Abstract
Objectives
SLE is a severe autoimmune disease characterized by autoreactive B cells and IC formation, which causes systemic inflammation. B cell–targeted therapy could be a promising treatment strategy in SLE patients; nevertheless, randomized clinical trials have not always been successful. However, some groups have demonstrated beneficial effects in severe SLE patients with off-label rituximab (RTX) with belimumab (BLM), or bortezomib (BTZ), which targeted different B cells subsets. This study assembled sera from SLE cohorts treated with RTX+BLM (n = 15), BTZ (n = 11) and RTX (n = 16) to get an in-depth insight into the immunological effects of these therapies on autoantibodies and IC formation.
Methods
Autoantibodies relevant for IC formation and the avidity of anti-dsDNA were determined by ELISA. IC-mediated inflammation was studied by complement levels and ex vivo serum-induced neutrophil extracellular trap formation.
Results
Reductions in autoantibodies were observed after all approaches, but the spectrum differed depending upon the treatment. Specifically, only RTX+BLM significantly decreased anti-C1q. Achieving seronegativity of ≥1 autoantibody, specifically anti-C1q, was associated with lower disease activity. In all SLE patients, the majority of anti-dsDNA autoantibodies had low avidity. RTX+BLM significantly reduced low-, medium- and high-avidity anti-dsDNA, while RTX and BTZ only significantly reduced medium avidity. IC-mediated inflammation, measured by C3 levels and neutrophil extracellular trap formation, improved after RTX+BLM and RTX but less after BTZ.
Conclusion
This study demonstrated the impact of different B cell–targeted strategies on autoantibodies and IC formation and their potential clinical relevance in SLE.
Keywords: SLE, autoantibodies, immune-complex formation, neutrophil extracellular traps, B cell–targeted therapies
Rheumatology key messages
RTX, RTX+BLM and BTZ resulted in different targeting of anti-C1q, high-avidity anti-dsDNA and the autoantibody repertoire.
Reduction of anti-C1q and high-avidity anti-dsDNA by RTX+BLM led to reduced immune-complex–mediated inflammation.
Achievement of autoantibody negativity, specifically anti-C1q, after B cell–targeted strategies, was beneficial for SLE patients.
Introduction
SLE is a chronic autoimmune disorder characterized by a break of tolerance leading to the development of autoreactive B cells that produce ANAs [1, 2]. These autoantibodies can form ICs that deposit in affected tissues, leading to complement activation and a systemic inflammatory cascade. A severe manifestation of IC-mediated inflammation in SLE patients is LN, which is histologically represented by a ‘full-house’ pattern of IC deposition in glomeruli [3, 4]. Typically, activation of the classical pathway of the complement system is an indirect biomarker of IC-mediated inflammation in SLE. Additionally, SLE-specific ICs can trigger excessive neutrophil extracellular trap (NET) formation [5], an immunogenic and toxic release of DNA by neutrophils [6–8]. In addition, NETs themselves are an important source of nuclear autoantigens, creating a pathological vicious cycle of perpetuating inflammation in SLE [5–9].
A reduction in autoantibodies, or even reversal to negativity, upon immunosuppressive treatment has been found to be associated with a beneficial clinical outcome in SLE [10–14]. These autoantibodies can be secreted by two distinct antibody-secreting cell (ASC) populations: short-lived proliferating plasma blasts (PBs) and non-dividing long-lived plasma cells (PCs) [15–17]. Typically, autoreactive PBs, originating from an activated naïve B cell population [16, 18] or the autoreactive memory compartment [15], are associated with increasing autoantibody (anti-dsDNA, anti-C1q) levels and disease flares. Autoreactive PCs are thought to maintain the persistence of circulating autoantibodies, and specifically those against ENAs that are refractory to immunosuppressive treatment [15, 19]. Additionally, B cells and PCs also have antibody-independent functions, such as antigen presentation, T cell activation, and cytokine production, which makes them an even more valuable therapeutic target in SLE [20].
Currently, a variety of therapeutic strategies can be used to target B cells, PBs and PCs [20], but the optimal approach for SLE has not yet been elucidated. Strategies that could be beneficial include: direct targeting of the ASC population by proteasome inhibition or by TACI-Ig (atacicept) [20], which blocks both proliferation-inducing ligand (APRIL) and B cell activating factor (BAFF) by binding to the TACI receptor, or by targeting of precursors of ASCs, including PBs, by B cell–depleting mAbs (e.g. directed against CD20, CD21 or CD19) or neutralization of B cell survival factors with anti-cytokine mAbs against APRIL or BAFF. Currently, anti-BAFF mAb Belimumab (BLM) is the only officially approved B cell–targeted therapy for SLE patients without renal and/or neurological manifestations, and it is currently being tested in the BLISS-LN trial for LN (NCT01639339). BLM decreased naïve and transitional B cells, but not switched memory B cells, whereas PCs did not decrease until after 2 years of BLM [21]. Rituximab (RTX), an anti-CD20 mAb, was not effective in two large randomized controlled trials in SLE patients; however, off-label use of RTX was promising, with strong effects on autoantibodies and the complement system [10, 22, 23]. The exact subpopulation of B cells targeted and eliminated by RTX remains uncertain [24]. Some B cell populations can also escape RTX, such as switched memory B cells, despite CD20 expression [24] and tissue-resident CD20– PCs [25]. In general, it is thought that RTX does not decrease CD20– PCs; however, presumably by targeting of their precursors, all CD27brCD38brPBs/PCs (CD20–/+) were significantly depleted after RTX in SLE [26]. Importantly, B cell depletion with RTX results in an increase in serum BAFF levels [9, 27], which can effectively be decreased with sequential BLM treatment after RTX [9]. This approach of combining RTX and BLM led to clinical responses in refractory SLE patients and significantly decreased memory B cells, transitional B cells and CD27brCD38br PBs/PCs [9]. During monthly BLM, PCs started to repopulate from week 24 onwards, while anti-dsDNA, anti-C1q and even ENAs remained suppressed. On the other hand, bortezomib (BTZ), a proteasome inhibitor targeting PCs, was effective in refractory SLE [28, 29]. BTZ decreased CD20– PCs, including both HLA-DR +/− in the peripheral blood and bone marrow, but did not target their precursors.
Altogether, B cell–targeted therapies may be effective in the treatment of SLE and LN, because different off-label approaches have demonstrated potential clinical benefit, but it is unclear which approach in SLE patients would be most optimal from an immunological perspective. To address this issue, the present study assembled previously published cohorts of SLE patients treated with experimental B cell–targeted strategies: RTX+BLM [9], BTZ, [28, 29] and (as a comparator) RTX [22] and investigated the immunological effects of these B cell–targeted strategies on autoantibodies and IC-mediated NET inflammation to increase the understanding for B cell–targeting in SLE.
