Abstract
Background
Metabolic syndrome is identified as a risk factor for the development of several systemic cancers, but its frequency among patients with glioblastoma and its association with clinical outcomes have yet to be determined. The aim of this study was to investigate metabolic syndrome as a risk factor for and affecting survival in glioblastoma patients.
Methods
A retrospective cohort study, consisting of patients with diagnoses at a single institution between 2007 and 2013, was conducted. Clinical records were reviewed, and clinical and laboratory data pertaining to 5 metabolic criteria were extrapolated. Overall survival was determined by time from initial surgical diagnosis to date of death or last follow-up.
Results
The frequency of metabolic syndrome among patients diagnosed with glioblastoma was slightly greater than the frequency of metabolic syndrome among the general population. Within a subset of patients (n = 91) receiving the full schedule of concurrent radiation and temozolomide and adjuvant temozolomide, median overall survival was significantly shorter for patients with metabolic syndrome compared with those without. In addition, the presence of all 5 elements of the metabolic syndrome resulted in significantly decreased median survival in these patients.
Conclusions
We identified the metabolic syndrome at a slightly higher frequency in patients with diagnosed glioblastoma compared with the general population. In addition, metabolic syndrome with each of its individual components is associated with an overall worse prognosis in patients receiving the standard schedule of radiation and temozolomide after adjustment for age.
Keywords: glioblastoma, hyperglycemia, metabolic syndrome, obesity, survival
Metabolic syndrome (MetS) is a well-established risk factor for nonneoplastic disorders such as type 2 diabetes mellitus and atherosclerotic cardiovascular disease.1 It is also identified as a risk factor for systemic cancers, notably breast and prostate cancer.2–5 In addition, there is evidence that MetS is associated with a more aggressive tumor biology and worse outcome in systemic cancers.6,7 The frequency of MetS and the association of MetS with clinical outcome in glioblastoma (GBM) patients have not yet been determined. We retrospectively reviewed the clinical and outcome data of newly diagnosed GBM (nGBM) adult patients at our center to determine the frequency of MetS and to compare the clinical outcome of patients with and without MetS.
Methods
We retrospectively reviewed the clinical records of all patients (N = 146) with nGBM at University Hospitals Cleveland Medical Center (UHCMC) Seidman Cancer Center from 2007 to 2013 enrolled in the Ohio Brain Tumor Study, an institutional review board‒approved clinical and tissue procurement study, with data updated through 2016.8 Clinical and laboratory criteria for the diagnosis of MetS were based on the consensus report by Alberti et al1 and included ≥3 of the following: hyperglycemia, hypertension, elevated triglycerides, reduced high-density lipoprotein C, and obesity. The criteria are identified as follows: for patients in whom fasting blood sugar levels were not available, hyperglycemia was determined by a history of diabetes and/or drug treatment of elevated glucose. Hypertension was determined by systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg or drug treatment for hypertension and a history of hypertension. Hyperlipidemia was identified by elevated triglycerides (≥150 mg/dL) and reduced high-density lipoprotein C (HDLC) (<40 mg/dL in males, <50 mg/dL in females) or drug treatment for elevated triglycerides or reduced HDLC. The criteria include a definition of obesity based on waist circumference which was not available in the medical records, and we substituted body mass index (BMI) ≥30 kg/m2, which is an accurate surrogate for waist circumference.9–11 Criteria were obtained prior to the diagnosis of GBM and the administration of steroids. Time to progression (TTP) and overall survival (OS) were determined by the interval from initial surgical diagnosis to imaging progression and to date of death or last follow-up.
Characteristics of nGBM patients with and without MetS were compared using chi-square tests or Fisher’s exact test for categorical variables. Differences in age were compared using t-tests, and Karnofsky performance status (KPS) scores were compared using the Wilcoxon rank sum test. Kaplan–Meier OS analysis stratified by MetS status (and by individual MetS factors) was performed generating median OS times, in months, with 95% CIs and log-rank tests, overall and for those who received standard therapy (surgery plus concurrent radiation and temozolomide and adjuvant temozolomide). In addition, adjusted median OS times with 95% CIs were generated, adjusting for age at diagnosis. Statistical significance was set at a P-value of 0.05.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review board at our institution. Informed consent was obtained from all participants.
