van der Heijde modified total Sharp score (mTSS; 0–528 scale) for PsA (including percentage with change from baseline ≤0, ≤0.5 and ≤smallest detectable change over 156 weeks) [21]. Radiographs were scored independently by two readers blinded to time point and clinical data. Radiographs at screening and weeks 52, 108 and 156 were used to examine radiographic progression over 3 years.
Statistical analysis
Four patient populations are presented: (i) patients randomized to ixekizumab at week 0 comprised the ixekizumab intent-to-treat population (ixekizumab ITT population); (ii) patients originally randomized to ixekizumab at week 0 who received at least one dose of study medication at or after week 24 and who had available efficacy and health outcome data summarized from week 24 to week 156 comprised the combined extension period population; (iii) patients originally randomized to ixekizumab at week 0 who received at least one dose of study medication at or after week 52 and who had available data summarized from week 52 to week 156 comprised the long-term extension period population; and (iv) all patients who received at least one dose of ixekizumab during the study where baseline was the time of first ixekizumab injection comprised the all ixekizumab exposure safety population.
Safety analyses were conducted using the all ixekizumab exposure safety population. The exposure-adjusted incidence rate (IR; or person-time-adjusted IR) over the entire time period is the total number of patients who experienced the treatment-emergent AE (TEAE) for each preferred term divided by the sum of all patients’ time (in 100 years) of ixekizumab exposure during the treatment periods.
For all categorical efficacy measures, missing data were imputed using non-responder imputation (NRI), multiple imputation (MI) and modified non-responder imputation (mNRI) methods. Additional statistical methods, including detailed definitions for each imputation method, are described in the Supplementary material, section Statistical Analysis, available at Rheumatology online.
SF-36 developers recommend scoring and interpretation of the eight domains using norm-transformed scores, which results in mean (s.d.) domain scores of 50 (10) and reduces the magnitude of possible change relative to a 0–100 transformation method [22]. Here we report norm-transformed scores [22].
Radiographic analyses were conducted using the long-term extension period population. Linear extrapolation was used to impute missing mTSS data if patients had baseline and at least one post-baseline value at week 52, 108 or 156. The percentage of patients without radiographic progression at weeks 52, 108 and 156 was also summarized using mTSS cut-off values of ≤0, ≤0.5 and ≤1.85 (smallest detectable change) [23].
Results
Patient disposition
The first participant was enrolled in January 2013, and the last study visit occurred in September 2017. In total, 381 of 417 (91.4%) randomized patients completed the double-blind period and entered the extension (Supplementary Fig. S1, available at Rheumatology online). Between week 32 and week 156, 70 of 381 (18.4%) patients met predefined criteria for mandatory discontinuation (i.e. failed to demonstrate ≥20% improvement from baseline in both tender joint count and swollen joint count). Of patients who entered the extension, 243 of 381 (63.8%) completed the 156-week study (Supplementary Fig. S1, available at Rheumatology online). One death in the IXEQ4W group is included in treatment discontinuations. A similar percentage of patients across treatment groups discontinued from the study early, most owing to lack of efficacy. Other reasons included an AE; sponsor, patient or physician decision; lost to follow-up; or entry criteria not met (Supplementary Fig. S1, available at Rheumatology online). Baseline demographics and disease characteristics for patients enrolled in the double-blind period and those entering the extension have been published previously [5, 7]. The percentage of patients who ceased or started csDMARDS or CSs during the 156-week study are reported in Supplementary Table S1, available at Rheumatology online.
Safety
A total of 386 patients (189 IXEQ2W and 197 IXEQ4W treated) were included in the safety analysis. By week 156, 88% of IXEQ2W- (IR, 38.0) and 87% of IXEQ4W-treated (IR, 38.1) patients had experienced at least one TEAE (Table 1). The most frequent TEAEs were upper respiratory tract infection, ISR, bronchitis and injection-site erythema (Table 1). The majority of events were mild or moderate in severity (investigator assessed). Severe events (investigator assessed) were reported in 11% of IXEQ2W- (IR, 4.8) and 9% of IXEQ4W-treated (IR, 4.0) patients. Serious adverse events were reported in 12% of IXEQ2W- (IR, 5.2) and 18% of IXEQ4W-treated (IR, 8.0) patients (Table 1).
Table 1.
