Highlights
-
•
Bone health should be prioritised when men with prostate cancer start long-term ADT.
-
•
Assessment by FRAX (±bone mineral density) identifies those at highest risk of fracture.
-
•
Treatment can minimise skeletal complications and reduce morbidity and mortality.
Keywords: Prostate cancer, Skeletal health, Osteoporosis, Fracture risk, Guidelines
Abstract
Context and objective
Incidence of prostate cancer (PC) is increasing, but androgen deprivation therapy (ADT) and other therapies are substantially improving survival. In this context, careful consideration of skeletal health is required to reduce the risk of treatment-related fragility fractures and their associated morbidity and mortality. This risk is currently not well-managed. ADT causes significant loss of bone mineral density (BMD). In the metastatic setting, systemic treatments (e.g. chemotherapy, abiraterone, enzalutamide) are used alongside ADT and may require concomitant glucocorticoids. Both ADT and glucocorticoids pose significant challenges to skeletal health in a population of patients already likely to have ongoing age-related bone loss and/or comorbid conditions. Current PC guidelines lack specific recommendations for optimising bone health. This guidance presents evidence for assessment and management of bone health in this population, with specific recommendations for clinical practitioners in day-to-day PC management.
Methods
Structured meetings of key opinion leaders were integrated with a systematic literature review. Input and endorsement was sought from patients, nursing representatives and specialist societies.
Summary of guidance
All men starting or continuing long-term ADT should receive lifestyle advice regarding bone health. Calcium/vitamin D supplementation should be offered if required. Fracture risk should be calculated (using the FRAX® tool), with BMD assessment included where feasible. BMD should always be assessed where fracture risk calculated using FRAX® alone is close to the intervention threshold. Intervention should be provided if indicated by local or national guidelines e.g. UK National Osteoporosis Guideline Group (NOGG) thresholds. Men requiring bone protection therapy should be further assessed (e.g. renal function), with referral to specialist centres if available and offered appropriate treatment to reduce fracture risk. Those near to, but below an intervention threshold, and patients going on to additional systemic therapies (particularly those requiring glucocorticoids), should have FRAX® (including BMD) repeated after 12–18 months.
Patient summary
Modern treatments for prostate cancer have led to significant improvements in survival and quality of life. However, some of these treatments may lead to weakening of patient’s bones with risk of fracture and it is therefore important to monitor patients’ bone health and provide bone protection where needed. This paper provides specific guidance to clinical teams, based on the most recent research evidence, to ensure optimal bone health in their patients.
1. Introduction
Bone health is emerging as one of the most important considerations for men receiving treatment for prostate cancer (PC). Projected to be the commonest cancer by 2030, 1 in 8 men will receive a diagnosis of PC in their lifetime. There are more than 400,000 new cases of PC in Europe each year. Despite the fact that PC is the second leading cause of cancer-related mortality in men [1], survival rates have improved considerably over the past four decades as a result of both earlier diagnosis and newer therapies (current 5-year survival is 85% in all patients, compared with 71% in 1980) [2].
As a result, many patients with PC now live with their disease for many years, and consideration of the long-term consequences of treatment is of increasing importance. Men with PC are not routinely referred to bone specialists for optimisation of their bone health, despite the fact that cancer treatment induced bone loss (CTIBL) and the resulting increased risks of fragility fractures (often requiring hospitalisation) represent substantial problems for patients and healthcare systems [3], in addition to those posed by pathological fractures in men with metastatic bone involvement [4]. New PC therapies, whilst improving survival, often add to this risk [5]. There is an urgent need for increased focus on these issues. This guidance aims to provide non-bone specialists with evidence-based recommendations to support the assessment and management of bone health in men receiving PC treatment.
2. Methodology
2.1. Expert group and specialist society involvement
This guidance was developed by a group of key opinion leaders in the management of PC and bone disorders. The group included medical and clinical oncologists, urologists, endocrinologists, rheumatologists, metabolic bone disease specialists, general practitioners and uro-oncology nurse specialists, with input from patient representatives. Input/endorsement was also sought from specialist societies (see acknowledgements).
2.2. Current guidelines
Current national and international guidelines lack detailed, recommendations developed specifically for managing CTIBL in men receiving treatment for PC. The UK National Institute for Health and Care Excellence (NICE) Clinical Guideline makes only general recommendations; that fracture risk is considered for all men receiving ADT and that treatment is offered to all those with osteoporosis [6]. Joint European Association of Urology, European Society for Radiotherapy and Oncology (ESTRO) and International Society for Geriatric Oncology (SIOG) PC guidelines suggest that BMD assessment is undertaken prior to the initiation of long-term ADT, and that the FRAX® tool should be used to estimate individual fracture risk [7]. Recent ASCO guidance is based on endorsement of a 2017 Cancer Care Ontario Programme [8] and Guidance from the National Comprehensive Cancer Network (NCCN) recommends screening and treatment for osteoporosis according to guidelines for the general population from the National Osteoporosis Foundation [9].
There is no current guidance as to the intervention thresholds that should be used to initiate treatment, or the most appropriate pharmacological therapy. It is often unclear who should have overall responsibility for managing bone health in PC patients, many of whom will be managed in a multi-disciplinary setting across both primary and secondary care.
2.3. Definition of scope
The scope of the guidance was defined as follows:
-
•
To address the need for specific guidance for the management of CTIBL in PC (including intervention thresholds) in a European setting
-
•
To summarise the evidence supporting the management of bone health during PC treatment for non-bone specialists (including general practitioners, urologists, oncologists and specialist nurses) involved in the care of patients with PC at risk of CTIBL.
-
•
Using the UK as an exemplar, to sit the PC guidance alongside the NICE-accredited National Osteoporosis Guidance Group Clinical Guideline (2017) for the prevention and treatment of osteoporosis and the NICE guidance for the diagnosis and management of prostate cancer (NICE Clinical Guideline 175).
2.4. Search strategy and selection of evidence
A literature search was undertaken using PubMed and Ovid MEDLINE databases for relevant peer-reviewed articles published in English between January 2000 and July 2019. Randomised controlled trials, observational studies and meta-analyses were included. Search terms included prostate cancer, prostate carcinoma and prostate adenocarcinoma, and were mapped to the following subject headings for bone; bone health, bone density, bone mineral density, osteoporosis, osteopenia, bone turnover markers, bone biomarkers, fracture, skeletal related event, bisphosphonates, denosumab and exercise. For ADT, prostate cancer was used to search in combination with ADT, androgen deprivation therapy, androgen suppression, hormone therapy, GnRH agonist, luteinising hormone releasing hormone antagonist, and antiandrogens.
