Abstract
Objective
Low disease activity (LDA) and remission are emerging treat-to-target (T2T) endpoints in SLE. However, the rates at which these endpoints are met in patients with high disease activity (HDA) are unknown. Atacicept, which targets B lymphocyte stimulator and a proliferation-inducing ligand, improved disease outcomes in SLE patients with HDA (SLEDAI-2K ≥10) at baseline in the phase 2b ADDRESS II study. This is a post hoc analysis of T2T endpoints in these patients.
Methods
Patients received weekly atacicept (75 or 150 mg s.c.) or placebo for 24 weeks (1:1:1 randomization). Attainment of three T2T endpoints, LDA (SLEDAI-2K ≤ 2), Lupus Low Disease Activity State (LLDAS) and remission (clinical SLEDAI-2K = 0, prednisone-equivalent ≤5mg/day and Physician’s Global Assessment <0.5), was assessed and compared with SLE Responder Index (SRI)-4 and SRI-6 response.
Results
Of 306 randomized patients, 158 (51.6%) had baseline HDA. At week 24, 37 (23.4%) HDA patients attained LDA, 25 (15.8%) LLDAS and 17 (10.8%) remission. Each of these endpoints was more stringent than SRI-4 (n = 87; 55.1%) and SRI-6 (n = 67; 42.4%). Compared with placebo (n = 52), at week 24, patients treated with atacicept 150 mg (n = 51) were more likely to attain LDA [odds ratio (OR) 3.82 (95% CI: 1.44, 10.15), P = 0.007], LLDAS [OR 5.03 (95% CI: 1.32, 19.06), P = 0.018] or remission [OR 3.98 (95% CI: 0.78, 20.15), P = 0.095].
Conclusion
At week 24, LDA, LLDAS and remission were more stringent than SRI-4 and SRI-6 response, were attainable in the HDA population and discriminated between treatment with atacicept 150 mg and placebo. These results suggest that T2T endpoints are robust outcome measures in SLE clinical trials and support further evaluation of atacicept in SLE.
Trail registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT01972568.
Keywords: SLE, atacicept, low disease activity, remission, treat to target, lupus low disease activity state
Rheumatology key messages
The utility of treat-to-target endpoints in SLE trials with high baseline disease activity is unknown.
Low disease activity and remission were attainable, and more stringent than SLE Responder Index-4 and -6 response.
Low disease activity endpoints discriminated between atacicept and placebo treatment.
Introduction
SLE is a multisystem autoimmune disease associated with significant morbidity and mortality that have changed little in recent decades [1, 2]. With current (mostly legacy) treatments, incomplete control of disease activity is common, and patients endure progressive organ damage accrual and poor quality of life [3, 4]. As new and more effective treatments emerge, following the example set in other rheumatic diseases, treat-to-target (T2T) approaches are likely to become part of the standard of care in SLE [5, 6]. In support of this, T2T endpoints for SLE have begun to be defined and validated. In SLE, both disease activity and treatment, especially corticosteroids (CS), contribute to long-term adverse outcomes [7]. As a result, T2T endpoints in SLE should combine the attainment of lower disease activity with lower-dose CS use [8].
A consensus on definitions of remission in SLE is emerging [9, 10]. Although further validation studies are still required, achieving remission-endpoints, defined by the absence of clinical activity and very low or zero oral CS doses, is associated with protection from damage accrual [11, 12]. With currently available treatments, the frequency of achieving sustained remission is variable, but generally low [13, 14]. Low disease activity (LDA) endpoints such as the Lupus Low Disease Activity State (LLDAS) are more attainable, yet still associated with reduced damage accrual [11, 15–17] and improved health-related quality of life [18].
The few studies reported so far suggest that these potential T2T definitions have utility as endpoints in SLE clinical trials. LLDAS has been shown to be feasible and clinically meaningful [19–21], although to date, remission has not been examined in the clinical trial setting. Whether these endpoints can be realistically attained in a sufficient number of patients, who begin a trial with high disease activity (HDA), to allow discrimination of placebo and active treatment in these patients, warrants study.
Activation and differentiation of B lymphocytes to autoantibody-producing plasma cells are hallmarks of SLE. This process is regulated by two cytokines: B lymphocyte stimulator (BLyS) of the TNF family (also known as B cell Activating Factor, or BAFF) and a proliferation-inducing ligand (APRIL) [22]. BLyS and APRIL are elevated in the serum of SLE patients and correlate with disease activity [23, 24], severe organ manifestations [25], organ damage [26] and autoantibody production [27]. BLyS and APRIL ligate a common receptor, transmembrane activating factor and cyclophilin ligand interactor [22]. Atacicept is a recombinant human transmembrane activating factor and cyclophilin ligand interactor-immunoglobulin fusion protein that binds to both BLyS and APRIL. Atacicept treatment has been associated with reductions in SLE disease activity in two large phase 2 studies [28, 29]. In the phase 2b ADDRESS II study, the primary endpoint was not met. However, in a pre-specified analysis of patients with baseline HDA [defined as SLEDAI-2K [30] ≥10 at screening], atacicept treatment resulted in significant improvements in SLE Responder Index (SRI)-4 and SRI-6 response rates and flares [28].