Safety overview across all treatment periods up to week 156 (all ixekizumab exposure safety population)
| Ixekizumab Q4W (total n = 197; PY = 449) |
Ixekizumab Q2W (total n = 189; PY = 440) |
|||
|---|---|---|---|---|
| n (%) | IR | n (%) | IR | |
| TEAEs | 171 (87) | 38.1 | 167 (88) | 38.0 |
| Viral URI | 29 (15) | 6.5 | 25 (13) | 5.7 |
| URI | 31 (16) | 6.9 | 22 (12) | 5.0 |
| Injection-site reaction | 21 (11) | 4.7 | 24 (13) | 5.5 |
| Bronchitis | 14 (7) | 3.1 | 17 (9) | 3.9 |
| Injection-site erythema | 10 (5) | 2.2 | 18 (10) | 4.1 |
| Diarrhoea | 8 (4) | 1.8 | 13 (7) | 3.0 |
| Hypertension | 11 (6) | 2.4 | 10 (5) | 2.3 |
| Headache | 12 (6) | 2.7 | 9 (5) | 2.0 |
| Back pain | 13 (7) | 2.9 | 8 (4) | 1.8 |
| Urinary tract infection | 14 (7) | 3.1 | 7 (4) | 1.6 |
| TEAE severitya | ||||
| Mild | 58 (29) | 12.9 | 77 (41) | 17.5 |
| Moderate | 95 (48) | 21.1 | 69 (37) | 15.7 |
| Severe | 18 (9) | 4.0 | 21 (11) | 4.8 |
| Serious adverse eventsb | 36 (18.3) | 8.0 | 23 (12.2) | 5.2 |
| Serious infections | 8 (4) | 1.8 | 3 (2) | 0.7 |
| Deaths | 1 (<1) | 0.2 | 0 | – |
| Discontinued owing to AEs (including death) | 18 (9) | 4.0 | 25 (13) | 5.7 |
| AEs of special interestc | ||||
| Infections | 110 (56) | 24.5 | 109 (58) | 24.8 |
| Candida infections | 6 (3) | 1.4 | 5 (3) | 1.1 |
| Injection-site reactions | 42 (21) | 9.3 | 46 (24) | 10.5 |
| Hepatic event | 19 (10) | 4.2 | 26 (14) | 5.9 |
| Allergic reaction/hypersensitivity events | 11 (6) | 2.4 | 20 (11) | 4.5 |
| Cerebrocardiovascular eventsd | 9 (5) | 2.0 | 11 (6) | 2.5 |
| Depression | 4 (2) | 0.9 | 6 (3) | 1.4 |
| Interstitial lung disease | 0 | – | 0 | – |
| Malignancies | 3 (2) | 0.7 | 2 (1) | 0.5 |
| Ulcerative colitis | 0 | – | 1 (<1) | 0.2 |
More than one event could be reported in the same patient. Patients may be counted in more than one category. The baseline for the all ixekizumab exposure safety population was the time of the first ixekizumab dose.
Investigator assessed.
Regulatory defined.
Reported as AEs according to the high-level term in MedDRA, v.20.0. Groups of AEs of special interest are shown.
Events were adjudicated.
AEs: adverse events; IR: exposure-adjusted incidence rates (n × 100/total patient-years); PY: patient-years; Q4W/Q2W: 80 mg once every 4 weeks/2 weeks; TEAE: treatment-emergent adverse events; URI: upper respiratory tract infection.
One death occurred during the study. A 59-year-old male with a history of dyslipidaemia, diabetes mellitus, hypertension and previous transient ischaemic attack experienced a fatal cerebrovascular accident while taking IXEQ4W; the event occurred 556 days after initiating IXEQ4W treatment. Twenty-five IXEQ2W- and 18 IXEQ4W-treated patients discontinued owing to AEs (Supplementary Table S2, available at Rheumatology online).
No active or reactivated tuberculosis cases were reported. One case of iritis was reported. Infections occurred in 58% of IXEQ2W- (IR, 24.8) and 56% of IXEQ4W-treated (IR, 24.5) patients (Table 1). Serious infections occurred in three IXEQ2W- (2%; IR, 0.7) and eight IXEQ4W-treated patients (4%; IR, 1.8). Pneumonia was the only serious infection reported in at least one patient (n = 2; IXEQ4W) (Supplementary Table S3, available at Rheumatology online). Eleven patients had a Candida infection. Among IXEQ2W-treated patients, two (1.0%; IR, 0.4) had oral candidiasis, two (1.0%; IR, 0.4) had skin candidiasis, and one (0.5%; IR, 0.2) had nail candidiasis. Among IXEQ4W-treated patients, five (2.6%; IR, 1.1) had oral candidiasis and one (0.5%; IR, 0.2) had oesophageal candidiasis. All but one case of Candida (oral) had resolved at study conclusion. The duration of Candida infection ranged from 12 to 246 days for oral candidiasis and from 9 to 20 days for skin Candida; the duration was 6 days for oesophageal Candida and 35 days for nail Candida.
ISRs occurred at a similar rate in IXEQ2W- (24%; IR, 10.5) and IXEQ4W-treated patients (21%; IR, 9.3) (Table 1). The majority of ISRs were mild in severity [IXEQ2W: 20% (IR, 8.6); IXEQ4W: 17% (IR, 7.6)]. Two patients (both IXEQ2W) discontinued treatment owing to an ISR. Allergic reactions/hypersensitivity events were reported in 11% (IR, 4.5) of IXEQ2W- and 6% (IR, 2.4) of IXEQ4W-treated patients. A case of mild dyspnoea was reported as ‘possible anaphylaxis’ 1 day after the first ixekizumab injection in a 52-year-old female. This event resolved within 1 day without any treatment, did not result in ixekizumab discontinuation and did not reoccur during the remainder of the study despite IXEQ2W injections for 19 additional months.
The rates of hepatic events [14% (IR, 5.9) and 10% (IR, 4.2)], adjudicated cerebrocardiovascular events [6% (IR, 2.5) and 5% (IR, 2.0)], depression [3% (IR, 1.4) and 2% (IR, 0.9)] and malignancies [1% (IR, 0.5) and 2% (IR, 0.7)] were also similar in IXEQ2W- and IXEQ4W-treated patients, respectively (Table 1). One case of ulcerative colitis was reported in a 55-year-old male 351 days after starting IXEQ2W. He had no self-reported history of IBD; no relevant medical history was reported. Ixekizumab was discontinued owing to latent tuberculosis (mandatory discontinuation per protocol) before the resolution of ulcerative colitis.
Two patients (one IXEQ2W, one IXEQ4W) experienced Grade 3 neutropenia. Neither case of Grade 3 neutropenia was reported within a week of an infection. No Grade 4 neutropenia was reported.
