Abstract
Inflammatory IL-6/STAT3 signaling is constitutively activated in diverse cancers and is associated with malignant cell proliferation, invasion and escape of antitumor immunosurveillance. Liraglutide, a glucagon-like peptide-1 (GLP-1) analog, is commonly used to treat insulin-resistant diabetes. In this study, for the first time, we showed that liraglutide remarkably improved the antitumor immune responses in hepatocellular carcinoma (HCC). Furthermore, we showed that the antitumor activity was mediated by nature killer cells (NKs) but not CD8+ T cells. Finally, we showed that liraglutide enhanced NK-mediated cytotoxicity by suppressing the IL-6/STAT3 signaling pathway in HCC cells. Our findings unveil a novel therapeutic role of liraglutide by manipulating the innate immunity in cancer therapy.
Introduction
Hepatocellular carcinoma (HCC) ranks the sixth most common cancer in the world [1]. HCC responds poorly to anticancer drugs and radiotherapy [2], and more than 90% of HCC cases are associated with chronic inflammation [3]. Accumulating evidence has shown that the inflammatory microenvironment of HCC contributes to antitumor immunosuppression. Signal transducer and activator of transcription 3 (STAT3) acts a pivotal role in determining and maintaining a procarcinogenic inflammatory microenvironment during tumor progression [[4], [5], [6], [7]], and in HCC, the interleukin (IL)-6/STAT3 pathway has important roles in tumor progression [8,9]. In tumor microenvironment, IL-6/STAT3 pathway plays to promote the survival, proliferation, and invasiveness of tumor cells, while intensively suppressing the antitumor immunity [10]. Recent studies have shown that anti-inflammatory strategies hold promise in cancer treatment [11]. We and others have shown that inhibition of aberrant STAT3 activation or deletion of STAT3 in mice suppresses tumor progression by improving antitumor immune responses [[12], [13], [14]]. Besides, STAT signaling also plays important roles in regulation of innate immunity of human population especially in people with tumors [15]. Furthermore, many clinical and/or preclinical studies have also shown that the anti-inflammatory agents used to target cancer-related inflammation enhance the effects of immunotherapies and suppress cancer progression [16].
Liraglutide is a glucagon-like peptide-1 (GLP-1) mimetic with 97% structural homology to GLP-1 [17] that promotes insulin secretion [18] and reduces post-prandial glucose levels [19] in people with type 2 diabetes. Interestingly, liraglutide has also been shown to have anti-inflammatory activity in non-alcoholic steatohepatitis [20]. Furthermore, liraglutide pretreatment was shown to significantly reduce IL-1β levels and effectively inhibit the formation of the NLRP3 inflammasome [21]. In line with these results, GLP-1 was observed to markedly inhibit ovalbumin-induced airway inflammation and NF-κB p65 activation [22]. Therefore, we hypothesized that liraglutide may possess antitumor efficacy by modulating the inflammatory microenvironment of HCC.
In our experiment, we showed liraglutide enhances the antitumor activity of NK cells by inhibiting IL-6/STAT3 signaling. NK cells (also known as natural killer cells) play important roles in innate immune system and are responsible for lysing cells such as transformed, virus infected cells or stressed cells without prior sensitization or MHC class restriction. In human body, NK cells are identified as CD3−CD16+CD56+ and NKp46 (natural cytotoxicity receptor) positive lymphocytes [23].
In this research, we studied the therapeutic role of liraglutide in manipulating the antitumor immune responses in HCC and attempted to elucidate the underlying mechanism.
Materials and methods
Cell lines
The hepatocellular carcinoma cell lines HCC-LM3 and Hepa1-6 were purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences. Cells were cultured in complete DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin and 2 mM l-glutamine (all from Life Technology, NY, USA) and kept at 37 °C in a humidified atmosphere containing 5% CO2.
Generation of NKT cells
Human peripheral blood (50 mL) was obtained from healthy male volunteers, and all volunteers have signed the informed consent. Human PBMCs were isolated freshly via density gradient centrifugation using Ficoll (Hao Yang Biological Products Technology, Tianjin, China) as described elsewhere [24,25]. Cells were then washed with PBS and propagated at 1 × 106 cells/mL in GT-T551 (Takara Bio, Shiga, Japan) containing 2000 IU/mL recombinant human IFN-γ (Chemo Wanbang Biopharma, Shanghai, China), 10 mg/mL OK432 (Shanghai Chugai Pharmaceutical, Chome, Japan), 1% penicillin-streptomycin and 2% heat-inactivated autologous plasma for 24 h. After that, cells were transferred to a cell culture flask with 5 μg/mL anti-CD3 antibody coated (T&L Biological Technology, Beijing, China). Then, GT-T551 medium containing 700 IU/mL recombinant human IL-2 was added (BD Biosciences, CA, USA). After 14 days, cells were detected by flow cytometry, collected and kept at −80 °C for further use. The experiment was approved by the Ethics Committee of Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, China.
Flow cytometry
The quality and phenotype of NKT cells were determined by flow cytometry. Briefly, cells were harvested and cultured for 30 min at 4 °C using four fluorescence-conjugated antibodies: anti-CD4-APC, anti-CD3-FITC, anti-NKG2D-APC and anti-CD8-PE (all from BD Biosciences, CA, USA). Then, the NKT cells were detected using a FACS-Caliber System (from BD Biosciences, CA, USA) before washed twice with PBS. The results were analyzed using FlowJo software (Version 7.6.5, Tree Star Inc., OR, USA).
