Abstract
Objective
Clinical trials are increasingly globalized, and adverse event (AE) rates and treatment responses may differ by geographical region. This study assessed regional differences in AE reporting rates and ACR response rates (ACR20/50) in patients with RA who received placebo/standard-of-care treatment in clinical trials.
Methods
Patients from the placebo arms of 7 RA trials in the TransCelerate Biopharma Inc database were grouped into 5 geographical regions (Asia, Latin America, Russian Federation and Eastern Europe [RFEE], USA, and Western Europe). Differences in demographics, AE reporting rates and ACR response were evaluated using descriptive statistics and omnibus tests for significance; pairwise comparisons were made between regions, with false discovery rate correction for multiple comparisons.
Results
Among 970 patients included, week 12 AE rates were significantly lower in the RFEE than in Asia, Latin America and the USA (22% vs 51%, 49% and 53%, respectively; P < 0.05 after false discovery rate correction). Similar differences in AE rates across geographical regions were seen at week 52. Among 747 patients with ACR data, the lowest response rates were observed in the USA (ACR20, 22%) and RFEE (ACR50, 3%); the highest response rates were seen in Western Europe (ACR20, 43%) and Latin America (ACR50, 15%). Only the differences in ACR50 response between the RFEE and Latin America remained significant after false discovery rate correction.
Conclusion
These placebo/standard-of-care arm data revealed significant regional differences in AE reporting rates and ACR50 response rates. Regional distribution of patients should be considered when conducting RA clinical trials, particularly during recruitment.
Keywords: ACR response, adverse events, clinical trials, regional variation, rheumatoid arthritis
Rheumatology key messages
We assessed regional differences in adverse event rates/disease activity in placebo/standard-of-care arms of RA clinical trials.
Significant regional differences in adverse event reporting rates and ACR50 response rates were observed.
Regional distribution of patients should be considered when conducting future RA clinical trials.
Introduction
Clinical trials are increasingly globalized, with a growing number of trials being conducted in Asia, Latin America and the Russian Federation and Eastern Europe (RFEE) [1, 2]. In contrast, the number of applications for authorization of clinical trials of medicines in the European Union and the number of clinical trials in Western Europe have decreased [3, 4]. Furthermore, the USA’s share of global funding for research (public and private) decreased by 13% from 2004 to 2012, while Asia tripled its research investment over the same period [5]. The percentage of published clinical research manuscripts from USA-based corresponding authors that include international investigators as co-authors has also increased [6], suggesting that global collaborations are becoming the rule rather than the exception.
Globalization of clinical trials is driven by multiple factors, including the need to enrol a sufficient number of patients to meet recruitment targets in a timely manner and provide statistical power to clinical studies [1, 2, 7]. Patients outside of the USA and Western Europe may be more likely to enrol in clinical trials to gain access to healthcare and therapies, which has been shown to be a motivating factor for patient participation in clinical trials [8]. Globalization of clinical trials is also likely driven by cost concerns related to the increased costs of drug development and decreased funding of clinical research [1, 2, 7, 9]. In addition to cost concerns and recruitment needs, the desire for clinical trial populations to reflect clinical practice and the call for diversity in clinical trials may be driving clinical trial globalization. Furthermore, conducting trials in multiple countries and in an ethnically diverse group of patients may facilitate regulatory approval in some countries, thus providing increased physician and patient access [7, 10, 11].
RA trials are part of this globalization trend. An analysis of trials registered at ClinicalTrials.gov has shown that 49% (65/133) of RA studies were conducted in multiple countries [12]; another study reported that of 127 RA clinical trials ongoing in 2012, 54% were conducted outside the USA [13]. As new biologics and biosimilars become available for patients with RA, the resulting increase in clinical trials leads to increased competition for patients [14]. Furthermore, access to biologic therapy for RA varies globally, with inequities reported across European countries [15–18]; therefore, conducting trials outside the USA and Western Europe provides a larger pool of patients who may be eligible for RA clinical trials, especially if inclusion criteria require that patients be biologic naive.
One concern with the globalization of clinical trials is that efficacy or safety outcomes may differ based on the geographical region in which trials are conducted. Previous studies across multiple diseases (including mental illness, cardiovascular disease, gastrointestinal disease, breast cancer and Alzheimer’s disease) have reported that adverse event (AE) reporting rates and placebo (or standard-of-care arm) response may differ by geographical region and level of economic development [19–25]. However, it remains uncertain how broadly such geographical differences in AE reporting rates and outcomes may be generalizable across therapeutic indications, and only limited data are available related to the effect of regional differences in RA clinical trials. This study used patient-level data from the placebo or standard-of-care arms of RA trials to assess potential regional differences in AE reporting rates and ACR responses.