TE-ADA developed in 53 patients over the 156-week treatment period (25 IXEQ2W, 28 IXEQ4W). Of these, 22 were neutralizing. The majority of TE-ADAs were low titre. There was no clear association between TE-ADA status and ACR20 response. There was no apparent association between development of antibodies to ixekizumab and allergic reactions/hypersensitivity events or ISRs.
Efficacy
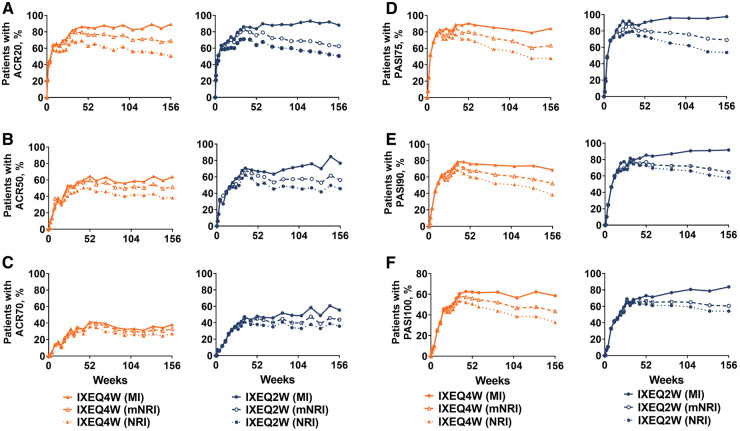
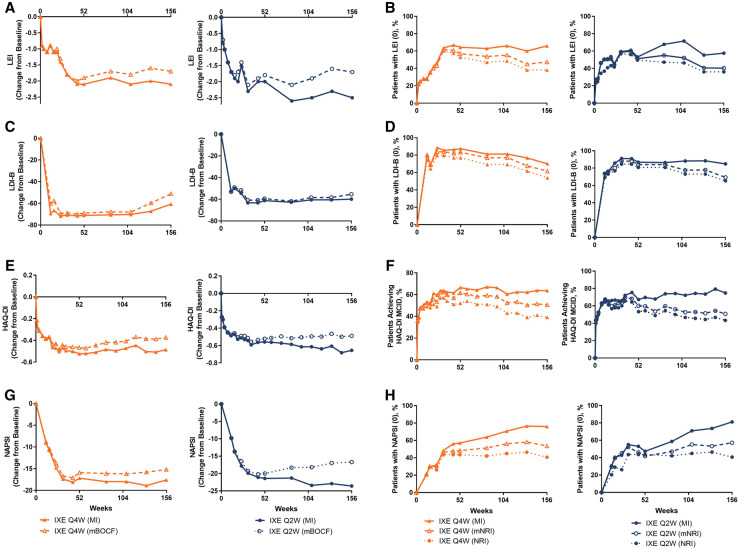
Efficacy outcomes at week 156 are presented in Table 2 for the ixekizumab ITT population; results were similar across treatment groups for the combined extension period population (Supplementary Table S4, available at Rheumatology online). In IXEQ2W- and IXEQ4W-treated patients, the ACR20, ACR50 and ACR70 response rates achieved by week 52 persisted through week 156 (Table 2, mNRI; Fig. 1A–C). At week 156, minimal disease activity response rates were numerically higher with IXEQ2W (48.9%) than with IXEQ4W (40.6%), and very low disease activity response rates were similar (21.0% with IXEQ2W and 17.3% with IXEQ4W) (Table 2, mNRI). Remission-to-low disease activity, measured by DAPSA >4 and ≤14, was achieved by 62.5% of IXEQ2W- and 63.6% of IXEQ4W-treated patients, and remission, measured by DAPSA ≤4, was achieved by 29.9% of IXEQ2W- and 24.8% IXEQ4W-treated patients at week 156 (Table 2, mNRI). Low disease activity, measured by PASDAS ≤3.2, was achieved by 57.6% of IXEQ2W- and 46.9% of IXEQ4W-treated patients and remission, measured by PASDAS ≤1.9, was achieved by 27.8% of IXEQ2W- and 21.4% IXEQ4W-treated patients at week 156 (Table 2, mNRI). Among patients with enthesitis, both IXEQ2W and IXEQ4W demonstrated persistent reductions from baseline on the LEI through week 156, with 40.3% of IXEQ2W- and 47.4% of IXEQ4W-treated patients achieving complete resolution of enthesitis at week 156 (Table 2, mNRI; Fig. 2A and B). Among patients with dactylitis, both IXEQ2W and IXEQ4W demonstrated persistent reductions from baseline on the LDI-B through week 156, and 69.3% of IXEQ2W- and 61.6% of IXEQ4W-treated patients achieved complete resolution of dactylitis at week 156 (Table 2, mNRI; Fig. 2C and D). At week 156, both IXEQ2W and IXEQ4W demonstrated persistent reductions from baseline on the HAQ-DI (Table 2; Fig. 2E). The percentage who achieved the MCID on the HAQ-DI also persisted to week 156 and was similar with IXEQ2W and IXEQ4W (Table 2 mNRI; Fig. 2F).
Table 2.
