Abstract
Encephalopathy with electrical status epilepticus in sleep (ESES) syndrome is characterized by a near-continuous spike-and-wave discharges during sleep with marked developmental regression, mainly in speech, and the presence of clinical seizures. Although the etiology ofESES is generally unknown, its resistance to antiseizure medication (ASM), and favorable responses to oral corticosteroids (OCS), support a role for inflammation. However, the prolonged use of OCS results in undesirable side effects and alternative treatment measures are needed. Herein, we present a patient with ESES who revealed responsed to a combination of immunomodulating agents other than OCS. The patient revealed 30, 50, and 100%, reduction in the ESES pattern on EEG with the sequential addition of anakinra (interleukin-1ß inhibitor), intravenous immunoglobulin (IVIg), and sirolimus, an inhibitor of mammalian target of rapamycin (mTOR) respectively, after discontinuation of OCS due to side effects. This combination of immune-modulating agents, that were selected based on monocyte cytokine profiles, also resulted in a gradual improvement of speech and behavioral symptoms. This case indicates a possible use of immunomodulating agents other than OCS for ESES syndrome.
Keywords: ESES, IL-1ß, mTOR inhibitor, Oral corticosteroid
Highlights
-
•
ESES syndrome can be controlled immunomodulating agents.
-
•
OCS was not requred for maintaing good control of ESES in the presented case.
-
•
Monocyte cytokine profiles may be useful for the selection of immunomodulating agents for treatment of ESES.
1. Introduction
Encephalopathy with electrical status epilepticus in sleep (ESES syndrome) mainly affects children, mostly present between 2 and 14 years of age [2], and is characterized by the ESES pattern in electroencephalogram (EEG) (originally described as > 85% of status epileptics during non-rapid eye movement sleep) and decline of cognitive functioning with or without clinical seizures [3]. ESES syndrome is often interchangeably called encephalopathy with continuous spikes and waves during sleep (CSWS), defined with prodromal EEG abnormalities followed by a CSWS pattern, and the improvement of EEG patterns with age [4,5]. ESES may include Landau–Kleffner syndrome (LKS) which was originally described as a clinical syndrome with acquired aphasia and seizures [6]. Spontaneous resolution of the ESES pattern tends to occur during puberty, but cognitive impairment generally persists [7,8]. Etiology of ESES syndrome is unknown in most cases, except for a small subset of ESES patients with identifiable brain malformations or gene mutations [9,10].
Most ESES patients without a known etiology have been treated with ASM1AEDs (mainly benzodiazepines), and oral corticosteroids (OCS). The therapeutic effects of ASM on ESES syndrome have been less than satisfactory [1]. OCS tend to provide better therapeutic effects than ASM, but OCS is not suitable for long-term use, given its significant side effects [1,7,11]. There is an urgent need of other treatment options for effectively controlling ESES syndrome. Immune mediated inflammation has been suspected to play a role in ESES with unknown etiology, considering favorable responses to OCS in ESES patients [12]. Other immunomodulating agents used for epileptic conditions other than ESES, such as intravenous immunoglobulin (IVIg) may be applicable for ESES. However, it should be noted that IVIg is not universally effective in controlling epilepsy [13].
This case report describes one ESES patient who responded unfavorably to ASM and had recurrence of ESES/cognitive impairment after discontinuation of OCS. However, a stepwise addition of immunomodulating agents, that were selected based on monocyte cytokine profiles, resulted in the complete resolution of the ESES pattern in his EEG and improvement in cognitive impairment. To our knowledge, anakinra (IL-1ß inhibitor) and sirolimus (mTOR inhibitor) are novel therapeutic approaches for ESES. The presented case indicates a possible application of immunomodulating agents other than OCS for ESES syndrome.
2. Materials and methods
2.1. The study subject
The signed consent form for preparation of this case report was obtained prior to submission of the paper per policy of our institution. For doing serial assays of monocyte cytokine production, he was enrolled in a research study approved by the IRB at our institution after obtaining a signed consent form.