Results
Table 1 shows the clinical characteristics, including prognostic factors of age, postoperative KPS, degree of resection, isocitrate dehydrogenase (IDH) mutational status, O6-methylguanine-DNA methyltransferase (MGMT) methylation, and treatment with radiation or radiation and temozolomide. Temozolomide was administered by the Stupp and Weber standard regimen.12 MetS was identified in 52 out of 146 total patients (35.6%), 38 men and 14 women. The median age in patients with MetS was not significantly different than that of patients without MetS (66.2 and 63.1 y, respectively, P = 0.1391).
Table 1.
Characteristics of nGBM patients with and without metabolic syndrome
| Overall | With MetS | Without MetS | P-valuea | ||||
|---|---|---|---|---|---|---|---|
| N = 146 | % | n = 52 | % | n = 94 | % | ||
| Treatment groups | 0.1983b | ||||||
| Surgery only | 31 | 21.2 | 14 | 26.9 | 17 | 18.1 | |
| Surgery + radiation only | 23 | 15.8 | 6 | 11.5 | 17 | 18.1 | |
| Surgery, concurrent radiation + temozolomide | 18 | 12.3 | 9 | 17.3 | 9 | 9.6 | |
| Surgery, concurrent radiation + temozolomide, and adjuvant temozolomide | 73 | 50.0 | 23 | 44.2 | 50 | 53.2 | |
| Extent of resection | 0.5324 | ||||||
| Biopsy | 11 | 7.5 | 5 | 9.6 | 6 | 6.4 | |
| Subtotal resection | 62 | 42.5 | 24 | 46.2 | 38 | 40.4 | |
| Gross total resection | 73 | 50.0 | 23 | 44.2 | 50 | 53.2 | |
| Sex | 0.1859 | ||||||
| Male | 89 | 61.0 | 38 | 73.1 | 51 | 54.3 | |
| Female | 57 | 39.0 | 14 | 26.9 | 43 | 45.7 | |
| Race | 0.2668 | ||||||
| White | 134 | 91.8 | 49 | 94.2 | 85 | 90.4 | |
| Nonwhite | 12 | 8.2 | 3 | 5.8 | 9 | 9.6 | |
| IDH1/2 mutation | 0.1899b | ||||||
| Wild type | 43 | 29.5 | 15 | 28.8 | 28 | 29.9 | |
| Mutant | 6 | 4.1 | 4 | 7.7 | 2 | 2.1 | |
| Not tested | 97 | 66.4 | 33 | 63.5 | 64 | 68.0 | |
| MGMT promoter methylation | 1.0000 b | ||||||
| Methylated | 19 | 13.0 | 7 | 13.5 | 12 | 12.8 | |
| Unmethylated | 16 | 11.0 | 5 | 9.6 | 11 | 11.7 | |