Efficacy overview at week 156 (ixekizumab intention-to-treat population)
| Ixekizumab Q4W (n = 107) |
Ixekizumab Q2W (n = 103) |
|||||
|---|---|---|---|---|---|---|
| Responder rate,n/Nx (%) | mNRI | NRI | MI | mNRI | NRI | MI |
| ACR20 | 75/107 (69.8) | 54/107 (50.5) | 95/107 (89.3) | 64/103 (62.5) | 52/103 (50.5) | 91/103 (88.1) |
| ACR50 | 55/107 (51.8) | 41/107 (38.3) | 68/107 (63.7) | 58/103 (56.1) | 47/103 (45.6) | 79/103 (76.7) |
| ACR70 | 36/107 (33.4) | 29/107 (27.1) | 41/107 (38.2) | 45/102 (43.8) | 37/103 (35.9) | 57/102 (55.6) |
| MDA | 43/106 (40.6) | 36/107 (33.6) | 52/106 (49.4) | 50/103 (48.9) | 44/103 (42.7) | 70/103 (68.0) |
| VLDA | 19/107 (17.3) | 18/107 (16.8) | 19/107 (17.9) | 22/103 (21.0) | 21/103 (20.4) | 25/103 (23.8) |
| LDAa | 68/107 (63.6) | 51/107 (50.5) | 85/107 (79.9) | 64/103 (62.5) | 54/103 (54.7) | 90/103 (87.2) |
| Remissionb | 27/107 (24.8) | 24/107 (22.4) | 29/107 (27.3) | 31/103 (29.9) | 29/103 (28.2) | 38/103 (36.6) |
| PASDAS LDA ≤3.2 | 49/104 (46.9) | 39/107 (36.4) | 63/103 (61.4) | 59/102 (57.6) | 51/103 (49.5) | 83/102 (80.9) |
| PASDAS ≤1.9 | 22/104 (21.4) | 20/107 (18.7) | 25/103 (24.7) | 28/102 (27.8) | 26/103 (25.2) | 36/102 (35.3) |
| LEI=0c | 32/68 (47.4) | 26/68 (38.2) | 45/68 (65.9) | 23/57 (40.3) | 19/57 (33.3) | 33/57 (57.5) |
| LDI-B=0d | 23/37 (61.6) | 21/39 (53.8) | 26/37 (70.1) | 17/25 (69.3) | 17/26 (65.4) | 21/25 (84.9) |
| PASI 75e | 46/73 (63.5) | 35/73 (47.9) | 61/73 (84.1) | 41/59 (69.1) | 36/59 (61.0) | 58/59 (97.5) |
| PASI 90e | 37/73 (51.2) | 28/73 (38.4) | 50/73 (68.7) | 38/59 (64.5) | 34/59 (57.6) | 54/59 (91.7) |
| PASI 100e | 32/73 (43.6) | 24/73 (32.9) | 43/73 (58.6) | 36/59 (60.5) | 32/59 (54.2) | 49/59 (83.7) |
| NAPSI (0)f | 36/68 (53.6) | 28/69 (40.6) | 52/68 (76.0) | 42/74 (57.1) | 38/74 (51.4) | 60/74 (81.1) |
| HAQ-DI MCIDg | 51/100 (51.1) | 39/100 (39.0) | 64/100 (63.7) | 46/90 (50.8) | 39/90 (43.3) | 67/90 (74.8) |
|
| ||||||
| Change from baseline h | mBOCF | MI | mBOCF | MI | ||
|
| ||||||
| LEIc | −1.7 (1.8) | −2.1 (0.2) | −1.7 (1.7) | −2.5 (0.2) | ||
| LDI-Bd | −51.2 (91.0) | −60.7 (15.6) | −55.4 (61.3) | −59.9 (11.9) | ||
| NAPSIf | −15.2 (19.9) | −17.6 (2.7) | −16.7 (21.9) | −23.6 (2.5) | ||
| HAQ-DI | −0.37 (0.57) | −0.49 (0.06) | −0.49 (0.59) | −0.66 (0.07) | ||
| SF-36 PCS score | 7.9 (10.2) | 10.3 (1.1) | 8.3 (9.6) | 10.9 (1.0) | ||
| SF-36 MCS score | 3.5 (11.6) | 4.1 (1.3) | 3.8 (10.3) | 5.2 (1.1) | ||
| EQ-5D VAS | 12.0 (24.8) | 14.7 (2.9) | 13.7 (21.1) | 18.3 (2.5) | ||
| WPAI absenteeism | −4.8 (18.8) | −8.7 (2.9) | 0.2 (27.7) | −2.5 (4.1) | ||
| WPAI activity impairment | −21.3 (26.1) | −28.3 (2.8) | −26.1 (26.0) | −32.1 (2.7) | ||
| WPAI presenteeism | −18.0 (27.2) | −28.4 (4.2) | −21.2 (21.8) | −25.0 (3.3) | ||
| WPAI work productivity | −17.5 (26.0) | −29.4 (4.1) | −18.2 (25.0) | −24.8 (3.5) | ||
DAPSA score >4 and ≤14.
DAPSA score ≤4.
Patients with baseline LEI > 0.
Patients with baseline LDI-B > 0.
Patients with baseline psoriasis of ≥3% body surface area.
Patients with baseline fingernail psoriasis present.
Patients with baseline HAQ-DI score ≥0.35.
Data are presented as the mean (s.e.m.) for MI analysis and mean (s.d.) for mBOCF analysis.
DAPSA: disease activity index for psoriatic arthritis; EQ-5D: EuroQoL 5 dimension questionnaire; LDA: low disease activity; LDI-B: Leeds dactylitis index-basic; LEI: Leeds enthesitis index; mBOCF: modified baseline observation carried forward; MCID: minimal clinically important difference; MCS: mental component summary; MDA: minimal disease activity; MI: multiple imputation; mNRI: modified non-responder imputation; NAPSI: nail psoriasis severity index; NR: near remission; Nx: number of patients with non-missing data; PASDAS: psoriatic arthritis disease activity score; PASI: psoriasis area and severity index; PCS: physical component summary; Q4W/Q2W: 80 mg once every 4 weeks/2 weeks; SF-36: 36-item short form health survey; VAS: visual analog scale; VLDA: very low disease activity; WPAI: work productivity and activity impairment questionnaire.