Methods
Data source
Seven of the 17 RA trials in the TransCelerate Biopharma Inc. database [26] that were available as of August 2017 [H9BMCBCDM (NCT01198002), H9BMCBCDO (NCT01202760), H9BMCBCDV (NCT01202773), IM126004 (NCT01404585), IM119015 (NCT00605735), IM101029 (NCT00048581), M10261 (NCT00647270); all Phase II or III] contained geographical information and were included in our analyses (Supplementary Table S1, available at Rheumatology online). Contributing companies voluntarily share their placebo or standard-of-care arm data from completed or discontinued clinical trials for the TransCelerate placebo and standard-of-care database. These data are compliant with existing Clinical Data Interchange Standards Consortium standards and only include previously published or otherwise disclosed information. Clinical trials completed after January 2008 are included to provide better uniformity in data standards and appropriate informed consent use language [27]. Contribution to the placebo and standard-of-care database is ongoing; as of June 2019, patient-level data from 136 clinical trials in 23 therapeutic areas involving >85 000 patients were available [28]. The TransCelerate database only contains data from the placebo/standard-of-care arms of these studies; treatment-arm data are not available. Trial information included AEs and severe AEs, joint counts (66/68 tender and swollen joint counts), patient-reported scores for global assessment, visual analogue pain scale scores and functional ability measures [HAQ–Disability Index (HAQ-DI)], as well as physician global assessment scores and CRP levels.
Patients and outcomes
Patients were grouped by geographical region (Asia, Latin America, RFEE, USA, and Western Europe). All patients were included for AE reporting. The ACR score analysis was restricted to patients with moderate to severe RA at baseline, defined by both ≥6 tender joints in the 68-joint count and ≥6 swollen joints in the 66-joint count at the day 1 visit. When multiple CRP results were associated with the same visit date, the highest value was used. This retrospective, observational analysis used only de-identified patient records and did not include the collection, use, or transmittal of individually identifiable data; therefore, institutional review board approval to conduct this study was not necessary.
Statistical methods
Differences in demographics, AE reporting rates and ACR response rates were evaluated using the Pearson χ2 omnibus test for differences between geographical regions, followed by pairwise comparisons using the Fisher exact test. False discovery rate (FDR) corrections were applied to multiple comparisons. Analyses were conducted in SAS (Cary, NC) and R Analytics (Vienna, Austria) analytics software.
Primary analysis and sensitivity analysis
The rates of 20% and 50% improvement in disease activity measures per ACR (ACR20 and ACR50) were calculated by comparing the baseline and week 12 scores. All the measurements were calculated as the percentage of the total of the score at baseline minus the score at week 12 and divided by the score at baseline. In the primary analysis, ACR scores were restricted to those patients with available data at week 12 for both tender and swollen joint counts and ≥3 of the 5 additional domains necessary to calculate ACR scores (physician global assessment, patient global assessment, visual analogue pain scale, HAQ-DI and CRP). To better understand the impact of this restriction on our results, imputation via last observation carried forward was conducted as a sensitivity analysis.
In last observation carried forward analyses, missing data at week 12 were imputed using either week 8 data (first choice) or, if data were missing at both weeks 12 and 8, week 16 data. In the primary analyses, patients with >1 measurement on the same visit date were excluded from the calculation. In the imputed analyses, if a patient had multiple measurements on the same visit date, the measurement with the highest value was used.
The disability score was calculated using the HAQ-DI, which contains 8 domains: activities, arising, eating, grip, hygiene, reach, walking, and dressing and grooming. The disability score was calculated as the sum of the domain scores, divided by the number of domains that have a score. If a domain consisted of more than one question, then the domain score was set as the highest score among all the scores in that domain. Patients were required to have valid scores for ≥6 of the 8 domains to be included in the analysis (in both the primary analysis and imputation). All data on ‘use of aids or devices’ and ‘help from another person’ were missing in trials IM101029 (NCT00048581) and M10261 (NCT00647270).
Results
Overall, 970 patients with moderate to severe RA were included in the AE analysis: 369 (38%) from the USA, 213 (22%) from the RFEE, 167 (17%) from Latin America, 164 (17%) from Asia and 57 (6%) from Western Europe (Table 1). The distribution of patients per country in each geographical region is shown in Supplementary Table S2, available at Rheumatology online. Across all geographical regions, the median age of patients was ≥51 years, and the majority of patients were female (Table 1). Exposure to previous biologic DMARDs and concurrent conventional synthetic DMARDs varied between trials (Supplementary Table S1, available at Rheumatology online).
Table 1.