| Not tested | 109 | 74.7 | 40 | 76.9 | 69 | 73.4 | |
| Elevated blood sugar | 5.68 × 10−10 | ||||||
| Elevated | 40 | 27.4 | 30 | 58.8 | 10 | 10.6 | |
| Not elevated | 105 | 71.9 | 21 | 41.2 | 84 | 89.4 | |
| Missing data | 1 | 0.6 | – | – | – | – | |
| Hypertension | 1.21 × 10−8 b | ||||||
| Hypertensive | 92 | 63.0 | 48 | 92.3 | 44 | 46.8 | |
| Not hypertensive | 54 | 37.0 | 4 | 7.7 | 50 | 53.2 | |
| Missing data | 0 | 0.0 | – | – | – | – | |
| Triglycerides | 4.95 × 10−17 | ||||||
| Increased triglycerides | 52 | 35.6 | 42 | 80.8 | 10 | 10.9 | |
| Triglycerides not increased | 92 | 63.0 | 10 | 19.2 | 82 | 89.1 | |
| Missing data | 2 | 1.4 | – | – | – | – | |
| HDLC | 2.10 × 10−17 | ||||||
| Decreased HDLC | 57 | 39.0 | 44 | 88.0 | 13 | 14.4 | |
| No decreased HDLC | 83 | 56.8 | 6 | 12.0 | 77 | 85.6 | |
| Missing data | 6 | 4.1 | – | – | – | – | |
| Obesity | 4.17 × 10−6 | ||||||
| Obese | 44 | 30.1 | 28 | 53.8 | 16 | 17.0 | |
| Not obese | 101 | 69.2 | 24 | 46.2 | 77 | 81.9 | |
| Missing data | 1 | 0.7 | – | – | 1 | 0.1 | |
| Age, y, mean | 64.2 | 66.2 | 63.1 | 0.1391 | |||
| Postoperative KPS (median) | 70 | 70 | 70 | 0.1687c | |||
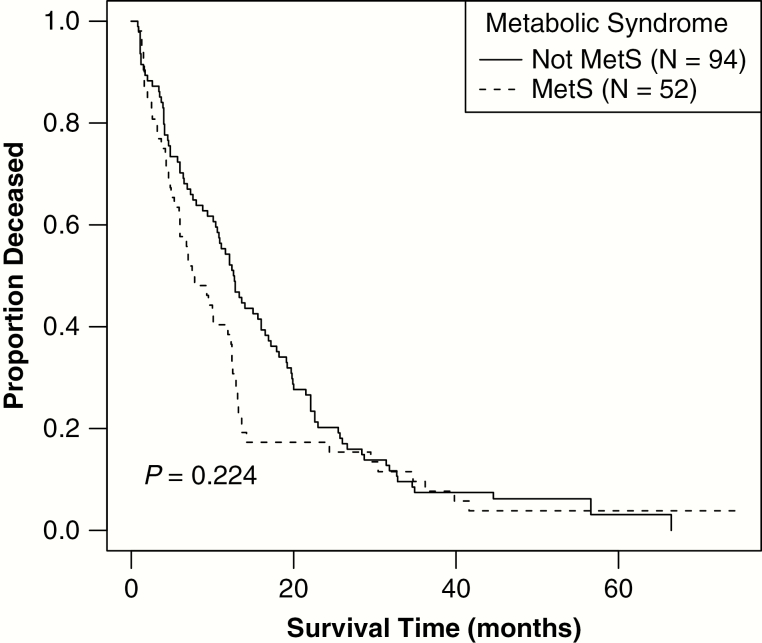
| Median survival, mo (95% CI) | 11.3 (9.3–12.8) | 7.7 (5.9–12.4) | 12.70 (10.8–16.9) | 0.224 | |||
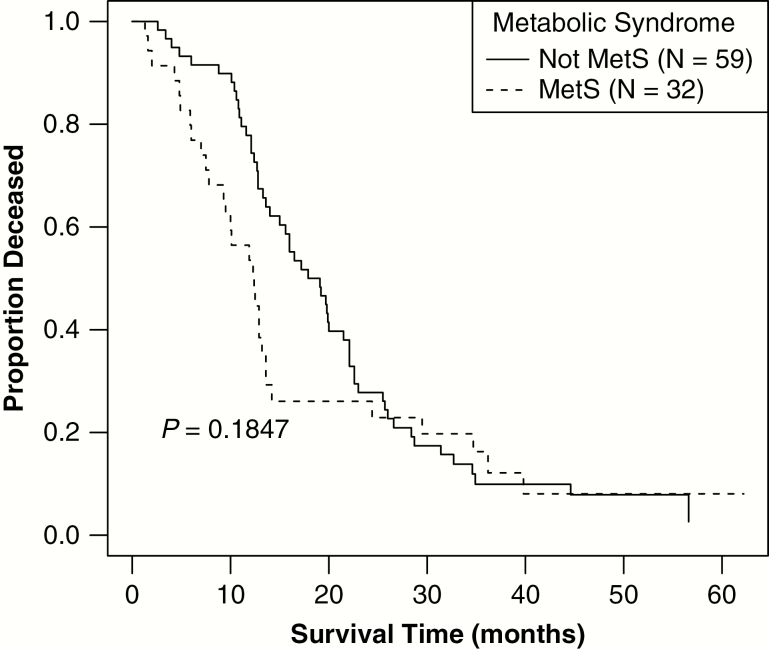
| Median survival [surgery, radiation + temozolomide only, adjusted for age], mo (95% CI) | 14.0 (12.8–19.7) | 12.4 (9.5–14.2) | 17.9 (15.0–22.1) | 0.1847d | |||
| Median TTP, mo (95% CI) | 11.0 (10.0–13.4) | 11.8 (8–..) | 11.0 (10.0–13.4) | 0.347 | |||
| Median TTP [surgery, radiation + temozolomide only, adjusted for age], mo (95% CI) | 10.3 (8.6–13.4) | 10.3 (8.0–..) | 11.0 (9.3–13.4) | 0.7000 d | |||
aTest of significance between MetS groups.
bFisher’s exact test.
cWilcoxon rank sum test.
d P-value for trait in Cox proportional hazards model adjusted for age at diagnosis.