Abstracts were screened for relevance by at least two members of the expert reference group, with any disagreement resolved by consensus after discussion.
3. Prostate cancer and bone loss
3.1. Androgen deprivation therapy (ADT)
ADT is offered to men with PC in several different clinical settings, including: men who present with or progress to metastatic disease (continuous ADT); men who receive radical radiotherapy for localised or locally advanced disease (temporary ADT); and men who progress during a period of watchful waiting who are not fit for radical treatment (palliative continuous ADT). These indications are based upon clear evidence from large randomised clinical trials [10], [11], [12], [13]. ADT may also be administered intermittently [14].
ADT is most commonly achieved by the administration of luteinising hormone releasing hormone (LHRH) agonists (such as goserelin and leuprorelin) and LHRH antagonists (such as degarelix). ADT causes a rapid and substantial reduction in circulating androgens and oestrogens, disrupting bone remodelling balance, stimulating osteoclast activity, decreasing osteoclast apoptosis, and increasing apoptosis of osteoblasts, all of which lead to net bone loss [15].
Even before ADT is initiated, PC patients may have lower baseline BMD than age-matched controls [16]. Prospective studies found that loss of BMD is most rapid during the first year of ADT (5–10% BMD loss) [17], [18] and is greater than both normal age-related bone loss (0.5–1.0% per annum) and bone loss during menopause. Bone loss, as well as disruption to bone microarchitecture [19] continues throughout the duration of ADT and ongoing CTIBL in men with PC is superimposed upon normal age-related bone loss (more than half of men diagnosed with PC are aged over 70 years). It is likely that older men will also have risk factors for fragility fracture other than ADT, such as risk of falls and comorbid conditions.
ADT also affects body composition. Adiposity is substantially increased along with a decrease in lean body mass within 3–12 months of ADT initiation [20]. ADT-induced sarcopenia, defined as a progressive impairment of muscle function due to loss of skeletal muscle mass, increases the risk of falls, fractures and consequent loss of function or independence [18].
3.2. Chemotherapy
The STAMPEDE and CHAARTED trials have demonstrated survival benefit when chemotherapy is given upfront alongside ADT in men with metastatic hormone-sensitive PC [21], [22]. Men in this situation who are fit enough are currently offered six cycles of docetaxel. In the STAMPEDE study, glucocorticoids were given alongside each cycle of docetaxel as pre-medication (dexamethasone) and as a daily dose of prednisolone (10 mg per day) throughout the treatment period of up to 18 weeks. In many (but not all) centres, this glucocorticoid regime is now adopted as standard of care, further challenging bone health.
3.3. Other systemic therapies
Abiraterone acetate is a selective androgen synthesis inhibitor which blocks androgen production in the testes, adrenals and prostate tumour tissue. It is recommended for use in men with metastatic castration resistant prostate cancer (mCRPC) [23] and has also been found to improve survival in men with newly diagnosed hormone-sensitive metastatic PC (compared to ADT alone) [24], [25]. It is currently approved in the USA, and recommended by ESMO and EAU for use in this setting [26]. As abiraterone also blocks the production of glucocorticoids, prednisolone (usual dose 10 mg/day) is given together with abiraterone, and may further challenge bone health.
Enzalutamide is an oral androgen receptor inhibitor that is currently used in men with mCRPC [27], which does not require concomitant glucocorticoids. Enzalutamide may also have a role in the management of non-metastatic CRPC [28] and also in metastatic castrate sensitive disease [29]. Other anti-androgen agents are in development or newly-approved (eg darolutamide [30] and apalutamide [31]) with currently unknown effects on fracture rate and assessment of bone health will be of growing and continuing importance. Radium-223 monotherapy, given IV, is widely used for treatment of mCRPC which has metastasised only to the skeleton, following trials which showed an overall survival benefit of 3.6 months [32] and a reduced risk of symptomatic skeletal related events (SSREs) [33]. However, in the REASURE trial, new fractures were commonly seen on imaging in men with mCRPC during and after treatment with Ra-223 monotherapy [34] and, when radium-223 is used in combination with other agents, increased fracture risk has been observed as reported in a combination study where the addition of radium-223 to abiraterone acetate plus prednisone or prednisolone did not improve SSRE-free survival in patients with castration-resistant prostate cancer and bone metastases, and was associated with an increased frequency of bone fractures compared with placebo [5].
Interim data from the ongoing EORTC1333/PEACEIII study, showed that addition of radium-223 to enzalutamide increased the 1-year cumulative fracture rate from 12.4% (already significant with enzalutamide alone) to 27.4%, but remarkably no fractures occurred when patients started treatment with a bone-protecting agent at least 6 weeks before radium-223 administration [35]. These are interim data, but they dramatically illustrate the importance of considering bone protection and following appropriate guidance. When full data become available, they may well justify all such mCRPC patients routinely receiving bone protection. In patients with bone metastases from CRPC at high risk for clinically significant SREs, ESMO guidelines recommend denosumab or zoledronate at doses higher than those required for protection against CTIBL alone.
4. Glucocorticoids and bone loss
The long-term use of glucocorticoids is the commonest cause of iatrogenic osteoporosis and one of the commonest causes of secondary osteoporosis. As a consequence of increased bone resorption, decreased formation and interruption of regulatory pathways, there is an early and rapid loss of BMD and bone quality. The risk of hip and vertebral fractures increases up to 7- and 17-fold respectively when doses equivalent to 10–12 mg prednisolone are given for more than 3 months [36]. There is a need for further studies to investigate the impact of the combination of docetaxel and glucocorticoids on bone health and risk of fracture in men with PC.
5. Osteoporosis
Osteoporosis is a progressive systemic skeletal disorder, characterised by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture [37]. Its prevalence increases with age, due to both age-related loss of BMD (0.5–1.0% per year) and the presence of factors that accelerate bone loss, such as the menopause, lifestyle factors, presence of comorbid conditions and use of medications affecting bone.
