Abstract
Objectives
Infection exerts a major burden in ANCA-associated vasculitis (AAV), however, its precise extent and nature remains unclear. In this national study we aimed to longitudinally quantify, characterize and contextualize infection risk in AAV.
Methods
We conducted a multicentre matched cohort study of AAV. Complementary data on infections were retrieved via data linkage with the population-based Scottish microbiological laboratory, hospitalization and primary care prescribing registries.
Results
A total of 379 AAV patients and 1859 controls were followed up for a median of 3.5 years (interquartile range 1.9–5.7). During follow-up, the proportions of AAV patients with at least one laboratory-confirmed infection, severe infection and primary care antibiotic prescription were 55.4%, 35.6% and 74.6%, respectively. The risk of infection was higher in AAV than in matched controls {laboratory-confirmed infections: incidence rate ratio [IRR] 7.3 [95% confidence interval (CI) 5.6, 9.6]; severe infections: IRR 4.4 [95% CI 3.3, 5.7]; antibiotic prescriptions: IRR 2.2 [95% CI 1.9, 2.6]}. Temporal trend analysis showed that AAV patients remained at a higher risk of infections throughout the follow-up period, especially year 1. Although the Escherichia genus was the most commonly identified pathogen (16.6% of AAV, 5.5% of controls; P < 0.0001), AAV patients had the highest risk for Herpes [IRR 12.5 (95% CI 3.7, 42.6)] and Candida [IRR 11.4 (95% CI 2.4, 55.4)].
Conclusion
AAV patients have up to seven times higher risk of infection than the general population and the overall risk remains significant after 8 years of follow-up. The testing of enhanced short- to medium-term prophylactic antibiotic regimes should be considered.
Keywords: granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, microscopic polyangiitis, infections, longitudinal study
Rheumatology key messages
AAV patients experience up to seven times greater risk of infection compared with matched controls.
This risk is greatest in the first year but persists after 8 years of follow-up.
The broad range of identified microbes indicates the potential need for broader-spectrum prophylactics.
Introduction
ANCA-associated vasculitides (AAVs) are a group of multisystem autoimmune diseases comprising granulomatosis with polyangiitis, microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis. Until the adoption of immunosuppressive therapy in the 1980s, AAVs were almost always fatal; however, they are now considered chronic, relapsing diseases with estimated 5 year survival rates of 80% [1]. Patient prognosis in AAV remains poor—an observation that is often attributed to the consequences of drug toxicity, specifically infections [2–5].
The risk of infections in AAV is considered high; however, this has yet to be robustly quantified. Studies of infection in AAV report variable risks ranging from 6 to 67% [6–14]. This wide range is mainly ascribed to diverse sampling frames, with many studies focussing on uncontrolled, highly selected tertiary care populations. Moreover, different approaches to infection identification have been employed, with most limited to those resulting in hospitalization.
Currently there is no ideal method for assessing infection risk in a patient population. To attain a thorough understanding of the infection risk, it is essential to utilize complementary approaches involving different levels of healthcare, such as hospitalizations, microbiological laboratory records and antibiotic prescriptions, each measure bringing different methodological strengths and clinical insights. In Scotland, population-based healthcare records are routinely collected electronically and are centralized. Since the records of individuals are indexed under unique identification numbers, records in various healthcare registries can be linked. Capitalizing on this extensive data linkage capacity, in this large, matched-cohort study we aimed to contextualize infections in AAV by comparing them with those in the general population and, uniquely, to characterize infections in AAV in terms of severity, temporal trends and causative pathogens.
Methods
Routinely collected healthcare registries and data linkage in Scotland
National Health Services (NHS) Scotland holds rich population-based healthcare registries, with almost complete population coverage [15]. These centralized registries are routinely linked to follow up individuals within the healthcare system [16]. In this study we used the hospital episode data (SMR01), which records information on all day-case and inpatient hospitalizations in Scotland; the Electronic Communication of Surveillance in Scotland (ECOSS) registry, which collects information on all positive microbiological test results (from both primary and secondary care); and the Prescribing Information System database, which records all medications dispensed in primary care [17–20]. Data linkage was conducted by NHS Scotland via deterministic linkage methods using unique identification numbers. This process was previously demonstrated to be of high quality [21, 22].