Animals
Male C57BL/6J mice (6–8 weeks old) were purchased from the Model Animal Research Center of Nanjing University. All mice were contained in an idea microenvironment (temperature: 23 ± 2 °C, humidity: 60 ± 10%, and a 12/12 h light/dark cycle) with free access to food and water. Mice were acclimatized for 7 days before further experiments. All animal experiments were approved by the Research Ethical Committee on Animal Experimentation of Nanjing University in accordance with the Guide for the Care and Use of Laboratory Animals.
Reagents
Liraglutide was obtained from Novo Nordisk (Bagsvaerd, Denmark). The Luciferase Assay System was obtained from Promega (Madison, WI, USA). Human IFN-γ ELISA kit was purchased from MABTECH (Stockholm, Sweden).
Antibodies
The anti-GAPDH antibody (MB001) was purchased from Bioworld (MN, USA). The anti-STAT3 (#9139) and anti-phospho-STAT3 (#9145) antibodies were obtained from Cell Signaling Technology (Boston, MA, USA).
NKT cell cytotoxicity assay
HCC-LM3 cells expressing luciferase served as target cells in NKT cell cytotoxicity assays. HCC-LM3 cells were seeded into 96-well plates and cultured overnight. Then, NKT cells were added with different ratios (effector:target, E:T). Twelve hours later, the wells were washed with PBS twice. Target cell viability was determined by luminescence spectrometry after adding 150 μg/mL luciferin (Gold Biotechnology, St. Louis, MO, USA) in accordance with the instruction manuals. In addition, HCC-LM3 cells (tumor cells) were incubated with or without liraglutide for 12 h followed by adding NKT cells (E:T = 5:1) for another 12 h. Cell activity was detected by luminescence spectrometry.
Western blot analysis
Cells were lysed in RIPA buffer (Beyotime Biotechnology, Shanghai, China) with a protease inhibitor table (Roche, Mannheim, Germany), and the protein concentration was detected by a BCA kit (Beyotime Biotechnology, Shanghai, China) as described elsewhere [26]. Targeted protein were migrated on a SDS-PAGE followed by transferring to PVDF membranes (Roche, Basel, Switzerland), followed by adding 5% nonfat milk. Then, the membranes were incubated with targeting antibodies overnight. After being incubated with appropriate horseradish peroxidase-conjugated secondary antibodies, the bands were detected by densitometry using the Tanon 5200 imaging system (Shanghai, China).
RNA isolation and quantitative real-time PCR
Quantitative RT-PCR (qRT-PCR) was conducted in accordance with the instruction manuals. In briefly, total RNA was extracted with TRIzol (Invitrogen, CA, USA), while cDNA was reverse-transcribed by the cDNA synthesis kit (TaKaRa, Shija, Japan). Real-time quantification was conducted via a SYBR Green Kit (Bio-Rad Laboratories, Inc., CA, USA) and an ABI 7300 Detection System (Advanced Biosystems, CA, USA). Targeted gene level was detected by the comparative Ct method while normalized to GAPDH.
The primer sequences were listed below: human GAPDH, forward (5′-CCATGTTCGTCATGGGTGTGAACCA-3′ and reverse (5′-CAGTAGAGGCAGGGATGATGTTC-3′); human IL-6, forward (5′-ACTCACCTCTTCAGAACGAATTG-3′) and reverse (5′-CCATCTTTGGAAGGTTCAGGTTG-3′); human IFN-γ, forward (5′-GAATTGGAAAGAGGAGAGTGACAGA-3′) and reverse (5′-CTCTTTTGGATGCTCTGGTCATCT-3′); mouse GAPDH, forward (5′-TCTCCTGCGACTTCAACA-3′) and reverse (5′-TGTAGCCGTATTCATTGTCA-3′); mouse IL-6, forward (5′-CCACCAAGAACGATAGTCA-3′) and reverse (5′-TTGTCACCAGCATCAGTC-3′); and mouse IFN-γ, forward (5′-ACACCTGATTACTACCTTCTTCAGCA-3′) and reverse (5′-TGACCTCAAACTTGGCAATACTCA-3′).
IFN-γ enzyme-linked immunosorbent spot (ELISpot) assay
IFN-γ secreted from in vivo tumor-derived cells was analyzed by the mouse IFN-γ ELISpot Kit (Mabtech, Stockholm, Sweden) in accordance with the manufacturer's protocol. In brief, after collagenase digestion of the tumors, cells were cultured in IFN-γ antibody-coated 96-well plates with a density of 2 × 105 cells/well. After 24 h, the cells were removed, washed and the biotinylated monoclonal anti-IFN-γ antibody was added followed by incubating for 2 h at room temperature. After that, the plates were washed with PBS followed by incubating with Streptavidin-Alkaline Phosphatase (Streptavidin-ALP) for 60 min at RT. Then, the BCIP/NBT-plus was added until distinct spots appeared. Finally, the reaction was stopped by tap water.
Enzyme-linked immunosorbent assay (ELISA)
The IFN-γ concentration of the cellular supernatant was measured by a human IFN-γ ELISA kit (ExCell Bio, Shanghai, China) according to the instruction manuals. In brief, after mixing NKT cells with HCC cells with or without liraglutide for 12 h, the media was collected for centrifuging at 5000 rpm for 5 min. Complete media with 10% FBS served as control.
