Abstract
Background
Meningiomas are the most common primary tumor of the CNS. Studies investigating the impact of a brain tumor diagnosis on mental health disorders (MHDs) in patients have been limited. The objective of this work is to characterize the incidence and factors affecting the presence of MHDs in untreated meningiomas.
Methods
Using a large-scale private payer database, MarketScan, we performed a retrospective study of patients with an untreated meningioma and corresponding MHD.
Results
We found that in patients diagnosed with an untreated meningioma, approximately 16% were diagnosed with an MHD within 1 year of the diagnosis of the meningioma.
Conclusion
By identifying risk factors, appropriate screening can focus on patients at high-risk for the development of MHD.
Keywords: CNS, meningioma, mental health disorders, MHD
Meningiomas are the most common primary tumor of the CNS, with an estimated incidence of 7.86 per 100 000 population per year.1,2 When symptomatic, the primary presenting complaints include headaches, seizures, or focal neurologic deficits; however, as many as three-quarters of meningiomas are discovered incidentally and are asymptomatic.3–6 The vast majority of these lesions are histologically benign, and therefore many asymptomatic meningiomas are left untreated, meaning that they are not surgically resected or treated with radiosurgery, but are instead monitored through serial imaging and do not require further intervention. With the dramatic increase of advanced cranial imaging in the United States in recent decades, there has been a 3-fold increase in the discovery of asymptomatic meningiomas and a proportionate increase in patients with untreated meningiomas.7
Studies investigating the impact of a brain tumor diagnosis on mental health disorders (MHDs) in patients have been limited. Existing studies have primarily examined patients with malignant gliomas and have consistently shown a significant psychological cost. Fifteen percent of glioma patients will meet diagnostic criteria for depression, with an equal number suffering from subclinical depressive symptoms.8 Similarly, rates of anxiety in brain tumor patients range between 30% and 63%.9–11 These psychiatric comorbidities are associated with decreased functional status, impaired quality of life, poor cognitive status, and reduced overall survival.12–16
In contrast to malignant gliomas, the impact of untreated meningiomas on MHDs is neither described nor discussed in the literature. Here, we characterize the incidence and factors affecting the presence of MHDs in untreated meningiomas in a large-scale private payer database.
Methods
Study Design
This retrospective observational cohort study used administrative claims data collected from the IBM Health MarketScan Research Database to characterize MHDs in patients with untreated meningiomas. This commercial database contains the inpatient and outpatient administrative medical data of approximately 225 million privately insured patients since 199517. Because we used a deidentified, external (outside our institution) database for this study, no institutional review board approval or patient consent was needed. The database provides detailed cost, use, and outcomes data for health-care services performed both in inpatient and outpatient settings across a variety of health plans. This includes preferred provider organizations, point of service plans, indemnity plans, and health maintenance organizations. The medical claims are linked to outpatient claims and person-level enrollment data using unique enrollee identifiers.
Patient Selection
Eligible patients for the cohort were identified from the database by querying International Classification of Diseases, Ninth Revision (ICD-9) codes corresponding to meningioma (192.1, 2252, 237.6). Eligible patients had continuous enrollment in the database for 1 year prior and 1 year following the diagnosis. Treatment status was determined through presence of corresponding Current Procedural Terminology (CPT) codes. Given the limitations of the database, presenting symptoms were inferred from a query of ICD-9 codes diagnosed in the 6 months prior to diagnosis of the meningioma but that had not been present prior (Table 1).
Table 1.