Governance
The research was approved by the Public Benefit and Privacy Panel for Health and Social Care, who scrutinize applications for access to anonymized routine data (1516-0194/Sarica). Information governance, confidentiality and data protection were undertaken according to the Data Protection Act (1998). Ethical approval was granted by the national Scotland ‘A’ Research Ethics Committee (15/SS/0152).
Study design
We used a matched cohort study using routinely collected health data covering a 9 year period from 1 January 2008 to 28 February 2017. Approval was received from the Scotland ‘A’ Research Ethics Committee (reference 15-SS-0152) and the Public Benefit and Privacy Panel for Health and Social Care (reference 1516-0194).
AAV cohort
An inception cohort of AAV patients was identified using the European Medicines Agency criteria in seven hospitals across Scotland [23]. To be eligible, patients were required to be diagnosed from 1 January 2008 onwards and be at least 16 years of age at diagnosis. Clinical information was collected on the date of diagnosis, including ANCA status and AAV type. The date of AAV diagnosis was assigned as the index date.
General population controls
NHS Scotland matched each patient with AAV with up to five general population controls by age (±2 years), sex and geography, i.e. post code. Each patient in the general population cohort was assigned the same index date as their corresponding AAV patient.
Follow-up period
Patients were followed from the index date until death or 28 February 2017, whichever came first. Information on death was collected through data linkage with the National Records System Death Registry, which records all deaths in Scotland [24].
Identifying infections in routinely collected healthcare registries
Depending on the registry from which they were identified, infections were categorized as laboratory-confirmed infections, severe infections or primary care antibiotics prescriptions. Laboratory-confirmed infections were identified using free-text records in the ECOSS registry. Uniquely, this registry contains information on pathogens identified in microbiological specimens. Only information on the genus of each identified pathogen was feasibly extractable. We excluded pathogens identified using serological testing, as it was not possible to distinguish IgG positivity and thus reliably ascertain whether associated infective events actually occurred during follow-up. Severe infections were identified in the hospitalization database using the International Classification of Diseases, Tenth Revision algorithms developed by Inada-Kim et al. [25]. These algorithms were originally developed using administrative datasets for surveillance of patients with a risk of sepsis. Primary care antibiotics prescriptions were identified using relevant generic drug names and British National Formulary (BNF) paragraphs, the professional guidelines used for prescriptions in Scotland [26]. Details on relevant BNF paragraphs are available in Supplementary Table S1, available at Rheumatology online. To account for common prophylaxis against infections in AAV patients, we excluded azithromycin, co-trimoxazole and nystatin prescriptions in this analysis.
To minimize misclassification of prolonged infections as recurrent infections, we assumed that infection or antibiotics prescribing records within 28 days of each other represented a continued infection [27]. When ascertaining laboratory-confirmed infections, we incorporated data on causative pathogens as well. Accordingly, positive test results within 28 days of each other were considered recurrent infections if they were due to different pathogens. It was not possible to consider causative pathogens for severe infections and primary care antibiotic prescriptions, as relevant information was not available.
Statistical analysis
The proportions of patients developing infections in each cohort were compared using chi-squared tests. Overall, infection rates in AAV and controls were compared using multilevel Poisson regression. The multilevel model introduces a ‘random effect’ that allows the risk of an event to vary randomly within each patient and hence permits repeated infections in the same patient to be independent [28, 29]. Models were adjusted for age at index, sex and health board of residence to account for confounding.
Temporal trends analysis was conducted by stratifying the follow-up time at 30, 90 and 180 days and yearly afterwards using Lexis expansions [30]. These time points were selected based on the current treatment guidelines on the duration of induction and remission therapy in AAV to provide sufficient granularity to observe potential temporal changes in the incidence rates of infections [23]. Infection rates at each interval were calculated separately by dividing the number of infections observed in each interval by person-years of follow-up included in each interval. The 95% CIs were computed using the Poisson assumption [28]. Incidence rate ratios (IRRs) comparing the rates at each discrete interval were calculated by dividing the rate in AAV by that in the general population. The 95% CIs around these IRRs were computed using the Byar method [31]. All analyses were performed in Stata version 14 (StataCorp, College Station, TX, USA) [32].