In vivo experiments and subcutaneous transplantation tumor model
An in vivo experimental hepatic carcinoma model was created by subcutaneous injection of 5 × 106 Hepa1-6 cells suspended with 100 μL PBS containing 10% Matrigel (BD Bioscience, CA, USA) in the right flank. Tumor growth was detected every 3 days, and tumor volume was calculated using the caliper measurement: volume = length × width2 / 2. If the tumor reached 100–150 mm3 (day −1), the mice were randomly divided into 4 groups (n = 6 per group): Liraglutide (LIR), Liraglutide + anti-NK antibody (LIR + anti-NK), Liraglutide + anti-CD8 antibody (LIR + anti-CD8) and Control group. The LIR + anti-NK and LIR + anti-CD8 groups received a single intraperitoneal injection of 500 μg anti-NK antibody or anti-CD8 antibody (both from Bio X Cell, USA), respectively, on day −1. FACS analysis of peripheral blood was performed to confirm the depletion effect of NK or CD8+ T cells. The next day (day 0), the mice began receiving a once-daily intratumoral injection of 100 μL of 0.9% saline (Control group) or 300 μg/kg liraglutide (LIR, LIR + anti-NK and LIR + anti-CD8 groups). Body weight and tumor volume were detected every third days by caliper measurement. Mice were killed if the tumor volume reached 2 cm3 or when mice exhibited moribund behavior.
In order to evaluate the immune activation of liraglutide in vivo, Hepa1-6 tumor-bearing mice were randomly divided into two groups (n = 5 per group). Then, the mice received a daily intratumoral injection of liraglutide (300 μg/kg per mouse) or 100 μL of PBS as a control. After 7 days, all the mice were sacrificed, and the tumors were dissected for ELISpot assays.
All animal experiments were approved by the Animal Care Committee of Nanjing University in keeping with the Institutional Animal Care and Use Committee guidelines.
Statistical analysis
Student's t-test was used for data analysis. Multiple comparisons were analyzed via one-way ANOVA statistical analysis. Statistical analyses were conducted by Prism software (Version 5.0), and the p-values <0.05 were considered to have statistically differences.
Results
Liraglutide activates antitumor immunity both in vivo and in vitro
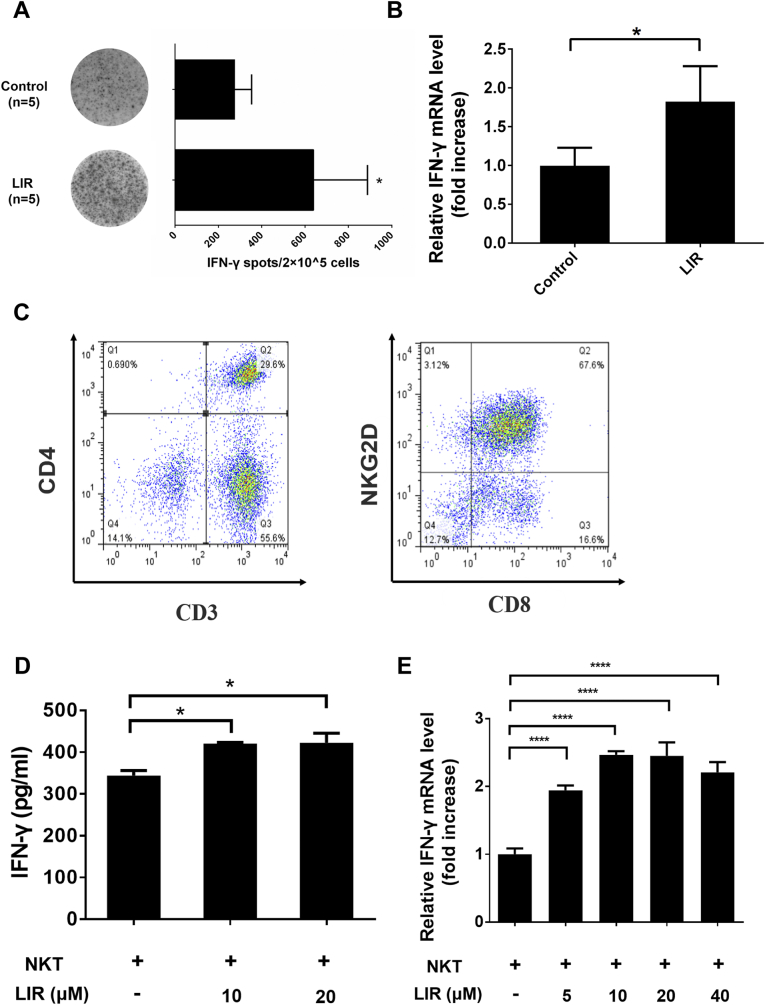
In immunocompetent C57BL/6 mice bearing subcutaneous HCC tumors, IFN-γ-producing cells in the tumors remarkably increased (up to 2.3-fold) by liraglutide treatment in comparison with that observed in the untreated group (Fig. 1A). Consistently, IFN-γ mRNA levels in tumor tissues were notably increased in the liraglutide group compared with that observed in the control group (Fig. 1B). Then, we isolated PBMCs and expanded these cells to NKT cells (approximately 67% pure), i.e., CD3+, CD8+ and NKG2D+ cells (Fig. 1C). Cognate ligands, MICA/B, for instance, which are often expressed in tumor cells without antigen-presenting cells could activate NKT cells. In line with the in vivo observations, IFN-γ production by NKT cells was significantly increased in the presence of liraglutide at doses of 10 and 20 μM (Fig. 1D). Consistently, IFN-γ expression also increased remarkably in NKT cells in the presence of liraglutide at a serial of doses (Fig. 1E). These data indicate that liraglutide can increase immune activity against HCC cells.
Fig. 1.