Methods
Study population
Serum samples were assembled from 42 severe, refractory SLE patients (each of whom met the 1997 revised ACR or 2012 SLICC criteria) from previously published cohorts treated with RTX [22] (n = 16), RTX+BLM [9] (n = 15) and BTZ [28, 29] (n = 11). All patients provided informed consent locally, and the study was approved by the local ethics committee at each centre. Demographics, clinical parameters, DASs, and medication use were recorded locally by the treating physicians. Local routine laboratory assessments were collected at each centre separately, including IgG, IgM and IgA, absolute count of CD19+ B cells, and C3 serum titres. Low C3 was defined as below the normal cut-off values as defined by each local laboratory; RTX <0.79 g/l, RTX+BLM <0.9 g/l, BTZ <0.9 g/l. Patients’ characteristics are summarized in Supplementary Table S1, available at Rheumatology online, and detailed treatment schedules in Supplementary Table S2, available at Rheumatology online.
Autoantibody measurements
To compare autoantibody titres of the SLE patients in different cohorts, serum levels of anti-dsDNA, -histones, -nucleosomes and -C1q IgG autoantibodies were measured centrally in the Leiden University Medical Centre (LUMC) in Leiden, the Netherlands. Details of the ELISAs are described in the supplementary materials, section Methods, available at Rheumatology online, and supplementary Fig. S1, available at Rheumatology online.
Preparation of neutrophils and quantification of NETs
Paul Karl Horan (PKH)-labelled (Sigma-Aldrich, USA) neutrophils from a healthy donor were stimulated with 10% SLE serum for 4 h to induce NET formation. After 3¾ h of stimulation, 1 µM SYTOXgreen (ThermoFisher, USA) was added for 15 min, after which neutrophils were fixed with 4% paraformaldehyde (AddedPharma, Netherlands). Hereafter, the NETs were visualized and quantified by 3 D confocal microscopy using the automated BD Pathway 855 (BD Biosciences, USA), or the Image Xpress Micro Confocal (Molecular Devices, USA), as described previously [30, 31].
Statistics
All clinical data are expressed as medians ± [interquartile ranges (IQRs)] for numerical data or given as percentages for nominal data. NET formation data are expressed as medians (IQRs) NET area per imaged neutrophil. NET formation ratios are expressed as medians (IQRs). To determine statistical differences between three independent groups the Kruskal–Wallis test for numerical data and the χ2 test for nominal variables was used. Statistical difference between two groups was determined with the Mann–Whitney U test, and Wilcoxon’s matched-pairs test was used for paired samples. Statistical analyses were performed with GraphPad software (La Jolla, USA) and SPSS version 32 (IBM, USA).
Results
Study population
The characteristics of SLE patients (n = 42) who were treated with RTX [22] (n = 16), a combination of RTX+BLM [9] (n = 15) or BTZ [28, 29] (n = 11) are summarized in Table 1. Briefly, most patients were females (86%), with a median age of ∼35 (IQR: 30–41) years and a median disease duration of ∼9 [6–16] years. SLE organ involvement included renal (69%), cardiorespiratory (64%) or neurologic (31%) disease. All SLE patients had comparable high DASs at baseline of ∼14 (10–19), which decreased significantly for all approaches (Table 1, Supplementary Fig. S2A, available at Rheumatology online). The refractory nature of the SLE was illustrated by previously used immunosuppressants (Supplementary Table S1, available at Rheumatology online). Both RTX+BLM-treated and BTZ-treated cohorts included a comparable number of patients that were refractory to RTX therapy (27% and 55%, respectively). Forty-seven per cent of RTX-treated patients had complement consumption, which was less than 91% of BTZ-treated (P = 0.03) and less than 87% of RTX+BLM-treated SLE patients (P = 0.01). Sixty-nine per cent of RTX-treated SLE patients were positive for anti-dsDNA autoantibodies, compared with 100% in both BTZ and RTX+BLM cohorts (P = 0.01). The measured autoantibody repertoire included autoantibodies against dsDNA in 88%, histones in 88%, nucleosomes in 95% and C1q in 81% of all SLE patients. Importantly, the serum levels of anti-dsDNA, anti-histone, anti-nucleosome and anti-C1q autoantibodies, our primary objective in this study, and the number of patients per cohort positive for these autoantibodies, other than anti-dsDNA, were comparable between the cohorts at baseline.
Table 1.
Patient characteristics
| Total (n = 42) | RTX (n = 16) | RTX+BLM (n = 15) | BTZ (n = 11) | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 35 (30–41) | 39 (33–47) | 31 (25–42) | 34 (30–36) | |
| Females (%) | 86% | 88% | 87% | 82% | |
| Ethnicity | |||||
| Caucasian (%) | 46% | 27% | 33% | 91% | |
| Black (%) | 44% | 53% | 60% | 9% | |
| Asian (%) | 10% | 20% | 7% | 0% | |
| SLE parameters | |||||
| Disease duration (years) | 9 (6–16) | 9 (7–17) | 10 (6–16) | 9 (5–15) | |
| Disease activity score | 14 (10–18) | 13 (10–16) | 18 (12–21) | 13 (10–18) | |
| Disease activity score after therapy | 4 (1–6) | 2 (2–6) | 2 (0–4) | 7 (5–7) | |
| Low C3 (%) | 73% | 47% | 87% | 91% | |
| CD19+ (10 × 10^6/L) | 110 (50–206) | 157 (72–221) | 117 (80–228) | 41 (15–53) | |
| Follow-up (weeks) | 24 (10–26) | 30 (24–34) | 24 (24–24) | 6 (3–7) | |
| Autoantibody profile | |||||
| Anti-dsDNA positivity (%) | 88% | 69% | 100% | 100% | |
| Anti-dsDNA AU/ml | 230 (86–585) | 123 (99–230) | 255 (67–455) | 438 (168–1116) | |
| Anti-histones positivity (%) | 88% | 75% | 93% | 100% | |
| Anti-histone AU/ml | 227 (71–535) | 167 (39–428) | 190 (67–467) | 227 (85–981) | |
| Anti-nucleosomes positivity (%) | 95% | 100% | 93% | 82% | |
| Anti-nucleosome AU/ml | 47 (15–207) | 47 (13–127) | 29 (15–163) | 208 (21–617) | |
| Anti-C1q positivity (%) | 81% | 69% | 87% | 91% | |
| Anti-C1q IU/ml | 61 (34–117) | 55 (28–115) | 85 (47–107) | 60 (36–125) |
BLM: belimumab; BTZ: bortezomib; RTX: rituximab; Low C3 was defined as below normal values according to local laboratory.