Fig. 1.
ACR and PASI responses
The intent-to-treat population was randomized to IXE at week 0 (ixekizumab intent-to-treat population). Starting at week 32, and at all subsequent visits during the extension period, patients were discontinued from study treatment if they failed to demonstrate ≥20% improvement from baseline in both tender and swollen joint counts. IXE Q4W/Q2W: 80 mg ixekizumab every 4 weeks/2 weeks; MI: multiple imputation; mNRI: modified non-responder imputation; NRI: non-responder imputation; PASI: psoriasis area and severity index.
Fig. 2.
Enthesitis, dactylitis, HAQ-DI and nail psoriasis
The intent-to-treat population was randomized to IXE at week 0 (ixekizumab intent-to-treat population). Starting at week 32, and all subsequent visits during the extension, patients not demonstrating ≥20% improvement from baseline in both tender and swollen joint counts were discontinued. mNRI and MI applied to categorical endpoints and mBOCF and MI applied to continuous endpoints. DI: [HAQ] disability index; IXE Q4W/Q2W: 80 mg ixekizumab every 4 weeks/2 weeks; LDI-B: Leeds dactylitis index-basic; LEI: Leeds enthesitis index; mBOCF: modified baseline observation carried forward; MCID: minimal clinically important differences; MI: multiple imputation; mNRI: modified non-responder imputation; NAPSI: nail area psoriasis severity index; NRI: non-responder imputation.
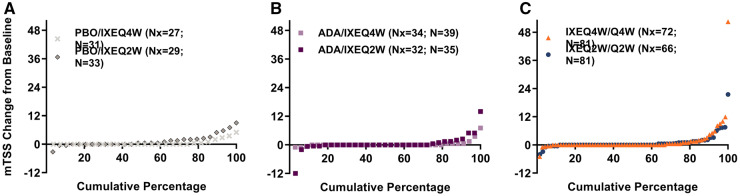
Over the 156-week period, mTSS mean (s.d.) change from baseline was similar with IXEQ2W [1.0 (3.2)] and IXEQ4W [1.1 (5.0)] (Table 3) and was minimal across the four treatment assignment groups (Supplementary Table S5, available at Rheumatology online). Cumulative probability plots show observed patient-level data of mTSS change from baseline to week 156 (Fig. 3). The percentage without radiographic progression at week 156 (mTSS ≤0) was numerically smaller with total IXEQ2W (61%) than total IXEQ4W (71%) (Table 3). A similar pattern was observed in patients with mTSS ≤0.5 (total IXEQ2W: 69%; total IXEQ4W: 79%) and with mTSS ≤1.85 (total IXEQ2W: 81%; total IXEQ4W: 87%).
Table 3.
Progression of structural joint damage (long-term extension period population)
| Week 52 |
Week 108 |
Week 156 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IXEQ4W /Q4W (N = 81) | IXEQ2W /Q2W (N = 81) | Total IXEQ4W (N = 151) | Total IXEQ2W (N = 149) | IXEQ4W/ Q4W (N = 81) | IXEQ2W/ Q2W (N = 81) | Total IXEQ4W (N = 151) | Total IXEQ2W (N = 149) | IXEQ4W/ Q4W (N = 81) | IXEQ2W /Q2W (N = 81) | Total IXEQ4W (N = 151) | Total IXEQ2W (N = 149) | ||
| mTSS change from baseline* | |||||||||||||
| Linear Extrap. | Mx | 72 | 65 | 132 | 126 | 72 | 66 | 133 | 127 | 72 | 66 | 133 | 127 |
| Mean | 0.6 | 0.3 | 0.4 | 0.3 | 1.1 | 0.6 | 0.8 | 0.7 | 1.7 | 1.0 | 1.1 | 1.0 | |
| s.d. | 2.3 | 1.5 | 1.8 | 1.9 | 4.1 | 2.1 | 3.1 | 2.5 | 6.6 | 3.2 | 5.0 | 3.2 | |
| mTSS cut-offs a | |||||||||||||
| 0 | Mx | 72 | 65 | 132 | 126 | 72 | 66 | 133 | 127 | 72 | 66 | 133 | 127 |
| % | 75 | 75 | 82 | 71 | 68 | 73 | 74 | 67 | 67 | 62 | 71 | 61 | |
| 0.5 | Mx | 72 | 65 | 132 | 126 | 72 | 66 | 133 | 127 | 72 | 66 | 133 | 127 |
| % | 82 | 82 | 87 | 79 | 74 | 83 | 80 | 77 | 74 | 70 | 79 | 69 | |
| 1.85b | Mx | 72 | 65 | 132 | 126 | 72 | 66 | 133 | 127 | 72 | 66 | 133 | 127 |
| % | 89 | 94 | 92 | 90 | 86 | 91 | 89 | 86 | 83 | 85 | 87 | 81 | |
The last visit of the long-term extension period was week 156. The IXEQ4W/Q4W group includes patients who received IXEQ4W during the 156-week treatment period; IXEQ2W/Q2W group includes patients who received IXEQ2W during the 156-week treatment period; the total IXEQ2W group includes patients who received IXEQ2W at any point during the study (regardless of initial treatment assignment); and the total IXEQ4W group includes patients who received IXEQ4W at any point during the study (regardless of initial treatment assignment).