Patient demographics by region
| Asia | Latin America | RFEE | USA | Western Europe | |
|---|---|---|---|---|---|
| (n = 164) | (n = 167) | (n = 213) | (n = 369) | (n = 57) | |
| Female, n (%) | 144 (87.8) | 148 (88.6) | 172 (80.8) | 298 (80.8) | 44 (77.2) |
| Age, median (range), years | 52 (18–83) | 51 (22–76) | 51 (25–79) | 55 (21–78) | 54 (25–81) |
| Race, n (%) | |||||
| Unknown | 0 (0.0) | 7 (4.2) | 0 (0.0) | 2 (0.5) | 0 (0.0) |
| American Indian or Alaskan | 0 (0.0) | 30 (18.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| Asian | 164 (100.0) | 13 (7.8) | 0 (0.0) | 3 (0.8) | 1 (1.8) |
| Black or African American | 0 (0.0) | 7 (4.2) | 0 (0.0) | 50 (13.5) | 0 (0.0) |
| Multiple | 0 (0.0) | 3 (1.8) | 0 (0.0) | 3 (0.8) | 0 (0.0) |
| Other | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | 0 (0.0) | 106 (63.5) | 213 (100.0) | 310 (84.0) | 56 (98.2) |
| Ethnicity, n (%) | |||||
| Unknown | 9 (5.5) | 38 (22.8) | 8 (3.8) | 31 (8.4) | 19 (33.3) |
| Hispanic or Latino | 0 (0.0) | 97 (58.1) | 0 (0.0) | 42 (11.4) | 3 (5.3) |
| Not Hispanic or Latino | 38 (23.2) | 9 (5.4) | 117 (54.9) | 296 (80.2) | 3 (5.3) |
| Not reported | 117 (71.3) | 23 (13.8) | 88 (41.3) | 0 (0.0) | 32 (56.1) |
RFEE: Russian Federation and Eastern Europe.
Adverse events
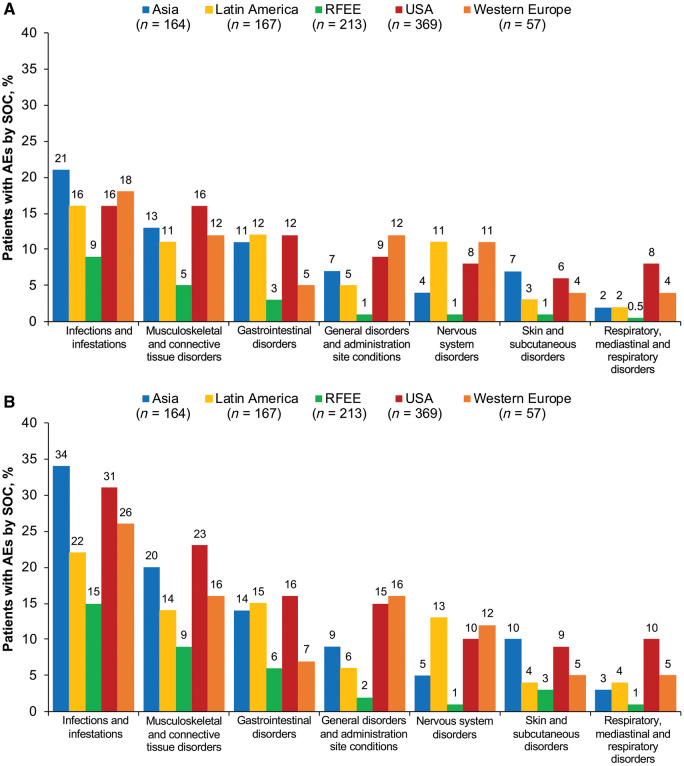
Omnibus tests for a statistically significant difference between the proportion of patients with AEs in any one region vs the overall mean for all regions indicated a significant difference at both weeks 12 (P = 8.5 × 10−13) and 52 (P = 2.9 × 10−13). After FDR correction, AE reporting rates were significantly lower in the RFEE than in Asia, Latin America and the USA at week 12 (22% vs 51%, P = 4.8 × 10−9; 49%, P = 5.9 × 10−8 and 53%, P = 6.5 × 10−14, respectively) and week 52 (39% vs 65%, P = 3.1 × 10−7; 58%, P = 1.9 × 10−4 and 68%, P = 4.6 × 10−12) (Fig. 1). The most common AEs overall by Medical Dictionary for Regulatory Activities System Organ Class at weeks 12 and 52 were ‘infections and infestations’ (15.3% and 26%, respectively), musculoskeletal and connective tissue disorders (12.0% and 17.4%) and gastrointestinal disorders (9.3% and 12.8%) (Fig. 2). At week 12, severe AEs occurred in 4.3% of patients, with the lowest rates in the RFEE (0.9%) and the highest in Western Europe (7.0%). At week 52, severe AEs occurred in 8.5% of patients, with the lowest rates in the RFEE (4.2%) and the highest rates in the USA (12.5%). Musculoskeletal and connective tissue disorders were the most common severe AEs at weeks 12 (2.5%) and 52 (4.2%). The number and proportion of patients in each region who withdrew from trials at week 12 due to AEs or death or due to any cause are shown in Supplementary Table S4, available at Rheumatology online.
Fig. 1.