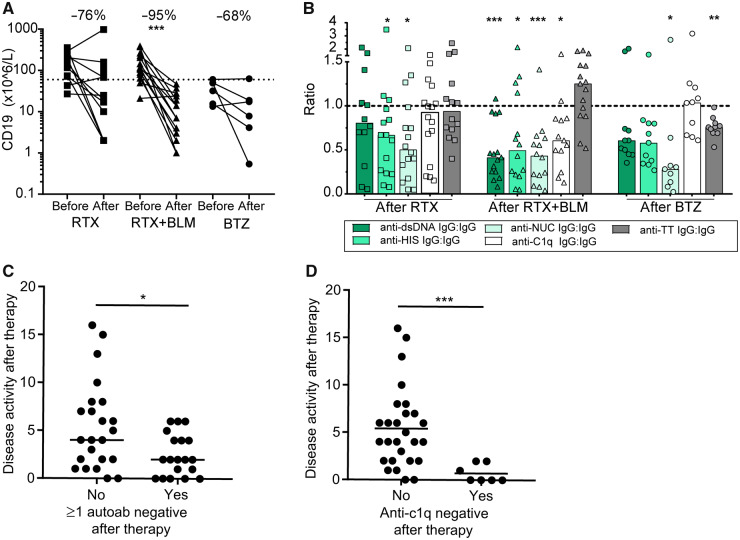
B cell depletion was associated with reduced autoantibody levels
To assess the effects of the different treatment strategies on humoral autoimmunity, the depleting effects on circulating CD19+ B cells were evaluated (Table 2, Fig. 1A). After RTX, circulating CD19+ B cells were ∼23 × 106 cells/litre (2–109), corresponding to a median of –76% (–7;–93) change from baseline (P = 0.08). After RTX+BLM, circulating CD19+ B cells were ∼7 × 106 cells/litre (3–24), corresponding to a median of –95% (–79; –97) change from baseline (P = 0.0001). After BTZ, circulating CD19+ B cells were ∼13 × 106 cells/litre (3–30), corresponding to a median of –68% (–81;+11) change as compared with baseline (P = 0.16). More detailed impact on B cell subsets by each therapy has been published separately for RTX [10, 22, 23, 26], RTX+BLM [9] and BTZ [28, 29]. Of interest, RTX+BLM was previously demonstrated to result in a more persistent reduction in B cells as compared with RTX alone in a comparative study [32].
Table 2.
Immunological parameters
| RTX |
RTX+BLM |
BTZ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | Before | After | P | ||
| CD19+ B cells (×10^6/l ) | 157 [72–221] | 23 [2–109] | 0.08 | 117 [80–228] | 7 [3–24] | 0.0001 | 41 [15–53] | 13 [3–30] | 0.16 | |
| Immunoglobulins | ||||||||||
| IgG (g/l) | 9.5 [8.3–13.1] | 10.8 [9.5–11.8] | 0.2 | 11.6 [10.4–13.4] | 9.6 [7.0–12.5] | 0.07 | 12.6 [10.9–13.3] | 11.0 [8.4–12.8] | 0.41 | |
| IgM (g/l) | 0.5 [0.4–1.0] | 0.4 [0.3–0.8] | 0.0002 | 0.7 [0.5–0.9] | 0.4 [0.3–0.6] | 0.0004 | 0.8 [0.6–1.1] | 0.7 [0.5–1.0] | 0.27 | |
| IgA (g/l) | 3.2 [2.5–3.9] | 2.9 [2.6–3.8] | 0.5 | 2.8 [1.9–4.3] | 2.7 [1.7–3.4] | 0.09 | 2.9 [2.4–4.0] | 2.2 [1.6–3.1] | 0.03 | |
| Anti-TT IgG (AU/ml) | 12 [6–16] | 13 [6–16] | 0.95 | 14 [5–28] | 13 [7–22] | 0.76 | 17 [6–47] | 14 [5–50] | 0.03 | |
| Autoantibody profile | ||||||||||
| Anti-dsDNA | 69% | 63% | 100% | 87% | 100% | 100% | ||||
| Anti-histones | 75% | 56% | 94% | 80% | 100% | 100% | ||||
| Anti-nucleosomes | 100% | 88% | 87% | 60% | 82% | 73% | ||||
| Anti-C1q | 69% | 56% | 87% | 53% | 91% | 91% | ||||
| Repertoire | 4 [2–4] | 3 [2–4] | 0.03 | 4 [3–4] | 3 [2–4] | 0.03 | 4 [3–4] | 4 [3–4] | 0.99 | |
| Autoantibody titres | ||||||||||
| Anti-dsDNA (AU/ml) | 123 [99–230] | 129 [49–180] | 0.20 | 255 [67–455] | 68 [32–118] | 0.0003 | 438 [168–1116] | 355 [104–798] | 0.03 | |
| Anti-histones (AU/ml) | 167 [39–428] | 86 [27–321] | 0.03 | 190 [67–467] | 51 [39–198] | 0.003 | 227 [85–981] | 108 [66–371] | 0.005 | |
| Anti–nucleosomes (AU/ml) | 47 [13–127] | 17 [8–129] | 0.06 | 29 [15–163] | 13 [6–24] | 0.0004 | 208 [21–617] | 45 [15–113] | 0.02 | |
| Anti-C1q (IU/ml) | 55 [28–115] | 50 [24–117] | 0.28 | 85 [47–107] | 32 [19–57] | 0.0005 | 60 [36–125] | 68 [26–106] | 0.32 | |
Fig. 1.
B cell depletion associated with reduced autoantibody levels
(A) The absolute values of CD19+ B cells are shown for each individual patient per cohort before and after treatment. Percentages indicate the median change per cohort. (B) The change in (auto) antibodies for each individual patient per cohort is expressed as a ratio of the normalized ratio of anti-TT IgG (grey bars), anti-dsDNA (dark green), anti-histones (middle green), anti-nucleosomes (light green) and anti-C1q (white) to total IgG after therapy and compared with the ratio at the baseline of these (auto)antibodies to total IgG. Bars indicate the median per cohort. (C) SLE disease activity was assessed in SLE patients who did not achieve seronegativity of any of the autoantibodies compared with SLE patients who did achieve seronegativity of at least one autoantibody. (D) SLE disease activity was assessed in anti-C1q–positive SLE patients who achieved seronegativity of these anti-C1q autoantibodies and compared with the patients who did not achieve anti-C1q seronegativity. The Wilcoxon matched-pairs signed rank test was used to test statistical differences between baseline and after different targeted therapies in paired patient serum samples. The Mann–Whitney U test was used to test statistical difference between two groups. *P <0.05, **P < 0.01, ***P < 0.001. BLM: belimumab; BTZ: bortezomib; HIS: histones; NUC: nucleosomes; RTX: rituximab.