Liraglutide enhances antitumor immune activities both in vivo and ex vivo. (A & B) 5 × 106 Hepa1-6 cells were subcutaneous injected to 6–8-week C57BL/6 mice in the right flank. Then HCC tumor-bearing mice received an intratumoral injection of PBS (control, n = 5) or liraglutide (300 μg/kg per mouse, once a day for 7 days, n = 5). After 7 days, we killed the mice, and the tumor masses were isolated.
(A) IFN-γ-producing immune cells were identified by a mouse IFN-γ ELISpot assay kit. (B) qRT-PCR was conducted to analyze the mouse IFN-γ mRNA level which was extracted from tumor tissues. (C) Isolated PBMCs were cultured ex vivo as mentioned in the Materials and Methods. After 2 weeks, cells were detected via flow cytometry with anti-CD3, anti-CD4, anti-CD8 and anti-NKG2D antibodies. (D) HCC-LM3 cells were treated in the presence or absence of liraglutide (10 or 20 μM) for 12 h, and the cells were then further co-incubated with NKT cells. After 12 h, the supernatants were collected and IFN-γ secreted by NKT cells was quantified by ELISA. (E) IFN-γ mRNA levels were determined by qRT-PCR. HCC-LM3 cells were treated as described above in (D). Then, mRNA was obtained and analyzed by qRT-PCR. The data are shown as the means ± SD. *p < 0.05 and ****p < 0.0001.
NK cells mediate the antitumor effect of liraglutide in HCC
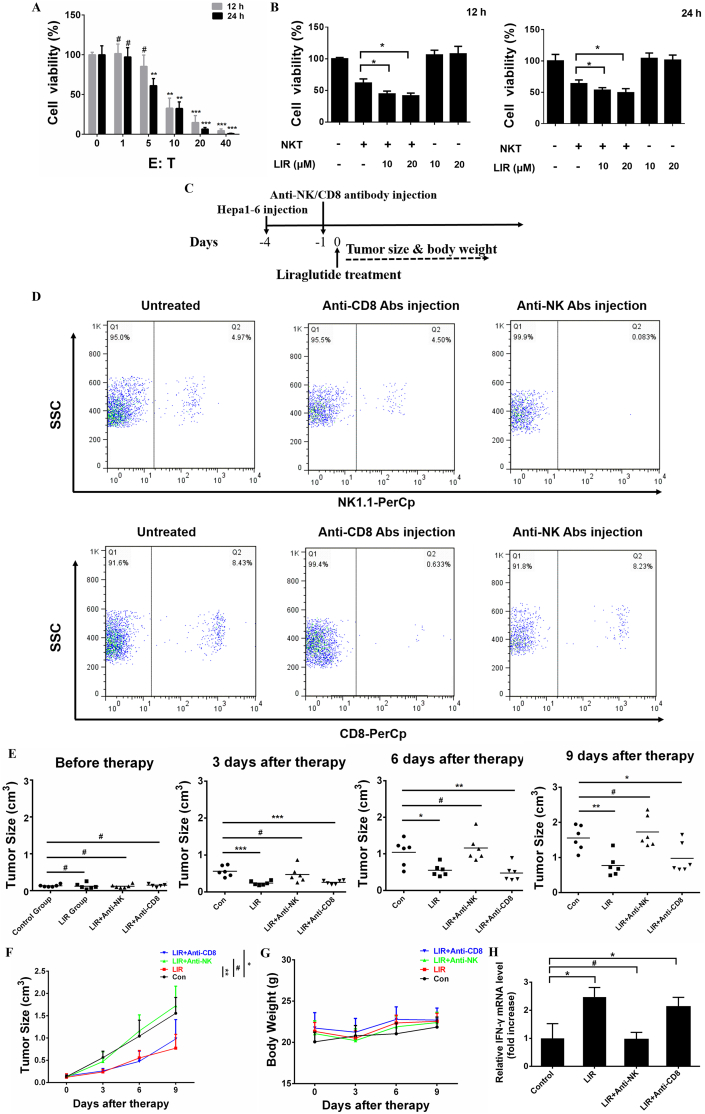
We subsequently investigated the antitumor efficacy of liraglutide. First, we determined the oncolytic activity of NKT cells toward HCC. As shown in Fig. 2A, NKT cells caused considerable oncolysis when the ratio of E:T was over 5 to 1. Then we choose the E:T ratio of 5:1 for the further experiments. We observed that liraglutide-treated HCC cells showed greater sensitivity to NKT-mediated oncolysis. However, liraglutide alone showed no apparent cytotoxicity toward HCC cells, suggesting that the enhanced antitumor activity was primarily depended on NKT cells (Fig. 2B). These data indicate that liraglutide sensitizes HCC to NKT-mediated oncolysis. Then, we further investigated the antitumor effect of liraglutide in a subcutaneous HCC immunocompetent mouse model with either NK or CD8+ T cell depletion using appropriate antibodies. The therapeutic schema is illustrated in Fig. 2C. The depletion efficacy of NK or CD8+ T cells was monitored 1 day after the depletion antibodies were administered (Fig. 2D). While liraglutide significantly inhibited tumor growth, its antitumor activity was entirely abolished by eliminating NK cells rather than by eliminating CD8+ T cells (Fig. 2E & F). There are no therapy-induced side effects or mice weight loss occurred during the experiment (Fig. 2G). Consistently with the tumor volumes, the IFN-γ level was also significantly increased in LIR and LIR + Anti-CD8 group (Fig. 2H). Taken together, our data shows liraglutide activates NK cells-mediate antitumor response.