International Classification of Diseases, Ninth Revision, and Current Procedural Terminology (CPT) Codes
| Cohort | Code |
|---|---|
| Benign neoplasm of cerebral meninges | 225.2 |
| Malignant neoplasm of cerebral meninges | 192.1 |
| Neoplasm of meninges of uncertain behavior | 237.6 |
| Treatment (CPT codes) | |
| Supratentorial meningioma resection | 61512, 61519 |
| Infratentorial meningioma resection | 61519 |
| Presentation (identified in the 6 mo prior to diagnosis, but not prior to that) | |
| Headache | 339, 346 |
| Seizure | 345, 780.3 |
| Visual symptoms (papilledema, field cut, visual disturbance) | 368, 369, 377.00 |
| Hemiplegia/Hemiparesis | 342 |
| Hearing loss | 389 |
| Dizziness/Vertigo | 780.4, 386.1, 386.2, 386.9 |
| Nonspecific neurologic symptoms (gait, smell, taste, coordination, aphasia, dysarthria, alterations in consciousness etc) | 780.0, 780.97, 780.2, 781.0, 781.1, 781.2, 781.3, 781.4, 784.3, 784.5 |
For assessment of MHDs, we used ICD-9 codes corresponding to episodic mood disorders, including depression (296.0-296.9), anxiety (300.0-300.9), nonalcohol drug dependence (304.0-304.9), adjustment reaction (309.0-309.1), and depressive disorder not otherwise specified (311.0-311.9). To capture those without an official MHD diagnosis, dispensing records were used for the psychotropic medications listed in the Supplement. This combined cohort represented the primary analysis. A subgroup analysis was performed for those patients who developed only depression and only anxiety. History of tobacco use was obtained from using a combination of ICD-9 Clinical Modification (ICD-9-CM) codes and CPT codes related to smoking behavior and history of alcohol use from the ICD-9-CM codes for alcohol dependence. This methodology is consistent with our previously published work.18 For a subgroup analysis, we looked at those with ICD-9 codes for depression and for anxiety separately.
Statistical Analysis
All statistical analysis was conducted in SAS version 9.4 software (SAS Institute). Univariate analysis was conducted to assess statistical significance of differences in proportions using a chi-square test for categorical variables. In the multivariable logistic regression model, we adjusted for residence (rural or urban), age, sex, region (East, South, Midwest, Northwest or Unknown), history of smoking, history of alcohol dependence, headache, seizures, visual symptoms, vertigo, hemiplegia, nonspecific neurologic symptoms (each, yes/no), and Charlson Comorbidity Index. Descriptive results are presented as proportions and the multivariable regression results are presented as adjusted odds ratio (aOR) with 95% CI. The statistical tests were significant if the P value was less than .05 (2-sided). Similar univariate and multivariable analyses were performed for the depression and anxiety subgroups.
Results
A total of 18 415 patients with newly diagnosed meningioma were identified from the database. Of these, 15 320 patients did not undergo treatment within 1 year of diagnosis and were selected for further analysis. The demographic details for this cohort are detailed in Table 2. Of these patients, approximately one-third carried a diagnosis of an MHD at the time of their meningioma diagnosis (n = 5132).
Table 2.
Baseline Characteristics of Patients With Untreated Meningiomas
| Characteristic | Variable | n (%) |
|---|---|---|
| Residence | Rural Urban | 2233 (14.58) 13087 (85.42) |
| Age, y | 18-34 35-44 45-54 55-65 | 918 (5.99) 2142 (13.98) 5106 (33.33) 7154 (46.70) |
| Sex | Male Female | 3643 (23.78) 11677 (76.22) |
| Region | South West Midwest Northeast Unknown | 5899 (38.51) 2768 (18.07) 3751 (24.48) 2701 (17.63) 201 (1.31) |
| Smoking | Yes No | 1511 (9.86) 13809 (90.14) |
| Alcohol abuse | Yes No | 343 (2.24) 14977 (97.76) |
| Presenting symptom | Headache Seizures Visual Symptoms Vertigo Hemiplegia Nonspecific | 1225 (8.0) 739 (4.82) 799 (5.22) 1660 (10.84) 60 (0.39) 1412 (9.22) |
| Preexisting mental health disorder | Yes No | 5132 (33.50) 10188 (66.50) |
Of the patients without an MHD diagnosis at the time they were diagnosed with a meningioma, 1639 (16.09%) went on to develop an MHD in the year following diagnosis of their meningioma. Of the 1639 patients with newly diagnosed MHDs following meningioma diagnosis, 661 (40%) were anxiety and 681 (42%) were depression. The remainder were those with a mixed diagnosis or substance misuse disorder.
