Abstract
Purpose
The presence of microvascular invasion (MVI) is an unfavorable prognostic factor for hepatocellular carcinoma (HCC). This study aimed to construct a nomogram-based preoperative prediction model of MVI, thereby assisting to preoperatively select proper surgical procedures.
Methods
A total of 714 non-metastatic HCC patients undergoing radical hepatectomy were retrospectively selected from Zhongshan Hospital between 2010 and 2018, followed by random assignment into training (N = 520) and validation cohorts (N = 194). Nomogram-based prediction model for MVI risk was constructed by incorporating independent risk factors of MVI presence identified from multivariate backward logistic regression analysis in the training cohort. The performance of nomogram was evaluated by calibration curve and ROC curve. Finally, decision curve analysis (DCA) was used to determine the clinical utility of the nomogram.
Results
In total, 503 (70.4%) patients presented MVI. Multivariate analysis in the training cohort revealed that age (OR: 0.98), alpha-fetoprotein (≥400 ng/mL) (OR: 2.34), tumor size (>5 cm) (OR: 3.15), cirrhosis (OR: 2.03) and γ-glutamyl transpeptidase (OR: 1.61) were significantly associated with MVI presence. The incorporation of five risk factors into a nomogram-based preoperative estimation of MVI risk demonstrated satisfactory discriminative capacity, with C-index of 0.702 and 0.690 in training and validation cohorts, respectively. Calibration curve showed good agreement between actual and predicted MVI risks. Finally, DCA revealed the clinical utility of the nomogram.
Conclusion
The nomogram showed a satisfactory discriminative capacity of MVI risk in HCC patients, and could be used to preoperatively estimate MVI risk, thereby establishing more rational therapeutic strategies.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the third leading cause of cancer-related mortality globally [1,2]. In Asia-Pacific region, particularly China, the age-standardized incidence of HCC is 14–36 per 100,000 men, much higher than the average global incidence [3]. Partial hepatectomy and liver transplantation are considered as the potentially curative approaches in patients with early- to intermediate-stage HCC [4,5]. Nevertheless, the long-term outcomes of HCC patients are far from satisfaction, largely due to the high recurrence rate, even in those with early-stage HCC undergoing partial hepatectomy or liver transplantation [6]. Therefore, it is urgent to explore effective pre-operative indicators to identify the aggressiveness of HCC, which could thereby facilitate in precise decision-making.
A variety of studies have demonstrated that the presence of microvascular invasion (MVI) is associated with post-operative recurrence of HCC [[7], [8], [9]]. MVI, an important histopathological prognostic factor correlated with lower survival rate and an indicator for aggressive behavior of HCC [7,10], is defined as microscopic tumor cell invasion into intrahepatic portal vein or hepatic vein branches, subsequently leading to tumor cell dissemination and metastasis [11,12]. In consideration of the significantly differential survival rate in HCC patients with or without MVI [7], it is critical to precisely identify patients with MVI prior to surgical intervention. In addition, the presence or absence of MVI could affect the clinical options between hepatic resection and liver transplantation [13,14]. To be specific, the absence of MVI is included within the new criteria for liver transplantation in HCC patients [14,15], while a wider resection margin is required for curative treatment in the presence of MVI [13]. Therefore, the accurate preoperative assessment of MVI presence can help clinicians to decide appropriate surgical procedures. However, histopathological examination of post-operative samples is currently considered as the only reliable diagnosis for MVI [7,16], which limits the clinical significance of MVI on management-related decision-making.
To this end, multiple studies have revealed a series of non-invasive clinical indicators for preoperative estimation of MVI. Serum tumor biomarkers and inflammatory biomarkers have been proposed for MVI risk estimation [17,18]. Unfortunately, tumor biomarker can also be abnormally elevated in patients with benign hepatic diseases. In a prospective study enrolling 855 patients with hepatitis C, alpha-fetoprotein (AFP) was frequently elevated in those with chronic hepatitis C or cirrhosis [19]. Therefore, serum biomarkers alone are not sufficient to predict the risk of MVI. Additionally, radiomics signature has been reported as a predictive and prognostic biomarker for HCC [20]. Both computed tomography (CT) textural characteristics [21] and gadoxetic acid-enhanced magnetic resonance imaging (MRI) features [22] have been suggested to be associated with early postoperative recurrence of HCC. Nevertheless, these findings require prospective validation.