Missing mTSS data were imputed using linear extrapolation if patients had a baseline and at least one post-baseline value (i.e. week 52, 108 or 156). At week 52, 0% were imputed; at week 108, 1.6% (n = 4) were imputed; and at week 156, 10.8% (n = 28) were imputed using linear extrapolation.
Smallest detectable change for the overall 3-year time period.
IXEQ4W/Q2W: 80 mg ixekizumab once every 4 weeks/2 weeks; Linear Extrap: linear extrapolation; mTSS: van der Heijde modified total Sharp score; Mx: number of patients with non-missing data after linear extrapolation.
Fig. 3.
Cumulative probability plots for mTSS change from baseline to week 156
Patients are from the long-term extension period population. Missing data were linearly extrapolated if patients had a baseline and ≥1 post-baseline value (i.e. week 52, 108 or 156). At week 52, 0% were imputed, at week 108 1.6% (n = 4) were imputed, and at week 156 10.8% (n = 28) were imputed using linear extrapolation. ADA: adalimumab; IXEQ4W/Q2W: 80 mg ixekizumab every 4 weeks/2 weeks; mTSS: van der Heijde modified total Sharp score; N: total patient number in the long-term extension period population; Nx: number of patients with non-missing change from baseline data after linear extrapolation; PBO: placebo.
For patients with baseline psoriasis ≥3% body surface area, PASI 75, 90 and 100 response rates persisted over the extension to week 156 (Table 2, mNRI; Fig. 1D–F). At week 156, 69.1% of IXEQ2W- and 63.5% of IXEQ4W-treated patients reached PASI 75, 64.5% of IXEQ2W and 51.2% of IXEQ4W-treated patients reached PASI 90 and 60.5% of IXEQ2W- and 43.6% of IXEQ4W-treated patients reached PASI 100 (Table 2, mNRI). Among patients with fingernail psoriasis, both IXEQ2W and IXEQ4W demonstrated reductions from baseline on the NAPSI that persisted through week 156 (Table 2; Fig. 2G). At week 156, 57.1% of IXEQ2W- and 53.6% of IXEQ4W-treated patients achieved complete resolution of fingernail psoriasis (Table 2, mNRI; Fig. 2H).
At week 156, the SF-36 PCS and MCS change from baseline were similar with IXEQ2W (8.3 and 3.8, respectively) and IXEQ4W (7.9 and 3.5, respectively) [Table 2, modified baseline observation carried forward (mBOCF)]. The individual domains of the SF-36 are shown in Supplementary Table S6, available at Rheumatology online (mBOCF).
Discussion
In bDMARD-naïve PsA patients, ixekizumab demonstrated early clinical improvement (i.e. response rates vs placebo as early as week 1 for ACR20, week 4 for ACR50 and week 8 for ACR70) [5]. The clinical improvements largely persisted throughout 3 years of ixekizumab treatment without incremental safety risks.
Long-term studies are important for evaluating the enduring benefits and safety risks of therapies for chronic, progressive diseases, such as PsA. These results show that the efficacy and safety profile of ixekizumab remains stable in bDMARD-naïve PsA patients. The safety results show consistency with previous studies of ixekizumab in the overall incidence of TEAEs in addition to AEs of special interest (including infections, hepatic events, allergic reactions/hypersensitivity events, cerebrocardiovascular events, depression and malignancies) over time despite increased exposure with continuous treatment [5–7, 24–27]. Previously described early improvements in signs and symptoms of PsA across all domains, including low rates of radiographic progression and improvements in patient-reported outcomes, persisted with ≤3 years of ixekizumab treatment [5, 7].
With long-term data, it is important to assess whether sustained inhibition of specific targeted cytokines increases the risk for certain AEs. Individuals with deficiencies in Th17 cells, IL-17RA or IL-17F may have increased susceptibility to infections, such as candidiasis [28, 29]. Thus, treatment with an IL-17 inhibitor, such as ixekizumab, secukinumab or brodalumab, may predispose PsA patients to infections [24, 25, 30, 31]. However, throughout a 156-week treatment period, the types of infections reported were as expected, with no increase in the incidence of TEAEs. Candida was reported in 11 patients, generally mild in severity and successfully managed in all but one patient during the study. No active or reactivated tuberculosis cases were reported. Pneumonia was the only serious infection reported in at least one patient (n = 2); other serious infections (n = 1 each) were chronic tonsillitis, herpes zoster, oesophageal candidiasis, bacterial arthritis, cellulitis, gastroenteritis clostridial, gastroenteritis rotavirus, latent tuberculosis, lower respiratory tract infection, mycoplasma pneumonia and upper respiratory tract infection (Supplementary Table S3, available at Rheumatology online).
ISRs have been noted with some biologic treatments [32, 33]. Here, ISRs were generally mild to moderate in severity (investigator assessed), lessened over time and did not result in discontinuation. Hypersensitivity reactions, such as skin rash, pruritus, urticaria, angioedema and anaphylactic reaction, may be associated with an immune system response that may or may not occur immediately after drug administration. In this study, 31 patients reported allergic reactions/hypersensitivity events, of which one was a case of potential anaphylaxis.
Among psoriasis patients, an increased incidence of IBD [34–37] and increased or worsened IBD after treatment with an IL-17 inhibitor have been demonstrated [38, 39]. An integrated analysis of 4209 ixekizumab-treated patients (6480 patient-years of exposure) reported that IBD was uncommon (<1%) [26]. Here, one case of ulcerative colitis was reported. Of note, this study may not represent the full population of PsA at risk of IBD because of the controversial role of IL-17 in IBD. We did not exclude patients with a history of IBD; only those with active IBD were excluded.