**Confidence interval (CI) cannot be calculated.
Median OS in the study population was 11.3 months (95% CI = 9.3–12.8). Although the difference in median OS was not statistically significant in those with MetS compared with those without MetS, there was a trend toward decreased survival in those with MetS (7.7 mo [95% CI = 5.9–12.4]) compared with those without MetS (12.7 mo [95% CI = 10.8–16.9]) (log rank P = 0.224) (Fig. 1). An analysis was conducted of the subset of patients that received the full schedule of radiation and concurrent and adjuvant temozolomide (n = 91; 61.1% of all patients) using a Cox proportional hazards model adjusted for age at diagnosis. Median OS was significantly shorter for those with MetS (12.4 mo, 95% CI = 9.5–14.2) versus those without MetS (17.9 mo, 95% CI = 15.0–22.1) (log-rank P = 0.18) (Fig. 2). When survival models were additionally adjusted for whether patients underwent gross total resection, this factor was associated with decreased hazard of death but was not statistically significant (P = 0.62). The number of tumor tissues studied for MGMT methylation and IDH mutation was small and we were thus unable to analyze those as factors in outcome. There was no significant association between MetS and TTP (median TTP was 11.8 mo in those with MetS and 11.0 mo in those without) or between individual elements of MetS with TTP (Table 1).
Fig. 1.
Overall survival time based upon MetS status. Kaplan–Meier curve stratified by MetS status depicts that OS time, in months, did not vary significantly based upon MetS status (median OS for patients with MetS = 7.7 mo, 95% CI: 5.9–12.4, vs median OS in patients without MetS = 12.7 mo, 95% CI: 10.8–16.9, log rank P = 0.22).
Fig. 2.
Age-adjusted survival status for patients receiving concurrent radiation and temozolomide (N = 91). A Kaplan–Meier curve stratified by MetS status and adjusted for age at diagnosis depicts OS status, in months, for patients receiving concurrent radiation and temozolomide and adjuvant temozolomide. Patients with MetS had a significantly shorter median OS (12.4 mo, 95% CI: 9.5–14.2) compared with patients without MetS (17.9 mo, 95% CI: 15.0–22.1, P = 0.18).
Table 1 shows the frequency of individual MetS elements and other prognostic variables. Though MetS data were not routinely assessed at clinic visits, very few MetS data elements were missing from electronic health records. The most common element of MetS we identified was hypertension (63.0% of patient population). Within the subset of patients receiving standard of care and adjusted for age at diagnosis, the presence of all 5 MetS criteria resulted in significantly decreased median OS (Table 2).
Table 2.
Frequency of nGBM patient population with metabolic syndrome factors (N = 146) and correlation of MetS diagnostic criteria with OS in patients with and without nGBM
| All Individuals | Individuals Who Received Surgery, Concurrent Radiation, and Temozolomide | |||||
|---|---|---|---|---|---|---|
| N | Median survival, mo (adjusted for age) (95% CI) | P valuea | N | Median survival, mo (adjusted for age) (95% CI) | P-valuea | |
| Elevated blood sugar | ||||||
| Elevated blood sugar | 40 | 7.2 (4.3–12.4) | 0.0976 | 23 | 11.9 (9.3–14.2) | 0.0298 |
| Blood sugar not elevated | 105 | 12.6 (10.4–15.6) | 67 | 17.2 (13.6–22.1) | ||
| Hypertension | ||||||
| Hypertensive | 92 | 7.4 (5.9–11.1) | 0.001 | 49 | 12.9 (11.9–15.6) | 0.1945 |
| Not hypertensive | 54 | 18.2 (13.6–22.1) | 42 | 19.7 (16.0–23.0) | ||
| Triglycerides | ||||||
| Increased triglycerides | 52 | 7.0 (5.3–12.4) | 0.0640 | 31 | 12.4 (9.3–14.2) | 0.0945 |
| Triglycerides not increased | 92 | 12.9 (11.1–16.9) | 59 | 17.9 (14.0–22.1) | ||
| HDLC | ||||||
| Decreased HDLC | 57 | 9.4 (6.0–12.9) | 0.8840 | 33 | 13.2 (12.4–25.7) | 0.9633 |
| No decreased HDLC | 83 | 12.4 (10.4–16.0) | 54 | 16.0 (13.3–19.9) | ||
| Obesity | ||||||
| Obese | 44 | 11.5 (7.8–13.6) | 0.7600 | 30 | 12.9 (11.1–19.7) | 0.1763 |
| Not obese | 101 | 11.6 (7.5–13.3) | 60 | 15.0 (12.8–22.1) | ||
a P-value for trait in Cox proportional hazards model adjusted for age at diagnosis.