No significant change in total serum IgG was observed after any of the treatment strategies, whereas anti-TT-IgG serum levels decreased significantly upon BTZ treatment to a median of 18% less (–4; –31) (P = 0.03), but not after RTX or RTX+BLM (Table 2). In contrast, total serum IgM levels decreased significantly after RTX and RTX+BLM, but not after BTZ (Table 2). Only BTZ decreased total serum IgA significantly, which was not decreased by RTX nor by RTX+BLM. These data confirmed the premise that BTZ targeted long-lived PCs, while RTX with or without BLM targeted short-lived PBs without directly affecting long-lived PCs.
Next, we investigated the effects of the different B cell–targeted strategies on SLE-specific autoantibodies, as summarized in Table 2 and Supplementary Fig. S3, available at Rheumatology online. Anti-dsDNA antibodies decreased significantly after RTX+BLM to a median of –68% (–48; –84) (P = 0.0003) and upon BTZ to a median of –48% (–29; –57) (P = 0.03), but not following RTX to a median of –22% (–10; –62) (P = 0.20) (Table 2). Anti-histone autoantibody titres decreased significantly following RTX to a median of –52% (–23; –62) (P = 0.03), following RTX+BLM to a median of –57% (–17; –85) (P = 0.003), and following BTZ to a median of –51% (–19; –63) (P = 0.005). Anti-nucleosomes autoantibodies titres decreased significantly after RTX+BLM to a median of –62% (–48; –86) (P = 0.0004) and after BTZ to a median of –77% (–42; –91) (P = 0.02), but non-significantly after RTX to a median of –39% (–77; +8) (P = 0.06). Anti-C1q antibodies decreased only significantly following RTX+BLM to a median of –45% (–69; –27) (P = 0.0005), but not after RTX to a median of –9% (–47; +19) (P = 0.28), nor after BTZ to a median of –14% (–26; +14) (P = 0.32). Specific targeting of autoantibody levels was assessed by comparing relative changes of IgG autoantibodies to total IgG antibodies per individual patient (Fig. 1B). Following RTX, anti-TT IgG remained stable, whereas significant reductions in anti-histone and anti-nucleosome autoantibodies were observed. Also, after RTX+BLM, anti-TT IgG remained unaffected, while preferential reductions in anti-dsDNA, anti-histone, anti-nucleosome and anti-C1q autoantibodies were observed. After BTZ, both anti-histone autoantibodies and anti-TT IgG were significantly reduced, while anti-C1q antibodies were unaffected.
IC formation is influenced by the extent of the autoantibody repertoire in SLE patients [33, 34]. SLE patients in all cohorts had a similar median number of positive autoantibodies, ∼4 (3–4) out of 4 measured specificities (Table 2, Supplemental Fig. S4A, available at Rheumatology online). Overall, we observed that 16 out of 42 SLE patients achieved seronegativity of ≥1 autoantibody specificity after treatment, RTX: 6/16, RTX+BLM: 8/15, and BTZ: 2/11(Supplemental Fig. S4B, available at Rheumatology online). This resulted in a significantly smaller autoantibody repertoire for RTX and RTX+BLM patients (Table 2). Interestingly, SLE patients who achieved negativity of one or more autoantibodies after therapy had a significantly lower DAS of ∼2 (0–4) compared with those who did not (4 (2–8); P = 0.02) (Fig. 1C). This was predominantly reflected in anti-C1q-positive SLE patients who achieved anti-C1q negativity after RTX (2/11), RTX+BLM (5/13), and BTZ (0/10), who had significantly lower DASs of ∼0 (0–2) compared with SLE patients who remained anti-C1q positive [∼4 (2–7); P = 0.0005)] (Fig. 1D).
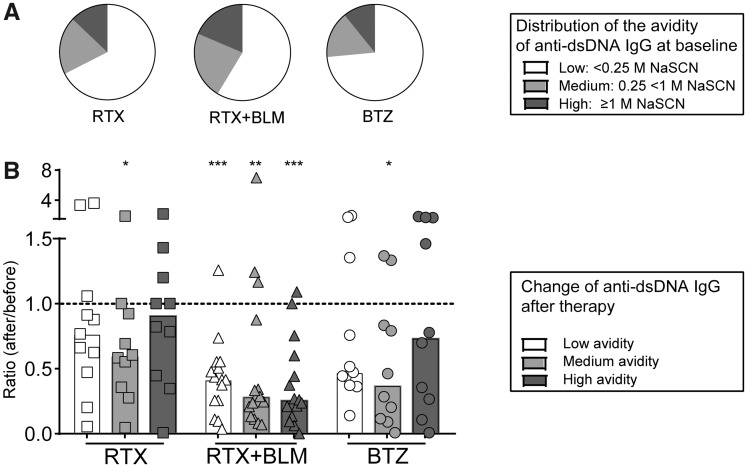
RTX+BLM targeted low-, medium- and high-avidity anti-dsDNA, whereas RTX and BTZ targeted only medium-avidity anti-dsDNA
The avidity of anti-dsDNA antibodies is thought to be an important contributor to their pathogenic potential [35], and especially high-avidity anti-dsDNA antibodies closely associated with SLE disease activity complement activation, thereby contributing to LN [36, 37]. To further dissect the effects of each treatment strategy on specific ASC populations, the avidity of anti-dsDNA autoantibodies, with anti-TT-IgG as a control, was determined (Fig. 2), as previously described [38]. An example of avidity curves for anti-dsDNA and anti-TT IgG and the definition of low-, medium- and high-avidity anti-dsDNA are displayed in supplementary Fig. S1, available at Rheumatology online. In summary, in 37 anti-dsDNA–positive SLE patients, the majority (59–74%) of anti-dsDNA autoantibodies were of low avidity (eluted from antigen with 0.25 M NaSCN or less) (Fig. 2A). Medium-avidity autoantibodies (eluted with ≥0.25 M and <1 M NaSCN) constituted 16–23% of anti-dsDNA in the cohorts, whereas high-avidity autoantibodies (eluted with ≥1 M NaSCN) encompassed 11–19%. The distribution of low-, medium- and high-avidity anti-dsDNA within total anti-dsDNA antibodies did not differ significantly between the cohorts before treatment (Fig. 2A). Anti-TT-IgG consisted entirely of high-avidity antibodies (eluted with ≥1 M NaSCN) (data not shown). Next, we analysed the absolute serum titres of low-, medium- and high-avidity anti-dsDNA autoantibodies (supplementary Fig. S5, available at Rheumatology online) and observed that medium-avidity anti-dsDNA decreased significantly after all three treatment strategies, whereas RTX+BLM significantly also decreased the low- and high-avidity anti-dsDNA (Fig. 2B, supplementary Fig. S5, available at Rheumatology online).