The development of TE-ADA was generally infrequent; the majority were of low titre and transient. There was no apparent association between TE-ADA development and efficacy, nor was there an association with ISRs or allergic reactions/hypersensitivity events.
Clinical efficacy, as assessed by ACR response, PASI response and improvement in key clinical domains for PsA, including improvement in enthesitis and dactylitis, persisted to week 156. Improvement in patient-reported outcomes and HRQoL also persisted to week 156.
PsA is a debilitating disorder, and structural joint damage, which is likely to result in irreversible joint deformity and disability, is present in 47% of patients within 2 years of disease onset [40, 41]. Low rates of progression of radiographic joint damage was observed throughout a 3-year period in all groups (Fig. 3; Table 3; Supplementary Table S5, available at Rheumatology online). The majority did not have radiographic progression beyond the smallest detectable change, with IXEQ2W and IXEQ4W showing a similar pattern overall. During ixekizumab treatment, low rates of structural joint damage progression were observed.
This study did not have a placebo or active control group beyond week 24, was not powered to compare ixekizumab dosing regimens and was limited to bDMARD-naïve patients. Additionally, drop-out rate and imputation with non-response should be considered when evaluating efficacy over 3 years.
Ixekizumab demonstrates early and persistent clinical improvement without incremental safety risks. Data from this 3-year study support the use of ixekizumab in PsA patients based on a consistent safety profile and stable, persistent efficacy across all disease domains.
Supplementary Material
Acknowledgements
The authors thank the patients, investigators and study staff who made this study possible. We also thank Matthew Hufford, PhD, Eli Lilly and Company, for his review and input on manuscript content; Kelly Guerrettaz, MA, Syneos Health, for writing support; and Teri Tucker, MA and Sarah Becker-Marrero, MA, Syneos Health, for editorial support. Some of the data within this manuscript have been presented at The European League Against Rheumatism (EULAR) conference (13–16 June 2018). Chandran V, Fleischmann R, Lespessailles E, et al. THU0333 Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: three year results from a phase 3 study (SPIRIT-P1). Ann Rheum Dis 2018; 77: 385. V.C. and R.M.F. contributed to acquisition, analysis and interpretation of the data. P.S.H. contributed to design and acquisition, analysis and interpretation of the data. J.S.E. and S.S.R. contributed to analysis and interpretation of the data. C.L.S. contributed to conception, design and interpretation of the data. E.L. contributed to the design, analysis and interpretation of the data. D.H., H.K., R.B.-V., J.A.B., A.T.S. and G.G. contributed to interpretation of the data. All authors revised the manuscript and read and approved the final version of the document. Lilly provides access to relevant anonymized patient-level data from studies on approved medicines and indications as defined by the sponsor-specific information at www.clinicalstudydatarequest.com. Additional study-related documents will be made available, including the study protocol, statistical analysis plan, clinical study report, and an annotated case report form. These materials will be available beginning 6 months after the publication is accepted, given approval of the indication in the US and EU. These materials will be provided to achieve the aims in the provided proposal.
Funding: This work was supported by funding provided by Eli Lilly and Company
Disclosure statement: V.C. reports grants from Eli Lilly and Company during the conduct of the study; personal fees from Amgen, grants and personal fees from Abbvie, personal fees from Bristol-Myers Squibb (BMS), personal fees from Celgene, personal fees and other from Eli Lilly, personal fees from Janssen, personal fees from Novartis, personal fees from Pfizer and personal fees from UCB, outside the submitted work. D.H. reports consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Eli-Lilly, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB, and is Director of Imaging Rheumatology bv. R.M.F. reports grants and personal fees from AbbVie, grants and personal fees from Amgen, grants and personal fees from BMS, grants and personal fees from Pfizer and grants from UCB, outside the submitted work. E.L. has received honoraria and travel grants from Amgen, Expanscience, Eli Lilly and Company, Novartis, MSD and UCB and has received research grants from Celgene, MSD and UCB. P.S.H. reports grants and research support paid to charity from Novartis, AbbVie and Janssen, honoraria or consulting fees paid to charity from AbbVie, Amgen, Pfizer and UCB, and honoraria or consulting fees paid to self from Celgene and Galapagos. H.K. reports personal fees from AbbVie, Asahikasei Pharma, Astellas, Bristol-Myers Squibb, Chugai, Eli Lilly and Company, Janssen, Mitsubishi Tanabe Pharma, Novartis and Pfizer, and grants from AbbVie, Asahikasei Pharma, Astellas, Chugai, Eisai, Mitsubishi Tanabe Pharma and Novartis. J.S.E., S.S.R., A.T.S., G.G. and J.A.B. are employees and/or stockholders of Eli Lilly and Company. C.L.S. is a former employee of Eli Lilly and Company. The other author has declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. Erratum in: N Engl J Med 2017;376:2097. [DOI] [PubMed] [Google Scholar]
- 2. Kavanaugh A, Helliwell P, Ritchlin CT.. Psoriatic arthritis and burden of disease: patient perspectives from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Rheumatol Ther 2016;3:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Lu J, Allan BW. et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res 2016;9:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakkas LI, Bogdanos DP.. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev 2017;16:10–5. [DOI] [PubMed] [Google Scholar]