Nomogram is a user-friendly statistical model that estimates a numerical probability for a given individual by integrating various variables [23,24]. Thus, in the present study, we aimed to construct and to validate a preoperative prediction model for the presence of MVI based on nomogram analysis.
Materials and methods
Patients and data collection
This retrospective study was approved by the Ethics Committee of Zhongshan Hospital (No. 2018-430-T326). Eligible patients were selected from Zhongshan Hospital between 2010 and 2018 based on the following inclusion criteria: (1) histopathological diagnosis of HCC; (2) initially treated by radical hepatectomy; (3) no distant metastasis at diagnosis; and (4) no history of other malignant tumors. The exclusion criteria were as follows: (1) received any anti-cancer therapy prior to hepatectomy; (2) without complete clinicopathological data; (3) with macrovascular invasion; and (4) unknown survival or less than one month. Finally, a total of 714 pathologically-confirmed HCC patients who underwent hepatectomy were selected in this study.
Clinicopathological variables were retrieved from medical records and imaging examinations. The pre-operative laboratory indexes included alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), total bilirubin (TB), albumin, AFP and hepatitis B serology. The assessment of cirrhosis was mainly based on ultrasonography or imaging examinations of liver. Additionally, patient demographics (age and sex) and tumor features (tumor size, tumor number and tumor capsule) were also collected and analyzed. To be specific, tumor features were analyzed and determined based on preoperative CT independently by two experienced radiologists.
After radical hepatectomy, all surgical samples were pathologically examined, especially for the presence of MVI. In the present study, MVI was defined as the microscopic presence of tumor emboli in hepatic veins, portal veins and lymphatic ducts lined by endothelium [10,25].
Construction and validation of nomogram
Patients were randomly into to the training cohort (N = 520) and the validation cohort (N = 194) by setting seed in R software. According to univariate logistic regression analysis and multivariate backward logistic regression in the training cohort, five independent risk factors (including age, AFP level, tumor size, cirrhosis and GGT level) were used to formulate a nomogram model for MVI prediction. Afterwards, the nomogram model was assessed in the validation cohort.
The performance of nomogram-based prediction of MVI risk was evaluated by calibration curves in both training and validation cohorts. Besides, receiver operating characteristics (ROC) curves were plotted, followed by calculation of areas under curve (AUCs) of ROC curves, aiming to investigate the overall performance of this nomogram model. Finally, decision curve analysis (DCA) was conducted to assess the net benefit and clinical utility of the prediction model. In DCA, the decision curve was compared with extreme cases of treating all or none patients. And greater net benefit over treating all and none patients of a certain model indicated its clinical utility [26,27]. Because hepatitis B virus (HBV) infection is a dominant etiology of HCC in China [28], we further investigated the discriminative capacity of this nomogram model in HBV-positive and HBV-negative HCC.
Statistical analysis
Continuous variables were shown means ± standard deviation, which were compared by Student's t-test. Chi-square test was used to compare categorical variables. Univariate logistic regression model was used to explore possible risk factors associated with the presence of MVI. And variables with P value < 0.1 were subsequently incorporated into multivariate backward logistic regression to identify independent risk factors of MVI presence. The odds ratio (OR) and 95% confidence intervals (CIs) were presented. Statistical analysis was performed using IBM SPSS Statistics software version 13.0 (SPSS, Chicago, IL) and R software for Windows (version R-3.4.3, the R Foundation for statistical computing). A P < 0.05 indicated statistical significance and all statistical comparisons were two-sided.
Results
Clinicopathological characteristics
During the study period, a total of 714 HCC patients who received partial hepatectomy were enrolled. Of them, 520 and 194 patients were categorized into the training cohort and validation cohort, respectively. The baseline clinicopathological characteristics of all HCC patients were summarized in Table 1. Generally, the clinicopathological data were similar between the two cohorts. Pathologically confirmed MVI was identified in 363 (69.8%) and 140 (72.2%) patients in the training and validation cohorts, respectively.
Table 1.
