Introduction
Folliculitis decalvans is a rare neutrophilic inflammation of the scalp, characterized by painful follicular papules, pustules, crusting, and tufting of hairs. It results in the destruction of hair follicles and finally in cicatricial alopecia. The precise pathophysiology remains vague; inherited immune abnormalities and staphylococcal superantigens are presumed causes. The latter rationalizes the use of conventional antimicrobial treatments, such as prolonged antibiotics and dapsone, and of anti-inflammatory drugs as part of the therapeutic armamentarium.1 However, unclear etiology makes treatment of refractory folliculitis decalvans challenging.
Apremilast is approved for the treatment of psoriasis and Behçet disease, both characterized by neutrophilic inflammation. It acts as a selective intracellular inhibitor of phosphodiesterase 4 to regulate the production of multiple inflammatory mediators.
Case report
A 28-year-old man, originally from Iraq, presented at our clinic with a 5-year history of painful folliculitis decalvans. We observed large oval patches of hair loss with erythema, follicular pustules, crusting, and hair tufts distributed along the scalp, with a cutis verticis gyrata (Fig 1, A). The clinical diagnosis was confirmed by histology (Fig 2). Swabs from pustules showed moderate presence of Staphylococcus aureus and Citrobacter koseri. A deep mycosis was excluded clinically and by histology. The patient did not report past or present signs of acne and hidradenitis suppurativa. Treatments with topical corticosteroids, antibiotics and dapsone gel, and systemic rifampicin combined with clindamycin, lymecyclin, dapsone, and isotretinoin failed; also, photodynamic light therapy and adalimumab, intermittently together with systemic corticosteroids, were ineffective. Remaining off-label treatment options—namely, interleukin (IL) 17 inhibitors—as well as radiotherapy were relegated as last options, considering costs and, respectively, the possible secondary effects and definite scarring of the whole scalp after irradiation. Apremilast in monotherapy was subsequently initiated as for treatment of psoriasis, supported only by shampooing with 2% chlorhexidine at the patient's discretion.
Fig 1.
A, Oval patches of hair loss with erythema, follicular pustules, crusting, and hair tufts distributed along the scalp. B, Three weeks and 4 months after start of apremilast, with nearly complete regression of the inflammation.
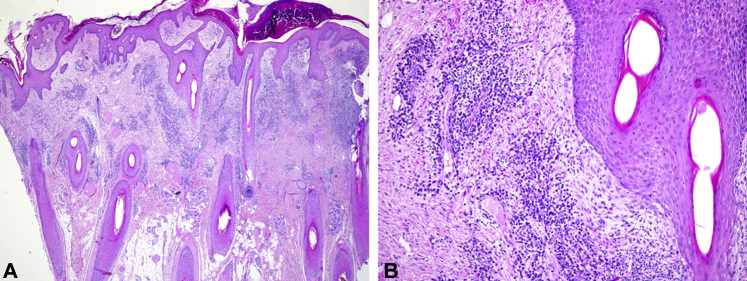
Fig 2.
A and B, Mostly superficial perifolliculitis with a mixed infiltrate composed of neutrophils, plasma cells, and eosinophils in a top-heavy pattern.
Rapid and continuous improvement of the inflammatory lesions and of the pain was reported within the first few days by the patient. Clinical control after 3 weeks showed a nearly complete remission (Fig 1, B). By then, pain and itching had subjectively ceased completely. Clinical remission was ascertained by the nearly complete resolution of erythema and of all follicular pustules, oozing, and crusts. This was underlined by trichoscopy, with abolition of any follicular hyperkeratosis and perifollicular erythema; tufted hairs, hair diameter diversity, cicatricial white patches, and yellow dots did not change significantly.2 Because this treatment had no insurance coverage, therapy was discontinued after 7 weeks, resulting in a rapid flareup. On approval by the health insurance company, apremilast monotherapy was resumed, with repeated prompt and full remission that continues (now >25 weeks).