Univariate analysis was then performed for the subset of patients who did not carry a preexisting MHD diagnosis to determine factors related to developing an MHD in the setting of a newly diagnosed, untreated meningioma (Table 3). Of note, those patients who developed an MHD after meningioma diagnosis were more likely to be female (78.22% vs 71.14%, P < .001), younger (P = .013), smokers (11.71% vs 7.98%, P < .001), or to have a history of alcohol abuse (2.50% vs 1.45%, P = .004). Furthermore, patients who developed an MHD were more likely to have presented with headaches (5.84% vs 8.66%, P < .001) or nonspecific neurological complaints (7.67% vs 9.58%, P = .011) and have higher Charlson Comorbidity Index scores (P = .001). We subsequently separated those patients diagnosed with depression and those diagnosed with anxiety and performed univariate analyses on these subgroups (Table 4). As seen with the combined cohort, these results revealed female sex, smoking history, and presenting symptoms (headache, vertigo, and nonspecific neurological symptoms) to be associated with the development both of anxiety and depression. Rural area of residence was associated with a higher chance of depression, whereas urban residence was associated with a higher chance of anxiety.
Table 3.
Univariate Analysis of Patients With Untreated Meningiomas
| Characteristic | Variable | No MHD n (%) | Developed MHD n (%) | P |
|---|---|---|---|---|
| Residence | Rural Urban | 1205 (14.10) 7344 (85.90) | 236 (14.40) 1403 (85.60) | .757 |
| Age, y | 18-34 35-44 45-54 55-65 | 568 (6.64) 1205 (14.10) 2843 (33.26) 3933 (46.01) | 119 (7.26) 278 (16.96) 528 (32.21) 714 (43.56) | .013 |
| Sex | Male Female | 2467 (28.86) 6082 (71.14) | 357 (21.78) 1282 (78.22) | < .001 |
| Region | South West Midwest Northeast Unknown | 3265 (38.19) 1471 (17.21) 2075 (24.27) 1616 (18.90) 122 (1.43) | 653 (39.84) 299 (18.24) 370 (22.57) 291 (17.75) 26 (1.59) | .322 |
| Smoking | Yes No | 682 (7.98) 7867 (92.02) | 192 (11.71) 1447 (88.29) | < .001 |
| Alcohol abuse | Yes No | 124 (1.45) 8425 (98.55) | 41 (2.50) 1598 (97.50) | .004 |
| Presenting symptom | Headache Seizures Visual symptoms Vertigo Hemiplegia Nonspecific | 499 (5.84) 373 (4.36) 453 (5.30) 831 (9.72) 27 (0.32) 656 (7.67) | 142 (8.66) 71 (4.33) 92 (5.61) 166 (10.13) 9 (0.55) 157 (9.58) | < .001 1.000 .590 .618 .168 .011 |
| Charlson Comorbidity Index score | 0 1 ≥ 2 | 5614 (65.57) 1562 (18.27) 1373 (16.06) | 1008 (61.50) 312 (19.04) 319 (19.46) | .001 |
Abbreviation: MHD, mental health disorder.
Table 4.
Univariate Analysis of Anxiety and Depression Subgroups in Patients With Untreated Meningiomas
| Characteristic | Variable | No MHD | Developed anxiety | P | Developed depression | P | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||||
| Residence | Rural Urban | 1205 7344 | 14.10 85.90 | 85 576 | 12.86 87.14 | .032 | 103 578 | 15.1284.88 | .034 |
| Age, y | 18-34 35-44 45-54 55-65 | 568 1205 2843 3933 | 6.64 14.10 33.26 46.01 | 39 123 216 283 | 5.90 18.61 32.68 42.81 | .014 | 54 101 204 322 | 7.93 14.83 29.96 27.28 | .252 |
| Sex | Male Female | 2467 6082 | 28.86 71.14 | 133 528 | 20.12 79.88 | < .001 | 149 532 | 21.88 78.12 | < .001 |
| Region | South West Midwest Northeast Unknown | 3265 1471 2075 1616 122 | 38.19 17.21 24.27 18.90 1.43 | 240 121 162 128 10 | 36.31 18.31 24.51 19.36 1.51 | .892 | 290 119 145 115 11 | 42.58 17.47 21.44 16.89 1.62 | .146 |
| Smoking | Yes No | 682 7867 | 7.98 92.02 | 67 594 | 10.14 89.86 | .009 | 79 602 | 11.60 88.40 | < .001 |
| Alcohol abuse | Yes No | 124 8425 | 1.45 98.55 | 12 649 | 1.82 98.18 | .093 | 11 670 | 1.62 98.38 | .119 |
| Presenting symptom | Headache Seizures Visual symptoms Vertigo Hemiplegia Nonspecific | 499 373 453 831 27 656 | 5.84 4.36 5.30 9.72 0.32 7.67 | 49 27 37 71 3 61 | 7.41 4.08 5.60 10.74 0.45 9.23 | .017 .076 .066 .037 .201 .021 | 62 28 31 60 6 67 | 9.10 4.11 4.55 8.81 0.88 9.84 | < .001 .076 .052 .041 .022 .008 |
| Charlson Comorbidity Index score | 0 1 ≥ 2 | 5614 1562 1373 | 65.57 18.27 16.06 | 419 114 128 | 63.39 17.25 19.36 | .084 | 406 139 136 | 59.62 20.41 19.97 | .004 |
Abbreviation: MHD, mental health disorder.