2.2. Cell cultures
Purified peripheral blood monocytes (PBMo), obtained with the use of an immunoaffinity column (monocyte separation kit II – human, MILTENYI BIOTEC, Cambridge, MA, United States), were used for assessing the production of monocyte cytokines [interleukin-1ß (IL-1ß), IL-6, IL-10, IL-12p40, tumor necrosis factor-α (TNF-α), soluble TNF receptor II (sTNFRII), and CC motif ligand 2 (CCL2)] by enzyme linked immunosorbent assay (ELISA) [14]. ELISA reagents were obtained from BD Biosciences (San Diego, CA, USA) and R & D (Minneapolis, MN, USA). PBMo were incubated overnight with and without stimuli of innate immunity [lipopolysaccharide (LPS) (0.1 μg/ml, GIBCO-BRL, Gaithersburg, MD, USA), zymosan (50 μg/ml, Sigma-Aldrich, St. Luis, Mo, USA), CL097 (20 μM, Invivogen, San Diego, CA, USA), and candida heat extract (HCKA, 107 cells/ml, Invivogen, as a source of ß-glucan) in the same culture conditions detailed previously [15].
3. Results
3.1. Case presentation
The patient initially presented at 6 years of age for evaluation of possible immune abnormalities in association with ESES to the Pediatric Allergy/Immunology Clinic. He was born at 37 weeks gestational age via cesarean section to a mother with pre-eclampsia. His birth weight was 5 pounds 11 oz. He was noted to have hyper-echolalia, mild muscle wasting/hypotonia, and chronic constipation around 2 years of age. An extensive metabolic and genetic workup excluded primary mitochondrial/metabolic disorders.
3.1.1. ESES diagnosis
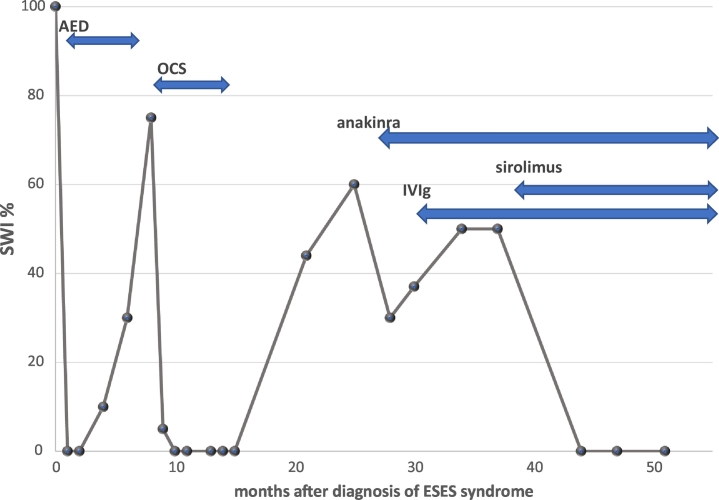
He developed normally except for mild speech delay (hyperlexia and echolalia) until around 5 years-old, when he regressed developmentally. Regression mainly affected speech/language, along with onset of disturbed sleep and hyperactivity, followed by a clinical seizure (nocturnal seizure) at 5.5 years. Neurology workup at John Hopkins led to a diagnosis of ESES syndrome [spike-wave index (SWI) 100%] with unremarkable findings in the magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) (Fig. 1).
Fig. 1.
Changes in percentage (%) of SWI by vEEG after the diagnosis of ESES syndrome.
3.1.2. Initial treatment measures
He revealed only temporary improvement in ESES pattern with ASM (diazepam, divalproex, and clobazam). Three months after the diagnosis of ESES, marked fluctuation of behavioral symptoms sets in, triggered by microbial infection: his symptoms were similar to those of acute-onset neuropsychiatric symptoms (PANS) [16]. He was then tried on OCS (prednisone 2 mg/kg/day), which resulted in the normalization of his EEG and improvement in cognitive functioning/behavioral symptoms (Fig. 1). His neuropsychiatric symptoms slowly returned, after tapering off the OCS protocol. The parents elected not to repeat OCS therapy due to assoicated lack of growth, excessive weight gain, and mood swing. Diffuse decreases in metabolic activity in the left temporal lobe by PET/SPECT scan and the ESES pattern (55% SWI) recurred after tapering off OCS (Fig. 1). He returned to our clinic at 7.5 years old to explore other treatment options.