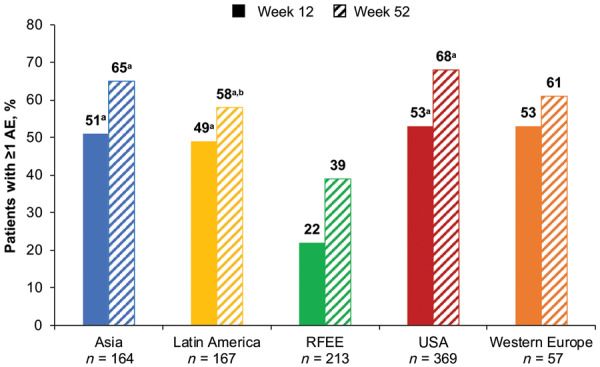
Proportion of patients with ≥1 reported AE at weeks 12 and 52 across geographical regions
All P values corrected for multiple comparisons. aP < 0.05 vs RFEE. bP < 0.05 vs the USA. AE: adverse event; RFEE: Russian Federation and Eastern Europe.
Fig. 2.
Proportion of patients reporting AEs, by MedDRA System Organ Class
(A) week 12; (B) week 52. AE: adverse event; RFEE: Russian Federation and Eastern Europe; SOC: Medical Dictionary for Regulatory Activities System Organ Class.
ACR response
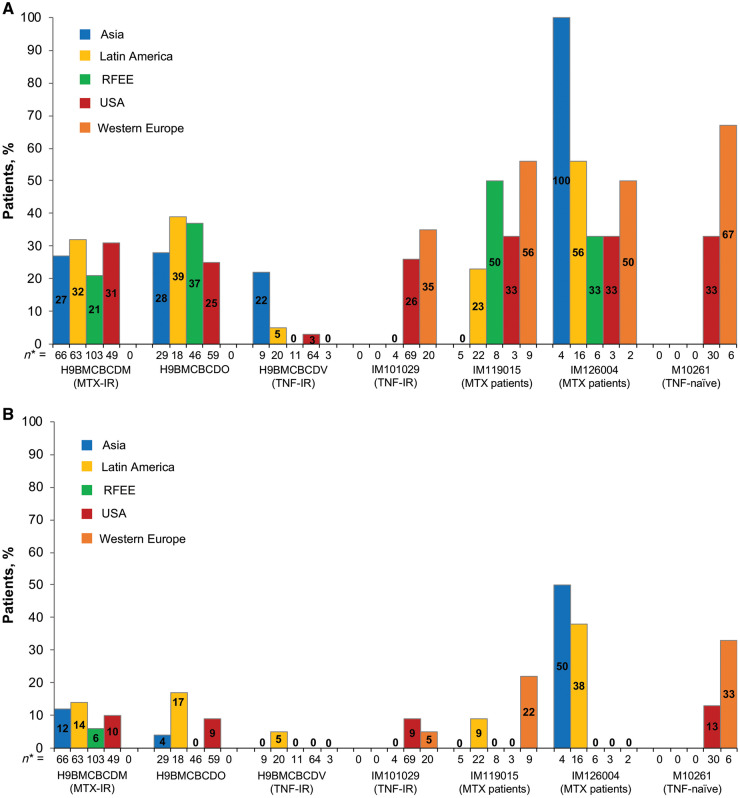
A total of 747 patients had ACR data (Table 2). For ACR20, the lowest response rates were observed in the USA and the highest in Western Europe. For ACR50, the lowest response rates were observed in the RFEE and the highest in Latin America (Table 2;Fig. 3). Omnibus tests for a statistically significant difference between the proportion of patients attaining an ACR response at week 12 in any one region and the overall mean across regions showed no difference for ACR20 (P = 0.3197) but did show a difference for ACR50 (P = 0.0018). Consequently, pairwise comparisons were carried out for ACR50 (Supplementary Table S3, available at Rheumatology online) and corrected for multiple comparisons using FDR. After FDR correction, ACR50 response rates at week 12 were significantly lower in the RFEE than in Latin America (3% vs 15%; P = 0.0004). ACR20/50 response rates at week 12 across all clinical trials are shown in Fig. 4.
Table 2.