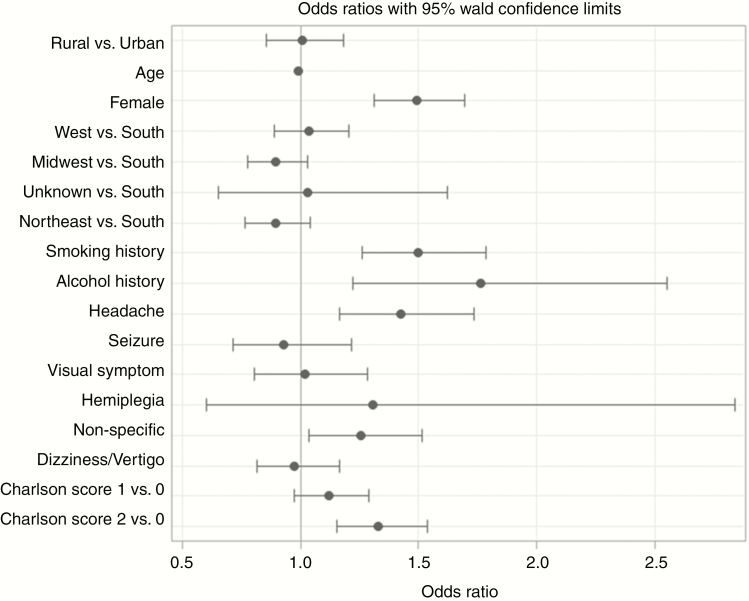
In multivariable analysis (Figure 1), patients who developed MHD were more likely to be female (aOR 1.49; CI, 1.31-1.70), have a smoking history (aOR 1.5; CI, 1.26-1.78), or alcohol history (aOR 1.77; CI, 1.22-2.55). Additionally, patients who developed an MHD were more likely to have presented with headaches (aOR 1.42; CI, 1.17-1.74), nonspecific neurologic symptoms (aOR 1.25; CI, 1.04-1.52), or to have a higher Charlson Comorbidity Index score (aOR 1.33; CI, 1.15-1.54). Of these factors, history of alcohol abuse carried the strongest association with the development of an MHD. Geographic setting, community type, and age did not have any significant effect on the odds of developing an MHD. These findings support the results of the univariate analysis. Multivariable analysis of depression and anxiety subgroups aligned with the results of the larger MHD group with a few notable differences. Female sex (aOR 1.64; CI, 1.35-2.00 and aOR 1.46; CI, 1.21-1.76, respectively) and Charlson Comorbidity Index (aOR 1.30; CI, 1.05-1.60 and aOR 1.36; CI, 1.10-1.68. respectively) were associated with increased risk for development both of anxiety and depression. The depression subgroup analysis did reveal a significant association with smoking (aOR 1.52; CI, 1.18-1.95) and headaches (aOR 1.551; CI, 1.17-2.05) that was not seen in the anxiety subgroup.
Figure 1.
Multivariable Analysis Describing Factors Associated With Mental Health Disorder and Comorbidities.
