Abstract
Background
Treatment for glioblastoma (GBM) in elderly (age > 65 years) patients can be affected by multiple geographic and socioeconomic parameters. Correspondingly, the aim of this study was to determine trends in treatment of elderly GBM patients in the United States.
Methods
All GBM patients in the U.S. National Cancer Database between 2005 and 2016 were retrospectively reviewed. Status of treatment by triple therapy (resection, chemotherapy, and radiation) were summarized and analyzed by U.S. Census region.
Results
There were 44 338 GBM patients included, with 21 573 (49%) elderly and 22 765 (51%) nonelderly patients with median ages 72 years (range, 65-90 years) and 47 years (range, 40-64 years), respectively. Compared to nonelderly patients, elderly patients had significantly lower odds of being treated by triple therapy (odds ratio, OR = 0.54) as a whole, and its individual elements of resection (OR = 0.78), chemotherapy (OR = 0.46), radiation therapy (OR = 0.52). This was reflected in each U.S. Census region, with the lowest odds of being treated with triple therapy, surgical resection, chemotherapy, and radiation therapy in New England (OR = 0.51) Mountain (OR = 0.66), West North Central (OR = 0.38), and the Middle Atlantic (OR = 0.44), respectively. Multivariable analysis revealed multiple socioeconomic parameters that significantly predicted lower odds of triple therapy in the elderly.
Conclusions
In the United States alone, there exists geographic disparity in the treatment outcomes of elderly GBM patients. Multiple socioeconomic parameters can influence access to treatment modalities for elderly patients compared to younger patients in different geographic regions, and public health initiatives targeting these aspects may prove beneficial conceptually to optimize and homogenize clinical outcomes.
Keywords: access, disparity, elderly, geographic, glioblastoma
Glioblastoma (GBM) is a devastating malignant brain tumor despite standard of care treatment including maximal safe resection followed by chemotherapy and radiation therapy.1 Issues facing our attempts in improving prognosis for this disease include homogenizing access to these different modalities of treatment. Whether access to treatment for GBM patients is vulnerable to age, geographic, and socioeconomic biases is not clear, especially in the setting of older patients in the United States.
Elderly patients (age > 65 years) diagnosed with GBM represent a growing demographic population known to follow a more vulnerable clinical course than younger patients.2 They are known to be less tolerant of chemoradiation,2 as well as less amenable to aggressive surgical resection.3 Yet views on optimal treatment for these patients are heterogeneous among clinicians, and therefore, ultimate treatment plans are likely to vary.4 Whether this variation can be mapped geographically at a national, public health level with possible predictors of treatment has yet to be attempted. Doing so would provide future efforts and direction as to which aspects of GBM treatment in the elderly can be targeted further in this population. Correspondingly, we sought to interrogate a national cancer database to determine whether indeed elderly GBM patients are treated at odds different from that of younger patients, as well as to identify whether any geographic or socioeconomic variations exist in the likelihood of accessing care.
Methods
Patient Selection
All data used for this study were extracted from the 2016 iteration of the National Cancer Database (NCDB), a database maintained by the Commission on Cancer and the American Cancer Society since 2004 that describes more than 70% of new cancer diagnoses from 1500 hospitals in the United States.5 The database was queried for all patients that satisfied the following criteria; inclusion criteria of 1) surgically obtained (biopsy or resection only) histological diagnosis of World Health Organization Grade IV malignant GBM (ICD-0-3 9440/3), 2) in an intracranial location, 3) in adults older than 18 years, 4) from known geographic locations with known treatment (surgical resection, chemotherapy, and radiation therapy) statuses, 5) diagnosed from 2005 onward; and exclusion criteria of 1) postmortem surgical diagnosis, that is, autopsy, 2) nonintracranial locations, 3) pediatric patients younger than 18 years, 4) diagnosed in 2004, 5) and incomplete treatment or Hispanic status data. The cutoff year of 2005 was decided to represent the year in which the Stupp protocol1 (ie, triple therapy) was established as standard of care.