- 5. Mease PJ, van der Heijde D, Ritchlin CT. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gottlieb AB, Papp KA, Birbara CA, Shuler CL. et al. Effect of psoriatic arthritis on ixekizumab clinical outcomes in moderate-to-severe psoriasis patients: a post hoc analysis. J Am Acad Dermatol 2018;79:593–5. [DOI] [PubMed] [Google Scholar]
- 7. van der Heijde D, Gladman DD, Kishimoto M. et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol 2018;45:367–77. Erratum in: J Rheumatol 2018;45:1608. [DOI] [PubMed] [Google Scholar]
- 8.American College of Rheumatology Committee to Reevaluate Improvement Criteria. A proposed revision to the ACR20: the hybrid measure of American College of Rheumatology response. Arthritis Rheum 2007;57:193–202. Erratum in: Arthritis Rheum 2007;57:1574. [DOI] [PubMed] [Google Scholar]
- 9. Fredriksson T, Pettersson U.. Severe psoriasis – oral therapy with a new retinoid. Dermatologica 1978;157:238–44. [DOI] [PubMed] [Google Scholar]
- 10.National Psoriasis Foundation. The psoriasis and psoriatic arthritis pocket guide. Portland, OR: National Psoriasis Foundation, 2009. [Google Scholar]
- 11. Coates LC, Fransen J, Helliwell PS.. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 12. Coates LC, Lubrano E, Perrotta FM. et al. What should be the primary target of “treat to target” in psoriatic arthritis? J Rheumatol 2019;46:38–42. [DOI] [PubMed] [Google Scholar]
- 13. Schoels M, Aletaha D, Funovits J. et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. [DOI] [PubMed] [Google Scholar]
- 14. Coates LC, Helliwell PS.. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheum 2016;43:371–5. [DOI] [PubMed] [Google Scholar]
- 15. Healy PJ, Helliwell PS.. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 16. Helliwell PS, Firth J, Ibrahim GH. et al. Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J Rheumatol 2005;32:1745–50. [PubMed] [Google Scholar]
- 17. Mease PJ, Woolley JM, Bitman B. et al. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol 2011;38:2461–5. [DOI] [PubMed] [Google Scholar]
- 18.SF-36v2 Health Survey [Internet]. Eden Prairie: Optum, Inc.; c2019 (updated 2019). https://campaign.optum.com/optum-outcomes/what-we-do/health-surveys/sf-36v2-health-survey.html (12 March 2019, date last accessed).
- 19.EuroQol Research Foundation. EQ-5D Instruments (updated 2017). https://euroqol.org/eq-5d-instruments/ (12 March 2019, date last accessed).
- 20.Reilly Associates. WPAI General Information (updated 2013). http://www.reillyassociates.net/WPAI_General.html (12 March 2019, date last accessed).
- 21. van der Heijde D, Sharp J, Wassenberg S, Gladman DD.. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64(Suppl 2):ii61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ware JE, Kosinski M, Dewey JE.. How to score version 2 of the SF-36© health survey (standard and acute forms). Lincoln, RI: QualityMetric Incorporated, 2000. [Google Scholar]
- 23. Bruynesteyn K, Boers M, Kostense P, van der Linden S, van der Heijde D.. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis 2005;64:179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Papp KA, Bachelez H, Blauvelt A. et al. Infections from seven clinical trials of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriasis. Br J Dermatol 2017;177:1537–51. [DOI] [PubMed] [Google Scholar]
- 25. Strober B, Leonardi C, Papp KA. et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol 2017;76:432–40.e17. [DOI] [PubMed] [Google Scholar]
- 26. Reich K, Leonardi C, Langley RG. et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: a presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol 2017;76:441–8.e2. [DOI] [PubMed] [Google Scholar]
- 27. Nash P, Kirkham B, Okada M. et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017;389:2317–27. [DOI] [PubMed] [Google Scholar]
- 28. Cypowyj S, Picard C, Maródi L, Casanova JL, Puel A.. Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol 2012;42:2246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J, Casanova JL, Puel A.. Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunol 2018;11:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saunte DM, Mrowietz U, Puig L, Zachariae C.. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol 2017;177:47–62. [DOI] [PubMed] [Google Scholar]
- 31. van de Kerkhof PC, Griffiths CE, Reich K. et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol 2016;75:83–98.e4. [DOI] [PubMed] [Google Scholar]
- 32.Enbrel (etanercept) [package insert]. Thousand Oaks, CA: Amgen, 2016.
- 33. Shear NH, Paul C, Blauvelt A. et al. Safety and tolerability of ixekizumab: integrated analysis of injection-site reactions from 11 clinical trials. J Drugs Dermatol 2018;17:200–6. [PubMed] [Google Scholar]
- 34. Skroza N, Proietti I, Pampena R. et al. Correlations between psoriasis and inflammatory bowel diseases. Biomed Res Int 2013;2013:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ouyang W, Kolls JK, Zheng Y.. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen AD, Dreiher J, Birkenfeld S.. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol 2009;23:561–5. [DOI] [PubMed] [Google Scholar]
- 37. Egeberg A, Mallbris L, Warren RB. et al. Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study. Br J Dermatol 2016;175:487–92. [DOI] [PubMed] [Google Scholar]
- 38. Hueber W, Sands BE, Lewitzky S. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Targan SR, Feagan BG, Vermeire S. et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol 2016;111:1599–607. [DOI] [PubMed] [Google Scholar]
- 40. McArdle A, Flatley B, Pennington SR, FitzGerald O.. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther 2015;17:141.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kane D, Stafford L, Bresnihan B, FitzGerald O.. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology 2003;42:1460–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.