Results
Infections in AAV and general population controls
A total of 379 AAV cases were matched with 1859 general population controls. Patients were followed up for a median of 3.5 years. Baseline patient characteristics are shown in Table 1. The characteristics of patients included in the analysis pertaining to antibiotic prescription can be found in Supplementary Table S2, available at Rheumatology online.
Table 1.
Patient characteristics at baseline
| Characteristics | AAV (n = 379) | Controls (n = 1859) |
|---|---|---|
| Male sex, n (%) | 196 (51.7) | 965 (51.9) |
| Age at index, years, median (IQR) | 61.6 (51.3–70.4) | 61.5 (51.1–70.2) |
| Follow-up, years, median (IQR) | 3.5 (1.9–5.7) | 3.5 (2.0–5.7) |
| AAV type, n (%) | NA | |
| GPA | 205 (54.1) | |
| MPA | 131 (34.6) | |
| EGPA | 41 (10.8) | |
| Missing | 2 (0.5) | |
| ANCA seropositivity, n (%) | NA | |
| PR3-ANCA | 185 (48.8) | |
| MPO-ANCA | 149 (39.3) | |
| ANCA negative | 43 (11.4) | |
| Missing | 2 (0.5) |
EGPA: eosinophilic granulomatosis with polyangiitis; GPA: granulomatosis with polyangiitis; MPA; microscopic polyangiitis; PR-3: proteinase-3.
The proportions of AAV patients with at least one laboratory-confirmed infection, severe infection and primary care antibiotic prescription at follow-up were 55.4%, 35.6% and 74.6%, respectively (Table 2). Over the duration of the study, AAV patients were more likely than controls to develop laboratory-confirmed infections [adjusted IRR 7.3 (95% CI 5.6, 9.6)], develop severe infections [adjusted IRR 4.4 (95% CI 3.3, 5.7)] and receive primary care antibiotic prescriptions [adjusted IRR 2.2 (95% CI 1.9, 2.1)]. During follow-up, 35 cases died; none of these had infection listed as their main cause of death.
Table 2.
Comparing overall infection incidence in AAV and the general population at follow-up
| Factor | AAV, n (%) | Controls, n (%) | P for χ2 test | IRR (unadjusted) (95% CI) | P for IRR | IRR (adjusted)a (95% CI) | P for IRRa |
|---|---|---|---|---|---|---|---|
| Laboratory-confirmed infections | 210 (55.4) | 294 (15.8) | <0.0001 | 6.7 (5.2, 8.6) | <0.0001 | 7.3 (5.6, 9.6) | <0.0001 |
| Severe infections | 131 (35.6) | 202 (10.9) | <0.0001 | 3.8 (2.9, 5.1) | <0.0001 | 4.4 (3.3, 5.7) | <0.0001 |
| Primary care antibiotic prescriptionsb | 258 (74.6) | 902 (53.0) | <0.0001 | 2.1 (1.7, 2.5) | <0.0001 | 2.2 (1.9, 2.6) | <0.0001 |
General population controls were used as the reference group when calculating IRR.
Adjusted for age, sex and health board of residence.
Primary care prescribing data were available for 2009 onwards, therefore analysis included patients with an index date on or after 1 January 2009.