To determine the utility of potential T2T endpoints in the analysis of treatment responses, specifically in subpopulation of patients who enter clinical trials with HDA, we conducted a post hoc analysis in the predefined ADDRESS II HDA subpopulation. For completeness, results from the whole [modified intention-to-treat (mITT)] population are also reported.
Methods
Study design
The design of the ADDRESS II study has been described previously [28]. Briefly, ADDRESS II was a 24-week, multicentre, randomized, double-blind, placebo-controlled phase 2b study to evaluate safety and efficacy of atacicept in patients with active, autoantibody-positive SLE (ClinicalTrials.gov NCT01972568). The study was performed in accordance with the Declaration of Helsinki, International Conference on Harmonization Guidance on Good Clinical Practice and local regulatory requirements. No patient consent was necessary or obtained for this specific analysis, which was a post hoc analysis of trial data. Patient consent for the trial was described in the original report. Patients received weekly s.c. injections of atacicept 75 or 150 mg or placebo for 24 weeks (1:1:1 randomization). The primary endpoint was SRI-4 response [31] at week 24 in the mITT population. The HDA subpopulation was prespecified and defined as patients with SLEDAI-2K ≥10 at screening.
Assessment of potential T2T endpoints
Potential T2T endpoints analysed included LLDAS, remission and LDA. LLDAS was defined according to the recent prospective validation study as a state comprising all of the following: SLEDAI-2K ≤4 without major organ activity; no new features of lupus disease activity compared with previous assessment measured on SLEDAI-2K; Physician’s Global Assessment (PGA) ≤1 (0–3); prednisone-equivalent ≤7.5 mg/day; and standard maintenance doses of immunosuppressants [15]. Remission was defined using the Definitions of Remission in SLE (DORIS) framework [9] (clinical SLEDAI-2K = 0; prednisone-equivalent ≤5 mg/day; PGA <0.5). In addition, a simple definition of LDA was defined as SLEDAI-2K ≤2.
Statistical analysis
Attainment of LDA, LLDAS and remission was assessed at each post-baseline visit up to week 24. Patients with missing data were imputed as non-responders for each of the analysed endpoints. The association of LDA, LLDAS and remission with SRI-4 and SRI-6 at week 24 was explored in the mITT population and HDA subpopulation using descriptive statistics. The proportions of patients attaining LDA, LLDAS and remission were compared with SRI-4 and SRI-6 attainment using the McNemar test. The attainment of the LDA, LLDAS, remission and SRI-6 response over 24 weeks according to treatment group was graphically presented using heat maps [19, 32]. Differences in LDA, LLDAS and remission attainment at week 24 between HDA patients treated with atacicept 75 or 150 mg vs placebo were analysed using odds ratios (ORs) estimated from logistic regression. These analyses were considered exploratory and no multiplicity adjustments were made.
Results
Study population
The ADDRESS II study included 306 patients fulfilling the revised ACR classification criteria for SLE [33] who were autoantibody-positive with active disease (SLEDAI-2K ≥6), despite standard of care. Patients received treatment with placebo (n = 100), atacicept 75 mg (n = 102) or atacicept 150 mg (n = 104) from day 1 to week 24 [28]. There were 158 (51.6%) patients with HDA at screening who were included in this post hoc analysis: 52 received placebo, 55 atacicept 75 mg and 51 atacicept 150 mg. Baseline characteristics in the HDA subpopulation were balanced except for some minor differences in race and ethnicity, and mean disease duration between treatment arms (Table 1). Safety data have been reported previously [28].
Table 1.