3.1.3. Changes in vEEG findings following immunomodulating agents
Anakinra, was started (100 mg/day subcutaneous (SQ) injection) at 7.6 years, due to increase in production of IL-1ß (Fig. 2). Despite improvement of behavioral symptoms with anakinra, the ESES pattern (> 60% SWI in all areas) persisted (Fig. 1). IVIg (0.6 g/kg/dose every 3 weeks) was added as a preventive measure for recurrent viral syndrome, since behavioral symptoms (hyperactivity, irritability, and worsening speech) worsened in the convalescence stage of viral syndrome. The ESES patterns persisted after the addition of IVIg to his regimen (Fig. 1). Sirolimus (1 mg/day) was then added and after 7 weeks of treatment, his vEEG revealed resolution of the ESES pattern. Normal EEG findings observed at 3 and 7 months were observed after the addition of sirolimus(Fig. 1).
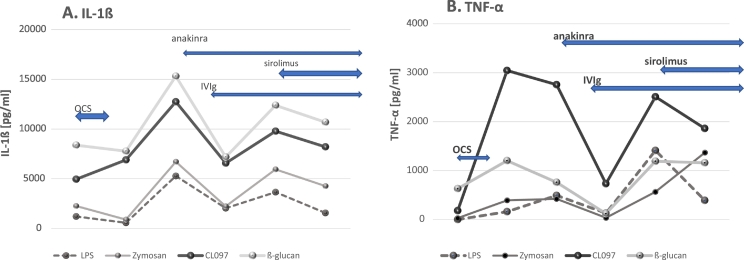
Fig. 2.
Changes in production of IL-1ß [panel A] and TNF-α [panel B] by PBMo from the obtained case.
3.1.4. Cognitive impairment and behavioral symptoms with immunomodulating agents
His mother who is a linguist, observed improved sleep pattern/speech/social interactions with anakinra despite the lack of improvement in vEEG. It is of note that the behavioral symptoms (especially motor tics) fluctuated following microbial infection and social stress, even after starting IVIg, and then sirolimus. IVIg was later switched to the SQ rout (0.2 g/kg/dose every week), due to increasingly difficult IV access: this change eliminated his need for OCS as pre- and post-medications. Despite fluctuating behavioral symptoms, with these immunomodulating agents, he retained gains in cognitive functioning. He would typically lose once acquired cognitive functions following immune insults prior to treatment. Then his behavioral symptoms slowly improved. Our attempt of reducing the anakinra dose after starting sirolimus was not tolerated with noticeable worsening behavioral symptoms and speech impairment. The patient continues to have hyperlexia and echolalia with infrequent novel utterance that were present prior to onset of ESES.
3.2. Laboratory findings
3.2.1. Metabolic and genetic workup
Results of an extensive metabolic workup, with the use of CSF, plasma, and urine were unremarkable. Array comparative genomic hybridization (CGH), epilepsy gene panel, mitochondrial genome sequencing, and whole exom sequencing (WES) did not reveal any abnormalities.
3.2.2. Conventional immune workup
He had a negative allergy workup with total IgE level at 11, and normal immunoglobulin (Ig) and pneumococcal antibody titers prior to start of supplemental Ig treatment. Quantiferon-TB test and hepatitis B screening were both negative before starting sirolimus. IgM and IgA and remained within normal range after starting anakinra and sirolimus. Sequential examination of his complete blood cell (CBC) count revealed normal levels of hemoglobin, WBC and platelet counts.