Fig. 2.
NK cells mediate the antitumor activity of liraglutide in HCC. (A) The oncolytic activity of NKT cells toward HCC-LM3 cells was determined by a Luciferase Assay System. The luciferase-expressing HCC-LM3 cells were cultured in 96-well plates overnight, and NKT cells were then added to each well at different ratios (E:T). After 12 or 24 h, the plates were analyzed by adding luciferin using luminescence spectrometry. (B) The luciferase-expressing HCC-LM3 cell line was treated with or without liraglutide (10 or 20 μM). After 12 or 24 h, these cells were co-cultured with NKT cells at a ratio (E:T) of 5:1 for another 12 h. (C–G) Six- to eight-week-old male C57BL/6 mice received a subcutaneous injection of 5 × 106 Hepa1-6 cells in the right flank. When the tumor size reached an average of 100–150 mm3, the mice were randomly divided into four groups. The LIR + anti-CD8 and LIR + anti-NK groups received an intraperitoneal injection of anti-CD8 or anti-NK antibodies on day −1. On the next day, all the mice, except those in the control group, received an intratumoral injection of liraglutide. The mice were killed when the tumor volume was greater than 2.0 cm3 or if they became moribund. (C) A schema shows the process of HCC cell injection and the following treatments. (D) Mouse peripheral blood was obtained 1 day after intraperitoneal injected of either anti-NK or anti-CD8 depletion antibodies, after which the cells were treated with target antibodies followed by FACS to identify the depletion efficiency. (E&F) Tumor growth of each group was monitored at a series of time points as indicated. (G) Body weight variation among the mice during treatment. (H) qRT-PCR was performed to measure the mouse IFN-γ mRNA level which was extracted from tumor tissues. The results are shown as the means ± SD of each group. #p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001.
Liraglutide downregulates IL-6/STAT3 signaling both in vivo and in HCC cells
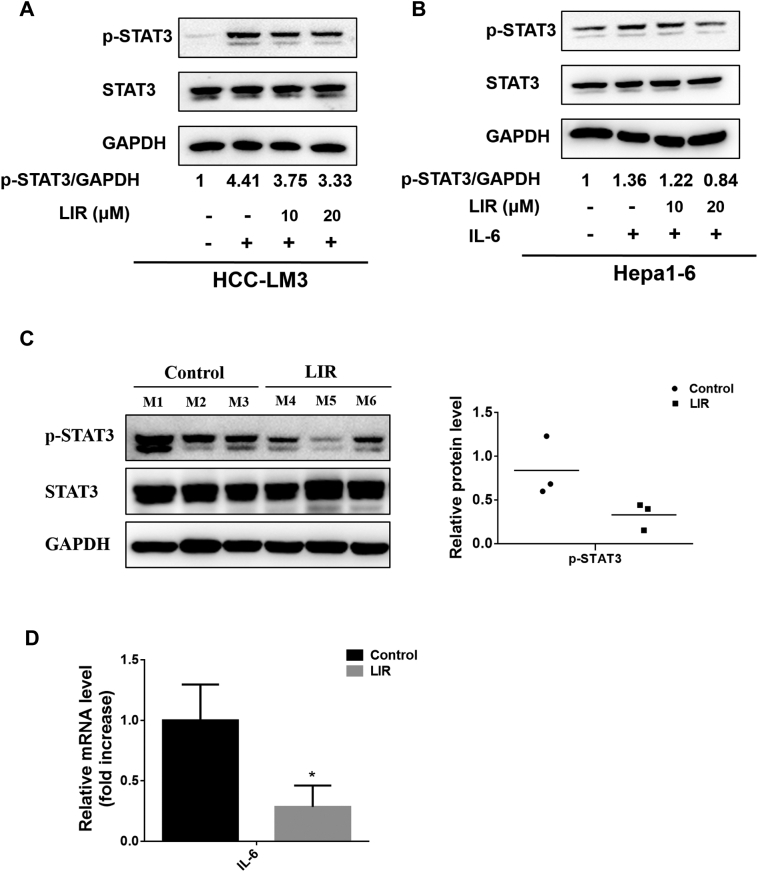
Having shown that liraglutide induces an NK-mediated antitumor effect in HCC, we sought to investigate the underlying mechanisms. As it has been demonstrated that IL-6 /STAT3 signaling activation inhibits innate antitumor immunity by suppressing NK cell activity [27], we postulated that the enhanced antitumor activity of NK cells by liraglutide may involve the downregulation of IL-6/STAT3 signaling. Therefore, we subsequently tested the impact of liraglutide on the IL-6/STAT3 signaling pathway. We observed that liraglutide (10 and 20 μM) markedly reduced the protein levels of p-STAT3 (the activated form of STAT3), which were activated by IL-6 in HCC-LM3 and Hepa1-6 cells in vitro (Fig. 3A & B). Moreover, the p-STAT3 levels in tumor tissues were also markedly reduced by liraglutide treatment (Fig. 3C). In line with this observation, liraglutide remarkably reduced the expression of IL-6 in HCC tumor tissue (Fig. 3D). These data show that liraglutide suppresses IL-6/STAT3 signaling pathway in HCC.
Fig. 3.