Fig. 2.
RTX+BLM targets low-, medium- and high-avidity anti-dsDNA autoantibodies, whereas RTX and BTZ target only medium-avidity anti-dsDNA
(A) Distribution of low-, medium- and high-avidity anti-dsDNA autoantibodies is displayed as mean percentages within the total of anti-dsDNA autoantibodies per cohort at baseline. (B) The ratio of the absolute serum titres of low-, medium- and high-avidity anti-dsDNA autoantibodies as compared with baseline are shown for each patient. Bars indicate the median per cohort. *P < 0.05, **P < 0.01, ***P < 0.001. BLM: belimumab; BTZ: bortezomib; RTX: rituximab.
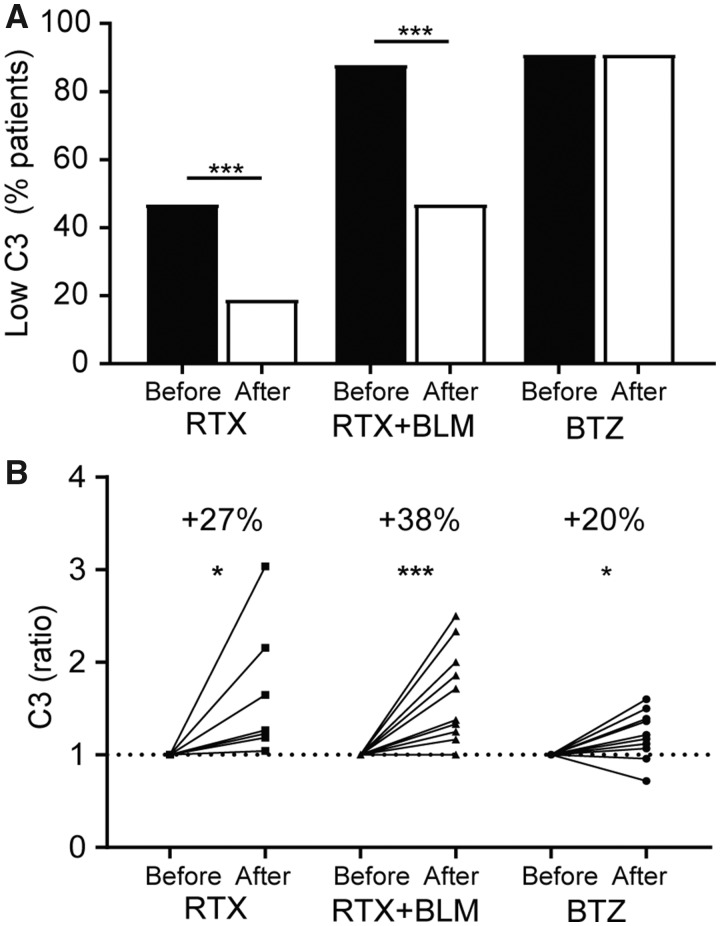
IC-mediated inflammation was targeted by RTX and RTX+BLM, but not by BTZ
Finally, we investigated the treatment effects on functional parameters of IC formation, i.e. complement consumption and ex vivo serum-induced NET formation. After RTX, C3 levels normalized in 5/7 SLE patients (Fig. 3A, supplementary Fig. S2B, available at Rheumatology online) in whom C3 serum levels increased by ∼27% (19–116%) (P = 0.02) (Fig. 3B). After RTX+BLM, C3 levels normalized in 6/13 SLE patients, in whom C3 serum levels increased by ∼38% (21–93) (P = 0.001). After BTZ, 0/10 SLE patients normalized C3 serum levels, however absolute C3 levels increased significantly by ∼20% (4–42) (P = 0.04).
Fig. 3.
Complement consumption is normalized after RTX and RTX+BLM, but not after BTZ
(A) The percentage of SLE patients that had reduced C3 levels (see supplementary figure 2B) in sera before (black bars) and after (white bars) rituximab (RTX), rituximab with belimumab (RTX+BLM) or bortezomib (BTZ) are displayed. (B) The change of C3 serum titres after RTX, RTX+BLM and BTZ as ratios compared with baseline are displayed. The Wilcoxon matched-pairs signed rank test was used to test statistical differences between baseline and after different targeted therapies in paired patients. *P < 0.05, ***P < 0.001, RTX: rituximab; BLM: belimumab; BTZ: bortezomib.
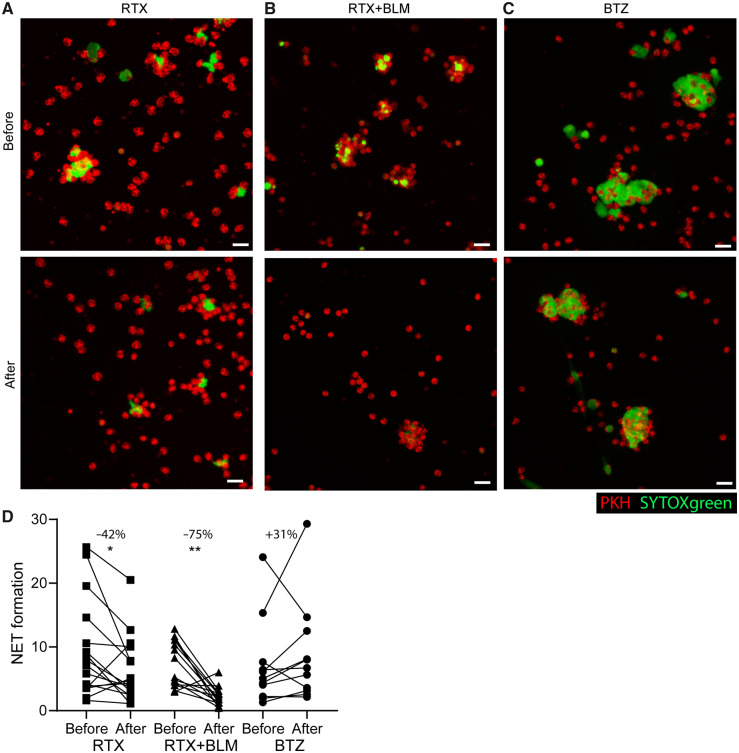
SLE-specific ICs can induce excessive NET formation [5–7]. Therefore, NET formation induced by SLE sera as a quantitative measurement of IC formation was quantified. Representative illustrations of the 3D NET quantification assay before and after RTX (A), RTX+BLM (B) and BTZ (C) are shown in Fig. 4. Typical SLE-induced clusters of NET-ting neutrophils are observed at baseline [5] (Fig. 4A–C). NET formation significantly decreased to a median of –75% (–88;–45) (P = 0.008) after RTX+BLM and to a median of –42% (–54; 10) (P = 0.03) after RTX, but not after BTZ, when it increased to a median of +31% (–36; 104) (P = 0.15) (Fig. 4D).