Baseline characteristics of ADDRESS II patients with HDA (SLEDAI-2K ≥10) at screening
| Placebo (n = 52) | Atacicept 75 mg (n = 55) | Atacicept 150 mg (n = 51) | |
|---|---|---|---|
| Age, mean (s.d.), years | 39 (13.7) | 35 (11.1) | 37 (10.4) |
| Female, n (%) | 47 (90.4) | 50 (90.9) | 49 (96.1) |
| Race or ethnicity, n (%) | |||
| White | 40 (76.9) | 35 (63.6) | 31 (60.8) |
| Black/African American | 2 (3.8) | 3 (5.5) | 4 (7.8) |
| Hispanic or Latino | 30 (57.7) | 30 (54.5) | 23 (45.1) |
| Disease characteristics | |||
| SLE disease duration, mean (s.d.), years | 6.8 (7.6) | 7.1 (7.6) | 5.5 (5.0) |
| SLEDAI-2K score, mean (s.d.) | 12 (2.3) | 12 (3.0) | 12 (2.4) |
| Serologically active disease,an (%) | 38 (73.1) | 43 (78.2) | 35 (68.6) |
| Medication history | |||
| Prednisone-equivalent dose at screening, mean (s.d.), mg/day | 10.4 (8.0) | 11.8 (8.6) | 11.1 (8.5) |
| >7.5 mg/day prednisone equivalent, n (%) | 30 (57.7) | 35 (63.6) | 29 (56.9) |
| Antimalarial, n (%) | 39 (75.0) | 42 (76.4) | 38 (74.5) |
| AZA, n (%) | 11 (21.2) | 13 (23.6) | 7 (13.7) |
| MTX, n (%) | 9 (17.3) | 7 (12.7) | 7 (13.7) |
| MMF, n (%) | 8 (15.4) | 11 (20.0) | 9 (17.6) |
| Serum biomarkers, n (%) | |||
| ANA ≥1:80 | 49 (94.2) | 53 (96.4) | 48 (94.1) |
| Anti-dsDNA ≥15 IU/ml | 36 (69.2) | 39 (70.9) | 32 (62.7) |
| Low C3 <LLN (0.9 g/l) | 23 (44.2) | 29 (52.7) | 20 (39.2) |
| Low C4 <LLN (0.1 g/l) | 16 (30.8) | 14 (25.5) | 15 (29.4) |
Positive anti-dsDNA antibodies or low complement at screening. C: complement; HDA: high disease activity, LLN: lower limit of normal; SLEDAI-2K: SLEDAI 2000.
Post hoc analysis of T2T endpoint attainment in the HDA subpopulation
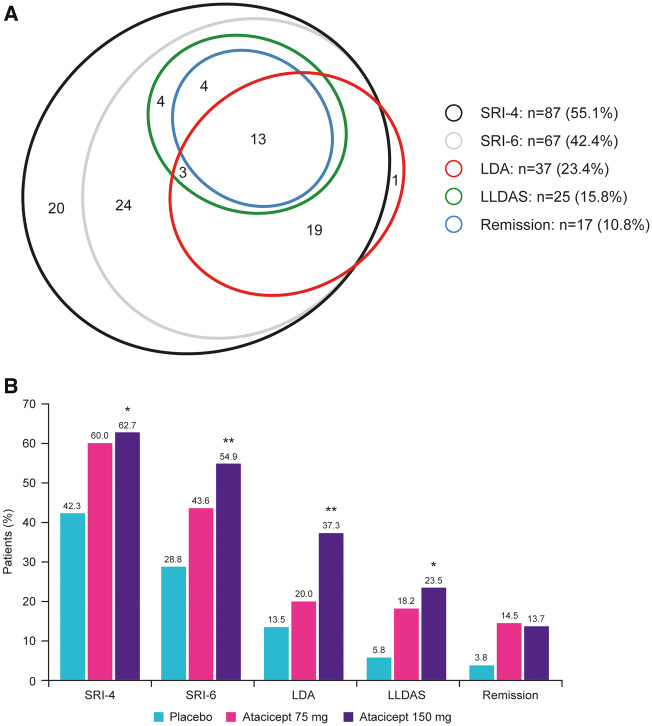
SRI-4 and SRI-6 responses in the HDA subpopulation have been reported previously [28]. Across treatment arms, 87 (55.1%) and 67 (42.4%) patients had an SRI-4 and SRI-6 response at week 24, respectively. LDA, LLDAS and remission were attained at week 24 by 37 (23.4%), 25 (15.8%) and 17 (10.8%) HDA patients, respectively (Fig. 1A). Almost all patients attaining LDA, LLDAS and remission also had an SRI-4 and SRI-6 response (94.6, 96.0 and 100% for both endpoints, respectively). One patient with LDA and one patient who attained both LDA and LLDAS were not SRI-4 or SRI-6 responders. Conversely, only 40.2, 27.6 and 19.5% of SRI-4 responders and 52.2, 35.8 and 25.4% of SRI-6 responders also attained LDA, LLDAS and remission, respectively. Thus, each of the T2T endpoints were significantly more stringent than SRI-4 and SRI-6 (P < 0.0001; Fig. 1A). Of note, all patients in remission also met the definition for LLDAS, consistent with the concept of these states being concentric [8]. In contrast, four subjects who attained remission did not meet the definition for LDA (SLEDAI-2K ≤2) because they had clinical SLEDAI = 0, but SLEDAI-2K >2 due to positive anti-dsDNA antibodies and low complement.