Liraglutide suppresses IL-6/STAT3 pathway in HCC. (A & B) Hepa1-6 and HCC-LM3 cells were treated with serial concentrations of liraglutide for 12 h, followed by adding IL-6 (25 ng/mL) for another 30 min. Then, cytosolic protein was isolated, STAT3 and p-STAT3 levels were detected by western blot analysis. The ratios of p-STAT3/GAPDH were quantified by densitometric analysis. (C & D) Mice bearing subcutaneous Heap1–6 HCC tumors received intratumoral injections of liraglutide (300 μg/kg) once a day for one week (n = 5), and mice that received PBS were used as controls (n = 5). Seven days later, the mice were killed and the tumor masses were dissected. (C) Protein levels of p-STAT3 and STAT3 in tumor tissues were detected by western blot analysis. The average ratios of p-STAT3/GAPDH were quantified by densitometric analysis. (D) The mRNA level of mouse IL-6 extracted from tumor tissues was analyzed by qRT-PCR. The results are shown as the means ± SD. *p < 0.05.
Liraglutide enhances the oncolytic efficacy of NKT cells by suppressing the IL-6/STAT3 signaling pathway
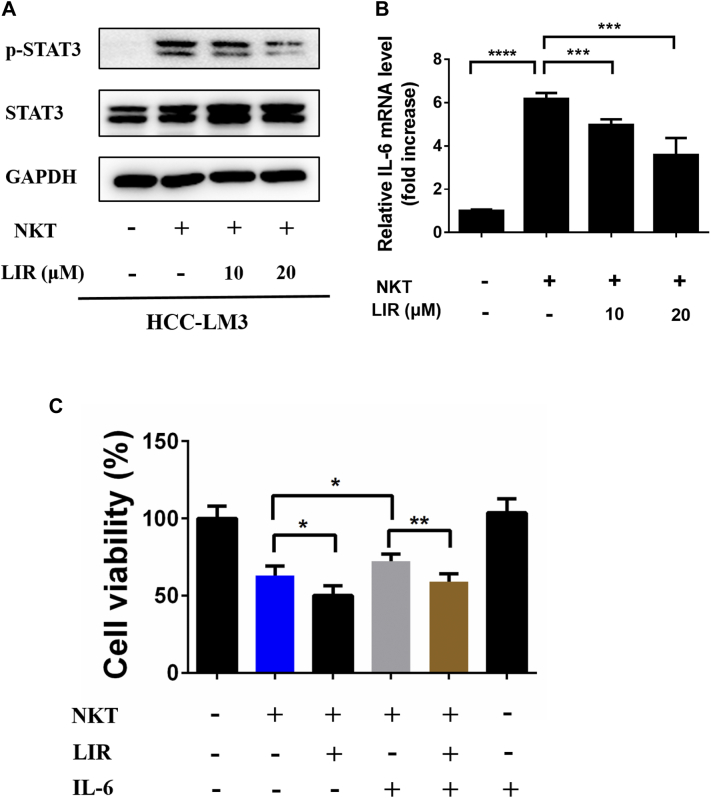
Interestingly, while NKT cells exhibited an antitumor effect toward HCC cells as shown in Fig. 2, we observed that NKT treatment robustly triggered the activation of STAT3 (Fig. 4A). Again, liraglutide markedly inhibited NKT-induced STAT3 activation (Fig. 4A) and IL-6 expression which were upregulated by NKT cells (Fig. 4B). Finally, we observed that the NKT-mediated oncolysis was indeed significantly weakened by the addition of IL-6 (Fig. 4C, the gray bar vs the blue bar), whereas this impairment was reverted by the addition of liraglutide (Fig. 4C, the gray bar vs the brown bar). Taken together, our data show that liraglutide inhibits the IL-6/STAT3 signaling pathway, resulting in enhanced NKT-mediated antitumor efficacy.
Fig. 4.
Liraglutide improves the oncolysis of NKT cells via suppressing IL-6/STAT3 signaling. Firstly, HCC-LM3 cells were treated with 10 or 20 μM liraglutide for 12 h and then were incubated with NKT cells for another 12 h. Then, cell lysates were harvested. (A) The protein levels of STAT3 and p-STAT3 were detected by western blot. (B) The mRNA levels of IL-6 were quantified by qRT-PCR analysis. (C) NKT-mediated oncolysis was detected using a Luciferase Assay System. The luciferase-expressing HCC-LM3 cells were cultured in 96-well plates and cultured overnight followed by adding liraglutide (20 μM) for 12 h. After that, cells were co-incubated with NKT cells with or without IL-6 (25 ng/mL) for 12 h. Then, luciferin was added, and cell activity was detected by luminescence spectrometry. The results are shown as the means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Discussion
Liraglutide has been widely applied in type II diabetes treatment. However, its antitumor activity, particularly in cancer immunotherapy has not been reported. In this study, we show that liraglutide improved the antitumor immune responses in HCC. Moreover, we observed that liraglutide-induced antitumor efficacy was mediated by NK cells rather than CD8+ T cells. Finally, we clearly confirmed that liraglutide inhibits the IL-6/STAT3 signaling pathway, thereby enhancing NK-mediated oncolysis in HCC. Our findings shed light on a previously unknown function of liraglutide in eliciting innate antitumor responses.
In the HCC subcutaneous model, we observed that liraglutide significantly enhanced antitumor immune responses, which was further confirmed in vitro using NKT cells. Generally, antitumor immune responses are often mediated by CD8+ T cells. To our surprise, NK cells rather than CD8+ T cells contributed to the liraglutide-mediated antitumor effect in HCC. Up to now, no report has shown the role of liraglutide in antitumor immunotherapy.