Fig. 4.
Serum-induced NET formation is significantly decreased after RTX and RTX+BLM, but is not affected by BTZ
Healthy PKH-labelled neutrophils were stimulated with sera of SLE patients at baseline and after treatment with B cell–targeted therapies to induce NET formation. NET formation was quantified three-dimensionally with immunofluorescence microscopy through analyzing the cumulative extracellular DNA area (SYTOX green) over Z-stacks per imaged neutrophils (red). Representative pictures of the quantitative assay of SLE serum-induced NET formation at baseline and after (A) RTX, (B) RTX+BLM and (C) BTZ. White scale bar = 20µM. (D) NET formation is expressed as absolute NET area per imaged neutrophil before and after B cell–targeted therapy for each individual SLE patient (individual lines) per cohort. Percentages indicate the median change per cohort. Wilcoxon matched-pairs signed rank test was used to test statistical differences between baseline and after different targeted therapies in paired patient serum samples. *P < 0.05, ** P < 0.01. RTX: rituximab; BLM: belimumab; BTZ: bortezomib.
Discussion
RTX, RTX+BLM and BTZ are novel B cell–targeted strategies that differentially target B cell and PC subsets, the sources of autoantibodies in SLE. In this reverse translational study, we demonstrated that autoantibody levels decreased upon each treatment strategy, but the extent of targeted autoantibodies was most significant for RTX+BLM in a quantitative manner (reduced autoantibody repertoire) as well as a qualitative manner (reduced low-, medium- and high-avidity anti-dsDNA autoantibodies). These effects were less pronounced for RTX only and not observed in BTZ-treated patients. Especially the reversal of anti-C1q to seronegative was associated with reduced IC-mediated inflammation and clinical disease activity, which happened most frequently after RTX+BLM, less after RTX and not after BTZ treatment. These observations collectively demonstrated the relevance of in-depth monitoring of the immunological effects of B cell–targeted strategies that have potential implications for the clinic.
Immunomonitoring of the humoral autoimmune response in SLE is challenging because a wide variety of autoantibodies mediate organ damage directly or indirectly through the formation of ICs [2], which subsequently trigger the complement system and NET formation [6, 9]. Anti-dsDNA [39], -histones [40], -nucleosomes [41] and -C1q [42] autoantibodies were shown to be involved in IC formation, correlated with disease activity, and thereby are closely linked to the pathogenesis of SLE. Specifically, anti-C1q antibodies have a pathogenic role in LN, because they activated the classical and lectin pathway of the complement system [43], and C1q-containing ICs are found in the glomeruli [44]. Increased anti-C1q titres predicted renal flares [42, 45], and after successful treatment, titres decreased [46]. Of interest, immunoadsorption of anti-C1q on C1q columns led to depletion of ICs and anti-C1q autoantibodies and was shown to be beneficial in SLE patients [47].
Overall, a reduction in pathogenic autoantibody levels after any treatment was associated with beneficial clinical outcome in SLE patients [11–13]. Therefore, achieving a reduction in autoantibody load, and ultimately achieving negativity of autoantibodies, could be a key treatment target in SLE patients. In this study, some B cell–targeted strategies were not always found to effectively eradicate pathogenic IC formation in SLE patients.
An important premise within this retrospective study of three uniquely treated cohorts of SLE patients is that RTX and RTX+BLM do not directly target long-lived PCs, but cause depletion of their precursors (i.e. B cells and short-lived PBs) [9, 22], whereas BTZ does predominantly target long-lived PCs [28, 29]. Additionally, B cell depletion was sustained longer after RTX+BLM as compared with RTX alone [32]. Different groups have demonstrated that both RTX and RTX+BLM reduced CD27brCD38br PBs [9, 26], although their maturation stage remained unclear. On the other hand, BTZ was shown to cause a significant depletion of CD20– PCs in peripheral blood (PB) and bone marrow (BM) in SLE patients [29], whereas their pre-cursor B cells and T cells remained largely unaffected [28]. After BTZ withdrawal, a rapid repopulation of short-lived HLA-DR+PCs, but not long-lived HLA-DR-PCs, occurred, accompanied by increasing autoantibody levels [28]. Indeed, our study further corroborated these previously published data by demonstrating a significant effect of BTZ on anti-TT-antibodies typically derived from long-lived PCs, which remained unaffected by RTX+BLM or RTX only. It is therefore of great interest that BTZ did not affect anti-C1q autoantibody levels in our study, whereas RTX+BLM did, strongly suggesting anti-C1q autoantibody production is predominantly derived from short-lived PBs susceptible to BLM treatment. Moreover, RTX+BLM also effectively targeted anti-dsDNA autoantibodies with low to high avidity. It is reasonable to assume that these phenomena underpinned the reduction in IC-mediated inflammation and the associated amelioration of clinical disease activity in SLE patients treated with RTX+BLM. This study showed similar effects in SLE patients treated with RTX only, but to a lesser extent, whereas IC-mediated inflammation was not affected in BTZ-treated SLE patients. Taken together, our study suggests that an important source of the pathogenic, IC-forming autoantibodies are derived from short-lived PBs. As such, these autoreactive PBs could be relevant immunomonitoring markers for the evaluation of novel, experimental therapies in SLE and LN patients.
We also demonstrated that most anti-dsDNA autoantibodies were of low avidity. The role of low-avidity anti-dsDNA in the pathophysiology of SLE is uncertain; however, high-avidity anti-dsDNA antibodies detected by the Farr assay and the Crithidia luciliae immunofluorescence test (CLIFT) were demonstrated to associate closely with disease activity and complement consumption, and to contribute to LN [36]. Therefore, it is of interest that RTX+BLM targeted significantly high-avidity anti-dsDNA autoantibodies, in contrast to RTX and BTZ, which did not affect the levels of high-avidity anti-dsDNA antibodies according to the previous publication of RTX+BLM treatment in SLE [9], which demonstrated that 7/12 patients had reversal of anti-dsDNA autoantibodies, measured by CLIFT, from positive to negative.