Socioeconomic Data
Basic demographic and socioeconomic data were directly abstracted from the database listings. These included age, sex, treating facility, insurance status, yearly income, education level, metropolitan residence, and comorbidity status. Treating facilities were coded as either academic or community settings. The insurance variable in the NCDB describes the insurance status at treatment. The income and education variables describe the average household income and proportion of non–high school graduates, respectively, in the patient’s residential zip code at time of diagnosis based on the 2000 U.S. Census data. The income variable brackets reported were less than $40 227, $40 227 to $50 353, $50 354 to $63 332, and more than $63 333, and education was reported as less than 6.3%, 6.3% to 10.8%, 10.9% to 17.5%, and more than 17.6% population being non–high school graduates. The metropolitan residence variable was defined by indexing the patient’s residential zip code at diagnosis to the 2003 files Rural-Urban Continuum Codes as developed by the U.S. Department of Agriculture Economic Research Services. The variable brackets were categorized as metropolitan (> 250 000 population) vs urban (2500-250 000 population), and rural (< 2500 population). Comorbidity was defined by the Charlson-Deyo score, where a score of 1 or more was defined as a patient having at least one comorbidity. Patients were included only when all data elements were provided, and excluded if any data element was absent or listed as missing.
Geographical Region Data
We abstracted the location of all patient data to 1 of 9 US Census regions used by the U.S. Bureau of the Census that categorizes all 50 states as 1) New England: Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, and Vermont; 2) Middle Atlantic: New Jersey, New York, and Pennsylvania; 3) South Atlantic: District of Columbia, Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, and West Virginia; 4) East North Central: Illinois, Indiana, Michigan, Ohio, and Wisconsin; 5) East South Central: Alabama, Kentucky, Mississippi, and Tennessee; 6) West North Central: Iowa, Kansas, Minnesota, Missouri, North Dakota, Nebraska, and South Dakota; 7) West South Central: Arkansas, Louisiana, Oklahoma, and Texas; 8) Mountain: Arizona, Colorado, Idaho, Montana, New Mexico, Nevada, Utah, and Wyoming; and 9) Pacific: Alaska, California, Hawaii, Oregon, and Washington.
Outcomes of Interest
Patients were defined in the elderly cohort if they were 65 years or older at diagnosis, with patients diagnosed at younger than 65 years defined into the nonelderly cohort. Primary outcomes of interest for these groups were the statuses of treatment—surgical resection, chemotherapy and radiation therapy. Positive surgical resection was defined as either subtotal or gross total resection as listed in the “cs_sitespecific_factor_7” variable. Positive chemotherapy was defined as patients receiving either single- or multiple-agent chemotherapy agent regimens as listed in the “rx_summ_chemo” variable. Positive radiation therapy was defined as treatment by any beam radiation regimen as listed in the “rx_summ_radiation” variable. Triple therapy was defined as simultaneous positive statuses for surgical resection, chemotherapy, and radiation therapy. Because our focus was on triple therapy and its individual components that make it up, and as such, dual combinations were not considered.
Statistical Analyses
Outcome comparisons between elderly vs nonelderly cohorts were conducted using the chi-square exact test and Wilcoxon rank-sum test for categorical and continuous data, respectively. Kaplan-Meier estimations and univariable followed by multivariable Cox proportional regression analyses were conducted to evaluate overall survival (OS) and determine hazard ratio (HR) of potential predictors. Odds of treatment with a particular modality vs without that modality for elderly vs nonelderly were then calculated by means of proportion and reported as odds ratio (OR) with 95% CI. Univariable followed by multivariable logistic regression analyses were then performed to analyze access to each treatment modality based on elderly status. For all regression analyses, univariable analysis was first conducted to identify candidate predictive parameters; based on these results, variables demonstrating a between-groups test statistic of P less than .10 were included in multivariable analysis to determine the independence of these factors. All analyses were conducted using STATA 14.1 (StataCorp); tests were 2-sided, and statistical significance was defined using the α threshold of .05.