Fig. 1.
Endpoint stringency of LDA, LLDAS and remission attainment vs SRI responses in HDA patients
(A) Euler diagram to illustrate the distributional shift for SRI-4 and SRI-6 responder vs attainment of each of the three evaluated T2T endpoints at week 24 in all HDA patients regardless of treatment. (B) Attainment of SRI and T2T endpoints by treatment arm at 24 weeks. *P < 0.05 (vs placebo); **P < 0.01 (vs placebo). HDA: high disease activity; LDA: low disease activity; LLDAS: Lupus Low Disease Activity State; SRI: SLE Responder Index; T2T: treat-to-target.
Post hoc analysis of T2T endpoint attainment with atacicept vs placebo in the HDA subpopulation
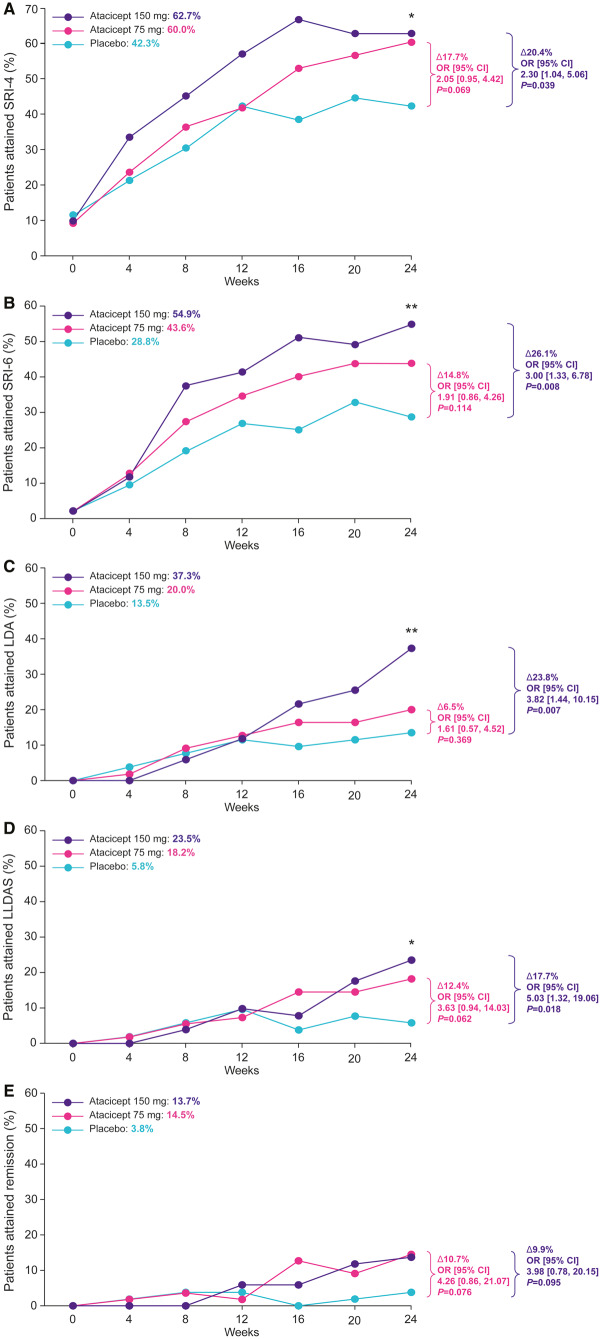
As previously reported [28], SRI-4 and SRI-6 responses at week 24 in the HDA subpopulation were significantly increased with atacicept 150 mg vs placebo [SRI-4: 62.7 vs 42.3%, OR 2.30 (95% CI: 1.04, 5.06), P = 0.039; SRI-6: 54.9 vs 28.8%, OR 3.00 (95% CI: 1.33, 6.78), P = 0.008]; the treatment effect of atacicept was evident from week 4 (SRI-4) and week 8 (SRI-6) (Fig. 1B). Both LDA and LLDAS at week 24 were also significantly increased with atacicept 150 mg vs placebo [LDA: 37.3 vs 13.5%, OR 3.82 (95% CI: 1.44, 10.15), P = 0.007; LLDAS: 23.5 vs 5.8%, OR 5.03 (95% CI: 1.32, 19.06), P = 0.018] (Fig. 1B). Increased remission attainment with atacicept 150 mg vs placebo at week 24 was also observed, but this did not reach statistical significance [13.7 vs 3.8%, OR 3.98 (95% CI: 0.78, 20.15), P = 0.095] (Fig. 1B). Differences between atacicept 150 mg and placebo in LDA, LLDAS and remission were observed from 16 weeks of treatment (Fig. 2). Increased attainment of all three T2T endpoints was also observed with atacicept 75 mg vs placebo at week 24, but the differences did not reach statistical significance.