Several limitations inherent in the design and analysis of this study need to be mentioned. First, the study was limited by the relatively small number of patients treated worldwide with off-label RTX+BLM, BTZ, or RTX. Despite in-depth studies in these small patient cohorts, the serendipity of some of the observations should be considered. Second, because this study assembled three independent cohorts (which were therefore without randomization), baseline differences were inevitable (Table 1, Supplementary Table S1, available at Rheumatology online), and this might have indirectly influenced our results. Although the cohorts were different with respect to the distribution of patients; ethnicities [48] (fewer black patients included in the BTZ-cohort), renal involvement (more patients in the RTX+BLM cohort), reduced C3 levels at baseline (less patients in RTX cohort) and anti-dsDNA positivity at baseline (less patients in RTX cohort), all baseline autoantibody serum titres and the number of patients per cohort positive for the autoantibodies, except anti-dsDNA were comparable between the cohorts (supplementary Fig. S3, available at Rheumatology online). As such, relevant changes in unbiased, immunological end points could be monitored in this study. Moreover, to avoid the issue of comparability, we only compared individual patients with their own baseline, and direct comparisons between cohorts were withheld. Third, we cannot exclude the possibility that some observed effects were due to concomitant immunosuppression within these separate treatment strategies. However, even though the cohorts differed significantly with respect to the use of MMF and CYC (Supplementary Tables S1 and S2, available at Rheumatology online), previous studies have shown that MMF and CYC were comparable with respect to the reduction in IgG levels or anti-dsDNA in SLE patients [49].
Additionally, we did not study anti-ENA autoantibodies in this study. But we have previously shown significant decreases after RTX+BLM for anti-Sm (–35%), anti-RNP70 (–48%) and anti-U1RNP (–58%) antibodies [9]. After BTZ, two-fifths of SLE patients had decreased anti-RNP or anti-Sm levels [28], while RTX did not affect these anti-ENAs [12]. Overall, these data underscored indirectly the effects of the studied B cell–targeted strategies on ENA-specific PCs, which are in line with the results of our study.
Lastly, it is important to note that reported data on BTZ-treated patients were evaluated at a significantly shorter follow-up time as compared with RTX and RTX+BLM-treated patients. This was a well-considered, deliberate part of the study’s design, because the immunological effects of proteasome inhibition by BTZ arise more quickly and last less time than the effects of B cell depletion by RTX±BLM. For the sake of clarity, we also investigated follow-up sera after a median of 12 weeks after the start of BTZ treatment, which demonstrated that autoantibody levels, B cells, complement levels and NET formation did not further improve, while some even worsened over time (Supplementary Fig. S6, available at Rheumatology online).
In conclusion, this reverse translational study monitored the differential effects of RTX, RTX+BLM and BTZ on the humoral autoimmune response of severe SLE patients. Immunological effects of each B cell–targeted strategy elucidated the relevance of reducing, and even eradicating, SLE-relevant autoantibodies, in particular anti-C1q. These findings were related to a reduction in IC-mediated inflammation and translated to clinical benefit. These results support the relevance of immunomonitoring in the context of emerging B cell–targeted strategies for severe SLE patients.
Funding: This work was supported by FOREUM (SLE project); the Dutch Kidney Foundation (KJPB12.028 &17OKG04); and a Clinical Fellowship from the Netherlands Organization for Scientific Research (90713460).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Rahman A, Isenberg DA.. Systemic lupus erythematosus. N Engl J Med 2008;358:929–39. [DOI] [PubMed] [Google Scholar]
- 2. Yaniv G, Twig G, Shor DB. et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev 2015;14:75–9. [DOI] [PubMed] [Google Scholar]
- 3. Sterner RM, Hartono SP, Grande JP.. The pathogenesis of lupus nephritis. J Clin Cell Immunol 2014;5(2):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rijnink EC, Teng YKO, Wilhelmus S. et al. Clinical and histopathologic characteristics associated with renal outcomes in lupus nephritis. Clin J Am Soc Nephrol 2017;12:734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Dam LS, Kraaij T, Kamerling SWA. et al. Intrinsically distinct role of neutrophil extracellular trap formation in antineutrophil cytoplasmic antibody–associated vasculitis compared to systemic lupus erythematosus. Arthritis Rheumatol 2019;71:2047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia-Romo GS, Caielli S, Vega B. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011;3:73ra20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lande R, Ganguly D, Facchinetti V. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011;3:73ra19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Dam LS, Rabelink TJ, van Kooten C, Teng Y.. Clinical implications of excessive neutrophil extracellular trap formation in renal autoimmune diseases. Kidney Int Rep 2019;4:196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kraaij T, Kamerling SWA, de Rooij ENM. et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun 2018;91:45–54. [DOI] [PubMed] [Google Scholar]
- 10. Lazarus MN, Turner-Stokes T, Chavele KM, Isenberg DA, Ehrenstein MR.. B-cell numbers and phenotype at clinical relapse following rituximab therapy differ in SLE patients according to anti-dsDNA antibody levels. Rheumatology 2012;51:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linnik MD, Hu JZ, Heilbrunn KR. et al. Relationship between anti-double-stranded DNA antibodies and exacerbation of renal disease in patients with systemic lupus erythematosus. Arthritis Rheum 2005;52:1129–37. [DOI] [PubMed] [Google Scholar]
- 12. Cambridge G, Stohl W, Leandro MJ. et al. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum 2006;54:723–32. [DOI] [PubMed] [Google Scholar]
- 13. Bootsma H, Spronk P, Derksen R. et al. Prevention of relapses in systemic lupus erythematosus. Lancet 1995;345:1595–9. [DOI] [PubMed] [Google Scholar]
- 14. Coremans IE, Spronk PE, Bootsma H. et al. Changes in antibodies to C1q predict renal relapses in systemic lupus erythematosus. Am J Kidney Dis 1995;26:595–601. [DOI] [PubMed] [Google Scholar]
- 15. Hiepe F, Radbruch A.. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol 2016;12:232–40. [DOI] [PubMed] [Google Scholar]