Results
Demographics and Socioeconomic Characteristics
A total cohort size of 44 338 GBM patients satisfied criteria for inclusion into our analyses (Table 1). There were 21 573 (49%) age 65 years or older in the elderly cohort, and 22 765 (51%) in the nonelderly cohort, and median ages were 72 years (range, 65-90 years) and 47 years (range, 40-64 years), respectively. Overall, there were 18 471 (42%) female and 25 867 (58%) male patients, 20 305 (46%) treated in an academic facility, and 1663 (8%) Hispanic patients. Those in the elderly cohort had statistically more male patients, more patients treated at an academic facility, and fewer Hispanic patients. With respect to census region overall, the most and least represented regions were the South Atlantic (20%) and Mountain (5%), respectively. This distribution was statistically different between elderly and nonelderly cohorts, with the elderly being most represented in the South Atlantic (19%), but least represented in the New England (5%) and Pacific (5%) regions. In terms of other socioeconomic parameters, the elderly (vs nonelderly) cohort was statistically more likely insured by Medicare (82% vs 8%), reside in a metropolitan area (82% vs 80%), and present with a comorbidity (36% vs 25%).
Table 1.
Demographic and Socioeconomic Characteristics of Overall Cohort With Comparison of Proportions Between Elderly and Nonelderly Cohorts
| Parameter | Overall (n,%) | Elderly | Nonelderly | P |
|---|---|---|---|---|
| Size, No. | 44 338 | 21 573 | 22 765 | |
| Sex | < .01 | |||
| Female | 18 471 (42%) | 9269 (43%) | 9202 (40%) | |
| Male | 25 867 (58%) | 12 304 (57%) | 13 563 (60%) | |
| Age (median, range), y | 64 (40-90) | 72 (65-90) | 47 (40-64) | NA |
| Hispanic | ||||
| Yes | 3615 (8%) | 1663 (8%) | 1952 (9%) | < .01 |
| No | 40 723 (92%) | 19 910 (92%) | 20 813 (91%) | |
| Treating facility | < .01 | |||
| Academic | 20 305 (46%) | 9234 (43%) | 11 071 (49%) | |
| Community | 24 033 (54%) | 12 339 (57%) | 11 694 (51%) | |
| Census region | < .01 | |||
| New England | 2354 (5%) | 1245 (6%) | 1109 (5%) | |
| Middle Atlantic | 7198 (16%) | 3579 (17%) | 3519 (16%) | |
| South Atlantic | 8940 (20%) | 4511 (21%) | 4429 (19%) | |
| East North Central | 7544 (17%) | 3607 (17%) | 3937 (17%) | |
| East South Central | 2677 (6%) | 1317 (6%) | 1360 (6%) | |
| West North Central | 3313 (7%) | 1589 (7%) | 1724 (8%) | |
| West South Central | 4102 (9%) | 1874 (9%) | 2228 (10%) | |
| Mountain | 2218 (5%) | 1047 (5%) | 1171 (5%) | |
| Pacific | 5992 (14%) | 2804 (6%) | 1109 (5%) | |
| Insurance status | < .01 | |||
| Medicare | 18 489 (44%) | 17 739 (82%) | 1750 (8%) | |
| Private insurance | 19 324 (44%) | 2853 (13%) | 16 471 (72%) | |
| Medicaid | 2605 (6%) | 317 (1%) | 2288 (10%) | |
| Not insured | 1481 (3%) | 150 (1%) | 1331 (6%) | |
| Other | 1439 (3%) | 514 (2%) | 925 (4%) | |
| Yearly income, $ | < .01 | |||
| > 63 333 | 17 492 (39%) | 8303 (38%) | 9189 (40%) | |
| 50 354-63 332 | 10 449 (24%) | 5105 (24%) | 5344 (23%) | |
| 40 227-50 353 | 9289 (21%) | 4685 (22%) | 4604 (20%) | |
| < 40 227 | 7108 (16%) | 3480 (16%) | 3528 (16%) | |
| Education level, % | .06 | |||
| < 6.3 | 12 637 (29%) | 6183 (29%) | 6454 (28%) | |
| 6.3-10.8 | 13 345 (30%) | 6483 (30%) | 6862 (30%) | |
| 10.9-17.5 | 10 781 (24%) | 5318 (25%) | 5463 (24%) | |
| > 17.6 | 7575 (17%) | 3589 (17%) | 3986 (18%) | |
| Metropolitan residence | < .01 | |||
| Yes | 35 871 (81%) | 17 234 (80%) | 18 637 (82%) | |
| No | 8467 (19%) | 4339 (20%) | 4128 (18%) | |
| Comorbidity | < .01 | |||
| Yes | 12 425 (30%) | 7787 (36%) | 5638 (25%) | |
| No | 30 913 (70%) | 13 786 (64%) | 17 127 (75%) |
Abbreviation: NA, not available.