Discussion
In this large, retrospective study, we found that in patients diagnosed with an untreated meningioma, approximately 16% were diagnosed with an MHD within 1 year of the diagnosis of meningioma. Independent risk factors included female sex and substance misuse. Headaches, nonspecific neurologic symptoms, and higher Charlson Comorbidity Index scores were also independently associated with a subsequent diagnosis of MHD. On subgroup analysis, patients with depression were more likely to have a history of smoking and to have presented with headaches; however, these risk factors were not observed to be significant in the anxiety subgroup.
Mental health disorders are a major public health problem in the United States and globally.19 Estimated costs for depression alone exceeded $200 billion in the United States in 201020. Furthermore, the incidence and prevalence of MHDs are increasing.21 Well-established risk factors for the development of an MHD include low self-esteem, substance misuse, past trauma, low education, and poor social support.22 Furthermore, stressful life events are associated with an increased risk of developing MHDs.23
Naturally, it follows that the diagnosis of a malignant neoplasm of the CNS, an event that carries a substantial amount of personal stress, is associated with a high risk of developing an MHD. A review by Rooney et al of 4089 glioma patients found between 6% and 28% of patients screen positive for depression.8 Similarly, a recent large review by Huang and colleagues found 21.7% of brain tumor patients had depression or depressive symptoms.9 Further studies in this group have placed rates of anxiety between 30% and 60%.10–13
Other studies have demonstrated significant differences in MHDs between patients with malignant and benign CNS neoplasms. Mainio et al found that although anxiety is initially high in patients following the diagnosis of a benign meningioma, this anxiety normalizes within 5 years.24 Further, rates of depression appear to increase over time in those with a malignant glioma, but not with histologically benign neoplasms.17,25 These differences can be attributed to the often-grim prognosis of malignant entities, demands of treatment, and frequent decline in neurologic function.
Meningiomas carry a far more favorable prognosis, and the burden of treatment is often less demanding. However, because meningioma is the most frequently diagnosed primary CNS neoplasm, the potential burden of concurrent MHDs may be significant. Here, we found approximately 16% of patients with newly diagnosed, untreated meningiomas developed an MHD. This finding is in keeping with previous small series examining the relationship between MHDs and meningioma. Goebel and Mehdorn10 examined 52 patients undergoing neurosurgical treatment for meningioma and found that 20% screened positive for anxiety after their diagnosis and 8% for depression. However, the rate of anxiety dropped to 10% following surgery, suggesting at least part of their anxiety was related to their upcoming surgical treatment.
In Goebel and Mehdorn,10 the authors found that rates of anxiety and depression were similar to the general German population from which the cohort was pulled. Similarly, our own cohort reflects the prevalence of MHDs in the US population. We found that close to one-third (33.5%) of our cohort had an MHD diagnosis prior to the meningioma diagnosis. Large US population studies have observed a lifetime prevalence of depression and anxiety to be approximately 29% (18% and 11%, respectively20,26,27). This may not be universal because a recent study from Thurin et al focusing on return to work following meningioma surgery in the Swedish population found that premorbid rates of depression were significantly higher in meningioma patients compared to controls (21.3% vs 16.5%, P < .001).28 One should also note a nearly 8% increase in history of depression at the time of surgery compared to 1 year prior, which is in line with our findings that meningioma diagnosis can have an impact on diagnosis of MHDs.
Characteristics identified in our study with increased odds of developing an MND included female sex and history of alcohol or tobacco use. This is not surprising given these variables are associated with increased prevalence of MHDs in the general population.22,29–32 Recent data further underscore the role of MHD, specifically depression, in return to work and long-term quality of life in patients with meningioma.28 Understanding the prevalence of MHD development following meningioma diagnosis and appreciating patient and tumor factors associated with a higher risk of MHD development will be of importance when considering screening and survivorship protocols. Incorporation of generic depression and anxiety batteries and early referral to mental health practitioners could be considered in the highest-risk groups.