Discussion
A variety of criteria to diagnose MetS are proposed. We used the criteria recommended by the multidisciplinary task force of Alberti and colleagues,1 except that we used BMI (patient weight divided by height) as a surrogate measure of obesity because waist circumference data were not available. We required that MetS elements be identified prior to the diagnosis of GBM because nGBM patients are typically treated pre- and postoperatively with a corticosteroid to reduce vasogenic edema. Corticosteroids can increase blood pressure, serum glucose, and, over time, BMI, resulting in a patient meeting diagnostic criteria that are due to an extrinsic pharmacologic effect.
In this retrospective review, the frequency of MetS in nGBM patients was 35.6%, slightly greater than the national prevalence of 34.2% as documented based on an analysis of NHANES (National Health and Nutrition Examination Survey) data from 2007‒2012.13 However, our study was conducted in Ohio, which has a slightly higher prevalence of diabetes (in 2013, Ohio = 10.4% vs US = 9.7%), of overweight or obesity (in 2013, Ohio = 65.1% vs US = 64.3%), and of hypertension (in 2013, Ohio = 33.5% vs US = 31.4%).14
No other published report details the frequency of MetS and its impact on survival in GBM, though individual elements of MetS in relation to the development of GBM have been studied by others. Obesity in particular has been studied as a potential risk factor in glioma development. Several meta-analyses have produced conflicting results. Selected studies included in these meta-analyses are summarized in Table 3. Niedermaier and colleagues conducted a systematic review and meta-analysis of adiposity and physical activity and their relation to glioma.15 Elevated BMI was not found to be associated with increased risk of glioma. Sergentanis and colleagues reported that while elevated BMI was associated with an increased risk of glioma in females, the relationship was not statistically significant in males.16 Dai and colleagues determined that obesity was an overall risk factor in the development of glioma,17 whereas in a large prospective study by Wiedemann obesity was not associated with risk for development of any glioma subtype.18
Table 3.
A review of selected studies and meta-analyses examining obesity as a risk factor for the development of GBM
| Reference | Study Location and Time | Subjects | Risk Estimate (95% CI) | Included in Dai et al13? | Included in Niedermaier et al31? | Included in Sergentanis et al34? |
|---|---|---|---|---|---|---|
| Benson et al (2008)33 | United Kingdom 1996–2001 | N = 646 women with diagnosis of glioma and 1 249 670 population controls | 1.07 (0.84–1.34)1 | X | X | X |
| Cabaniols et al (2011)34 | France 2005 | N = 122 men and women with diagnosis of glioma and 122 hospital controls | 0.70 (0.41–1.18)2 | X | X | |
| Edlinger et al (2012)28 | Sweden, Austria, and Norway 1972–2006 | N = 436 men and women with diagnosis of high-grade glioma and 580 000 population controls | 1.03 (0.85–1.26)3 | X | ||
| Jones et al (2010)29 | United States, 1991–2008 | N = 1259 men and women with diagnosis of glioblastoma multiforme | 1.08 (0.91 –1.28)3 | X | ||
| Little et al (2013)35 | United States 2004–2012 | N = 1111 men and women with diagnosis of glioma and 1096 community controls | 1.06 (0.70–1.60)2 | X | ||
| Michaud et al (2011)36 | Europe 1992–2000 | N = 340 men and women with diagnosis of glioma and 380 775 community controls | 1.06 (0.76–1.48)3 | X | X | X |
| Moore et al (2009)37 | United States 1995–2003 | N = 257 men and women with diagnosis of glioma and 305 681 controls | 1.29 (0.89–1.86)1 | X | X | X |
| Siegel et al (2003)16 | United States 2005–2012 | N = 853 men and women with diagnosis of high-grade glioma | 1.24 (1.00–1.54)3 | X | ||
| Wiedmann et al (2017)38 | Norway 1984–2008 | N = 148 men and women with diagnosis of glioma and 74 242 population controls | 1.04 (0.58–1.85)3 | X | X |
1RR, relative risk; 2OR, odds ratio; 3HR, hazard ratio.