Clinicopathological characteristics of patients.
| Characteristics | Training cohort (N = 520) |
Validation cohort (N = 194) |
||||
|---|---|---|---|---|---|---|
| Total | MVI (+) | MVI (−) | Total | MVI (+) | MVI (−) | |
| Age (year) | 55 ± 11.4 | 54.9 ± 11.5 | 52.0 ± 11.1 | 55 ± 10.0 | 55.6 ± 10.0 | 51.9 ± 11.1 |
| Sex | ||||||
| Female | 86(0.1654) | 59(0.1625) | 27(0.172) | 24(0.1237) | 23(0.1643) | 1(0.0185) |
| Male | 434(0.8346) | 304(0.8375) | 130(0.828) | 170(0.8763) | 117(0.8357) | 53(0.9815) |
| ALT (U/L) | ||||||
| ≤44 | 373(0.7173) | 259(0.7135) | 114(0.7261) | 137(0.7062) | 101(0.7214) | 36(0.6667) |
| >44 | 147(0.2827) | 104(0.2865) | 43(0.2739) | 57(0.2938) | 39(0.2786) | 18(0.3333) |
| TB (umol/L) | ||||||
| ≤17.1 | 421(0.8096) | 296(0.8154) | 125(0.7962) | 160(0.8247) | 118(0.8429) | 42(0.7778) |
| >17.1 | 99(0.1904) | 67(0.1846) | 32(0.2038) | 34(0.1753) | 22(0.1571) | 12(0.2222) |
| Albumin (g/dL) | ||||||
| ≤3.5 | 501(0.9635) | 353(0.9725) | 148(0.9427) | 189(0.9742) | 135(0.9643) | 54(1) |
| >3.5 | 19(0.0365) | 10(0.0275) | 9(0.0573) | 5(0.0258) | 5(0.0357) | 0(0) |
| AFP (ng/mL) | ||||||
| ≤20 | 218(0.4192) | 171(0.4711) | 47(0.2994) | 88(0.4536) | 68(0.4857) | 20(0.3704) |
| 20–400 | 156(0.3) | 110(0.303) | 46(0.293) | 58(0.299) | 43(0.3071) | 15(0.2778) |
| ≥400 | 146(0.2808) | 82(0.2259) | 64(0.4076) | 48(0.2474) | 29(0.2071) | 19(0.3519) |
| Cirrhosis | ||||||
| No | 129(0.2481) | 101(0.2782) | 28(0.1783) | 51(0.2629) | 34(0.2429) | 17(0.3148) |
| Yes | 391(0.7519) | 262(0.7218) | 129(0.8217) | 143(0.7371) | 106(0.7571) | 37(0.6852) |
| HBsAg | ||||||
| Negative | 108(0.2077) | 82(0.2259) | 26(0.1656) | 48(0.2474) | 32(0.2286) | 16(0.2963) |
| Positive | 412(0.7923) | 281(0.7741) | 131(0.8344) | 146(0.7526) | 108(0.7714) | 38(0.7037) |
| Tumor size (cm) | ||||||
| ≤2 | 92(0.1769) | 76(0.2094) | 16(0.1019) | 38(0.1959) | 33(0.2357) | 5(0.0926) |
| 2–5 | 251(0.4826) | 188(0.5179) | 63(0.4013) | 77(0.3969) | 64(0.4571) | 13(0.2407) |
| >5 | 177(0.3404) | 99(0.2727) | 78(0.4968) | 79(0.4072) | 43(0.3071) | 36(0.6667) |
| Tumor number | ||||||
| Solitary | 447(0.8596) | 319(0.8788) | 128(0.8153) | 162(0.8351) | 119(0.85) | 43(0.7963) |
| Multiple | 73(0.1404) | 44(0.1212) | 29(0.1847) | 32(0.1649) | 21(0.15) | 11(0.2037) |
| Tumor capsule | ||||||
| Yes | 186(0.3577) | 119(0.3278) | 67(0.4268) | 67(0.3454) | 40(0.2857) | 27(0.5) |
| No | 334(0.6423) | 244(0.6722) | 90(0.5732) | 127(0.6546) | 100(0.7143) | 27(0.5) |
| GGT (U/L) | ||||||
| ≤50 | 256(0.4923) | 200(0.551) | 56(0.3567) | 98(0.5052) | 78(0.5571) | 20(0.3704) |
| >50 | 264(0.5077) | 163(0.449) | 101(0.6433) | 96(0.4948) | 62(0.4429) | 34(0.6296) |
Abbreviations: MVI, microvascular invasion; ALT, alanine aminotransferase; TB, total bilirubin; AFP, α-fetoprotein; HBsAg, hepatitis B surface antigen; GGT, γ-glutamyltransferase.