Temporal trends of infections in AAV and general population controls
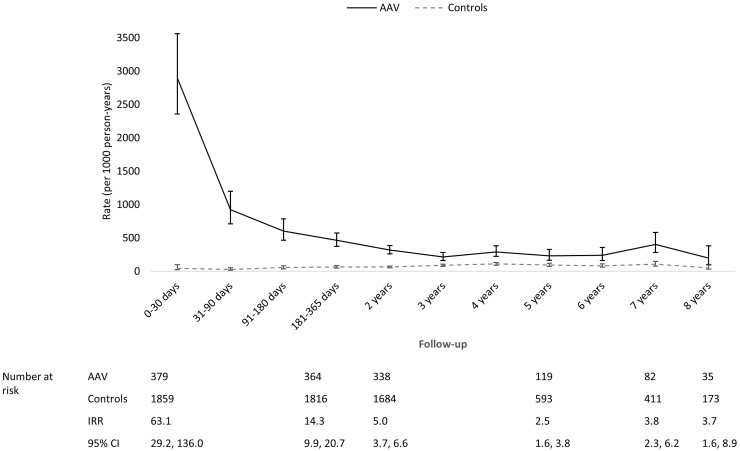
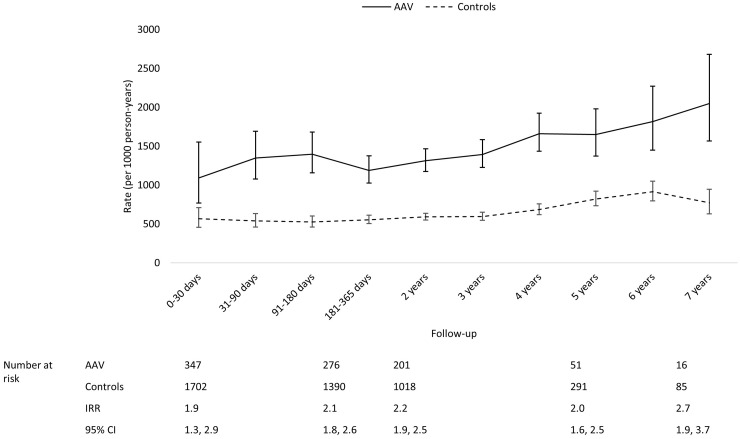
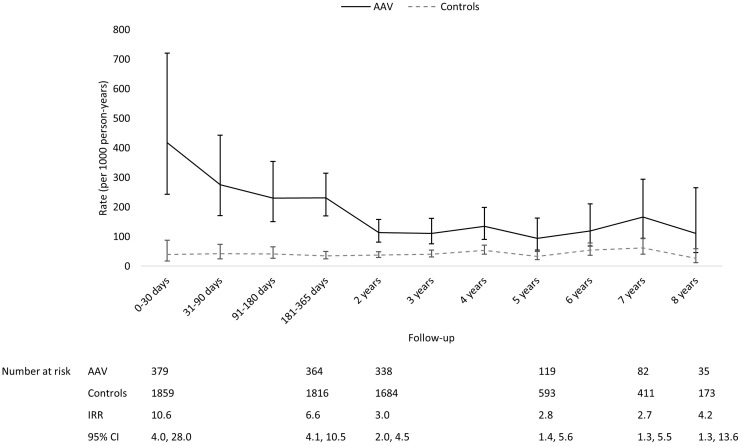
AAV patients had the highest rates of laboratory-confirmed infections and severe infections during the first year of follow-up, especially during the first 30 days (Figs 1 and 2). Both the rates of laboratory-confirmed infections and severe infections fell thereafter; however, AAV patients remained at a higher risk of both types of infections than controls throughout follow-up. Although the difference in the rate of primary care antibiotics prescriptions between AAV and controls was not as substantial as the differences observed with the other metrics, throughout follow-up AAV patients had a higher rate of relevant prescriptions than controls (Fig. 3).
Fig. 1.
Comparing temporal trends in the rate of laboratory-confirmed infections in AAV and control
The rate in controls was used as the reference when calculating IRR.
Fig. 3.
Comparing temporal trends in the rate of primary care antibiotic prescriptions in AAV and controls
The rate in controls was used as the reference when calculating IRR.
Fig. 2.
Comparing temporal trends in the rate of serious infections in AAV and controls
The rate in controls was used as the reference when calculating IRR.
Pathogens in AAV and the general population controls
Sixty-one pathogen types were identified during follow-up. AAV pathogens commonly involved the urinary tract (27%), followed by infections identified from the blood (22.3%) and lower respiratory tract (19.9%) (Supplementary Table S3, available at Rheumatology online). The risks of the most frequently observed pathogens in AAV are reported in Table 3. Results for rarer infections (n < 5) were not disclosed to preserve patient confidentiality; note that these included Pneumocystis jirovecii. Overall, Escherichia was the most commonly observed genus in both AAV and controls (16.2 vs 5.5%, P < 0.001). However, the greatest disparity in confirmed pathogens between AAV and controls were for Herpes [IRR 12.5 (95% CI 3.7, 42.6)], Candida [IRR 11.4 (95% CI 2.4, 55.4)], Clostridium [IRR 9.2 (95% CI 2.7, 30.7)], Enterococcus [IRR 5.3 (95% CI 2.2, 12.4)] and Enterobacter [IRR 5.2 (95% CI 1.0, 14.0)].