The World Health Organization (WHO) definition of osteoporosis, is based upon BMD. Using dual energy X-ray absorptiometry (DXA), a BMD T-score of 2.5 SD or more below the mean value for young healthy adults is diagnostic of osteoporosis [38]. The proximal femur (total hip or femoral neck) is an important site to assess due to its higher predictive risk for hip fracture. Measurement of lumbar spine (LS) BMD should also be considered in all patients, though the presence of degenerative changes, vascular calcification and fractures may affect interpretation of the result. In Europe, there are 22 million women and 5.5 million men living with osteoporosis, which is responsible for 3.5 million fragility fractures per year. The economic burden of both incident and prior fragility fractures has been estimated at €37 billion [39].
Fragility fractures arise as a consequence of low energy mechanical forces that would not ordinarily cause fracture (equivalent to a fall from standing height or less), and most commonly affect the proximal femur (hip), vertebrae and distal radius [40]. Hip fractures, in particular, pose a considerable challenge to health and social care provision. Significant morbidity arises from the consequent pain, disability and loss of independence, with over 50% of patients unable to live independently, and only 30% recovering fully. The association between hip fracture and mortality is well established, with around one third of patients dying within 12 months [41]. Importantly, mortality following hip fracture is significantly higher in men [42].
Several other factors that are BMD-independent may also contribute to fracture risk including; age, sex, increased risk of falls, previous fracture, family history of fracture, and other lifestyle factors. Fracture risk assessment tools such as FRAX® and QFracture have been developed, to integrate these variables with other information in order to better determine the risk of fracture.
A range of bone turnover markers (BTMs), mostly related to collagen metabolism, can be measured in urine or serum, to indicate the status of bone formation and bone resorption. While not used widely in clinical practice, they may be particularly useful in monitoring the response of bone turnover to pharmacological treatments [43].
6. Management of CTIBL in prostate cancer
6.1. Patient and clinician education
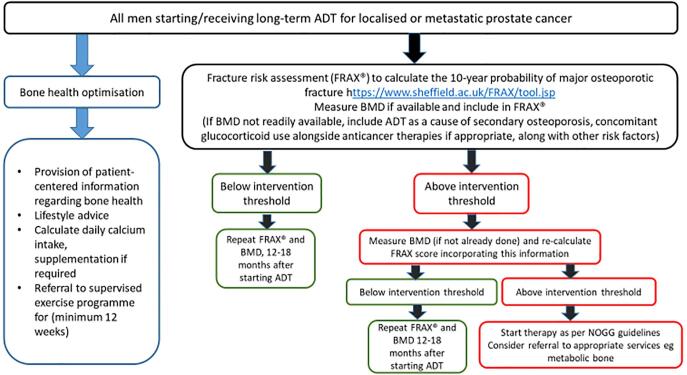
Evidence suggests that men with PC receiving ADT often lack basic osteoporosis knowledge and do not actively seek to take measures to optimise their bone health [44]. Provision of individualised, patient-centred information can improve knowledge and engagement with appropriate lifestyle modifications. Published surveys of urologists and oncologists have found that clinicians lack confidence in providing self-management advice to patients to optimise bone health, and do not feel able to effectively manage men who are identified as having abnormal BMD [45], [46]. We recommend this is addressed by clinicians following Fig. 3, Fig. 4 of this guidance which will be made available as a simple downloadable guide.
Fig. 3.
Algorithm for assessment of bone health in prostate cancer patients receiving ADT. Note regarding patients with mCRPC: Around 80% mCRPC patients develop bone metastases. In patients with bone metastases from CRPC at high risk for clinically significant SREs, ESMO guidelines recommend denosumab or zoledronate at doses higher than those required for protection against CTIBL alone. However, where mCRPC patients do not already receive bone protection for prevention of metastatic morbidity, in view of emerging data referred to in Section 3, it is strongly recommended that all such patients should be considered for bone protection to prevent osteoporotic fragility fractures.
Fig. 4.
Guidance for clinicians. The following guidance is given for management of bone health in patients with prostate cancer starting ADT or for patients already receiving ADT who have not previously had a bone health assessment.
6.2. Lifestyle factors
Both smoking and excessive alcohol intake are associated with lower BMD in men with PC, and should be avoided [47]. It has been demonstrated that exercise improves muscular strength, cardiorespiratory fitness, lean body mass, fatigue, and quality of life in men receiving treatment for PC [48]. NICE Clinical Guideline 131 [49] for PC recommends that all men starting or having ADT should be offered supervised resistance and aerobic exercise at least twice a week for 12 weeks. Further advice is provided by many organisations, including the Royal Osteoporosis Society in the UK [50], the European Society of Medical Oncology (ESMO) [51] and a compendium of EU-specific reports [52].
6.3. The role of calcium and vitamin D
Daily calcium intake (DCI) is inadequate in the majority of older men with PC [53]. The NOGG recommends a DCI of 700–1200 mg, if possible through dietary intake with supplements if needed. DCI may be calculated using an online tool (http://www.cgem.ed.ac.uk/research/rheumatological/calcium-calculator).
Optimal bone health requires vitamin D (serum 25-hydroxyvitamin D 25-OHD) levels of at least 50 nmol/L, and levels below 30 nmol/L significantly increase the likelihood of bone disease [54]. Vitamin D deficiency affects more than a quarter of older men, with up to three quarters found to have insufficiency (25–50 nmol/L). NOGG recommends vitamin D supplementation with 800 IU daily in all men aged over 50 at increased risk of fracture [55].
6.4. Bone protective agents
In randomised studies (Table 1) [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], bisphosphonates including pamidronate, neridronate, risedronate, zoledronate and alendronate have been shown to be effective in the prevention of BMD loss associated with ADT at the lumbar spine (LS), femoral neck and total hip, with accompanying significant reduction in BTMs.
Table 1.