Interestingly, patients presenting with symptoms of headache and nonspecific symptoms had a higher likelihood of developing an MHD in multivariable analysis. It is surprising that hemiplegia, often a more debilitating symptom then headaches, was not independently associated with risk of MHD. A consideration was made to include cognitive changes in the symptoms analyzed; however, from previous experience with this data set, it was evident that cognitive changes were notoriously undercoded and therefore the decision was made to forego the inclusion of cognitive changes as a symptom. To our knowledge, no other study of mental health in brain tumor patients has examined an association between presenting symptom and the odds of developing a subsequent MHD.
Although unable to determine this from a database study such as this, it is feasible that specific individual patient factors related to the tumor and diagnosis may have a profound effect on the likelihood of subsequent MHD. For instance, the psychological effect of counseling provided by the patient’s neurosurgeon could have a heavy impact on the patient’s outlook about his or her diagnosis. It is entirely conceivable that a young patient with a large meningioma who is counseled that surgery might someday be necessary would have a different psychological burden than an older counterpart with a small meningioma who is counseled that necessary intervention is exceedingly unlikely. Unfortunately, the inherent limitations of this study design make any inference on this possibility impossible but certainly further studies to explore this would be valuable.
The primary limitation of this study is inherent limitations to the MarketScan database. As such, errors in clinical coding may have resulted in inaccurate data. However, given the incentive to maximize billing revenue, it is likely that the coding data is reasonably accurate. Additionally, the MarketScan database captures data only from private payers and therefore information regarding meningioma patients covered by Medicare or Medicaid is not included. Our study may therefore underestimate the true prevalence of MHDs because of the high prevalence of MHDs in patients of low socioeconomic status. Efforts are under way to address this limitation with a more diverse cohort. Additionally, patients with MHDs underuse mental health services and may not be accurately captured by a claims-based database. Furthermore, we included only depression, anxiety, adjustment disorders, and nonalcohol substance misuse in our query of MHDs and therefore may not have captured patients with diagnoses such as bipolar or primary psychosis. Unfortunately, brain tumor location could not be gleaned from the database and was not included in the analysis. A final limitation is our decision to exclude meningioma patients undergoing treatment. These patients are unavoidably subject to a different set of physiologic and psychosocial stressors, which may skew results given the distinct association pattern between MHDs and meningiomas altogether. Further population-based studies are needed to accurately illustrate the association between MHDs and patient undergoing treatment for meningiomas.
Conclusion
Our study explores the relationship between MHDs and untreated meningiomas across a large and broad population-based cohort. We found a significant proportion of patients with untreated meningiomas will develop an MHD. By identifying risk factors, appropriate screening can focus on patients at high risk for the development of an MHD.
Supplementary Material
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: B.E.Z. has received speakers fees from NICO Corp and has served as a consultant for Medtronic Inc. N.G. has received grant funding from Medrobotics Corp and book royalties from Thieme Inc. The other authors have nothing to declare.
References
- 1. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. [DOI] [PubMed] [Google Scholar]
- 4. Annegers JF, Schoenberg BS, Okazaki H, Kurland LT. Epidemiologic study of primary intracranial neoplasms. Arch Neurol. 1981;38(4):217–219. [DOI] [PubMed] [Google Scholar]
- 5. Nakasu S, Hirano A, Shimura T, Llena JF. Incidental meningiomas in autopsy study. Surg Neurol. 1987;27(4):319–322. [DOI] [PubMed] [Google Scholar]
- 6. Islim AI, Mohan M, Moon RDC, et al. Incidental intracranial meningiomas: a systematic review and meta-analysis of prognostic factors and outcomes. J Neurooncol. 2019;142(2):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Radhakrishnan K, Mokri B, Parisi JE, O’Fallon WM, Sunku J, Kurland LT. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37(1):67–73. [DOI] [PubMed] [Google Scholar]