Significant P values appear in bold.
Treatment Predicts Overall Survival in Elderly Cohort
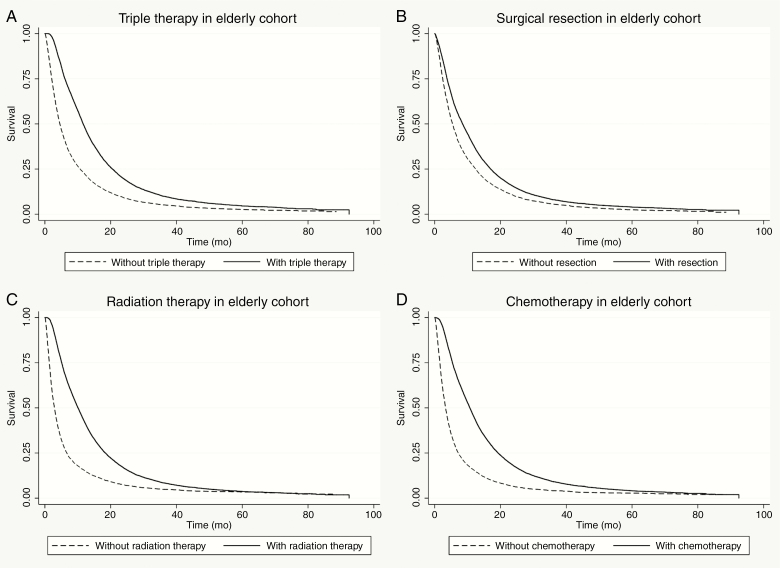
The median OS in the elderly vs nonelderly cohort was 7.3 months (95% CI, 7.2-7.5) vs 15.3 months (95% CI, 15.2-15.6), respectively (P < .01). In the elderly cohort, triple therapy resulted in significantly longer OS compared to those treated without triple therapy (Figure 1). Similarly, elderly GBM patients treated with surgical resection, chemotherapy, and radiation therapy individually all had superior OS compared to patients treated without those modalities (Figure 1). Multivariable analysis, which incorporated significant univariable demographic and socioeconomic parameters, confirmed that all elements of triple therapy independently predicted longer OS in the elderly cohort, that is, surgical resection (HR = 0.81), chemotherapy (HR = 0.57), and radiation therapy (HR = 0.69) (Supplementary Table 1).
Figure 1.
Kaplan-Meier plots of overall survival within the elderly cohort treated with A, vs without triple therapy (11.7 vs 4.6 months; log-rank P < .01); B, resection (8.5 vs 5.4 months; log-rank P < .01); C, radiation therapy (10.1 vs 2.9 months; log-rank P < .01); and D, chemotherapy (10.7 vs 3.3 months; log-rank P < .01).
Treatment Access Outcomes by Elderly Status
With respect to treatment access in the overall cohort, 52% were treated with triple therapy, 73% with surgical resection, 71% with chemotherapy, and 75% with radiation therapy (Table 2). Compared to the nonelderly cohort, elderly patients were treated at significantly lower proportions in terms of triple therapy (45% vs 59%, OR = 0.54), as well as surgical resection (71% vs 76%, OR = 0.78) chemotherapy (63% vs 79%, OR = 0.46), and radiation therapy (69% vs 81%, OR = 0.52).
Table 2.
Outcomes for Entire Cohort With Comparison of Proportions by Odds Ratio Between Elderly and Nonelderly Groups
| Parameter | Overall | Elderly | Nonelderly | OR (95% CI) | P |
|---|---|---|---|---|---|
| Size, No. | 44 338 | 21 573 | 22 765 | ||
| Triple therapy | 0.56 (0.54-0.58) | < .01 | |||
| Yes | 23 160 (52%) | 9684 (45%) | 13 476 (59%) | ||
| No | 21 178 (48%) | 11 889 (55%) | 9289 (41%) | ||
| Surgical outcome | 0.78 (0.75-0.81) | < .01 | |||
| Resection | 32 435 (73%) | 15 234 (71%) | 17 201 (76%) | ||
| Biopsy | 11 903 (27%) | 6339 (29%) | 5564 (24%) | ||
| Chemotherapy | 0.46 (0.45-0.48) | < .01 | |||
| Yes | 31 634 (71%) | 13 681 (63%) | 17 953 (79%) | ||
| No | 12 704 (29%) | 7892 (37%) | 4812 (21%) | ||
| Radiation therapy | 0.52 (0.50-0.55) | < .01 | |||
| Yes | 33 391 (75%) | 14 935 (69%) | 18 456 (81%) | ||
| No | 10 947 (25%) | 6638 (31%) | 4309 (19%) |
Abbreviation: OR, odds ratio.