3.2.3. Monocyte cytokine profiling
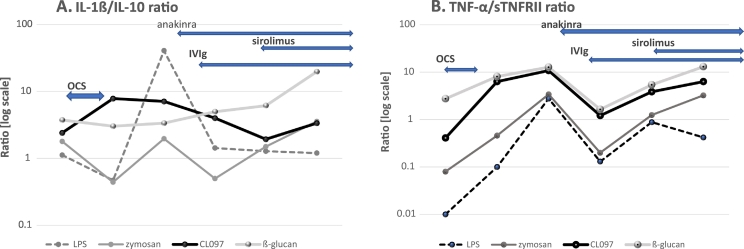
Monocyte cytokine profiles were tested repeatedly as summarized in Fig. 2, Fig. 3. When his neuropsychiatric symptoms and ESES pattern by vEEG became exacerbated after tapering off of OCS, the most notable finding in monocyte cytokine profiles was an increase in IL-1ß and TNF-α production (Fig. 2A and B). Production of these cytokines decreased after starting anakinra and IVIg, but increased again, after he suffered from a viral syndrome. These levels declined after starting sirolimus. Although IL-6 has been implicated with epilepsy and febrile seizures [17,18], we did not observe significant changes in IL-6 production by PBMo in his case. When we examined the ratio of inflammatory cytokine vs. counter-regulatory cytokines, as IL-1ß/IL-10 and TNF-α/sTNFRII ratios, we observed an increase in these ratios before starting anakinra (Fig. 3). The IL-1ß/IL-10 ratios under LPS or CL097 stimulated cultures revealed similar tendencies as compared to changes of production of IL-1ß and TNF-α (Fig. 3A and B).
Fig. 3.
Changes in IL-1ß/IL-10 and TNF-α/sTNFRII ratios produced by PBMo in this patient.
4. Discussion
A role of inflammatory cytokines in seizure disorders has been suspected in febrile seizures [19]. In animal models of febrile seizures, increased production of IL-1α, IL-1ß, IL-6, and TNF-α has been reported and also in humans, both centrally (the brain) and the peripherally [20]. Immune mediated inflammation has also been implicated with aafebrile seizures, indicating a possible role for anti-inflammatory treatment as therapeutic options for epilepsy [21,22]. A role of inflammation has also been suspected in ESES, given favorable responses to OCS [1,11]. Increase in serum cytokine levels (TNF-α, IL-6, IL-1α, and CCL2) has been shown in 11 ESES patients with unknown etiology [12]. In this study, the authors reported significant decrease in plasma IL-6 levels in 5 ESES patients when they were treated with either OCS in 3 patients, and monthly IVIg in 2 patients, without noticeable changes in plasma levels of other cytokines [12].
Serum levels of cytokines are easy to measure, but may not be sensitive biomarkers, since some cytokines that are implicated with clinical seizures [21], i.e. TNF-α and IL-1ß, are known to have short half-lives. In other neurodegenerative diseases, levels of cytokine have been shown to vary over time [23].
We have studied changes in monocyte cytokine profiles in treatment-resistant epilepsy as well as autism spectrum disorders [14,24]. In these conditions, production of IL-1ß and IL-1ß/IL-10 ratios revealed correlations with behavioral symptoms of autism spectrum disorder and responses to the anakinra for clinical seizures [14,24,25]. This is attributed to the fact that PBMo can migrate to the brain and differentiate into bone marrow derived microglial cells skewed to inflammatory responses [26,27].
After the patient failed to maintain remission following tapering off OCS, we assessed monocyte cytokine profiles, to address other treatment options. The results of increased production of IL-1ß, TNF-α, IL-1ß/IL-10 ratio, and TNF-α/sTNFRII ratios (Fig. 2, Fig. 3), indicated a use of immunomodulating agents for controlling the production of these cytokines. Anakinra was initially utilized because it has a good safety profile except for local reactions at the injection site [28]. IVIg was then added to prevent the recurrent viral syndromes, that often triggered his worsening behavioral symptoms. IVIg has been reported to have had favorable responses in 2 ESES subjects by others [12], and has also been used as a second line measure for treatment-resistant seizures [13,29].