Randomised studies of the effect of bisphosphonates and denosumab in men receiving ADT for Prostate Cancer.
| Year | Study population | N | Study groups | Follow-up | Key findings |
|---|---|---|---|---|---|
| 2001 [56] | Locally advanced or recurrent PC | 47 | Pamidronate + ADT vs ADT only | 48 weeks | No significant BMD change in pamidronate group Significant loss of BMD at LS and hip in ADT only group (−3.3% and −1.8%, p < 0.001) |
| 2001 [57] | Metastatic PC | 21 | MAB + pamidronate vs MAB | 12 months | Significant increase in LS (+7.8% vs −5.7% p = 0.0001) and femoral neck (+2.0% vs −2.3% p = 0.0007) BMD in pamidronate group compared to MAB group |
| 2003 [58] | Non-metastatic PC | 106 | Zoledronic acid + ADT vs ADT | 12 months | Zoledronic acid associated with increased LS BMD compared to ADT alone (5.6% Vs −2.2%, p < 0.001) |
| 2005 [59] | Locally advanced PC, OP at baseline | 60 | MAB vs MAB + neridronic acid vs bicalutamide vs bicalutamide + neridronic acid | 12 months | MAB group experienced significant BMD loss at LS and hip (−4.9% and −1.9%; p = 0.002 and 0.004 respectively) No BMD change in the MAB and neridronic acid group Non-significant BMD loss in bicalutamide groupBMD increase at LS (+2.5%; p < 0.05) and hip (+1.6%; p < 0.05) in bicalutamide + neridronic acid group |
| 2006 [60] | Non metastatic PC and received ADT <12 months | 120 | Zoledronic acid + ADT vs placebo + ADT | 12 months | Increase in LS and hip BMD in zoledronic acid group compared with placebo (p < 0.0001 for both) |
| 2007 [61] | Localised and metastatic PC receiving ADT <12 months | 42 | Zoledronic acid + ADT vs placebo + ADT | 12 months | Increase in LS (+4.9% vs −2.2% p < 0.0001) and femoral neck (0.9% vs −3.2% p < 0.0001) BMD in zoledronic acid group compared with placebo |
| 2007/8 [62,63] | Non-metastatic PC | 112 | Alendronic acid + ADT vs placebo + ADT (crossover at 12 months) |
24 months | BMD increased at LS and hip with alendronic acid, and decreased with placebo (p < 0.001) at 1 year At crossover, significant LS and hip BMD gains continued during second year of alendronic acid.BMD maintained at hip and spine in those who switched to placebo, but BMD loss at radius. |
| 2007 [64] | Non-metastatic PC receiving ADT | 44 | Zoledronic acid + ADT vs placebo + ADT | 12 months | Increase in hip (+4.0% vs −3.1% p < 0.001) and LS (+0.7% vs −1.9% p = 0.004) BMD with zoledronic acid compared with placebo |
| 2009 [65] | Non metastatic PC initiating or already receiving ADT | 93 | Zoledronic acid + ADT vs placebo + ADT | 12 months | Increased LS BMD with zoledronic acid in those receiving ADT for < 1 year (+5.12% vs −3.13% with placebo, p = 0.0029) and in those receiving ADT for more than 2 years (+4.82% vs +0.99% with placebo, p = 0.0013) |
| 2009 [66] | Non metastatic PC | 1468 | Denosumab + ADT vs placebo + ADT | 2 years | Significant increase in LS BMD by 5.6% in denosumab arm vs 1.0% reduction in placebo arm (p < 0.001). Denosumab reduced new vertebral fractures (1% vs 3.9%) and significantly reduced BTM levels vs placebo |
| 2011 [67] | ADT in patients with PC and osteoporosis | 234 | Denosumab + ADT vs alendronic acid + ADT | 2 years | Denosumab was superior to alendronic acid in improvement of LS BMD (+5.6% vs +1.1%). |
| 2013 [68] | ADT for 2–3 years and OP | 104 | Risedronate + ADT vs placebo + ADT | 2 years | Decreased LS BMD in both groups, no significant difference between groups |
| 2013 [69] | Localised PC | 186 | Alendronic acid + ADT vs placebo + ADT | 12 months | Significant increase in LS BMD with alendronic acid (+1.7% and −1.9% with placebo, p < 0.0001) |
In the above studies, significant reductions in BTMs were observed following administration of bone targeted agents Abbreviations: PC: prostate cancer; BM: bone metastasis; MAB: maximum androgen blockade; ADT: androgen deprivation; LS: lumbar spine; BMD: bone mineral density; OP: osteoporosis.
However, bisphosphonate studies to date have had important limitations including small patient numbers, heterogeneous populations, variation in type and frequency of bisphosphonate administration, and varying follow-up schedules. Importantly, no study has been sufficiently powered to detect differences in fracture incidence, though a meta-analysis of 15 randomised studies including 2,634 patients receiving ADT showed that treatment with bisphosphonates prevented fracture (RR 0.8, 95% CI 0.69–0.94, p = 0.005)[70]. Most studies that compared zoledronic acid with placebo used a 4 mg dose administered 3-monthly, a higher dose than the 5 mg infused annually for the treatment of osteoporosis. It is unlikely that large, prospective, randomised bisphosphonate trials for prevention of bone loss and reduction of fracture rate in men receiving ADT will be carried out and no bisphosphonate is currently approved for this specific purpose. However, it seems reasonable to use bisphosphonates in men with PC under the same rationale as they are used to manage other forms of osteoporotic bone loss.
Larger randomised studies have been performed for denosumab in men receiving ADT (details in Table 1). In a placebo-controlled study, denosumab significantly increased BMD and reduced incidence of new vertebral fractures [66]. In a randomised study in Italy, France and Switzerland comparing denosumab with alendronate [67], denosumab was superior in terms of improved LS BMD after 2 years. Although no significant difference was observed in fracture rate, on the basis of these results, denosumab has been authorised by the European Medicines Authority for use in the prevention of CTIBL associated with ADT. However, given that no significant difference if fracture rate has been observed, it should be noted that there are differences in both cost and side-effect profiles between denosumab and alendronate (and other bisphosphonates) and, as with all bone-targeted agents, the choice of agent will depend on patient need and local practice.
Bisphosphonates and denosumab are associated with similar adverse effects, the most serious of which is osteonecrosis of the jaw (ONJ). Studies of denosumab in both metastatic PC and non-metastatic CRPC reported ONJ incidence of ≤5% [71], [72]. Similarly, the frequency of ONJ with bisphosphonate use in men with PC is 1–2% [72]. Importantly, these are based on doses used in metastatic bone disease (4 mg zoledronic acid or 120 mg denosumab every 4 weeks). With lower doses, the incidence in osteoporosis patients is substantially lower, estimated at between 0.001% and 0.01% [73]. Both denosumab and bisphosphonates are associated with an increased risk of hypocalcaemia, but when denosumab was given twice yearly to men with PC on ADT, the incidence of hypocalcaemia was less than 1% [66].