Fig. 2.
Attainment of LDA, LLDAS, remission and SRI endpoints in HDA patients over 24 weeks
Rates of attainment of (A) SRI-4, (B) SRI-6, (C) LDA, (D) LLDAS and (E) remission over 24 weeks in HDA patients treated with placebo, atacicept 75 mg or atacicept 150 mg. *P < 0.05 (vs placebo); **P < 0.01 (vs placebo). HDA: high disease activity; LDA: low disease activity; LLDAS: Lupus Low Disease Activity State; OR: odds ratio; PGA: Physician’s Global Assessment; SLEDAI-2K: SLEDAI 2000; SRI: SLE Responder Index.
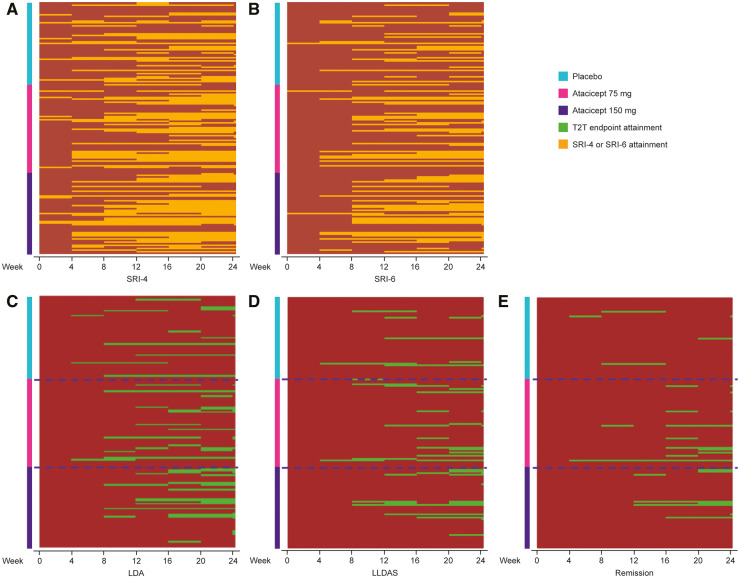
Fig. 3 shows heat maps of the attainment and retention of SRI-4, SRI-6, LDA, LLDAS and remission over 24 weeks in the HDA subpopulation in each treatment group: consistently more atacicept-treated than placebo-treated patients attained LDA, LLDAS and remission, and this was maintained over time.
Fig. 3.
Individual patient response heat maps for SRI, LDA, LLDAS and remission over 24 weeks
Individual patient attainment of (A) SRI-4, (B) SRI-6, (C) LDA, (D) LLDAS and (E) remission over 24 weeks in HDA patients treated with placebo, atacicept 75 mg or atacicept 150 mg. LDA: low disease activity; LLDAS: Lupus Low Disease Activity State; PGA: Physician’s Global Assessment; SLEDAI-2K: SLEDAI 2000; SRI: SLE Responder Index; T2T: treat-to-target.
The performance of the T2T endpoints was also examined in the whole trial (mITT) population (supplementary Fig. S1, available at Rheumatology online). As previously reported [28], SRI-4 and SRI-6 response rates increased with atacicept 150 mg vs placebo at week 24, but the differences did not reach statistical significance. Similarly, atacicept 150 mg treatment increased the proportion of patients achieving LDA, LLDAS and remission at week 24, but the differences did not reach statistical significance vs placebo.
Among patients in the HDA subpopulation who were serologically active (anti-dsDNA and low complement) at screening (n = 69), atacicept 150 mg treatment still resulted in an increase in SRI-4 and SRI-6 response rates and more frequent attainment of LDAS, LLDAS and remission vs placebo; none of these increases reached statistical significance (data not shown).