- 8. Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61–76. [DOI] [PubMed] [Google Scholar]
- 9. Huang J, Zeng C, Xiao J, et al. Association between depression and brain tumor: a systematic review and meta-analysis. Oncotarget. 2017;8(55):94932–94943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goebel S, Mehdorn HM. Development of anxiety and depression in patients with benign intracranial meningiomas: a prospective long-term study. Support Care Cancer. 2013;21(5):1365–1372. [DOI] [PubMed] [Google Scholar]
- 11. Mainio A, Hakko H, Niemelä A, Koivukangas J, Räsänen P. Depression and functional outcome in patients with brain tumors: a population-based 1-year follow-up study. J Neurosurg. 2005;103(5):841–847. [DOI] [PubMed] [Google Scholar]
- 12. Bunevicius A, Tamasauskas S, Deltuva V, Tamasauskas A, Radziunas A, Bunevicius R. Predictors of health-related quality of life in neurosurgical brain tumor patients: focus on patient-centered perspective. Acta Neurochir (Wien). 2014;156(2):367–374. [DOI] [PubMed] [Google Scholar]
- 13. Pringle AM, Taylor R, Whittle IR. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. Br J Neurosurg. 1999;13(1):46–51. [DOI] [PubMed] [Google Scholar]
- 14. van der Vossen S, Schepers VP, Berkelbach van der Sprenkel JW, Visser-Meily JM, Post MW. Cognitive and emotional problems in patients after cerebral meningioma surgery. J Rehabil Med. 2014;46(5):430–437. [DOI] [PubMed] [Google Scholar]
- 15. Gathinji M, McGirt MJ, Attenello FJ, et al. Association of preoperative depression and survival after resection of malignant brain astrocytoma. Surg Neurol. 2009;71(3):299–303, discussion 303. [DOI] [PubMed] [Google Scholar]
- 16. Mainio A, Tuunanen S, Hakko H, Niemelä A, Koivukangas J, Räsänen P. Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: a follow-up from 1990 to 2003. Eur Arch Psychiatry Clin Neurosci. 2006;256(8):516–521. [DOI] [PubMed] [Google Scholar]
- 17. IBM MarketScan Research Database for Health Services Researchers white paper. IBM 2019. https://www.ibm.com/products/marketscan-research-databases [Google Scholar]
- 18. Lee JH, Ba D, Liu G, Leslie D, Zacharia BE, Goyal N. Association of head and neck cancer with mental health disorders in a large insurance claims database. JAMA Otolaryngol Head Neck Surg. 2019;145(4):339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D’Angelo C, Mirijello A, Leggio L, et al. State and trait anxiety and depression in patients with primary brain tumors before and after surgery: 1-year longitudinal study. J Neurosurg. 2008;108(2):281–286. [DOI] [PubMed] [Google Scholar]
- 20. Kessler RC, Ormel J, Petukhova M, et al. Development of lifetime comorbidity in the World Health Organization world mental health surveys. Arch Gen Psychiatry. 2011;68(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162. [DOI] [PubMed] [Google Scholar]
- 22. Compton WM, Conway KP, Stinson FS, Grant BF. Changes in the prevalence of major depression and comorbid substance use disorders in the United States between 1991-1992 and 2001-2002. Am J Psychiatry. 2006;163(12):2141–2147. [DOI] [PubMed] [Google Scholar]
- 23. Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. Am J Psychiatry. 2006;163(1):115–124. [DOI] [PubMed] [Google Scholar]
- 24. Mainio A, Hakko H, Timonen M, Niemelä A, Koivukangas J, Räsänen P. Depression in relation to survival among neurosurgical patients with a primary brain tumor: a 5-year follow-up study. Neurosurgery. 2005;56(6):1234–1241; discussion 1241. [DOI] [PubMed] [Google Scholar]
- 25. Litofsky NS, Farace E, Anderson F Jr, Meyers CA, Huang W, Laws ER Jr; Glioma Outcomes Project Investigators Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery. 2004;54(2):358–366; discussion 366–367. [DOI] [PubMed] [Google Scholar]
- 26. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 27. Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38(3):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thurin E, Corell A, Gulati S, et al. Return to work following meningioma surgery: a Swedish nationwide registry-based matched cohort study. Neurooncol Pract. 2019:npz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163(1):109–114. [DOI] [PubMed] [Google Scholar]
- 30. Seedat S, Scott KM, Angermeyer MC, et al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66(7):785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. [DOI] [PubMed] [Google Scholar]
- 32. Steinberg ML, Williams JM, Li Y. Poor mental health and reduced decline in smoking prevalence. Am J Prev Med. 2015;49(3):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.