The continuous STAT3 activation acts an important role in tumor immunotolerance [[28], [29], [30]]. As shown in the present study, IL-6 weakened the NKT-mediated oncolysis of HCC, which was reverted by liraglutide. Moreover, liraglutide significantly blocked the IL-6/STAT3 signaling activated by NKT cells. Therefore, we speculate that the suppression of IL-6/STAT3 by liraglutide may contribute to NK-mediated oncolysis. Several recent studies have shown that blocking STAT3 activation in tumor cells enhances NK cell-mediated antitumor efficacy by upregulating the levels of NKG2D ligands, such as MULT1, RAE1, H60 and MICA/B, or by suppressing IL-10 and TGF-β, which negatively regulate NK cell function [[31], [32], [33], [34]]. STAT signaling especially constitutive STAT3 activation was associated with an impaired NK cell function in tumor patients not only in animal model [15,35]. Another study reported that STAT3 inhibition combined with the treatment of STING agonist increased the number of CD8+ T cells while decreasing those of regulatory T cells and myeloid-derived suppressor cells in TME [36]. Nevertheless, how liraglutide distinguishes NK cells from other immune cells (e.g., CD8+ T cells) and enhances NK-mediated antitumor activity deserves further intensive investigation.
It has been reported that inhibition of STAT3 sensitizes HCC cells to sorafenib induced tumor cell death [37]. Similarly, STAT3 activation contributed to regorafenib resistance of HCC [38]. Therefore, it makes the possibility of combining liraglutide with the current standard HCC targeted therapy to improve the clinical outcomes.
In addition to the inhibition of IL-6/STAT3, as a mimetic of GLP1, liraglutide probably alters the metabolic microenvironment by affecting process such as glycolysis, thereby improving antitumor immune responses. Accumulated evidence has confirmed the relationship between tumor metabolism and immune responses [39]. Therefore, liraglutide may bridge the glycolytic regulation and innate immunity of cancer therapy in HCC.
In summary, in our research, we showed, for the first time, that liraglutide enhances the antitumor activity of NK cells by inhibiting IL-6/STAT3 signaling. Given that liraglutide has already been used clinically, our findings may open a new era for recruiting liraglutide as a potential immunotherapeutic agent that can be readily translated into oncology clinical trials.
CRediT authorship contribution statement
Jiwu Wei and Junhua Wu: Conceptualization, Writing - review & editing. Xian Lu: Methodology, Validation, Writing - original draft. Chun Xu: Methodology, Writing - review & editing. Jie Dong and Shuguang Zuo: Visualization, Investigation. Hailin Zhang: Data curation. Chunping Jiang: Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81773255, 81472820, 81700037, 81972888), the Natural Science Foundation of Jiangsu Province of China (BK20171098), the Primary Research and Development Plan of Jiangsu Province (BE2018701), the Science and Education Project of Suzhou (KJXW2019066), and the Science and Technology Innovation Foundation of Nanjing University (14913414).
Contributor Information
Junhua Wu, Email: wujunhua@nju.edu.cn.
Jiwu Wei, Email: wjw@nju.edu.cn.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Huo X., Han S., Wu G., Latchoumanin O., Zhou G., Hebbard L. Dysregulated long noncoding RNAs (lncRNAs) in hepatocellular carcinoma: implications for tumorigenesis, disease progression, and liver cancer stem cells. Mol. Cancer. 2017;16:165. doi: 10.1186/s12943-017-0734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringelhan M., Pfister D., O'Connor T., Pikarsky E., Heikenwalder M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 4.Lee C., Cheung S.T. STAT3: an emerging therapeutic target for hepatocellular carcinoma. Cancers (Basel) 2019;11 doi: 10.3390/cancers11111646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura H., Ohno Y., Toyoshima Y., Ohtake J., Homma S., Kawamura H. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017;108:1947–1952. doi: 10.1111/cas.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.He G., Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lokau J., Schoeder V., Haybaeck J., Garbers C. Jak-Stat signaling induced by interleukin-6 family cytokines in hepatocellular carcinoma. Cancers (Basel) 2019;11 doi: 10.3390/cancers11111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todoric J., Antonucci L., Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev. Res. (Phila.) 2016;9:895–905. doi: 10.1158/1940-6207.CAPR-16-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye T.H., Yang F.F., Zhu Y.X., Li Y.L., Lei Q., Song X.J. Inhibition of Stat3 signaling pathway by nifuroxazide improves antitumor immunity and impairs colorectal carcinoma metastasis. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2016.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X., Wo G., Li B., Xu C., Wu J., Jiang C. The anti-inflammatory NHE-06 restores antitumor immunity by targeting NF-κB/IL-6/STAT3 signaling in hepatocellular carcinoma. Biomed. Pharmacother. 2018;102:420–427. doi: 10.1016/j.biopha.2018.03.099. [DOI] [PubMed] [Google Scholar]
- 14.Moreira D., Adamus T., Zhao X., Su Y.L., Zhang Z., White S.V. STAT3 inhibition combined with CpG immunostimulation activates antitumor immunity to eradicate genetically distinct castration-resistant prostate cancers. Clin. Cancer Res. 2018;24:5948–5962. doi: 10.1158/1078-0432.CCR-18-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirjačić M.K., Srdić-Rajić T., Babović N., Džodić R., Jurišić V., Konjević G. Decreased expression of pSTAT, IRF-1 and DAP10 signalling molecules in peripheral blood lymphocytes of patients with metastatic melanoma. J. Clin. Pathol. 2016;69:300–306. doi: 10.1136/jclinpath-2015-203107. [DOI] [PubMed] [Google Scholar]