Other pharmacological treatments for osteoporosis (including the selective oestrogen receptor modulators raloxifene and toremifene) are not currently recommended to prevent bone loss in PC patients receiving ADT. Teriparatide (recombinant PTH) is contraindicated in patients with metastatic bone disease and in those who have received prior radiotherapy.
Where patients with metastatic prostate cancer are already receiving anti-resorptive therapy for the management of their metastatic disease (usually at higher doses than required for prevention of osteoporosis), there is clearly no benefit from further bone health monitoring, as patients will already be receiving the appropriate therapy to prevent/treat bone loss.
7. Fracture risk assessment and interventions
Risk assessment tools are available to determine the risk of fragility fracture for individual patients. Additional clinical risk factors contribute to fracture risk, at least partially independently of BMD (indeed most fractures occur in individuals subsequently found to have non-osteoporotic BMD). The two most frequently used tools are the Fracture Risk Assessment Tool (FRAX® available at https://www.sheffield.ac.uk/FRAX), which calculates 10 year probability of fracture and, QFracture (https://qfracture.org) in the UK only, which can calculate 1–10 year incidence of fracture. Both tools estimate the risk of hip fracture alone and other major osteoporotic fractures (hip, clinical spine, wrist or humerus). Unlike QFracture, FRAX® may be used with or without BMD. In contrast to FRAX®, there are currently no published intervention thresholds using the QFracture tool. Neither has been specifically developed for use in men with PC.
7.1. Frax®
FRAX® is based on primary data from 12 prospectively studied population-based international cohorts, comprising more than 60,000 individuals and 5000 incident fractures, with subsequent external validation in cohorts comprising 230,486 individuals. Although men comprised only 25% of the original FRAX® cohorts, current evidence suggests that the risk is the same in both sexes and the tool has been shown to be of predictive value in both male and mixed gender cohorts [74].
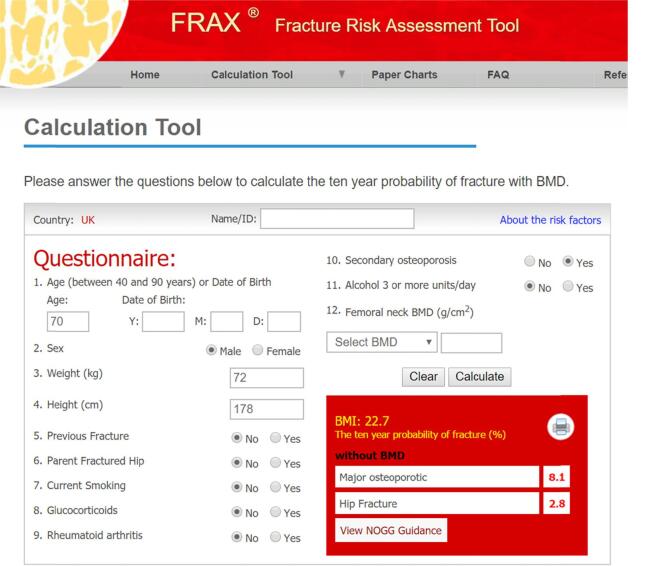
FRAX® does not require specialist knowledge and can be performed in general practice or outpatient settings. It incorporates a relatively small number of clinical risk factors (Fig. 1). Anticancer treatments are not currently included as a specific risk factor. FRAX® computes fracture probability taking both the risk of fracture and risk of death into account, important because some risk factors affect both. FRAX® is used in an increasing number of guidelines worldwide. In addition to providing an estimate of risk, the FRAX® website in some country models has a link to national guidance for the management of osteoporosis (such as the UK National Osteoporosis Guideline Group in the UK).
Fig. 1.
The UK FRAX® tool. Screenshot showing a FRAX®calculation of major fracture and hip fracture probability in a man aged 70 years with secondary osteoporosis (e.g. prostate cancer on ADT). Note that, because there is no BMD measurement included, the secondary osteoporosis factor has been checked as ‘Yes’ in recognition of the patient being on ADT. If a BMD measurement was included, the FRAX® risk calculation would take no account of whether the secondary osteoporosis box is checked or not as the BMD takes precedence.
FRAX has recently been shown to be predictive of falls in elderly men [75]. This is particularly significant in older men receiving ADT, which alters body composition. As well as increasing the risk of falls, sarcopenia also decreases rehabilitation potential in the post-fall setting [76].
7.2. FRAX® and ADT
The inclusion of ADT as a new, specific risk variable within FRAX® would require sufficient evidence that ADT is associated with fracture risk independently of the risk variables already included, particularly BMD. Current evidence suggests that this may not be the case [77], [78]. A conservative assumption, therefore, would be that the modification of fracture risk by ADT is captured almost completely by its impact on BMD. The secondary osteoporosis variable in FRAX® already serves this function and so should be ticked when patients receive ADT. This variable contains a number of risk factors that have been shown to be associated with fracture risk (RR 1.3–1.7) but with little or no evidence that this risk is truly independent of BMD. Thus, if and when BMD is entered to the calculation, no further weight is accorded to the presence of this risk factor. As for any clinical prediction tool, interpretation should be tempered by additional information of clinical significance; such as a high falls risk, multiple prior fractures, immobility and severe rheumatoid arthritis.
In addition to ADT, men with newly diagnosed metastatic prostate cancer may also receive additional systemic therapies, which are given with concurrent glucocorticoids. Docetaxel chemotherapy involves daily prednisolone and pre-treatment dexamethasone, for up to 18 weeks. Abiraterone acetate may also be given along with a daily dose of prednisolone for up to 2 years. All men undergoing prolonged periods of exposure to medium/high doses of glucocorticoids have a greater risk of fracture, which is reflected in its inclusion in the FRAX® risk factors.