Significant odds ratios with 95% CI and significant P values appear in bold.
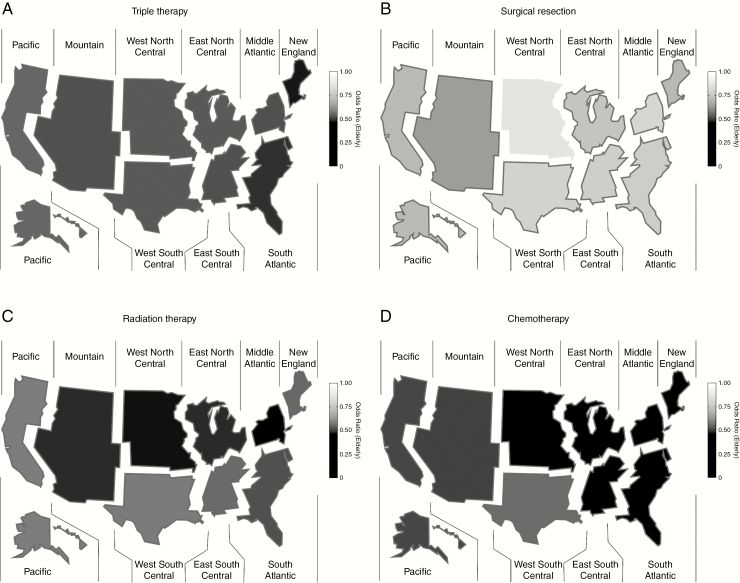
Regional Odds of Treatment Access by Elderly Status
The odds of treatment in the elderly cohort vs the nonelderly cohort were then compared for each US Census region (Table 3). The odds were significantly lower for the elderly cohort to be treated with triple therapy, as well as surgical resection, chemotherapy, and radiation therapy in all 9 Census regions, with the one exception of surgical resection in West North Central (Figure 2). The lowest odds of accessing triple therapy, and then surgical resection, chemotherapy, and radiation therapy, and triple therapy in the elderly cohort were in the New England (OR = 0.51), Mountain (OR = 0.66), West North Central (OR = 0.38), and Middle Atlantic (OR = 0.44) regions, respectively.
Table 3.
Treatment Outcomes for Entire Cohort by Census Region With Odds Ratios for Treatment Calculated Comparing Elderly to Nonelderly Cohorts
| Triple therapy | Surgical resection | Chemotherapy | Radiation therapy | |
|---|---|---|---|---|
| Census region | ||||
| New England | 0.51 (0.43-0.61) | 0.72 (0.59-0.88) | 0.39 (0.31-0.48) | 0.55 (0.43-0.69) |
| Middle Atlantic | 0.55 (0.50-0.61) | 0.83 (0.75-0.92) | 0.40 (0.36-0.45) | 0.44 (0.39-0.50) |
| South Atlantic | 0.54 (0.50-0.59) | 0.78 (0.70-0.85) | 0.46 (0.42-0.51) | 0.52 (0.47-0.57) |
| East North Central | 0.56 (0.51-0.61) | 0.76 (0.69-0.85) | 0.42 (0.38-0.47) | 0.48 (0.43-0.54) |
| East South Central | 0.57 (0.47-0.65) | 0.80 (0.67-0.94) | 0.44 (0.37-0.53) | 0.55 (0.46-0.65) |
| West North Central | 0.58 (0.51-0.67) | 0.90 (0.77-1.06) | 0.38 (0.32-0.45) | 0.47 (0.39-0.56) |
| West South Central | 0.58 (0.51-0.66) | 0.81 (0.70-0.93) | 0.56 (0.49-0.63) | 0.58 (0.51-0.65) |
| Mountain | 0.53 (0.45-0.63) | 0.66 (0.55-0.80) | 0.51 (0.42-0.62) | 0.48 (0.38-0.58) |
| Pacific | 0.57 (0.51-0.63) | 0.72 (0.64-0.81) | 0.51 (0.45-0.57) | 0.58 (0.52-0.66) |
Significant odds ratios with 95% CI appear in bold.