- 16.Crusz S.M., Balkwill F.R. Inflammation and cancer: advances and new agents. Nat. Rev. Clin. Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen L.B., Nielsen P.F., Huusfeldt P.O., Johansen N.L., Madsen K., Pedersen F.Z. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J. Med. Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 18.Nauck M.A., El-Ouaghlidi A., Hompesch M., Jacobsen J., Elbroend B. No impairment of hypoglycemia counterregulation via glucagon with the long-acting GLP-1 derivative, NN2211, in subjects with Type 2-diabetes. Diabetologia. 2003;462:A285. [Google Scholar]
- 19.Flint A., Kapitza C., Hindsberger C., Zdravkovic M. The once-daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv. Ther. 2011;28:213–226. doi: 10.1007/s12325-010-0110-x. [DOI] [PubMed] [Google Scholar]
- 20.Ipsen D.H., Rolin B., Rakipovski G., Skovsted G.F., Madsen A., Kolstrup S. Liraglutide decreases hepatic inflammation and injury in advanced lean non-alcoholic steatohepatitis. Basic Clin. Pharmacol. Toxicol. 2018;123:704–713. doi: 10.1111/bcpt.13082. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F., Zhang Y., Chen J., Hu X., Xu Y. Liraglutide attenuates lipopolysaccharide-induced acute lung injury in mice. Eur. J. Pharmacol. 2016;791:735–740. doi: 10.1016/j.ejphar.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Zhu T., Wu X.L., Zhang W., Xiao M. Glucagon like peptide-1 (GLP-1) modulates OVA-induced airway inflammation and mucus secretion involving a protein kinase a (PKA)-dependent nuclear factor-kappaB (NF-kappaB) signaling pathway in mice. Int. J. Mol. Sci. 2015;16:20195–20211. doi: 10.3390/ijms160920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurišić V., Vuletić A., Martinović K.M., Konjević G. The role of NK cells in cancer. Cancer Immunology. 2020:133–146. [Google Scholar]
- 24.Lee J.H., Lee J.H., Lim Y.S., Yeon J.E., Song T.J., Yu S.J. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 25.Maricic I., Marrero I., Eguchi A., Nakamura R., Johnson C.D., Dasgupta S. Differential activation of hepatic invariant NKT cell subsets plays a key role in progression of nonalcoholic steatohepatitis. J. Immunol. 2018;201:3017–3035. doi: 10.4049/jimmunol.1800614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurisic V., Srdic-Rajic T., Konjevic G., Bogdanovic G., Colic M. TNF-α induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. J. Membr. Biol. 2011;239:115–122. doi: 10.1007/s00232-010-9309-7. [DOI] [PubMed] [Google Scholar]
- 27.Konjević G.M., Vuletić A.M., Mirjačić M.K., Larsen A.K., Jurišić V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine. 2019;117:30–40. doi: 10.1016/j.cyto.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Shen Y., Wang S., Shen Q., Zhou X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018;415:117–128. doi: 10.1016/j.canlet.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdeil G., Lawrence T., Schmitt-Verhulst A.M., Auphan-Anezin N. Targeting STAT3 and STAT5 in tumor-associated immune cells to improve immunotherapy. Cancers (Basel) 2019;11 doi: 10.3390/cancers11121832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H., Kortylewski M., Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 31.Sui Q., Zhang J., Sun X., Zhang C., Han Q., Tian Z. NK cells are the crucial antitumor mediators when STAT3-mediated immunosuppression is blocked in hepatocellular carcinoma. J. Immunol. 2014;193:2016–2023. doi: 10.4049/jimmunol.1302389. [DOI] [PubMed] [Google Scholar]
- 32.Sun X., Sui Q., Zhang C., Tian Z., Zhang J. Targeting blockage of STAT3 in HCC cells augments NK cell functions via reverse HCC-induced immune suppression. Mol. Cancer Ther. 2013;12:2885–2896. doi: 10.1158/1535-7163.MCT-12-1087. [DOI] [PubMed] [Google Scholar]
- 33.Cacalano N.A. Regulation of natural killer cell function by STAT3. Front. Immunol. 2016;7:128. doi: 10.3389/fimmu.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady J., Carotta S., Thong R.P., Chan C.J., Hayakawa Y., Smyth M.J. The interactions of multiple cytokines control NK cell maturation. J. Immunol. 2010;185:6679–6688. doi: 10.4049/jimmunol.0903354. [DOI] [PubMed] [Google Scholar]
- 35.Wu J., Gao F.X., Wang C., Qin M., Han F., Xu T. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019;38:321. doi: 10.1186/s13046-019-1310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei J., Zhang Y., Luo Q., Zheng W., Li W., Zeng X. STAT3 inhibition enhances CDN-induced STING signaling and antitumor immunity. Cancer Lett. 2019;450:110–122. doi: 10.1016/j.canlet.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 37.Xie L., Zeng Y., Dai Z., He W., Ke H., Lin Q. Chemical and genetic inhibition of STAT3 sensitizes hepatocellular carcinoma cells to sorafenib induced cell death. Int. J. Biol. Sci. 2018;14:577–585. doi: 10.7150/ijbs.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W., Zhang S., Ma D., Yan D., Zhang G., Cao Y. Targeting SphK2 reverses acquired resistance of regorafenib in hepatocellular carcinoma. Front. Oncol. 2020;10:694. doi: 10.3389/fonc.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wegiel B., Vuerich M., Daneshmandi S., Seth P. Metabolic switch in the tumor microenvironment determines immune responses to anti-cancer therapy. Front. Oncol. 2018;8:284. doi: 10.3389/fonc.2018.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]