Finally, oral sirolimus was added to his regimen for the following reasons. First, mTOR inhibitors have been used for controlling treatment-resistant seizures in patients with mutations causing over-activation of the mTOR pathway [30]. mTOR inhibitors have also been used for patients with primary immunodeficiency syndromes which cause overactivation of mTOR pathway and subsequent neuropsychiatric symptoms [31]. In addition, mTOR inhibitors are known to shift T cell differentiation from inflammatory T-helper 17 (Th17) cells into regulatory T (Treg) cells and increase autophagy, potentially exerting multiple anti-inflammatory effects on various immune cells, including brain immune cells [32]. Because of its immunosuppressive effects, there is a potential risk of bone marrow suppression and activation of tuberculosis and hepatitis B may be possible. The presented case had both negative hepatitis B screening and quantiferon TB gold. He has been closely monitored and so far, there is no evidence of side effects. In the presented case, addition of sirolimus revealed resolution of the ESES pattern. TNF-α and IL-1ß production also tended to decrease with the addition of sirolimus to his treatment regimen, indicating a role of mTOR pathway activation in his ESES.
Our case revealed favorable effects of immunomodulating agents, further supporting a role of neuroinflammation in ESES syndrome with unknown etiology. OCS have been noted to exert favorable effects on ESES, but chronic OCS are not suitable as a maintenance medication for some patients, given their unfavorable side effects. On the other hand, the immunomodulating agents tried in the presented case (IVIg, anakinra, and sirolimus) can be used for prolonged periods with tolerable side effects. However, anakinra and IVIg are injectables and costly, as compared to oral sirolimus. Oral gate keeper inhibitors such as sirolimus may be a good first line choice when treatment measures other than OCS are required for ESES. The presented case also indicates a need for the blockage of other inflammatory pathways to fully control ESES other than just the mTOR pathway. Becuase this is a single case report, such possibilities need to be addressed in a larger number of ESES patients, preferentially in a double-blinded, placebo-controlled study design.
5. Conclusions
This study presents the case of a child with ESES syndrome who was reasonably controlled with a combination of immunomodulating agents other than OCS. Further studies will be necessary to elucidate which immunomodulating agents are suitable for each ESES patient, based on better characterization of clinical features and laboratory findings.
Abbreviation used
- AEDs
anti-epileptic drugs1 Use ASM as the abbreviation for antiseizure medication
- CGH
comparative genomic hybridization
- CSF
cerebral spinal fluid
- CSWS
continuous spikes and waves during sleep
- EEG
electroencephalogram
- ESES
encephalopathy with electrical status epilepticus in sleep
- Ig
immunoglobulin
- IL
interleukin
- IVIg
intravenous immunoglobulin
- LKS
Landau–Kleffner syndrome
- MRI
magnetic resonance imaging
- mTOR
mammalian target of rapamycin
- OCS
oral corticosteroid
- PBMo
peripheral blood monocytes
- PANS
pediatric autoimmune neuropsychiatric syndrome
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- SPUH
Saint Peter's University Hospital
- SQ
subcutaneous
- SWI
spike wave index
- TGF
tumor growth
- TNF
tumor necrosis factor
- vEEG
video EEG
- WES
whole exome sequencing
Ethical statement
For a case report, our institution does not require IRB review, but request a written permission to prevent the detail of the case. A written permission (consent) was obtained before preparation of this case presentation. The signed consent form following the policy of our institution was obtained.
Author contributions
HJ was directly involved in the case of the presented case and was responsible for his clinical care, immune workup, and preparation of manuscript. LG was responsible for conducting immune assays and data preparation.
Declaration of competing interest
Authors have nothing to disclose.
Acknowledgement
This study was partly supported by funding from Jonty Foundation, St. Paul, MN, and Governor's Council for Medical Research and Treatment of Autism, DHHS, NJ (CAUT16APL007). Authors are thankful for generous support from the family of the presented case and their generous permission to presenting the case. We are also thankful to Dr. L. Huguenin for her critically reviewing the manuscript.
Footnotes
Please substitute AED with ASM throughout the text.