Table 3.
Comparing pathogens in AAV and the general population
| Infection typea | AAV (n = 379), n (%) | Control (n = 1859), n (%) | P for χ2 test | IRR (95% CI) | P for IRR | Adjusted IRRc (95% CI) | P for IRRc |
|---|---|---|---|---|---|---|---|
| Escherichia | 63 (16.6) | 102 (5.5) | <0.0001 | 4.9 (3.2, 7.5) | <0.0001 | 4.3 (2.7, 6.9) | <0.0001 |
| Staphylococcus | 49 (12.9) | 64 (3.4) | <0.0001 | 5.1 (3.1, 8.5) | <0.0001 | 5.0 (3.3, 7.5) | <0.0001 |
| Klebsiella | 26 (6.9 | 34 (1.8) | <0.0001 | 4.9 (1.7, 14.1) | 0.003 | 4.7 (2.1, 10.6) | <0.0001 |
| Haemophilus | 23 (6.1) | 30 (1.6) | <0.0001 | 4.3 (2.3, 8.1) | <0.0001 | 5.7 (3.3, 10.0) | <0.0001 |
| Streptococcus | 20 (5.3) | 29 (1.6) | <0.0001 | 4.8 (2.7, 8.8) | <0.0001 | 5.5 (3.2 ,9.3) | <0.0001 |
| Enterococcus | 19 (5.0) | 41 (2.2) | 0.002 | 5.3 (2.2, 12.4) | <0.0001 | 6.8 (2.9, 16.0) | <0.0001 |
| Candida | 9 (2.4) | 12 (0.7) | 0.001 | 11.4 (2.4, 55.4) | 0.002 | NA | NA |
| Enterobacter | 8 (2.1) | 7 (0.4) | <0.0001 | 5.2 (2.0, 14.0) | 0.001 | NA | NA |
| Herpes b | 7 (1.9) | <5 (<0.3)* | <0.0001 | 12.5 (3.7, 42.6) | <0.0001 | NA | NA |
| Pseudomonas | 7 (1.9) | 7 (0.4) | 0.001 | 2.0 (0.5, 7.6) | 0.326 | NA | NA |
| Citrobacter | 6 (1.6) | <5 (<0.3)* | <0.0001 | 4.8 (1.4, 16.7) | 0.014 | NA | NA |
| Clostridium | 6 (1.6) | <5 (<0.3)* | <0.0001 | 9.2 (2.7, 30.7) | <0.0001 | NA | NA |
| Campylobacter | 5 (1.3) | 7 (0.4) | 0.022 | 3.4 (1.6, 7.2) | 0.002 | NA | NA |
| Mycobacterium | 5 (1.3) | 6 (0.3) | 0.011 | 3.8 (0.8, 17.8) | 0.091 | NA | NA |
Infection types were determined using genus only. Therefore it is not possible to distinguish between different types of infections of the same genus, e.g. herpes simplex virus infections vs herpes zoster virus infections.
Includes all herpes viruses.
Adjusted for age at index, sex and health board of residence. Infection types are ordered from most to least common infections in AAV.
Cell values <5 suppressed in accordance with statistical disclosure process to preserve patient confidentiality.
NA: model adjustment was not applicable due to small numbers.
Discussion
In this study we are the first to longitudinally quantify and characterize the infection risk in AAV and compare it with that in the general population using three complementary infection measures. Our findings show that AAV patients are at a high risk of infections: they were seven times more likely than the general populations to have laboratory-confirmed events, four times more likely to develop infections requiring hospitalization and twice as likely to have been prescribed antibiotics in primary care. Crucially, our data also indicate that this increased risk persists in the long term. While Escherichia was the most common pathogen identified in both AAV and controls, the greatest proportional differences between the two groups were seen for Herpes, Candida, Clostridium, Enterococcus and Enterobacter.