Proportion of ACR responders, with and without imputation, by region and trial
| Region | Trial | Without imputation (n = 747) |
With imputation (n = 794) |
||||
|---|---|---|---|---|---|---|---|
| n | ACR20, % | ACR50, % | n | ACR20, % | ACR50, % | ||
| Asia | All | 113 | 28.3 | 9.7 | 121 | 26.5 | 9.1 |
| H9BMCBCDM | 66 | 27.3 | 12.1 | 72 | 25.0 | 11.1 | |
| H9BMCBCDO | 29 | 27.6 | 3.5 | 30 | 26.7 | 3.3 | |
| H9BMCBCDV | 9 | 22.2 | 0.0 | 10 | 20.0 | 0.0 | |
| IM119015 | 5 | 0.0 | 0.0 | 5 | 0.0 | 0.0 | |
| IM126004 | 4 | 100.0 | 50.0 | 4 | 100.0 | 50.0 | |
| Latin America | All | 139 | 30.2 | 15.1 | 147 | 29.9 | 15.7 |
| H9BMCBCDM | 63 | 31.8 | 14.3 | 65 | 30.8 | 13.9 | |
| H9BMCBCDO | 18 | 38.9 | 16.7 | 19 | 42.1 | 21.1 | |
| H9BMCBCDV | 20 | 5.0 | 5.0 | 21 | 4.8 | 4.8 | |
| IM119015 | 22 | 22.7 | 9.1 | 24 | 25.0 | 12.5 | |
| IM126004 | 16 | 56.3 | 37.5 | 18 | 50.0 | 33.3 | |
| RFEE | All | 178 | 25.3 | 3.4 | 188 | 24.5 | 3.2 |
| H9BMCBCDM | 103 | 21.4 | 5.8 | 106 | 20.8 | 5.7 | |
| H9BMCBCDO | 46 | 37.0 | 0.0 | 49 | 36.7 | 0.0 | |
| H9BMCBCDV | 11 | 0.0 | 0.0 | 15 | 0.0 | 0.0 | |
| IM101029 | 4 | 0.0 | 0.0 | 4 | 0.0 | 0.0 | |
| IM119015 | 8 | 50.0 | 0.0 | 8 | 50.0 | 0.0 | |
| IM126004 | 6 | 33.3 | 0.0 | 6 | 33.3 | 0.0 | |
| USA | All | 277 | 22.4 | 7.2 | 294 | 21.8 | 7.5 |
| H9BMCBCDM | 49 | 30.6 | 10.2 | 49 | 30.6 | 12.2 | |
| H9BMCBCDO | 59 | 25.4 | 8.5 | 62 | 24.2 | 8.1 | |
| H9BMCBCDV | 64 | 3.1 | 0.0 | 70 | 4.3 | 0.0 | |
| IM101029 | 69 | 26.1 | 8.7 | 74 | 25.7 | 9.5 | |
| IM119015 | 3 | 33.3 | 0.0 | 4 | 25.0 | 0.0 | |
| IM126004 | 3 | 33.3 | 0.0 | 3 | 33.3 | 0.0 | |
| M10261 | 30 | 33.3 | 13.3 | 32 | 31.3 | 12.5 | |
| Western Europe | All | 40 | 42.5 | 12.5 | 44 | 40.9 | 11.4 |
| H9BMCBCDV | 3 | 0.0 | 0.0 | 3 | 0.0 | 0.0 | |
| IM101029 | 20 | 35.0 | 5.0 | 22 | 31.8 | 4.6 | |
| IM119015 | 9 | 55.6 | 22.2 | 9 | 55.6 | 22.2 | |
| IM126004 | 2 | 50.0 | 0.0 | 2 | 50.0 | 0.0 | |
| M10261 | 6 | 66.7 | 33.3 | 8 | 62.5 | 25.0 | |
ACR20: 20% improvement in disease activity measures per ACR; ACR50: 50% improvement in disease activity measures per ACR; RFEE: Russian Federation and Eastern Europe.
Fig. 3.
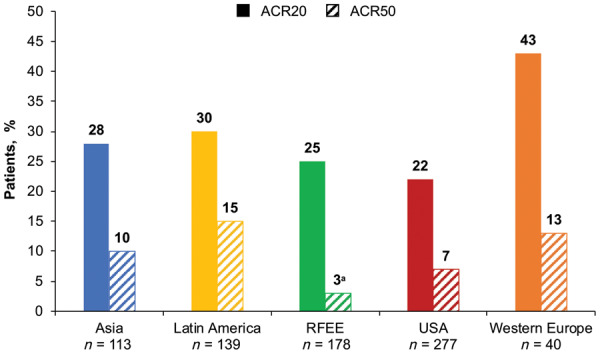
Proportion of ACR responders at week 12 (no imputation), by region
a P = 0.0004 vs Latin America (corrected for multiple comparisons). ACR20/50: 20% or 50% improvement in response, respectively, per ACR; RFEE: Russian Federation and Eastern Europe.
Fig. 4.
Proportion of ACR responders at week 12 by trial and region, without imputation
(A) ACR20; (B) ACR50. * Number of patients from each geographical region in the clinical trial. ACR20: 20% improvement in response per ACR; ACR50: 50% improvement in response per ACR; IR: inadequate response; RFEE: Russian Federation and Eastern Europe.
Imputation added an additional 47 patients (with imputation, n = 794; without imputation, n = 747) to the ACR analysis population. When stratified by region and trial, postimputation results for both ACR50 and ACR20 were similar to those obtained without imputation (Table 2). Missingness and the increased number of patients in the ACR analysis population following imputation, by region, are shown in Supplementary Table S5, available at Rheumatology online.