Univariate and multivariate analyses for risk factors of MVI presence
Univariate logistic regression analysis was used to determine the risk factors for MVI presence based on preoperative variables. As shown in Table 2, age (OR = 0.98, P = 0.007), AFP (OR = 2.84, P = 0.001), cirrhosis (OR = 1.78, P = 0.013), tumor size (OR = 3.74, P < 0.001), tumor number (OR = 1.64, P = 0.061), tumor capsule (OR = 1.53, P = 0.032) and GGT (OR = 2.21, P < 0.001) were associated with the presence of MVI in the training cohort. The above variables were further incorporated into multivariate backward logistic regression model according to the cutoff P value < 0.1 in univariate analysis. As a result, age (OR = 0.98, P = 0.032), AFP (OR = 2.34, P < 0.001), tumor size (OR = 3.15, P < 0.001), cirrhosis (OR = 2.03, P = 0.005) and GGT (OR = 1.61, P = 0.028) were found to be independently associated with the presence of MVI (shown in Table 3).
Table 2.
Univariate logistic regression analysis of MVI presence based on preoperative data in the training cohort.
| Variable | OR (95% CI) | P value |
|---|---|---|
| Age (year) | 0.98 (0.96–0.99) | 0.007 |
| Sex | ||
| Female | Reference | |
| Male | 0.93 (0.57–1.54) | 0.791 |
| ALT (U/L) | ||
| ≤44 | Reference | |
| >44 | 0.94 (0.62–1.43) | 0.769 |
| TB (umol/L) | ||
| ≤17.1 | Reference | |
| >17.1 | 1.13 (0.71–1.81) | 0.61 |
| Albumin (g/dL) | ||
| ≤3.5 | Reference | |
| >3.5 | 2.15 (0.85–5.39) | 0.11 |
| AFP (ng/mL) | ||
| ≤20 | Reference | |
| 20–400 | 1.52 (0.95–2.44) | 0.081 |
| ≥400 | 2.84 (1.79–4.50) | 0.001 |
| Cirrhosis | ||
| No | Reference | |
| Yes | 1.78 (1.11–2.84) | 0.013 |
| HBsAg | ||
| Negative | Reference | |
| Positive | 1.47 (0.90–2.39) | 0.114 |
| Tumor size (cm) | ||
| ≤2 | Reference | |
| 2–5 | 1.59 (0.86–2.93) | 0.135 |
| >5 | 3.74 (2.02–6.92) | <0.001 |
| Tumor number | ||
| Solitary | Reference | |
| Multiple | 1.64 (0.98–2.74) | 0.061 |
| Tumor capsule | ||
| Yes | Reference | |
| No | 1.53 (1.04–2.24) | 0.032 |
| GGT (U/L) | ||
| ≤50 | Reference | |
| >50 | 2.21 (1.50–3.26) | <0.001 |
Abbreviations: MVI, microvascular invasion; OR, odds ratio; CI, confidence interval; ALT, alanine aminotransferase; TB, total bilirubin; AFP, α-fetoprotein; HBsAg: hepatitis B surface antigen; GGT, γ-glutamyltransferase.
Table 3.
Multivariate backward logistic regression analysis of MVI presence based on preoperative data in the training cohort.
| Variable | β | OR (95% CI) | P value |
|---|---|---|---|
| Age (year) | −0.02 | 0.98 (0.96–1.00) | 0.032 |
| AFP (ng/mL) | |||
| ≤20 | Reference | ||
| 20–400 | 0.38 | 1.46 (0.89–2.4) | 0.132 |
| ≥400 | 0.85 | 2.34 (1.44,3.82) | <0.001 |
| Tumor size (cm) | |||
| ≤2 | Reference | ||
| 2–5 | 0.45 | 1.57 (0.83–2.97) | 0.167 |
| >5 | 1.15 | 3.15 (1.61–6.17) | <0.001 |
| Cirrhosis | |||
| No | Reference | ||
| Yes | 0.71 | 2.03 (1.22–3.36) | 0.005 |
| GGT (U/L) | |||
| ≤50 | Reference | ||
| >50 | 0.478 | 1.61 (1.05–2.47) | 0.028 |
Abbreviations: MVI, microvascular invasion; OR, odds ratio; CI, confidence interval; AFP, α-fetoprotein; GGT, γ-glutamyltransferase.