- 16. Tipton CM, Hom JR, Fucile CF, Rosenberg AF, Sanz I.. Understanding B-cell activation and autoantibody repertoire selection in systemic lupus erythematosus: a B-cell immunomics approach. Immunol Rev 2018;284:120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schrezenmeier E, Dorner JD.. Targeting B cells and plasma cells in glomerular diseases: translational perspectives. J Am Soc Nephrol 2018;29:741–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tipton CM, Fucile CF, Darce J. et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015;16:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng Q, Mumtaz IM, Khodadadi L. et al. Autoantibodies from long-lived ‘memory’ plasma cells of NZB/W mice drive immune complex nephritis. Ann Rheum Dis 2013;72:2011–7. [DOI] [PubMed] [Google Scholar]
- 20. Almaani S, Rovin BH.. B-cell therapy in lupus nephritis: an overview. Nephrol Dial Transplant 2019;34:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobi AM, Huang W, Wang T. et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum 2010;62:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cambridge G, Isenberg DA, Edwards JC. et al. B cell depletion therapy in systemic lupus erythematosus: relationships among serum B lymphocyte stimulator levels, autoantibody profile and clinical response. Ann Rheum Dis 2008;67:1011–6. [DOI] [PubMed] [Google Scholar]
- 23. Condon MB, Ashby D, Pepper RJ. et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis 2013;72:1280–6. [DOI] [PubMed] [Google Scholar]
- 24. Rehnberg M, Amu S, Tarkowski A, Bokarewa MI, Brisslert M.. Short- and long-term effects of anti-CD20 treatment on B cell ontogeny in bone marrow of patients with rheumatoid arthritis. Arthritis Res Ther 2009;11:R123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uzzan M, Ko HM, Rosenstein AK. et al. Efficient long-term depletion of CD20+ B cells by rituximab does not affect gut-resident plasma cells. Ann N Y Acad Sci 2018;1415:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vital EM, Dass S, Buch MH. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 2011;63:3038–47. [DOI] [PubMed] [Google Scholar]
- 27. Vallerskog T, Heimburger M, Gunnarsson I. et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther 2006;8:R167.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexander T, Cheng Q, Klotsche J. et al. Proteasome inhibition with bortezomib induces a therapeutically relevant depletion of plasma cells in SLE but does not target their precursors. Eur J Immunol 2018;48:1573–9. [DOI] [PubMed] [Google Scholar]
- 29. Alexander T, Sarfert R, Klotsche J. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis 2015;74:1474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kraaij T, Tengstrom FC, Kamerling SW. et al. A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun Rev 2016;15:577–84. [DOI] [PubMed] [Google Scholar]
- 31. Arends EJ, van Dam LS, Kraaij T. et al. A high-throughput assay to assess and quantify neutrophil extracellular trap formation. J Vis Exp 2019;143:e59150. [DOI] [PubMed] [Google Scholar]
- 32. Dall’Era M, Aranow C, Byron M. et al. Phase 2 trial of induction therapy with anti-CD20 (rituximab) followed by maintenance therapy with anti-BAFF (Belimumab) in patients with active lupus nephritis [abstract]. Arthritis Rheumatol 2018;70(suppl 10). [Google Scholar]
- 33. Moroni G, Quaglini S, Radice A. et al. The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsy. J Immunol Res 2015;10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papp K, Vegh P, Hobor R. et al. Immune complex signatures of patients with active and inactive SLE revealed by multiplex protein binding analysis on antigen microarrays. PLoS One 2012;7:e44824.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nossent JC, Huysen V, Smeenk RJ, Swaak AJ.. Low avidity antibodies to double stranded DNA in systemic lupus erythematosus: a longitudinal study of their clinical significance. Ann Rheum Dis 1989;48:677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andrejevic S, Jeremic I, Sefik-Bukilica M. et al. Immunoserological parameters in SLE: high-avidity anti-dsDNA detected by ELISA are the most closely associated with the disease activity. Clin Rheumatol 2013;32:1619–26. [DOI] [PubMed] [Google Scholar]
- 37. Oliveira RC, Oliveira IS, Santiago MB, Sousa Atta ML, Atta AM.. High avidity dsDNA autoantibodies in Brazilian women with systemic lupus erythematosus: correlation with active disease and renal dysfunction. J Immunol Res 2015;814748.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teng YK, Verburg RJ, Verpoort KN. et al. Differential responsiveness to immunoablative therapy in refractory rheumatoid arthritis is associated with level and avidity of anti-cyclic citrullinated protein autoantibodies: a case study. Arthritis Res Ther 2007;9:R106.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manson JJ, Isenberg DA.. The origin and pathogenic consequences of anti-dsDNA antibodies in systemic lupus erythematosus. Expert Rev Clin Immunol 2006;2:377–85. [DOI] [PubMed] [Google Scholar]
- 40. van Bavel CC, Dieker JW, Kroeze Y. et al. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis 2011;70:201–7. [DOI] [PubMed] [Google Scholar]
- 41. Dieker J, Schlumberger W, McHugh N. et al. Reactivity in ELISA with DNA-loaded nucleosomes in patients with proliferative lupus nephritis. Mol Immunol 2015;68:20–4. [DOI] [PubMed] [Google Scholar]
- 42. Matrat A, Veysseyre-Balter C, Trolliet P. et al. Simultaneous detection of anti-C1q and anti-double stranded DNA autoantibodies in lupus nephritis: predictive value for renal flares. Lupus 2011;20:28–34. [DOI] [PubMed] [Google Scholar]
- 43. Thanei S, Vanhecke D, Trendelenburg M.. Anti-C1q autoantibodies from systemic lupus erythematosus patients activate the complement system via both the classical and lectin pathways. Clin Immunol 2015;160:180–7. [DOI] [PubMed] [Google Scholar]
- 44. Trouw LA, Groeneveld TW, Seelen MA. et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J Clin Invest 2004;114:679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moroni G, Trendelenburg M, Del Papa N. et al. Anti-C1q antibodies may help in diagnosing a renal flare in lupus nephritis. Am J Kidney Dis 2001;37:490–8. [DOI] [PubMed] [Google Scholar]
- 46. Grootscholten C, Dieker JW, McGrath FD. et al. A prospective study of anti-chromatin and anti-C1q autoantibodies in patients with proliferative lupus nephritis treated with cyclophosphamide pulses or azathioprine/methylprednisolone. Ann Rheum Dis 2007;66:693–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hiepe F, Pfuller B, Wolbart K. et al. C1q: a multifunctional ligand for a new immunoadsorption treatment. Ther Apher 1999;3:246–51. [DOI] [PubMed] [Google Scholar]
- 48. Isenberg D, Appel GB, Contreras G. et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology 2010;49:128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fassbinder T, Saunders U, Mickholz E. et al. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther 2015;17:92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.