Hyperglycemia, hypertension, and dyslipidemia have also been studied as individual risk factors for glioma. Two retrospective case-control studies found no association between diabetes, hyperlipidemia, and risk for developing GBM.19,20 Another case-control study found an inverse association between long-term diabetes, chronic hyperglycemia, and glioma risk.21 Differing results were found in a prospective study conducted by Edlinger and colleagues that included 580 000 individuals, 1312 of whom had diagnoses of primary brain tumor over the course of 10 years.22 Increased diastolic blood pressure and triglycerides were found to be associated with increased risk of brain tumor, including high-grade glioma. An additional case-control study found a significantly higher prevalence of hypertension in glioma patients age 60 years and older compared with all other cancer patients.23
Individual elements of MetS in relation to clinical outcome and survival in patients with nGBM have been reported by others. Jones and colleagues prospectively studied the effect of BMI on mortality in 1259 patients with untreated GBM using self-reported height and weight or data abstracted from medical records to calculate BMI.24 There was no significant association between BMI and survival in nGBM patients. However, in a retrospective study of patients surgically treated for high-grade glioma, Chambless and colleagues found elevated BMI to be an independent risk factor for poor outcome.25 More recently, Siegel and colleagues identified pre-diagnostic obesity, defined as the presence of elevated BMI 1 to 5 years prior to diagnosis, as a significant independent predictor of poor outcome among high-grade glioma patients.26 A literature review by Barami found obesity to be associated with decreased OS in patients with GBM diagnosis.19 In our age-adjusted analysis of patients receiving standard therapy of concurrent radiation and temozolomide and adjuvant temozolomide, obesity was associated with lower median survival (12.9 mo in obese patients vs 15 mo in non-obese patients), though not statistically significant (P = 0.18).
Hyperglycemia was assessed in several retrospective studies, all of which confirmed worse survival in malignant glioma patients with persistent hyperglycemia after controlling for glucocorticoid dose and other confounding factors.27–29 However, a limitation of each of these studies is the use of random blood glucose to define hyperglycemia (not fasting levels or glycated hemoglobin levels). In addition, the degree of tumor resection was not assessed as a prognostic variable. A meta-analysis of published studies through January 2018 also concluded that hyperglycemia conferred a statistically significant poorer OS in patients with nGBM.30 Our study also found a significant correlation of hyperglycemia with decreased median OS in those patients receiving standard of care (11.9 mo in patients with elevated blood sugar vs 17.2 mo in patients without elevated blood sugar, P = 0.03).
Our study is the first to analyze hypertension and dyslipidemia as potential prognostic factors for survival in nGBM patients. We found an association between hypertension and decreased median survival in patients receiving standard radiation and temozolomide (12.9 mo in hypertensive patients vs 19.7 mo in non-hypertensive patients, P = 0.19). We also found an association between dyslipidemia and decreased median survival (12.4 mo in patients with elevated triglycerides vs 17.9 mo in patients without elevated triglycerides, P = 0.09 and 13.2 months in patients with decreased HDLC vs 16 mo in patients without decreased HDLC, P = 0.96). These data should be interpreted with caution as laboratory values of lipids were not routinely available and dyslipidemia in many patients was determined by prescribed medications, which may have been prescribed prophylactically.