7.3. Intervention thresholds
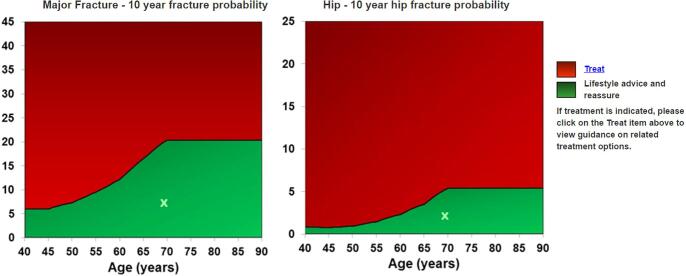
Approaches used to set intervention thresholds [79], [80] depend on local factors such as reimbursement policies, health economic assessment, willingness to pay for health care in osteoporosis and access to DXA. Most recommendations for intervention thresholds in osteoporosis are based on postmenopausal osteoporosis where there is an established evidence base, but NOGG has included the management of male osteoporosis in their most recent guidance [55]. Since it would be difficult to justify a different (i.e. lower) intervention threshold in men, it is logical to apply the same thresholds in men with PC. Thus, men with probabilities at or above the upper threshold (Fig. 2) should be offered treatment.
Fig. 2.
NOGG intervention thresholds. The thresholds depicted by the lines between the green and red areas above are the 10-year probabilities of a major osteoporotic fracture (left graph) or hip fracture (right graph) in women with a previous fracture. Applying the same criteria to men with PC, treatment should be strongly considered in those with fracture probabilities at or above the threshold. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As current evidence suggests that fracture risk in ADT users is BMD-dependent, it is reasonable to suggest that BMD should be included in the risk calculation by FRAX® for all PC patients, wherever this is feasible. Where access to BMD is limited, FRAX® should be performed and BMD measurements targeted to those men with FRAX® probabilities, calculated without BMD (but selecting the secondary osteoporosis box to recognise ADT), lying close to the intervention threshold (for example, the amber area on the chart available at https://www.sheffield.ac.uk/NOGG/result-nobmd.html?).
7.4. Further assessment prior to treatment
Men with a previous fracture and/or who are found to be osteoporotic should have further investigations to exclude other causes of secondary osteoporosis, as treatment of the latter (e.g. malabsorption or liver disease) forms part of overall management. This may be best achieved by referral to appropriate services (metabolic bone/rheumatology/endocrinology).
7.5. Dosing regimens
NOGG recommends oral bisphosphonates such as alendronate (10 mg daily) or risedronate (5 mg daily) for osteoporosis in men, reflecting the licensed doses. In clinical practice, however, the majority of men receive once weekly oral bisphosphonates (alendronic acid 70 mg or risedronate sodium 35 mg) as used in women with postmenopausal osteoporosis. Oral therapy can often be initiated by the patient’s GP following assessment of fracture risk. Where oral therapy is not feasible or tolerated, intravenous zoledronic acid may be used (5 mg once yearly), or denosumab can be given subcutaneously at a dose of 60 mg once every 6 months.
7.6. Reassessment of fracture risk
Fracture risk reassessment should be undertaken when there is a change in systemic therapy or a change in risk factor profile (e.g. commencing glucocorticoids, incident fracture, development of other cause of secondary osteoporosis etc). In men on ADT whose fracture risk lies below but close to the intervention threshold, a FRAX reassessment (including a repeat BMD) should be undertaken after 12–18 months. All men who have been on ADT for 5 years should be reassessed even if they were not deemed at risk on baseline FRAX®.
In those on bone protective therapy, reassessment should be undertaken in 3 years for those receiving intravenous zoledronate annually, or 5 years for oral bisphosphonate use. Denosumab use should also be reviewed at 5 years but not discontinued without review in a specialist bone service.
8. Conclusions
Maintenance of bone health is increasingly important in patients with PC where, even in advanced disease, survival is now typically several years. The range and numbers of lines of treatment men receive, especially in the advanced setting, is increasing and many have impacts on the skeleton. It is critical to embed consideration of bone health and its optimisation throughout the patient journey.
9. Recommendations
The clinical guidance summarised in Fig. 3, Fig. 4 should be followed for all men commencing ADT and for all men currently on ADT who have not had previous assessment of fracture risk. It is important that all care pathways, although they may vary locally, should identify at an early stage who in the care pathway carries responsibility for bone health monitoring and treatment. We recommend that these guidelines should be available as a quick reference guide, including a brief summary and algorithm, as an electronic download for use in routine practice.
CRediT authorship contribution statement
Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing - original draft, Writing - review & editing.
Acknowledgments
Acknowledgements
We are grateful to the following organisations who provided input to and/or endorsement of these guidelines. International Cancer and Bone Society (CABS), UK National Osteoporosis Guideline Group (NOGG); Royal Osteoporosis Society (ROS); British Geriatrics Society (BGS); British Uro-oncology Group (BUG); Association of Cancer Physicians (ACP); Prostate Cancer UK; and the British Association of Urological Nurses.
We also gratefully acknowledge the support of Amgen for providing an unrestricted educational grant to support travel costs for meetings of the Expert Group.
References
- 1.Cancer research UK mortality statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/mortality#heading-Zero.
- 2.Cancer Research UK. Cancer statistics. 2013 Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence#heading-One.
- 3.Thorstenson A., Bratt O., Akre O. Incidence of fractures causing hospitalisation in prostate cancer patients: Results from the population-based PCBaSe Sweden. Eur. J. Cancer. 2012;48:1672–1681. doi: 10.1016/j.ejca.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 4.D'Oronzo S., Coleman R., Brown J. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2018;6(15) doi: 10.1016/j.jbo.2018.10.004. 4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith M., Parker C., Saad F., Miller K., Tombal B., Ng Q.S. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–419. doi: 10.1016/S1470-2045(18)30860-X. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Clinical Excellence (NICE). Prostate cancer: diagnosis and management. Clinical guideline 175. London, UK: 2014. Available from: https://www.nice.org.uk/guidance/cg175.
- 7.Mottet N, Bellmunt J, Briers E, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. 2016. Available from: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Prostate-Cancer-2016.pdf.
- 8.Saylor P.J., Rumble R.B., Tagawa S., Eastham A., Finelli A., Reddy P.S. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a Cancer Care Ontario Guideline. J. Clin. Oncol. 2020;38:1736–1743. doi: 10.1200/JCO.19.03148. [DOI] [PubMed] [Google Scholar]
- 9.NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer Version 2.2020. Page 46.