Our findings are consistent with previous studies that have largely focussed on severe infections requiring hospitalization. For instance, a Swedish study showed an increased risk of severe infections in AAV compared with the general population using similar methods [11].
Our long-term assessment of infection risk in AAV is novel. While several studies agree that the risk of infection in AAV is greatest early after diagnosis when the burden of immunosuppression is greatest [33], we are the first to demonstrate that this risk remains elevated for several years. It is not uncommon for patients to remain on immunosuppression for this length of time. However, a large proportion of patients are completely weaned off such therapy after 2–5 years and it is interesting to observe similar infection risk ratios from year 3 onwards, suggesting persisting long-term immune dysfunction that may reflect chronic perturbation of the underlying disease or continued injury from past immunosuppression (although it is noteworthy that during the time frame of this study, rituximab was not commonly prescribed in Scotland).
Controlled study designs are crucial in infection epidemiology, and here we report the first comparisons of pathogens between AAV and the general population. We show that bacterial infections are the most commonly observed infections in AAV. Although limited in number, previous uncontrolled single-centre studies have indicated the same [7, 12, 13, 34, 35]. The largest of these studies was by Yang et al. [12], who reviewed the case notes of 248 AAV patients in China. Of the 87 infections observed within a 15 month period, Acinetobacter baumannii, Staphylococcus aureus and Pseudomonas aeruginosa constituted the most common bacterial pathogens, while Candida albicans and varicella-zoster virus were the most common fungal and viral pathogens, respectively. In our study, the most common bacterial pathogens were Escherichia, Staphylococcus and Klebsiella, while the most common viral and fungal pathogens were Herpes and Candida. Our study excluded episodes of varicella-zoster and many other viruses detected by Ig testing, as it was not possible to distinguish IgG from IgM responses. The differences in bacterial pathogens between the two studies may be due to differences in geography and follow-up times (a median of 3.2 years in our study vs 15 months).
Our study has several important strengths. First, we used three different methods, each with its advantages, and identified infections in rich healthcare databases with almost complete population coverage. In particular, the largely consistent temporal trends of infection risk across laboratory-confirmed and severe infections provide validity to our conclusions. We did not observe a similar disparity between the earlier rates of primary care antibiotics prescribing in AAV and the general population, an observation that most likely reflects the predominance of hospital-directed management during the initial post-diagnosis phase.
Several methodological limitations must be considered when interpreting our findings. Our study was not entirely population-based. However, we identified AAV patients from rheumatology and nephrology departments, the two departments primarily responsible for managing AAV patients in Scotland, and the sites involved in our study included teaching and general hospitals from almost every health catchment area in Scotland, thus the potential impact of selection bias on our findings is likely minimal. Second, the effect of potential surveillance bias on the results must also be considered. Infection among immunosuppressed patients is a common concern and so clinicians will have a lower threshold to investigate and treat AAV patients for this possibility compared with the general population. This explains some of the contextual disparity with the general population in regards to the laboratory-confirmed infections and antibiotic prescriptions, although such bias will be a less of an issue for serious infections. Finally, while data linkage of administrative databases offers an efficient and cost-effective way to develop and analyse disease cohorts, researchers with specific hypotheses are inevitably constrained to the analysis of variables originally determined for alternative purposes. For this reason, we were unable to report potentially interesting data such as numbers of hospital-acquired infections, immunosuppressant burden, prevalence of renal dysfunction and vasculitis relapse.
Even in the absence of quantitative data, physicians have long been familiar with the risk of infections in AAV. Indeed, international recommendations for the management of vasculitis advocate prophylaxis against infections (specifically co-trimoxazole for P. jirovecii, which was prescribed to 76% of this cohort) in patients taking cyclophosphamide and routine checks for hypoimmunoglobulinaemia prior to each course of rituximab, as well as vaccination against certain infections, if possible [33, 36]. Despite these efforts to tackle the infectious burden in AAV, the current study demonstrates that this risk remains substantial. It is highest immediately following diagnosis and the spectrum of pathogens at this time is broad. Moreover, the risk remains significantly greater than in the general population, even after 8 years. Taken together, the role of broader-spectrum antibiotic prophylaxis—at least during the first months—should be considered. For example, low-dose cephalexin has been safely and effectively used for infection prophylaxis in other populations [37] and its spectrum of action is known to target the salient microbes identified herewith. The gains of such an intervention must be balanced with their toxicity (e.g. higher rates of Clostridium) and so clinical trials are warranted to complement targeting of modifiable risk factors (e.g. corticosteroids, dosing regimens) in order to reduce this unacceptable infection burden.