A study of the association between MetS and GBM is clinically relevant because it may increase our understanding of the pathophysiology of GBM development. Human glial tumors possess insulin receptors with insulin-binding activities. Insulin has been shown to stimulate glucose uptake in cultures of human GBM cells.31 Thus, there is a rationale to suggest that insulin resistance, one characteristic of MetS, may be a factor in the growth of gliomas. Alternatively, hyperglycemia alone may promote tumor growth. Although we are not certain if MetS is more prevalent among nGBM patients compared with the general population, we did identify that individual criteria of MetS significantly affected survival in nGBM patients who received standard radiation and temozolomide and that the combination of all MetS factors carries an especially poor prognosis. Other potential mechanisms by which metabolic abnormalities might influence GBM outcome include the effects of obesity on the tumor microenvironment, including the increased levels and availability of growth factors such as insulin and insulin-like growth factor, altered adipocytokine levels, low-grade inflammation, and oxidative stress.32
The retrospective design is a limitation in our study because not all elements of MetS were routinely obtained at clinic visits. We extrapolated data on the use of antihypertensives and lipid-lowering agents as treatment for hypertension and hyperlipidemia, whereas these may have been used in a prophylactic fashion. However, the study design did allow us to include pretreatment BMI and glucose levels. In addition, we included patients who did not receive standard radiochemotherapy, typically because of low KPS or advanced age, and are thus representative of the typical nGBM population. To our knowledge, no prior studies have assessed the effect of hypertension or dyslipidemia as factors affecting survival outcome in patients with nGBM; it merits further investigation in a prospective study design. Because of small numbers of available tissue of MGMT methylation and IDH mutation status, we were not able to incorporate these variables into the outcome assessment.
Conclusions
Despite the limitations in our study, the association of MetS with a worse prognosis in GBM patients receiving standard radiation and temozolomide provides the rationale for a prospective study to determine clinical and laboratory evidence of MetS in nGBM compared with sex- and age-matched controls, and to correlate the individual factors of MetS with patient outcome. If MetS is found to impact treatment outcome, the potential exists that efforts to control MetS in nGBM patients could lead to improved survival by lifestyle changes and medications appropriate to the individual factors identified.
Funding
Dr Ostrom is supported by a Research Training Grant from the Cancer Prevention and Research Institute of Texas (CPRIT; RP160097T).
Conflict of interest statement.
The authors declare that they have no conflicts of interest or relevant financial relationships to disclose.
References
- 1. Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 2. Healy LA, Ryan AM, Carroll P, et al. Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol (R Coll Radiol). 2010;22(4):281–288. [DOI] [PubMed] [Google Scholar]
- 3. Irwin ML, Duggan C, Wang CY, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol. 2011;29(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhindi B, Locke J, Alibhai SMH, et al. Dissecting the association between metabolic syndrome and prostate cancer risk: analysis of a large clinical cohort. Eur Urol. 2015;67(1):64–70. [DOI] [PubMed] [Google Scholar]
- 5. Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147(1):159–165. [DOI] [PubMed] [Google Scholar]
- 6. Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bitzur R, Brenner R, Maor E, et al. Metabolic syndrome, obesity, and the risk of cancer development. Eur J Intern Med. 2016;34:89–93. [DOI] [PubMed] [Google Scholar]
- 8. Ostrom QT, McCulloh C, Chen Y, et al. Family history of cancer in benign brain tumor subtypes versus gliomas. Front Oncol. 2012;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gierach M, Gierach J, Ewertowska M, et al. Correlation between body mass index and waist circumference in patients with metabolic syndrome. ISRN Endocrinol. 2014;2014:514589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bozeman SR, Hoaglin DC, Burton TM, et al. Predicting waist circumference from body mass index. BMC Med Res Methodol. 2012;12:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farin HM, Abbasi F, Reaven GM. Comparison of body mass index versus waist circumference with the metabolic changes that increase the risk of cardiovascular disease in insulin-resistant individuals. Am J Cardiol. 2006;98(8):1053–1056. [DOI] [PubMed] [Google Scholar]
- 12. Stupp R, Weber DC. The role of radio- and chemotherapy in glioblastoma. Onkologie. 2005;28(6-7):315–317. [DOI] [PubMed] [Google Scholar]
- 13. Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prev Chronic Dis. 2017;14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Behavioral risk factor surveillance system prevalence & trends data 2016. https://www.cdc.gov/brfss/index.html. Accessed September 17, 2018.