Discussion
Robust treatment endpoints have been defined and validated for multiple rheumatic diseases, and in RA a T2T approach has been demonstrated to achieve meaningfully improved outcomes [34]. Obtaining the same goal in SLE is more challenging, partly because of the complexity of assessing disease activity, and also because of the lack of data from studies using highly effective therapies to help analyse the utility of potential T2T endpoints. It has been suggested that the development and validation of T2T endpoints for SLE [5, 6] may contribute discriminatory outcomes for use in trials and allow prospective metrics-based studies. A key step towards this goal is examination of the utility of potential T2T endpoints in clinical trial settings. Several potential endpoints for SLE have now been described. A multinational collaborative group proposed and preliminarily validated LLDAS as a T2T endpoint [15]. LLDAS attainment has now been shown in multiple cohort studies to be associated with both reduced damage accrual [11, 15, 16] and improved health-related quality of life [18], and was recently formally validated in a prospective study [17]. Recently, LLDAS has been shown to discriminate active treatment from placebo more robustly than most commonly used measures [19–21], although in the one study reported to date, remission did not [35]. Based on the concept that remission and LLDAS should represent concentric, increasingly stringent cutoffs, the DORIS working group has proposed a framework for remission definitions for SLE [9]. A large cohort study has confirmed that remission is relatively uncommon in SLE compared with LLDAS, but when attained—even for a short time—it confers protection from damage accrual [11]. However, it is unclear whether the use of potential T2T endpoints such as LLDAS and remission are feasible in patients who enter a clinical trial with HDA.
Our findings in the HDA subpopulation from the ADDRESS II trial at week 24 indicate that each of the three endpoints analysed is a robust outcome measure more stringent and discriminatory than SRI-4 or SRI-6. At week 24, of the three endpoints assessed, LLDAS yielded the highest OR for discrimination between active treatment and placebo. These results are consistent with findings reported by Ordi-Ros et al. [20] and Morand et al. [19] in studies of MMF and of anifrolumab, respectively. These data also support the utility of the DORIS remission definition used here in a trial setting. Remission and LLDAS were less frequently attained than a simple definition of LDA based on SLEDAI-2K alone, consistent with the observation in a large prospective cross-sectional study that all domains of LLDAS contribute to the validity of this definition [36]. The definitions of remission and LLDAS include PGA as an outcome measure, which may be critical to balance omissions in the SLEDAI-2K measure [37]. Of note, while LLDAS and remission were concentric in this study, differences in definitions meant that some patients who met the definitions of LLDAS and remission did not meet the definition of LDA because they had SLEDAI-2K >2 due to positive anti-dsDNA antibodies and low complement. While the simple LDA measure may be a useful, discriminatory endpoint for trials, its prognostic utility remains to be examined.
Compared with placebo, treatment with atacicept 150 mg was associated with an ∼4-fold relative increase in the odds of reaching LDA, a 5-fold relative increase in the odds of reaching LLDAS and a 4-fold relative increase in the odds of achieving remission in HDA patients at week 24, although the last of these was rarer and did not reach statistical significance. Parodis et al. also recently reported that remission did not significantly separate active treatment from placebo in post hoc analyses of the belimumab phase 3 trial datasets [35], whereas LLDAS did [21]. These findings are consistent with the treatment benefits previously reported with atacicept 150 mg compared with placebo in HDA patients, using SRI-4, SRI-6 and reduction in first severe flares as endpoints [28]. Atacicept benefit on these potential T2T endpoints was first observed at week 16, which contrasts with the improvements in SRI-4 and SRI-6 with atacicept vs placebo that were evident from weeks 4 and 8, respectively. However, further prospective studies with greater statistical power would be required to determine the timing of onset of benefit for potential T2T endpoints. Early onset of response may be informative for long-term outcomes, since a recent study demonstrated that meeting the LLDAS definition within 6 months of commencing treatment was associated with improved outcomes in SLE [38].
When examining the full (mITT) ADDRESS II trial population, we found that although atacicept 150 mg treatment increased the proportion of patients achieving all three T2T endpoints at week 24 vs placebo, the differences did not reach statistical significance. These results are consistent with the increases in SRI-4 and SRI-6 response rates observed with atacicept 150 mg vs placebo in the mITT population [28], which also showed a trend for treatment effect that did not reach statistical significance. These findings suggest that the potential T2T endpoints evaluated in this post hoc analysis may be more relevant to patients with HDA.
Several limitations apply to the interpretation of these data. The analysis of potential T2T endpoints was a post hoc analysis, suitable for generating hypotheses that must be confirmed by future testing in a formal T2T strategy trial. Response rates in the trial setting, as reported here, may under- or overestimate responses because the dataset analysed here was not powered for assessment of these endpoints and the duration of their attainment. However, in a previously reported analysis of LLDAS in a trial dataset, with a 12-month duration, significant differences in duration of LLDAS attainment were detected [19]. The 6-month duration of this study was insufficient for analysis of the impact of potential T2T endpoint attainment on damage accrual, but the association of LLDAS and remission attainment with protection from damage accrual has been well described [11, 12, 15, 16].