Figure 2.
Heat map of significant odds ratios for treatment calculated comparing elderly to nonelderly cohorts for A, triple therapy; B, resection; C, radiation therapy; and D, chemotherapy. Regions with significant ratios are outlined in red.
Within these results, the odds for elderly patients to receive chemotherapy in the New England, Middle Atlantic, and West North Central regions were significantly lower than the Pacific and West South Central regions. The odds for elderly patients to receive radiation therapy were significantly lower in the Middle Atlantic region than the West South Central and Pacific regions. There were no regions in which the elderly cohort had significantly higher odds of accessing any treatment modality compared to the nonelderly cohort.
Access to Treatment in the Elderly Cohort
We analyzed socioeconomic parameters within the elderly cohort that could predict treatment by triple therapy first, and then treatment modality separately, across all U.S. Census regions. Univariable analysis identified multiple parameters as potential predictors of access (Supplementary Table 2). Multivariable analyses revealed within the elderly cohort that the odds of treatment by triple therapy were significantly decreased by older age, female sex, Hispanic status, Medicaid insurance, no insurance, lower income bracket, lower proportions of high school graduates, and the presence of a comorbidity (Table 4). Furthermore, these parameters were found to affect all the individual components of triple therapy similarly (Supplementary Table 3).
Table 4.
Multivariable Logistic Regression Analysis of Socioeconomic Parameters to Predict Treatment by Triple Therapy in Elderly Group
| Parameter | Triple therapy | ||
|---|---|---|---|
| OR (95% CI) | Coefficient, SE | P | |
| Age, y | 0.93 (0.93-0.94) | –0.07, 0.01 | < .01 |
| Sex | |||
| Female | 0.94 (0.89-0.99) | –0.06, 0.03 | .04 |
| Male (REF) | . | . | . |
| Hispanic | |||
| Yes | 0.87 (0.79-0.97) | –0.13, 0.05 | .01 |
| No (REF) | . | . | . |
| Treating facility | |||
| Academic (REF) | . | . | . |
| Community | 0.96 (0.91-1.02) | –0.04, 0.03 | .16 |
| Insurance status | |||
| Medicare (REF) | . | . | . |
| Private insurance | 0.94 (0.86-1.02) | –0.07, 0.04 | .12 |
| Medicaid | 0.68 (0.53-0.86) | –0.39, 0.12 | < .01 |
| Not insured | 0.42 (0.29-0.60) | –0.87, 0.18 | < .01 |
| Other | 0.85 (0.71-1.02) | –0.17, 0.09 | .08 |
| Yearly income, $ | |||
| > 63 333 (REF) | . | . | . |
| 50 354-63 332 | 0.90 (0.84-0.98) | –0.10, 0.04 | .01 |
| 40 227-50 353 | 0.85 (0.78-0.93) | –0.16, 0.04 | < .01 |
| < 40 227 | 0.73 (0.66-0.81) | –0.32, 0.05 | < .01 |
| Education level, % | |||
| < 6.3% (REF) | . | . | . |
| 6.3-10.8 | 0.92 (0.85-0.99) | –0.09, 0.04 | .02 |
| 10.9-17.5 | 0.86 (0.78-0.94) | –0.15, 0.05 | < .01 |
| > 17.6 | 0.80 (0.72-0.89) | –0.22, 0.05 | < .01 |
| Comorbidity | |||
| Yes | 0.75 (0.71-0.79) | –0.29, 0.03 | < .01 |
| No (REF) | . | . | . |
Abbreviations: OR, odds ratio; REF, reference variable.
Significant odds ratios with 95% CI, coefficient, SE values, and significant P values appear in bold.