Discussion
Patient-level data from placebo or standard-of-care arms in the TransCelerate database revealed significant regional differences in both AE reporting rates and ACR20/50 response rates. The results of the present study are consistent with those evaluating AE reporting rates in breast cancer clinical trials [24] and gastrointestinal clinical trials [23], which showed variations in AE reporting rates across geographic regions. A large study of acute coronary syndrome showed that patients in Eastern Europe and Asia were less likely to report serious AEs; however, there was no variation in non-serious AEs [25]. In contrast with the present study, more patients with Alzheimer’s disease in North America and Europe reported AEs than those in the RFEE and Japan [22]. Differences in AE reporting rates may reflect cultural differences across different geographical regions, such as relationships between patients and healthcare providers, access to healthcare providers and tolerance of pain and discomfort [19, 21, 23]. In addition, differences may be related to variability in the quality of the standard-of-care health practices and overall health and lifestyle factors [3, 29]. Furthermore, there may be variation among clinical trial investigators across regions in adherence to clinical trial protocols related to AE reporting [30].
Overall, the proportion of patients reporting different categories of AEs—most commonly infections and infestations, musculoskeletal and connective tissue disorders, gastrointestinal disorders and nervous system disorders—was generally lower in the RFEE than in other regions, suggesting that observed differences are a general phenomenon rather than one limited to specific symptom categories. Reporting rates of severe AEs showed similar results.
Observed differences in ACR20/50 responses between regions may reflect differences in overall health and lifestyle factors between the different geographical regions, as well as potential variability in the assessment of outcomes by both patients and physicians in the various regions. For example, in low-income countries, worse physician-reported outcomes have been reported despite better patient-reported outcomes [31]. In contrast, a meta-analysis of 10 RA trials found that physician-reported outcomes in placebo-arm populations were more favourable than patient-reported outcomes, and higher rates of placebo-arm response were found in Latin American patients [32].
Baseline disease activity may also influence ACR response, such that patients with higher baseline disease activity may be more likely to achieve ACR response than those with lower disease activity. We addressed this possibility in two ways: first, we required all patients to have ≥6 tender and swollen joints; second, we adjusted for baseline disease severity by comparing patients above the median baseline tender and swollen joint counts with those below the median. These results did not show any consistent tendency towards better ACR response for patients in either category (Supplementary Table S6, available at Rheumatology online).
Regional differences in standard of care and patient access to biologic treatments for RA may also contribute to the variation in ACR response across regions. Patients who have an inadequate response to MTX are more likely to achieve an ACR70 response than patients who have an inadequate response to TNF inhibitors (TNFi-IR) [33]; thus, it is possible that differences in previous conventional synthetic DMARD and TNF inhibitor exposure in the patient populations may have contributed to some of the differences in ACR response between the geographical regions. For instance, in the trials including TNF-IR patients, 9 patients from Asia were involved compared with 133 patients from the USA. However, because ACR20 and ACR50 results stratified by trial and cross-tabulated by trial within region were similar across strata, we do not believe that observed differences are driven by overrepresentation of particular regions.
Regarding the missing data for ACR score calculation for 47 patients, the results of analyses conducted using imputation were similar to those obtained when excluding these patients. This suggests that the results of the main analysis are robust to missing data.
An analysis of 32 Phase II and III RA trials between 1999 and 2018 found an increase in placebo-arm ACR50 and ACR70 response over time [34]. The authors note that in their sample, patient enrolment in Latin America and Southeast Asia began to increase starting in 2008 and that in low-income settings, trial participation may improve adherence to therapy, possibly boosting placebo-arm response rates. Our analysis yielded no evidence for or against this hypothesis, but we find it plausible and note that we did observe high rates of ACR response in Latin American participants. Another explanation offered by the authors of the previously mentioned study is that the observed increase in placebo-arm response over time may reflect the wider availability of more effective therapies that decrease the severity of RA in prospective trial participants, which may lead investigators to report patients’ disease as being more severe than it actually is to enable them to meet trial inclusion criteria. Again, we were unable to address this explanation using our data, but we excluded participants with tender and swollen joint counts <6.
Limitations
One limitation of this study is that we did not examine potential drivers of regional differences and thus were limited to a descriptive analysis. This was due in part to limitations inherent in clinical trial data, including limited details that would enable thorough consideration of the factors that could drive regional differences in AE reporting and ACR response. As such, we can only speculate on potential drivers (e.g. health system quality, cultural practices, standard of care for RA and population differences). Additional patient-level data may enable such analyses in the future.