- 10.Denham J.W., Steigler A., Lamb D.S. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011:451–459. doi: 10.1016/S1470-2045(11)70063-8. [DOI] [PubMed] [Google Scholar]
- 11.Bolla M., De Reijke T.M., Tienhoven V. Duration of androgen suppression in the treatment of prostate cancer. N. Engl. J. Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 12.Hanks G.E., Pajak T.F., Porter A. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92–02. J. Clin. Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Hussain M., Tangen C.M., Berry D.L. Intermittent versus Continuous Androgen Deprivation in Prostate Cancer. N. Engl. J. Med. 2013;368:1314–1339. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonfill X., Arevalo-Rodriguez I., García L.M., Quintana M.J., Buitrago-Garcia D., Urbina D.L. Intermittent androgen deprivation therapy: recommendations to improve the management of patients with prostate cancer following the GRADE approach. Cancer Manage. Res. 2018;10:2357–2367. doi: 10.2147/CMAR.S164856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saylor P.J., Smith M.R. Bone health and prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:20–27. doi: 10.1038/pcan.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain S.A., Weston R., Stephenson R.N. Immediate dual energy X-ray absorptiometry reveals a high incidence of osteoporosis in patients with advanced prostate cancer before hormonal manipulation. BJU Int. 2003;92:690–694. doi: 10.1046/j.1464-410x.2003.04471.x. [DOI] [PubMed] [Google Scholar]
- 17.Berruti A., Dogliotti L., Terrone C. Changes in bone mineral density, lean body mass and fat content as measured by dual energy X-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J. Urol. 2002;167:2361–2367. [PubMed] [Google Scholar]
- 18.Daniell H.W., Dunn S.R., Ferguson D.W. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J. Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 19.Dalla Via J.D., Daly R.M., Owen P.J. Bone mineral density, structure, distribution and strength in men with prostate cancer treated with androgen deprivation therapy. Bone. 2019;127:367–375. doi: 10.1016/j.bone.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Gardner J.R., Livingston P.M., Fraser S.F. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: A systematic review. J. Clin. Oncol. 2014;32:335–346. doi: 10.1200/JCO.2013.49.5523. [DOI] [PubMed] [Google Scholar]
- 21.James N.D., Sydes M.R., Clarke N.W. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeney C.J., Chen Y.H., Carducci M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Excellence. Abiraterone for treating metastatic hormone-relapsed prostate cancer before chemotherapy is indicated. 2016. Available from: https://www.nice.org.uk/terms-and-.
- 24.James N.D., de Bono J.S., Spears M.R. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fizazi K., Tran N., Fein L. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 26.Parker C., Gillessen S., Heidenreich A. eUpdate 2017 – Cancer of the Prostate Treatment Recommendations: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;(Suppl 5):v69–v77. doi: 10.1093/annonc/mdv222. Available from: https://www.esmo.org/Guidelines/Genitourinary-Cancers/Cancer-of-the-Prostate/eUpdate-Treatment-Recommendation. [DOI] [PubMed] [Google Scholar]
- 27.Scher H.I., Fizazi K., Saad F. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 28.Hussain M., Fizazi K., Saad F. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis I.D., Martin A.J., Stockler M.R. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 30.Fizazi K., Shore N., Tammela T.L. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 31.Smith M.R., Saad F., Chowdhury S. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 2018;378:1408–1418. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 32.Parker C., Nilsson S., Heinrich D., Helle S.I., O'Sullivan J.M., Fosså S.D. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 33.Sartor O., Coleman R., Nilsson S. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–746. doi: 10.1016/S1470-2045(14)70183-4. [DOI] [PubMed] [Google Scholar]
- 34.Alonzi R., Parker C.C., Tunariu N. Fracture risk after radium-223 (Ra-223) in metastatic castration resistant prostate cancer (mCRPC) J. Clin. Oncol. 2019;37(15 suppl) [Google Scholar]
- 35.Tombal B., Loriot Y., Saad F. Decreased fracture rate by mandating bone protecting agents in the EORTC1333/PEACEIII trial combining Ra223 with enzalutamide versus enzalutamide alone. Early results. J. Clin. Oncol. 2019;37(15 suppl):5007. [Google Scholar]
- 36.Buehring B., Viswanathan R., Binkley N. Glucocorticoid-induced osteoporosis: An update on effects and management. J. Allergy Clin. Immunol. 2013;132:1019–1030. doi: 10.1016/j.jaci.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Kanis J.A., Melton L.J., Christiansen C. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 38.Kanis J.A., McCloskey E.V., Johansson H. A reference standard for the description of osteoporosis. Bone. 2008:467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Svedbom A., Hernlund E., Ivergård M. Osteoporosis in the European Union: a compendium of country-specific reports. Arch. Osteoporos. 2013;8:137. doi: 10.1007/s11657-013-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Osteoporosis Guideline Group. Osteoporosis Clinical guideline for prevention and treatment: Executive Summary. 2016. Available from: https://www.shef.ac.uk/NOGG/NOGG_Executive_Summary.pdf.
- 41.Neuburger J., Currie C., Wakeman R. The impact of a national clinician-led audit initiative on care and mortality after hip fracture in England. Med. Care. 2015;53:686–691. doi: 10.1097/MLR.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haentjens P., Magaziner J., Colón-Emeric C.S. Meta-analysis: excess mortality after hip fracture among older women and men. Ann. Intern. Med. 2010;152:380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown J.E., Coleman R.E. Biomarkers of bone turnover in oncology: applications in diagnosis and treatment. Expert Opin. Med. Diagn. 2010;4:125–138. doi: 10.1517/17530050903473147. [DOI] [PubMed] [Google Scholar]
- 44.Nadler M., Alibhai S., Catton P. Osteoporosis knowledge, health beliefs, and healthy bone behaviours in patients on androgen-deprivation therapy (ADT) for prostate cancer. BJU Int. 2013;118:1301–1309. doi: 10.1111/j.1464-410X.2012.11777.x. [DOI] [PubMed] [Google Scholar]
- 45.Damji A.N., Bies K., Alibhai S.M. Bone health management in men undergoing ADT: examining enablers and barriers to care. Osteoporos. Int. 2015;26:951–959. doi: 10.1007/s00198-014-2997-6. [DOI] [PubMed] [Google Scholar]
- 46.Ellsworth P., Providence R.I., Carithers G. Educational needs of urologists on bone health in prostate cancer. J. Urol. 2011;185(4Supp):e290. [Google Scholar]
- 47.Agarwal M.M., Khandelwal N., Mandal A.K. Factors affecting bone mineral density in patients with prostate carcinoma before and after orchidectomy. Cancer. 2005;103:2042–2052. doi: 10.1002/cncr.21047. [DOI] [PubMed] [Google Scholar]
- 48.Buffart L.M., Newton R.U., Chinapaw M.J. The effect, moderators, and mediators of resistance and aerobic exercise on health-related quality of life in older long-term survivors of prostate cancer. Cancer. 2015;121:2821–2830. doi: 10.1002/cncr.29406. [DOI] [PubMed] [Google Scholar]
- 49.https://www.nice.org.uk/search?q=NG131.