Supplementary Material
Acknowledgements
We wish to thank Information Division Services Scotland for assisting with data linkage and data access in the National Safe Haven. Information presented in this article was previously presented as a poster at the American College of Rheumatology Annual Conference 2018, Chicago, IL, USA. The study was conceived by S.H.S., A.M., C.B. and N.B. All authors contributed to the study design and data collection. Data analysis and interpretation and drafting of the manuscript were conducted by all authors. C.B. and N.B. were joint senior authors. All authors critically reviewed the manuscript and approved the final version.
Funding: S.H.S. and the study were funded by the Aberdeen Development Trust and the Farr Institute of Health Informatics Research. The Farr Institute is supported by a 10-funder consortium: Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the Medical Research Council, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Assembly Government), the Chief Scientist Office (Scottish Government Health Directorates) and the Wellcome Trust (Scotland MR/K007017/1).
Disclosure statement: L.E. is a GlaxoSmithKline employee. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Booth AD, Almond MK, Burns A. et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003;41:776–84. [DOI] [PubMed] [Google Scholar]
- 2. Westman KW, Bygren PG, Olsson H. et al. Relapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol 1998;9:842–52. [DOI] [PubMed] [Google Scholar]
- 3. Little MA, Nightingale P, Verburgh CA. et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010;69:1036–43. [DOI] [PubMed] [Google Scholar]
- 4. Le Guenno G, Mahr A, Pagnoux C. et al. Incidence and predictors of urotoxic adverse events in cyclophosphamide-treated patients with systemic necrotizing vasculitides. Arthritis Rheum 2011;63:1435–45. [DOI] [PubMed] [Google Scholar]
- 5. Boomsma MM, Stegeman CA, Kramer AB. et al. Prevalence of reduced bone mineral density in patients with anti-neutrophil cytoplasmic antibody associated vasculitis and the role of immunosuppressive therapy: a cross-sectional study. Osteoporosis Int 2002;13:74–82. [DOI] [PubMed] [Google Scholar]
- 6. Rottem M, Fauci AS, Hallahan CW. et al. Wegener granulomatosis in children and adolescents: clinical presentation and outcome. J Pediatr 1993;122:26–31. [DOI] [PubMed] [Google Scholar]
- 7. Krafcik SS, Covin RB, Lynch JP III. et al. Wegener’s granulomatosis in the elderly. Chest 1996;109:430–7. [DOI] [PubMed] [Google Scholar]
- 8. Charlier C, Henegar C, Launay O. et al. Risk factors for major infections in Wegener granulomatosis: analysis of 113 patients. Ann Rheum Dis 2009;68:658–63. [DOI] [PubMed] [Google Scholar]
- 9. Goupil R, Brachemi S, Nadeau-Fredette AC. et al. Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin J Am Soc Nephrol 2013;8:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGregor JG, Negrete-Lopez R, Poulton CJ. et al. Adverse events and infectious burden, microbes and temporal outline from immunosuppressive therapy in antineutrophil cytoplasmic antibody-associated vasculitis with native renal function. Nephrol Dial Transplant 2015;30(Suppl 1):i171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohammad AJ, Segelmark M, Smith R. et al. Severe infection in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol 2017;44:1468–75. [DOI] [PubMed] [Google Scholar]
- 12. Yang L, Xie H, Liu Z. et al. Risk factors for infectious complications of ANCA-associated vasculitis: a cohort study. BMC Nephrol 2018;19:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen M, Yu F, Zhang Y. et al. Clinical and pathological characteristics of Chinese patients with antineutrophil cytoplasmic autoantibody associated systemic vasculitides: a study of 426 patients from a single centre. Postgrad Med J 2005;81:723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Besada E, Koldingsnes W, Nossent J.. Characteristics of late onset neutropenia in rheumatologic patients treated with rituximab: a case review analysis from a single center. QJM 2012;105:545–50. [DOI] [PubMed] [Google Scholar]
- 15.ISD Scotland. National Data Catalogue. 2017. http://www.ndc.scot.nhs.uk/National-Datasets/Full-A-Z/index.asp (1 July 2019, date last accessed).