Discussion
The optimal treatment regimen for elderly GBM patients (age > 65 years) is not comprehensively defined. One challenge facing any standardization is the complicated nature of treating older vs younger patients. Our study of a US national database demonstrates that although treatment is clearly effective in improving outcomes in elderly GBM patients, treatment patterns have geographic variations across the country and across the modalities of surgical resection, chemotherapy, and radiation therapy. Furthermore, there are multiple socioeconomic factors that affect the likelihood of these specific patients being treated by standard GBM treatment modalities, providing public health initiatives with areas to target to homogenize treatment availability across the country.
The unique component of our study was that it highlighted the statistically significant lower treatment likelihoods for elderly GBM patients compared to younger patients being heterogeneous across U.S. Census regions. This follows a previous effort describing that, for GBM patients of all ages, prognosis varies across the United States based on geographic location.6 Superficially, our results could indicate that particular treatment modalities in particular regions of the United States are more accessible to elderly patients, or that particular socioeconomic factors affecting elderly patients accessing treatment are more prevalent in certain areas.7 However, perhaps mechanistically, it may also reflect anecdotally the heterogeneous nature of the public health infrastructure at a regional level to communicate, arrange, and facilitate treatment for cancers of this age group.8
Replicating treatment regimens designed primarily for younger GBM patients remains difficult to achieve in elderly GBM patients because of age-associated changes in physiology. Elderly patients typically present in poorer physical shape than younger patients, limiting to a degree their postoperative recovery potential and therefore amenability to more-aggressive resections.9 Fitness for chemoradiation can be affected by multiple chronic conditions, polypharmacy, frailty, and predispositions to neurocognitive decline, all aspects that are more prevalent in the older age groups.10,11 Therefore, it is not necessarily surprising that consistently throughout the United States, both at a national and census region level, the odds of all 3 treatment modalities were significantly lower in the elderly cohort compared to the nonelderly cohort.
The question remains, however, whether such lower odds compared to younger patients is detrimental to the prognosis of GBM in the elderly patients in the United States. Outside surgery when safe, multiple large randomized trials have provided conflicting results as to the optimal adjuvant combination for elderly patients, confounding any assumption that elderly patients require all modalities to achieve the best odds of survival.12 The NOA-08 trial13 showed temozolomide (TMZ) was comparable to radiation therapy alone in terms of survival in GBM patients younger than 65 years, but the NORDIC trial14 concluded that TMZ was superior to radiation therapy alone in patients older than 70 years. Perry et al15 demonstrated that concomitant TMZ with radiation therapy conferred a survival advantage to radiation therapy alone in those older than 65 years. Our finding that there are different odds of treatment in elderly GBM patients in different census regions for different modalities likely reflects an intrinsic heterogeneity in treatment paradigms in the absence of an established treatment consensus in this population.
There is little evidence suggesting the need to emphasize equality of access to GBM care within elderly patients across different geographic regions. This is in great contrast to the pediatric literature, which extensively describes efforts in which cancer care and access can be homogenized.16,17 Given our finding that odds of treatment are much less compared to younger patients, we encourage future efforts to truly start to consider elderly GBM as a niche GBM demographic requiring specific focus in terms of access and availability of treatment. This could include specialized clinical trials, for example, because many experimental, prospective efforts designed to treat GBM are focused more on patients younger than 65 (eg, the landmark European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada Clinical Trials Group study1 establishing TMZ and radiation therapy as convention had an inclusion criterion of < 70 years). Further socioeconomic analysis may reveal region-dependent biases affecting retirees in accessing care, which could also contribute to homogenizing care and standardizing GBM outcomes.