- 15. Niedermaier T, Behrens G, Schmid D, et al. Body mass index, physical activity, and risk of adult meningioma and glioma: a meta-analysis. Neurology. 2015;85(15):1342–1350. [DOI] [PubMed] [Google Scholar]
- 16. Sergentanis TN, Tsivgoulis G, Perlepe C, et al. Obesity and risk for brain/CNS tumors, gliomas and meningiomas: a meta-analysis. PLoS One. 2015;10(9):e0136974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dai Z, Huang Q, Liu H. Different body mass index grade on the risk of developing glioma: a meta-analysis. Chinese Neurosurg J. 2015;1(Oct):7. [Google Scholar]
- 18. Wiedmann MKH, Brunborg C, Di Ieva A, et al. The impact of body mass index and height on the risk for glioblastoma and other glioma subgroups: a large prospective cohort study. Neuro Oncol. 2017;19(7):976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barami K, Lyon L, Conell C. Type 2 diabetes mellitus and glioblastoma multiforme-assessing risk and survival: results of a large retrospective study and systematic review of the literature. World Neurosurg. 2017;106:300–307. [DOI] [PubMed] [Google Scholar]
- 20. Disney-Hogg L, Sud A, Law PJ, et al. Influence of obesity-related risk factors in the aetiology of glioma. Br J Cancer. 2018;118(7):1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seliger C, Ricci C, Meier CR, et al. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro Oncol. 2016;18(3):340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edlinger M, Strohmaier S, Jonsson H, et al. Blood pressure and other metabolic syndrome factors and risk of brain tumour in the large population-based Me-Can cohort study. J Hypertens. 2012;30(2):290–296. [DOI] [PubMed] [Google Scholar]
- 23. Houben MP, Louwman WJ, Tijssen CC, et al. Hypertension as a risk factor for glioma? Evidence from a population-based study of comorbidity in glioma patients. Ann Oncol. 2004;15(8):1256–1260. [DOI] [PubMed] [Google Scholar]
- 24. Jones LW, Ali-Osman F, Lipp E, et al. Association between body mass index and mortality in patients with glioblastoma mutliforme. Cancer Causes Control. 2010;21(12):2195–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chambless LB, Parker SL, Hassam-Malani L, et al. Type 2 diabetes mellitus and obesity are independent risk factors for poor outcome in patients with high-grade glioma. J Neurooncol. 2012;106(2):383–389. [DOI] [PubMed] [Google Scholar]
- 26. Siegel EM, Nabors LB, Thompson RC, et al. Prediagnostic body weight and survival in high grade glioma. J Neurooncol. 2013;114(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Derr RL, Ye X, Islas MU, et al. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27(7):1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGirt MJ, Chaichana KL, Gathinji M, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63(2):286–291; discussion 291. [DOI] [PubMed] [Google Scholar]
- 29. Adeberg S, Bernhardt D, Foerster R, et al. The influence of hyperglycemia during radiotherapy on survival in patients with primary glioblastoma. Acta Oncol. 2016;55(2):201–207. [DOI] [PubMed] [Google Scholar]
- 30. Lu VM, Goyal A, Vaughan LS, et al. The impact of hyperglycemia on survival in glioblastoma: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;170:165–169. [DOI] [PubMed] [Google Scholar]
- 31. Gong Y, Ma Y, Sinyuk M, et al. Insulin-mediated signaling promotes proliferation and survival of glioblastoma through Akt activation. Neuro Oncol. 2016;18(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benson VS, Pirie K, Green J, et al. ; Million Women Study Collaborators Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008;99(1):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cabaniols C, Giorgi R, Chinot O, et al. Links between private habits, psychological stress and brain cancer: a case-control pilot study in France. J Neurooncol. 2011;103(2):307–316. [DOI] [PubMed] [Google Scholar]
- 35. Little RB, Madden MH, Thompson RC, et al. Anthropometric factors in relation to risk of glioma. Cancer Causes Control. 2013;24(5):1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michaud DS, Bové G, Gallo V, et al. Anthropometric measures, physical activity, and risk of glioma and meningioma in a large prospective cohort study. Cancer Prev Res (Phila). 2011;4(9):1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore SC, Rajaraman P, Dubrow R, et al. Height, body mass index, and physical activity in relation to glioma risk. Cancer Res. 2009;69(21):8349–8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wiedmann M, Brunborg C, Lindemann K, et al. Body mass index and the risk of meningioma, glioma and schwannoma in a large prospective cohort study (The HUNT Study). Br J Cancer. 2013;109(1):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]