References
- 1.van den Munckhof B., van Dee V., Sagi L., Caraballo R.H., Veggiotti P., Liukkonen E. Treatment of electrical status epilepticus in sleep: a pooled analysis of 575 cases. Epilepsia. 2015;56(11):1738–1746. doi: 10.1111/epi.13128. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez Fernandez I., Chapman K.E., Peters J.M., Harini C., Rotenberg A., Loddenkemper T. Continuous spikes and waves during sleep: electroclinical presentation and suggestions for management. Epilepsy Res Treat. 2013;2013:583531. doi: 10.1155/2013/583531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patry G., Lyagoubi S., Tassinari C.A. Subclinical “electrical status epilepticus” induced by sleep in children. A clinical and electroencephalographic study of six cases. Arch Neurol. 1971;24(3):242–252. doi: 10.1001/archneur.1971.00480330070006. [DOI] [PubMed] [Google Scholar]
- 4.Caraballo R., Pavlidis E., Nikanorova M., Loddenkemper T. Encephalopathy with continuous spike-waves during slow-wave sleep: evolution and prognosis. Epileptic Disord. 2019;21(S1):15–21. doi: 10.1684/epd.2019.1052. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch E., Caraballo R., Bernardina B.D., Loddenkemper T., Zuberi S.M. Encephalopathy related to status epilepticus during slow sleep: from concepts to terminology. Epileptic Disord. 2019;21(S1):5–12. doi: 10.1684/epd.2019.1051. [DOI] [PubMed] [Google Scholar]
- 6.Hughes J.R. A review of the relationships between Landau-Kleffner syndrome, electrical status epilepticus during sleep, and continuous spike-waves during sleep. Epilepsy Behav. 2011;20(2):247–253. doi: 10.1016/j.yebeh.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Hempel A., Frost M., Agarwal N. Language and behavioral outcomes of treatment with pulse-dose prednisone for electrical status epilepticus in sleep (ESES) Epilepsy Behav. 2019;94:93–99. doi: 10.1016/j.yebeh.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez Fernandez I., Loddenkemper T., Peters J.M., Kothare S.V. Electrical status epilepticus in sleep: clinical presentation and pathophysiology. Pediatr Neurol. 2012;47(6):390–410. doi: 10.1016/j.pediatrneurol.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Galanopoulou A.S., Bojko A., Lado F., Moshe S.L. The spectrum of neuropsychiatric abnormalities associated with electrical status epilepticus in sleep. Brain Dev. 2000;22(5):279–295. doi: 10.1016/s0387-7604(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 10.Kessi M., Peng J., Yang L., Xiong J., Duan H., Pang N. Genetic etiologies of the electrical status epilepticus during slow wave sleep: systematic review. BMC Genet. 2018;19(1):40. doi: 10.1186/s12863-018-0628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Munckhof B., Alderweireld C., Davelaar S., van Teeseling H.C., Nikolakopoulos S., Braun K.P.J. Treatment of electrical status epilepticus in sleep: clinical and EEG characteristics and response to 147 treatments in 47 patients. Eur J Paediatr Neurol. 2018;22(1):64–71. doi: 10.1016/j.ejpn.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 12.van den Munckhof B., de Vries E.E., Braun K.P., Boss H.M., Willemsen M.A., van Royen-Kerkhof A. Serum inflammatory mediators correlate with disease activity in electrical status epilepticus in sleep (ESES) syndrome. Epilepsia. 2016;57(2):e45–e50. doi: 10.1111/epi.13274. [DOI] [PubMed] [Google Scholar]
- 13.Geng J., Dong J., Li Y., Ni H., Jiang K., Shi L.L. Intravenous immunoglobulins for epilepsy. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD008557.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jyonouchi H., Geng L., Davidow A.L. Cytokine profiles by peripheral blood monocytes are associated with changes in behavioral symptoms following immune insults in a subset of ASD subjects: an inflammatory subtype? J Neuroinflammation. 2014;11:187. doi: 10.1186/s12974-014-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jyonouchi H., Geng L. Associations between monocyte and T cell cytokine profiles in autism spectrum disorders: effects of dysregulated innate immune responses on adaptive responses to recall antigens in a subset of ASD children. Int J Mol Sci. 2019;20(19) doi: 10.3390/ijms20194731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang K., Frankovich J., Cooperstock M., Cunningham M.W., Latimer M.E., Murphy T.K. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2015;25(1):3–13. doi: 10.1089/cap.2014.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uludag I.F., Duksal T., Tiftikcioglu B.I., Zorlu Y., Ozkaya F., Kirkali G. IL-1beta, IL-6 and IL1Ra levels in temporal lobe epilepsy. Seizure. 2015;26:22–25. doi: 10.1016/j.seizure.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 18.de Vries E.E., van den Munckhof B., Braun K.P., van Royen-Kerkhof A., de Jager W., Jansen F.E. Inflammatory mediators in human epilepsy: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2016;63:177–190. doi: 10.1016/j.neubiorev.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Kwon A., Kwak B.O., Kim K., Ha J., Kim S.J., Bae S.H. Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure. 2018;59:5–10. doi: 10.1016/j.seizure.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Feng B., Chen Z. Generation of febrile seizures and subsequent epileptogenesis. Neurosci Bull. 2016;32(5):481–492. doi: 10.1007/s12264-016-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paudel Y.N., Shaikh M.F., Shah S., Kumari Y., Othman I. Role of inflammation in epilepsy and neurobehavioral comorbidities: implication for therapy. Eur J Pharmacol. 2018;837:145–155. doi: 10.1016/j.ejphar.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Rana A., Musto A.E. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15(1):144. doi: 10.1186/s12974-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Martinez L., Calvo A.C., Munoz M.J., Osta R. Are circulating cytokines reliable biomarkers for amyotrophic lateral sclerosis? Int J Mol Sci. 2019;20(11) doi: 10.3390/ijms20112759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jyonouchi H., Geng L. Intractable epilepsy (IE) and responses to anakinra, a human recombinant IL-1 receptor agonist (IL-1ra): case reports. J Clin Cell Immunol. 2016;7(5) [Google Scholar]
- 25.Jyonouchi H., Geng L., Streck D.L., Dermody J.J., Toruner G.A. MicroRNA expression changes in association with changes in interleukin-1ss/interleukin10 ratios produced by monocytes in autism spectrum disorders: their association with neuropsychiatric symptoms and comorbid conditions (observational study) J Neuroinflammation. 2017;14(1):229. doi: 10.1186/s12974-017-1003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKim D.B., Weber M.D., Niraula A., Sawicki C.M., Liu X., Jarrett B.L. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2018;23(6):1421–1431. doi: 10.1038/mp.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng L., Murugan M., Bosco D.B., Liu Y., Peng J., Worrell G.A. Microglial proliferation and monocyte infiltration contribute to microgliosis following status epilepticus. Glia. 2019;67(8):1434–1448. doi: 10.1002/glia.23616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettiol A., Lopalco G., Emmi G., Cantarini L., Urban M.L., Vitale A. Unveiling the efficacy, safety, and tolerability of anti-interleukin-1 treatment in monogenic and multifactorial autoinflammatory diseases. Int J Mol Sci. 2019;20(8) doi: 10.3390/ijms20081898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J.J., Wang Y., Lan S.Y., Chan O.W., Hsia S.H., Chou M.L. Combination of intravenous immunoglobulin and steroid pulse therapy improves outcomes of febrile refractory status epilepticus. Epilepsy Res. 2018;142:100–105. doi: 10.1016/j.eplepsyres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Saffari A., Brosse I., Wiemer-Kruel A., Wilken B., Kreuzaler P., Hahn A. Safety and efficacy of mTOR inhibitor treatment in patients with tuberous sclerosis complex under 2 years of age - a multicenter retrospective study. Orphanet J Rare Dis. 2019;14(1):96. doi: 10.1186/s13023-019-1077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coulter T.I., Cant A.J. The treatment of activated PI3Kdelta syndrome. Front Immunol. 2018;9:2043. doi: 10.3389/fimmu.2018.02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacella I., Piconese S. Immunometabolic checkpoints of Treg dynamics: adaptation to microenvironmental opportunities and challenges. Front Immunol. 2019;10:1889. doi: 10.3389/fimmu.2019.01889. [DOI] [PMC free article] [PubMed] [Google Scholar]