- 50.https://theros.org.uk/.
- 51.Coleman R., Body J.-J., Aapro M., Hadji P., Herrsted J. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014;25(Suppl 3):124–137. doi: 10.1093/annonc/mdu103. [DOI] [PubMed] [Google Scholar]
- 52.Svedbom A., Hernlund E., Ivergård M., Compston J., Cooper C., Stenmark J. Osteoporosis in the European Union: a compendium of country-specific reports. Arch. Osteoporos. 2013;8:137. doi: 10.1007/s11657-013-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Planas J., Morote J., Orsola A. The relationship between daily calcium intake and bone mineral density in men with prostate cancer. BJU Int. 2007;99:812–815. doi: 10.1111/j.1464-410X.2006.06695.x. [DOI] [PubMed] [Google Scholar]
- 54.Ross A.C., Manson J.E., Abrams S. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Osteoporosis Guideline Group. NOGG 2017: Clinical guideline for the prevention and treatment of osteoporosis 2017. Available from: https://www.sheffield.ac.uk/NOGG/NOGGGuideline 2017.pdf.
- 56.Smith M.R., McGovern F.J., Zietman A.L. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 57.Diamond T.H., Winters J., Smith A. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomized, placebo-controlled crossover study. Cancer. 2001;92:1444–1450. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 58.Smith M.R., Eastham J., Gleason D.M. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J. Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 59.Magno C., Anastasi G., Morabito N. Preventing bone loss during androgen deprivation therapy for prostate cancer: Early experience with neridronate. Eur. Urol. 2005;47:575–580. doi: 10.1016/j.eururo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Ryan C.W., Huo D., Demers L.M. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J. Urol. 2006;176:972–978. doi: 10.1016/j.juro.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 61.Ryan C.W., Huo D., Bylow K. Suppression of bone density loss and bone turnover in patients with hormone-sensitive prostate cancer and receiving zoledronic acid. BJU Int. 2007;100:70–75. doi: 10.1111/j.1464-410X.2007.06853.x. [DOI] [PubMed] [Google Scholar]
- 62.Greenspan S.L., Nelson J.B., Trump D.L. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann. Intern. Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 63.Greenspan S.L., Nelson J.B., Trump D.L. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J. Clin. Oncol. 2008;26:4426–4434. doi: 10.1200/JCO.2007.15.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michaelson M.D., Kaufman D.S., Lee H. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J. Clin. Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhoopalam N., Campbell S.C., Moritz T. Intravenous zoledronic acid to prevent osteoporosis in a veteran population with multiple risk factors for bone loss on androgen deprivation therapy. J. Urol. 2009;182:2257–2264. doi: 10.1016/j.juro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 66.Smith M.R., Egerdie B., Hernández Toriz N. Denosumab in men receiving androgen deprivation therapy for prostate cancer. N. Engl. J. Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doria C., Leali P.T., Solla F. Denosumab is really effective in the treatment of osteoporosis secondary to hypogonadism in prostate carcinoma patients? A prospective randomized multicenter international study. Clin. Cases Miner. Bone Metab. 2016;13(3):195–199. doi: 10.11138/ccmbm/2016.13.3.195. [cited 2017 May 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/28228781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choo R., Lukka H., Cheung P. Randomized, double-blinded, placebo-controlled, trial of risedronate for the prevention of bone mineral density loss in nonmetastatic prostate cancer patients receiving radiation therapy plus androgen deprivation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013;85:1239–1245. doi: 10.1016/j.ijrobp.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Klotz L.H., McNeill I.Y., Kebabdjian M. A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: The cancer and osteoporosis research with alendronate and leupr. Eur. Urol. 2013;63:927–935. doi: 10.1016/j.eururo.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Serpa Neto A., Tobias-Machado M., Esteves M.A. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2012;15:36–44. doi: 10.1038/pcan.2011.4. [DOI] [PubMed] [Google Scholar]
- 71.M.R. Smith, F. Saad, R. Coleman, et al. Denosumab and bone metastasis-free survival in men with castration-resistant prostate cancer: results of a global phase 3, randomised, placebo-controlled trial. [cited 2018 Sep 21]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3671878/pdf/nihms472298.pdf. [DOI] [PMC free article] [PubMed]
- 72.Fizazi K., Carducci M., Smith M. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan A.A., Morrison A., Hanley D.A. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 74.Hoff M., Meyer H.E., Skurtveit S. Validation of FRAX and the impact of self-reported falls among elderly in a general population: the HUNT study, Norway. Osteoporos. Int. 2017;28:2935–2944. doi: 10.1007/s00198-017-4134-9. [DOI] [PubMed] [Google Scholar]
- 75.Harvey N.C., Johansson H., Odén A. FRAX predicts incident falls in elderly men: findings from MrOs Sweden. Osteoporos. Int. 2016;27:267–274. doi: 10.1007/s00198-015-3295-7. [DOI] [PubMed] [Google Scholar]
- 76.Landi F., Calvani R., Ortolani E. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos. Int. 2017;28:1569–1576. doi: 10.1007/s00198-017-3929-z. [DOI] [PubMed] [Google Scholar]
- 77.Lin D., Smith M.R., Morton R.A.S.M. Use of age and BMD to predict fracture risk in men on androgen deprivation therapy. J. Clin. Oncol. 2009;27(15S):9517. [Google Scholar]
- 78.Ahlborg H.G., Nguyen N.D., Center J.R. Incidence and risk factors for low trauma fractures in men with prostate cancer. Bone. 2008;43:556–560. doi: 10.1016/j.bone.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Kanis J.A., Harvey N.C., Cooper C., Johansson H., Odén A., McCloskey E.V. A systematic review of intervention thresholds based on FRAX: A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch. Osteoporos. 2016;11(1) doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Compston J., Cooper A., Cooper C. Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas. 2009;62:105–108. doi: 10.1016/j.maturitas.2008.11.022. [DOI] [PubMed] [Google Scholar]