- 16.Scottish Public Health Observatory. Hospital discharges. 2016. http://www.scotpho.org.uk/publications/overview-of-key-data-sources/scottish-national-data-schemes/hospital-discharges (1 July 2019, date last accessed).
- 17.ISD Scotland. SMR01 – General/Acute Inpatient and Day Case. 2017. https://www.ndc.scot.nhs.uk/Data-Dictionary/SMR-Datasets/SMR01-General-Acute-Inpatient-and-Day-Case/ (1 July 2019, date last accessed).
- 18.Health Protection Scotland. ECOSS (Electronic Communication of Surveillance in Scotland). 2017.hps.scot.nhs.uk/data/ (1 July 2019, date last accessed).
- 19.Information Services Division Scotland. Guide to prescribing services. Edinburgh: NHS National Services Scotland, 2012. [Google Scholar]
- 20. Alvarez-Madrazo S, McTaggart S, Nangle C. et al. Data resource profile: the Scottish National prescribing information system (PIS). Int J Epidemiol 2016;45:714–715f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans J, MacDonald TM.. Record-linkage for pharmacovigilance in Scotland. Br J Clin Pharmacol 1999;47:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scottish Public Health Observatory. ISD linked database. 2015. http://www.scotpho.org.uk/publications/overview-of-key-data-sources/scottish-national-data-schemes/isd-linked-database (1 July 2019, date last accessed).
- 23. Watts R, Lane S, Hanslik T. et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2006;66:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Death Records. 2016. http://www.adls.ac.uk/national-records-of-scotland/death-records/?detail (1 July 2019, date last accessed).
- 25. Inada-Kim M, Page B, Maqsood I. et al. Defining and measuring suspicion of sepsis: an analysis of routine data. BMJ Open 2017;7:e014885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joint Formulary Committee. British National Formulary. 54th edn.London: BMJ Group and Pharmaceutical Press, 2007. [Google Scholar]
- 27. McDonald HI, Nitsch D, Millett ER. et al. New estimates of the burden of acute community-acquired infections among older people with diabetes mellitus: a retrospective cohort study using linked electronic health records. Diabet Med 2014;31:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirkwood BR, Sterne J.. Essential Medical Statistics. 2nd edn Oxford: Blackwell Science, 2003. [Google Scholar]
- 29. Hilbe JM. Negative Binomial Regression. 2nd edn.Cambridge: Cambridge University Press, 2011. [Google Scholar]
- 30.StataCorp. Stsplit — Split and join time-span records. College Station, TX: StataCorp, 2013.
- 31. Rothman K, Boice JD.. Epidemiologic analysis with a programmable calculator. Report 79-1649. Washington, DC: U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, 1979. [Google Scholar]
- 32.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp, 2015.
- 33. Yates M, Watts RA, Bajema IM. et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016;75:1583–94. [DOI] [PubMed] [Google Scholar]
- 34. Bonaci-Nikolic B, Nikolic MM, Andrejevic S. et al. Antineutrophil cytoplasmic antibody (ANCA)-associated autoimmune diseases induced by antithyroid drugs: comparison with idiopathic ANCA vasculitides. Arthritis Res Ther 2005;7:R1072–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koselj-Kajtna M, Koselj M, Rott T, Kandus A. et al. Infectious complications of immunosuppressive treatment for anti-neutrophil cytoplasm antibody-related vasculitis. Transplant Proc 2002;34:3001–2. [DOI] [PubMed] [Google Scholar]
- 36. van Assen S, Agmon-Levin N, Elkayam O. et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011;70:414–22. [DOI] [PubMed] [Google Scholar]
- 37. Fisher H, Oluboyede Y, Chadwick T. et al. Continuous low-dose antibiotic prophylaxis for adults with repeated urinary tract infections (AnTIC): a randomised, open-label trial. Lancet Infect Dis 2018;18:957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.