In conclusion, we report that LLDAS, LDA and remission are feasible endpoints in SLE trials, attainable in patients with highly active disease at baseline, which discriminate active treatment from placebo at week 24. LLDAS had the most optimal performance characteristics (based on ORs) of the three measures studied. Since LLDAS and remission have been demonstrated to be stepwise more stringent endpoints in terms of their attainability and association with protection from damage accrual, they may be particularly meaningful trial endpoints. In summary, these data indicate that atacicept 150 mg demonstrated a significant treatment effect vs placebo at week 24 on these potential T2T endpoints in patients with HDA, supporting the continued development of this treatment in SLE.
Supplementary Material
Acknowledgements
The authors thank the patients and their families, as well as the ADDRESS II study team, for their participation. EMD Serono (including EMD Serono authors) and all other authors approved the final version of the manuscript and were involved in the final decision to submit the manuscript for publication. Medical writing assistance was provided by Hannah Fleetwood, Bioscript Science, Macclesfield, UK and supported by Merck KGaA, Darmstadt, Germany.
Funding: The study was sponsored by EMD Serono, Inc., a business of Merck KGaA (Darmstadt, Germany), who were involved in the study design, the collection, analysis and interpretation of data, and the writing of the manuscript.
Disclosure statement: E.M. has received grants/research support from AstraZeneca/Medimmune, Janssen, Union Chimique Belge (UCB), EMD Serono (a business of Merck KGaA, Darmstadt, Germany) and Bristol Myers Squibb (BMS); and consultant fees for EMD Serono, AstraZeneca/Medimmune, Eli Lilly and Janssen. D.A.I. has received consultant fees for EMD Serono (a business of Merck KGaA, Darmstadt, Germany); consulting fees have been passed to a local arthritis charity. D.J.W has received consultant fees for Amgen, Lilly, EMD Serono (a business of Merck KGaA, Darmstadt, Germany), Merck KGaA, Celgene and Janssen. A.H.K., C.V.-M., P.C. and K.P. are employees of EMD Serono (a business of Merck KGaA, Darmstadt, Germany). C.A. has received grants/research support from EMD Serono (a business of Merck KGaA, Darmstadt, Germany), GlaxoSmithKline (GSK), Janssen, UCB, Xencor, Genentech; and consultant fees for GSK, AstraZeneca/Medimmune. J.T.M has received grants/research support from GSK and BMS (investigator-initiated studies); consultant fees for EMD Serono (a business of Merck KGaA, Darmstadt, Germany), Eli Lilly, GSK, Anthera and Biogen; and provided clinical trial data management/analysis/QA services for Celgene and Xencor.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Jorge AM, Lu N, Zhang Y, Rai SK, Choi HK.. Unchanging premature mortality trends in systemic lupus erythematosus: a general population-based study (1999–2014). Rheumatology (Oxford) 2018;57:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Croca SC, Rodrigues T, Isenberg DA.. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology (Oxford) 2011;50:1424–30. [DOI] [PubMed] [Google Scholar]
- 3. Nossent J, Kiss E, Rozman B. et al. Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus 2010;19:949–56. [DOI] [PubMed] [Google Scholar]
- 4. Lopez R, Davidson JE, Beeby MD, Egger PJ, Isenberg DA.. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology (Oxford) 2012;51:491–8. [DOI] [PubMed] [Google Scholar]
- 5. Franklyn K, Hoi A, Nikpour M, Morand EF.. The need to define treatment goals for systemic lupus erythematosus. Nat Rev Rheumatol 2014;10:567–71. [DOI] [PubMed] [Google Scholar]
- 6. van Vollenhoven RF, Mosca M, Bertsias G. et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014;73:958–67. [DOI] [PubMed] [Google Scholar]
- 7. Bruce IN, O’Keeffe AG, Farewell V. et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morand EF. Connective tissue diseases: remission in SLE - are we there yet? Nat Rev Rheumatol 2016;12:696–8. [DOI] [PubMed] [Google Scholar]
- 9. van Vollenhoven R, Voskuyl A, Bertsias G. et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis 2017;76:554–61. [DOI] [PubMed] [Google Scholar]
- 10. van Vollenhoven RF, Voskuyl A, Morand E, Aranow C.. Remission in SLE: closing in on the target. Ann Rheum Dis 2015;74:2103–6. [DOI] [PubMed] [Google Scholar]
- 11. Petri M, Magder LS.. Comparison of remission and Lupus Low Disease Activity State in damage prevention in a United States systemic lupus erythematosus cohort. Arthritis Rheumatol 2018;70:1790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zen M, Iaccarino L, Gatto M. et al. Prolonged remission in Caucasian patients with SLE: prevalence and outcomes. Ann Rheum Dis 2015;74:2117–22. [DOI] [PubMed] [Google Scholar]
- 13. Wilhelm TR, Magder LS, Petri M.. Remission in systemic lupus erythematosus: durable remission is rare. Ann Rheum Dis 2017;76:547–53. [DOI] [PubMed] [Google Scholar]