A second limitation is the uneven contribution of patients across trials and regions. Three trials [H9BMCBCDM (NCT01198002), H9BMCBCDO (NCT01202760) and H9BMCBCDV (NCT01202773)] contributed a disproportionate number of participants, and the geographical regions were not evenly distributed among the trials. The majority of patients from Asia were involved in only two trials (H9BMCBCDM and H9BMCBCDO), meaning that results seen in Asia could be influenced by trial-specific (including standard of care for RA and population differences) rather than region-specific factors. However, the significant difference in ACR50 response observed between the RFEE and Asia may indeed be driven primarily by region-specific factors, as the majority of patients from the RFEE were involved in those same two trials. Separately, the small number of patients from Western Europe limits interpretation of results from this region.
Third, unmeasured factors influencing AE reporting and ACR response may vary between countries within a region (e.g. the number of healthcare resources available in Sri Lanka vs Japan). Owing in part to small, country-specific sample sizes, we did not assess such differences within regions (e.g. between countries in the RFEE).
Fourth, rather than conducting a meta-analysis, all patients were combined for analyses, which may introduce a potential source of bias if there are systemic, study-level differences in AE reporting (e.g. because of the use of different concomitant medications).
Finally, data were limited to those available in the TransCelerate database, which does not include data from all sponsors of RA studies; therefore, results of this study may not be generalizable to all patients. An increased contribution of patient-level data from clinical trials to the TransCelerate database could provide more information and power for future analyses.
Despite these limitations, this analysis is one of the first to investigate regional differences in AE reporting rates and outcomes in patients with RA. In the literature, individual study results may be reported at different time points or using different regional categories, making comparisons between regions difficult. Accessing and pooling actual patient-level trial data using uniform standards and time points, as done in this study, is a strength of this analysis and provides an opportunity to better understand geographical differences and their potential implications.
Conclusion
Patient-level data from the placebo arms of patients with RA in the TransCelerate database revealed significant regional differences in AE reporting rates and ACR50 response rates but not in ACR20 response rates. These results suggest that analyses in which the regional population differs from the overall patient population should be interpreted with caution and may lead trial investigators and sponsors to consider limiting or balancing enrolment from certain geographical regions in RA clinical trials. Special care should be taken to avoid both over-reporting and under-reporting, and to ensure that favourable or unfavourable safety findings are not an artefact of geographic region but are instead generalizable and broadly applicable to a range of geographic areas.
Supplementary Material
Acknowledgements
The authors thank Daisy Lee for her contributions to this manuscript. Support for third-party writing assistance for this manuscript, furnished by Nicola Gillespie, DVM, of Health Interactions, Inc, was provided by Genentech, Inc.
Funding: This work was funded by Genentech, Inc.
Disclosure statement: D.K., E.T., J.C., J.G., J.P. and K.T. are employees of and have stock ownership in Genentech, Inc.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Drain PK, Robine M, Holmes KK, Bassett IV.. Trial watch: global migration of clinical trials. Nat Rev Drug Discov 2014;13:166–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drain PK, Parker RA, Robine M, Holmes KK.. Global migration of clinical research during the era of trial registration. PloS One 2018;13:e0192413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations Development Programme. Human development report 2010: the real wealth of nations. New York: United Nations Development Programme, 2010. [Google Scholar]
- 4.European Commission. Proposal for a regulation of the European parliament and of the council on clinical trials on medicinal products for human use, and repealing directive 2001/20/EC. Brussels: European Commission, 2012. [Google Scholar]
- 5. Moses H III, Matheson DHM, Cairns-Smith S. et al. The anatomy of medical research: US and international comparisons. JAMA 2015;313:174–89. [DOI] [PubMed] [Google Scholar]
- 6. Conte ML, Liu J, Schnell S, Omary MB.. Globalization and changing trends of biomedical research output. JCI Insight 2017;2:e95206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glickman SW, McHutchison JG, Peterson ED. et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med 2009;360:816–23. [DOI] [PubMed] [Google Scholar]
- 8. Lee SJ, Lenert L, Weisman S, Kavanaugh A.. Factors affecting rheumatoid arthritis patients’ decisions to participate in clinical trials. J Rheumatol 2005;32:2317–25. [PubMed] [Google Scholar]
- 9. Knepper TC, McLeod HL.. When will clinical trials finally reflect diversity? Nature 2018;557:157–9. [DOI] [PubMed] [Google Scholar]
- 10.Japan Pharmaceutical Manufacturers Association. Pharmaceutical administration and regulations in Japan. 2018. http://www.jpma.or.jp/english/parj/ (05 March 2019, date last accessed).