Outside the validation of increasing age and comorbidities as risk factors for lower odds of treatment even within the elderly, we also identified many significant socioeconomic predictors of treatment that resonated for all treatment modalities individually and collectively. Perhaps the domain of the most public health interest would be insurance status and income levels, which appear to significantly modulate the treatment modalities used. These trends have been alluded to in the GBM population across all ages previously,18 but the mechanisms by which they can be addressed in the elderly population requires special consideration. Given a greater majority of patients older than 65 years are retired, the intricate interaction between pension and insurance likely plays a large role in determining the affordability of treatment of GBM for these patients, contrasted to the younger population, who are receiving an active income.19
There are limitations to this study. First, being a retrospective analysis of a national database, there remain clinical data that cannot be determined or imputed to our analysis. It is entirely possible that absence of a particular treatment (hence not triple therapy) may have been influenced by inability or contraindication to another modality, which can be appreciated only with prospective data. Second, a better understanding of clinical decision making specific to patients older than 65 years would also be beneficial in interpreting the differences in treatment outcomes across the country. Third, the categorization of U.S. Census regions provides some indication of where possible geographic biases exist; however, state-by-state and institution-by-institution data would prove even more useful when determining where to focus public health initiatives. Unfortunately, that level of data is not available in the NCDB. Last, the elderly demographic is a unique niche in GBM management because, given the disease’s malignant course, it would not be surprising if a larger proportion of patients prefer to forgo any treatment compared to younger patients in pursuit of their own interpretation of quality of life. The bioethical considerations in this setting could possibly affect access to treatment and their outcomes, which is a confounder in the current methodologies used to present and analyze population data about elderly GBM.
Conclusions
The optimization of GBM outcomes includes homogenizing any possible access to care disparities. Within the United States, there are clear trends showing GBM patients diagnosed at age 65 or older experience lower odds of being treated by all staples of conventional therapy, including surgical resection, chemotherapy, and radiation therapy. Furthermore, these odds differ based on geographic region but collectively can be predicted based on numerous socioeconomic parameters. Public health initiatives and bioethical considerations targeting these aspects specific to this age group may prove effective in ensuring greater equality in treatment access among all GBM patients.
Supplementary Material
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement.
The authors have no conflicts to disclose.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Okada M, Miyake K, Tamiya T. Glioblastoma treatment in the elderly. Neurol Med Chir (Tokyo). 2017;57(12):667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen-Inbar O. Geriatric brain tumor management part II: glioblastoma multiforme. J Clin Neurosci. 2019;67:1–4. [DOI] [PubMed] [Google Scholar]
- 4. Lorimer CF, Saran F, Chalmers AJ, Brock J.. Glioblastoma in the elderly—how do we choose who to treat? J Geriatr Oncol. 2016;7(6):453–456. [DOI] [PubMed] [Google Scholar]
- 5. Lerro CC, Robbins AS, Phillips JL, Stewart AK.. Comparison of cases captured in the National Cancer Data Base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759–1765. [DOI] [PubMed] [Google Scholar]
- 6. Xu H, Chen J, Xu H, Qin Z. Geographic variations in the incidence of glioblastoma and prognostic factors predictive of overall survival in US adults from 2004–2013. Front Aging Neurosci. 2017;9:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Towne SD., Jr Socioeconomic, geospatial, and geopolitical disparities in access to health care in the US 2011–2015. Int J Environ Res Public Health. 2017;14(6):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and patterns of disparities in cancer mortality among US counties, 1980–2014. JAMA. 2017;317(4):388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Timmer M, Seibl-Leven M, Wittenstein K, et al. Long-term outcome and health-related quality of life of elderly patients after meningioma surgery. World Neurosurg. 2019;125:e697–e710. [DOI] [PubMed] [Google Scholar]
- 10. Presley CJ, Reynolds CH, Langer CJ. Caring for the older population with advanced lung cancer. Am Soc Clin Oncol Educ Book. 2017;37:587–596. [DOI] [PubMed] [Google Scholar]
- 11. DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–796. [DOI] [PubMed] [Google Scholar]
- 12. Nassiri F, Taslimi S, Wang JZ, et al. Determining the optimal adjuvant therapy for improving survival in elderly patients with glioblastoma: a systematic review and meta-analysis. Clin Cancer Res. 2020;26(11):2664–2672. [DOI] [PubMed] [Google Scholar]
- 13. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 14. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 15. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol. 2015;33(27):3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Currie ER, McPeters SL, Mack JW. Closing the gap on pediatric palliative oncology disparities. Semin Oncol Nurs. 2018;34(3):294–302. [DOI] [PubMed] [Google Scholar]
- 18. Brown DA, Himes BT, Kerezoudis P, et al. Insurance correlates with improved access to care and outcome among glioblastoma patients. Neuro Oncol. 2018;20(10):1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horner EM, Cullen MR. The impact of retirement on health: quasi-experimental methods using administrative data. BMC Health Serv Res. 2016;16:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.