Discussion
We present a patient with conventional therapy-refractory folliculitis decalvans, successfully treated with apremilast only. Because interruption of treatment and resumption of therapy resulted in recurrence and renewed remission, respectively, it is likely that apremilast suppressed the inflammatory reaction in folliculitis decalvans. The pathogenesis of this disease is not fully understood. Staphylococcus is thought to have a direct or indirect pathogenic role in the etiology and may be responsible for the dominant neutrophilic inflammatory infiltrate. A defective immune system also increases the risk of folliculitis decalvans, with several familial cases reported. Mechanical factors and structural abnormalities, such as the plicate scalp in this patient, may play an additional role.
T cells (CD3+ and CD4+), intercellular adhesion molecule 1, interferon gamma, and IL-4 and -8 are upregulated in folliculitis decalvans, being responsible for the neutrophil migration into the dermis. Gradually, fibroblast growth factor and transforming growth factor beta also become upregulated and are thought to mediate the scarring reaction.3, 4, 5 Apremilast, as a specific phosphodiesterase 4 inhibitor, suppresses innate and adaptive immune cells, including neutrophils and T cells. Reduced generation of spontaneous reactive oxygen species and of neutrophilic extracellular traps was observed in rheumatoid arthritis patients treated with apremilast.6 Apremilast also leads to downregulation of various proinflammatory cytokines, including interferon gamma, tumor necrosis factor α, and IL-6, -8, -12, -17, and -23, and to the upregulation of immunomodulatory cytokines such as IL-10.7 The inhibition of phosphodiesterase 4 therefore alters the cytokine composition and generates an anti-inflammatory state, likely leading to control of the inflammation. Because neutrophils are the predominant inflammatory cell in folliculitis decalvans, the inhibitory effect of apremilast on neutrophil activity could explain the rapid beneficial effect observed in this case.6
To our knowledge, this is the first report of a successful therapy of folliculitis decalvans with phosphodiesterase 4 inhibition; after prolonged treatment, a taper of apremilast will show whether full remission is possible in this patient who did not respond to conventional therapies. The rapid effectiveness of phosphodiesterase 4 inhibition introduces new therapeutic options for folliculitis decalvans and warrants further investigation. Prospective studies with bigger patient cohorts will verify the eventual general value of apremilast for folliculitis decalvans.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Rambhia P.H., Conic R.R.Z., Murad A., Atanaskova-Mesinkovska N., Piliang M., Bergfeld W. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794–801.e1. doi: 10.1016/j.jaad.2018.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Crehuet P., Vano-Galvan S., Molina-Ruiz A.M. Trichoscopic features of folliculitis decalvans: results in 58 patients. Int J Trichology. 2017;9:140–141. doi: 10.4103/ijt.ijt_85_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiarini C., Torchia D., Bianchi B., Volpi W., Caproni M., Fabbri P. Immunopathogenesis of folliculitis decalvans: clues in early lesions. Am J Clin Pathol. 2008;130:526–534. doi: 10.1309/NG60Y7V0WNUFH4LA. [DOI] [PubMed] [Google Scholar]
- 4.Harries M.J., Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol. 2010;177:2152–2162. doi: 10.2353/ajpath.2010.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyraud A., Milpied B., Thiolat D. Inflammasome activation characterizes lesional skin of folliculitis decalvans. Acta Derm Venereol. 2018;98:570–575. doi: 10.2340/00015555-2924. [DOI] [PubMed] [Google Scholar]
- 6.van Breda S., Rossi S., Hasler P. Regulation of neutrophil extracellular traps by apremilast (phosphodiesterase 4 inhibition) Arthritis Rheumatol. 2019;71(suppl 10) [Google Scholar]
- 7.Schafer P.H., Chen P., Fang L., Wang A., Chopra R. The pharmacodynamic impact of apremilast, an oral phosphodiesterase 4 inhibitor, on circulating levels of inflammatory biomarkers in patients with psoriatic arthritis: substudy results from a phase III, randomized, placebo-controlled trial (PALACE 1) J Immunol Res. 2015;2015:906349. doi: 10.1155/2015/906349. [DOI] [PMC free article] [PubMed] [Google Scholar]