- 11. Zammar G, Meister H, Shah J. et al. So different, yet so similar: meta-analysis and policy modeling of willingness to participate in clinical trials among Brazilians and Indians. PLoS One 2010;5:e14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan NA, Singh M, Spencer HJ, Torralba KD.. Randomized controlled trials of rheumatoid arthritis registered at ClinicalTrials.gov: what gets published and when. Arthritis Rheumatol 2014;66:2664–74. [DOI] [PubMed] [Google Scholar]
- 13. Paul JR, Ranganathan P.. Clinical trials in rheumatoid arthritis: a status report from the ClinicalTrials.gov website. Rheumatol Int 2012;32:1831–5. [DOI] [PubMed] [Google Scholar]
- 14. Gelinas L, Lynch HF, Bierer BE, Cohen IG.. When clinical trials compete: prioritising study recruitment. J Med Ethics 2017;43:803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergstra SA, Branco JC, Vega-Morales D. et al. Inequity in access to bDMARD care and how it influences disease outcomes across countries worldwide: results from the METEOR-registry. Ann Rheum Dis 2018;77:1413–20. [DOI] [PubMed] [Google Scholar]
- 16. Putrik P, Ramiro S, Kvien TK. et al. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country’s wealth? Ann Rheum Dis 2014;73:2010–21. [DOI] [PubMed] [Google Scholar]
- 17. Hodkinson B, Tikly M, Adebajo A.. Rheumatoid arthritis in the developing world: stepping up to the challenge. Clin Rheumatol 2014;33:1195–6. [DOI] [PubMed] [Google Scholar]
- 18. El Zorkany B, Alwahshi HA, Hammoudeh M. et al. Suboptimal management of rheumatoid arthritis in the Middle East and Africa: could the EULAR recommendations be the start of a solution? Clin Rheumatol 2013;32:151–9. [DOI] [PubMed] [Google Scholar]
- 19. Contopoulos-Ioannidis D, Tseretopoulou X, Ancker M. et al. Comparative rates of harms in randomized trials from more developed versus less developed countries may be different. J Clin Epidemiol 2016;78:10–21. [DOI] [PubMed] [Google Scholar]
- 20. Vieta E, Pappadopulos E, Mandel FS, Lombardo I.. Impact of geographical and cultural factors on clinical trials in acute mania: lessons from a ziprasidone and haloperidol placebo-controlled study. Int J Neuropsychopharmacol 2011;14:1017–27. [DOI] [PubMed] [Google Scholar]
- 21. Yusuf S, Wittes J.. Geographic variations in controlled trials. N Engl J Med 2017;376:1196–8. [DOI] [PubMed] [Google Scholar]
- 22. Grill JD, Raman R, Ernstrom K. et al. Comparing recruitment, retention, and safety reporting among geographic regions in multinational Alzheimer’s disease clinical trials. Alzheimers Res Ther 2015;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joelson S, Joelson IB, Wallander MA.. Geographical variation in adverse event reporting rates in clinical trials. Pharmacoepidemiol Drug Saf 1997;6(Suppl 3):S31–5. [DOI] [PubMed] [Google Scholar]
- 24. González V, Machado A, Fung H. et al. Geographic variation in adverse event reporting patterns in breast cancer clinical trials [abstract P3-17-01]. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium; 2017 Dec 5-9; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2018;78(4 Suppl):Abstract nr P3-17-01. [Google Scholar]
- 25. Guimarães PO, Lopes RD, Stevens SR. et al. Reporting clinical end points and safety events in an acute coronary syndrome trial: results with integrated collection. J Am Heart Assoc 2017;6:e005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gill D. Re-inventing clinical trials through TransCelerate. Nat Rev Drug Discov 2014;13:787–8. [DOI] [PubMed] [Google Scholar]
- 27. Bhuyan P, Chen C, Desai J. et al. Development and implementation of a pharma-collaborative large historical control database. TransCelerate Biopharma Inc 2015. http://www.transceleratebiopharmainc.com/wp-content/uploads/2015/04/TransCelerate-PSoC-Data-Sharing-White-Paper.pdf (02 November 2019, date last accessed). [Google Scholar]
- 28. Yin PT, Desmond J, Day J.. Sharing historical trial data to accelerate clinical development. Clin Pharmacol Ther 2019;106:1177–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murray CJ, Vos T, Lozano R. et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 30. Aizpuru F. Adverse events as end points: the need to account for both sides of the same coin. J Am Heart Assoc 2017;6:e006018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Putrik P, Ramiro S, Hifinger M. et al. In wealthier countries, patients perceive worse impact of the disease although they have lower objectively assessed disease activity: results from the cross-sectional COMORA study. Ann Rheum Dis 2016;75:715–20. [DOI] [PubMed] [Google Scholar]
- 32. Xu X, Dong B, Hsu CH. et al. Physician/site staff assessments contribute to high placebo response in rheumatoid arthritis clinical trials. Arthritis Rheumatol 2016;68(Suppl 10):abstract 2594. [Google Scholar]
- 33. Smolen JS, Aletaha D, Barton A. et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 34. Bechman K, Yates M, Norton S, Cope AP, Galloway JB.. Placebo response in rheumatoid arthritis clinical trials. J Rheumatol 2020;47:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.