- 14. Medina-Quiñones CV, Ramos-Merino L, Ruiz-Sada P, Isenberg D.. Analysis of complete remission in systemic lupus erythematosus patients over a 32-year period. Arthritis Care Res (Hoboken) 2016;68:981–7. [DOI] [PubMed] [Google Scholar]
- 15. Franklyn K, Lau CS, Navarra SV. et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann Rheum Dis 2016;75:1615–21. [DOI] [PubMed] [Google Scholar]
- 16. Tsang A, Bultink IE, Heslinga M, Voskuyl AE.. Both prolonged remission and Lupus Low Disease Activity State are associated with reduced damage accrual in systemic lupus erythematosus. Rheumatology (Oxford) 2017;56:121–8. [DOI] [PubMed] [Google Scholar]
- 17. Golder V, Kandane-Rathnayake R, Huq M. et al. Evaluation of remission definitions for systemic lupus erythematosus: a prospective cohort study. Lancet Rheumatol 2019;1:E103–10. [DOI] [PubMed] [Google Scholar]
- 18. Golder V, Kandane-Rathnayake R, Hoi AY. et al. Association of the Lupus Low Disease Activity State (LLDAS) with health-related quality of life in a multinational prospective study. Arthritis Res Ther 2017;19:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morand EF, Trasieva T, Berglind A, Illei GG, Tummala R.. Lupus Low Disease Activity State (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the Phase IIb MUSE trial of anifrolumab. Ann Rheum Dis 2018;77:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M. et al. Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Ann Rheum Dis 2017;76:1575–82. [DOI] [PubMed] [Google Scholar]
- 21. Oon S, Huq M, Golder V. et al. Lupus Low Disease Activity State (LLDAS) discriminates responders in the BLISS-52 and BLISS-76 phase III trials of belimumab in systemic lupus erythematosus. Ann Rheum Dis 2019;78:629–33. [DOI] [PubMed] [Google Scholar]
- 22. Vincent FB, Morand EF, Schneider P, Mackay F.. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 2014;10:365–73. [DOI] [PubMed] [Google Scholar]
- 23. Salazar-Camarena DC, Ortiz-Lazareno PC, Cruz A. et al. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus 2016;25:582–92. [DOI] [PubMed] [Google Scholar]
- 24. Hegazy M, Darwish H, Darweesh H, El-Shehaby A, Emad Y.. Raised serum level of APRIL in patients with systemic lupus erythematosus: correlations with disease activity indices. Clin Immunol 2010;135:118–24. [DOI] [PubMed] [Google Scholar]
- 25. Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF.. Association of serum B cell activating factor from the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) with central nervous system and renal disease in systemic lupus erythematosus. Lupus 2013;22:873–84. [DOI] [PubMed] [Google Scholar]
- 26. McCarthy EM, Lee RZ, Ni Gabhann J. et al. Elevated B lymphocyte stimulator levels are associated with increased damage in an Irish systemic lupus erythematosus cohort. Rheumatology (Oxford) 2013;52:1279–84. [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Roschke V, Baker KP. et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001;166:6–10. [DOI] [PubMed] [Google Scholar]
- 28. Merrill JT, Wallace DJ, Wax S. et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, Phase IIb study. Arthritis Rheumatol 2018;70:266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Isenberg D, Gordon C, Licu D. et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis 2015;74:2006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gladman DD, Ibañez D, Urowitz MB.. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 31. Furie RA, Petri MA, Wallace DJ. et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum 2009;61:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Heijde D, Dougados M, Landewé R. et al. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology (Oxford) 2017;56:1498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 34. Smolen JS, Landewé R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parodis I, Emamikia S, Gomez A. et al. Definitions of remission in systemic lupus erythematosus, a post-hoc analysis of two randomised clinical trials. Lancet Rheumatol 2019;1:E163–73. [DOI] [PubMed] [Google Scholar]
- 36. Golder V, Kandane-Rathnayake R, Hoi AY. et al. Frequency and predictors of the Lupus Low Disease Activity State in a multi-national and multi-ethnic cohort. Arthritis Res Ther 2016;18:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Golder V, Huq M, Franklyn K. et al. Does expert opinion match the operational definition of the Lupus Low Disease Activity State (LLDAS)? A case-based construct validity study. Semin Arthritis Rheum 2017;46:798–803. [DOI] [PubMed] [Google Scholar]
- 38. Piga M, Floris A, Cappellazzo G. et al. Failure to achieve Lupus Low Disease Activity State (LLDAS) six months after diagnosis is associated with early damage accrual in Caucasian patients with systemic lupus erythematosus. Arthritis Res Ther 2017;19:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.