Construction and validation of nomogram-based preoperative MVI prediction
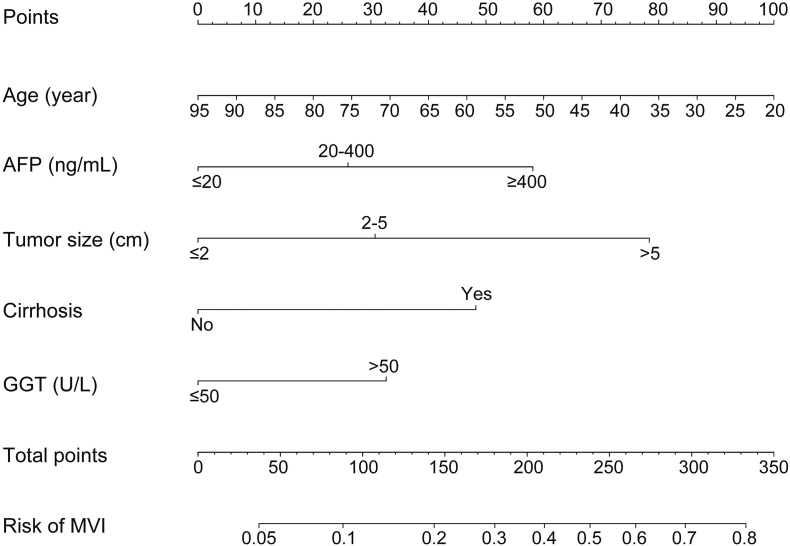
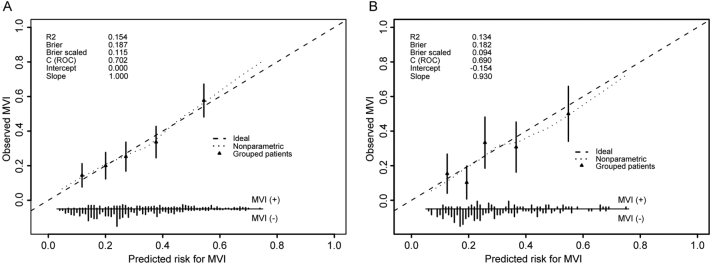
In multivariate analysis of the training cohort, we identified five independent risk factors with significant association with MVI presence. Thus, the five factors were used to construct a nomogram model for preoperative MVI estimation. As shown in Fig. 1, age exerted the largest effect on the presence of MVI, with a maximal score of 100 points. The points were approximately 60, 80, 50 and 35 for AFP ≥ 400 ng/mL, tumor size >5 cm, with the presence of cirrhosis and GGT > 50 U/L, respectively. The nomogram showed good accuracy in MVI risk prediction, with a C-index of 0.702. Calibration plot displayed good consistency between actual MVI risk confirmed by pathological examination and predicted risk of MVI (Fig. 2A).
Fig. 1.
Nomogram to preoperatively estimate the risk of MVI presence in hepatocellular carcinoma. To use the nomogram, find the position of each variable on the corresponding axis, draw a line to the points axis for the number of points, add the points from all of the variables, and draw a line from the total points axis to determine the MVI probabilities at the lower line of the nomogram. MVI, microvascular invasion; AFP, α-fetoprotein; GTT, γ-glutamyltransferase.
Fig. 2.
Validity of the predictive performance of the nomogram in estimating the risk of MVI presence in the training cohort (A, N = 520) and the validation cohort (B, N = 194). The distribution of the predicted probabilities of MVI presence is shown at the bottom of the graphs, separating those with MVI (+) and MVI (−). The triangles indicate the observed frequencies of MVI presence by the deciles of the predicted probability. MVI, microvascular invasion; C index, concordance index; and ROC, receiver operating characteristic.
In the validation cohort, the C-index was 0.690 for MVI prediction based on nomogram analysis. Similarly, the calibration curve showed good agreement of observed MVI risk with predicted MVI risk (Fig. 2B).
Assessment of the performance of nomogram model
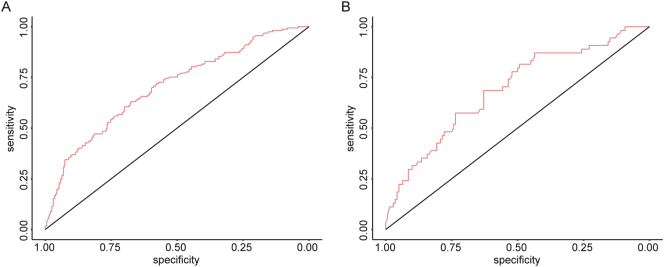
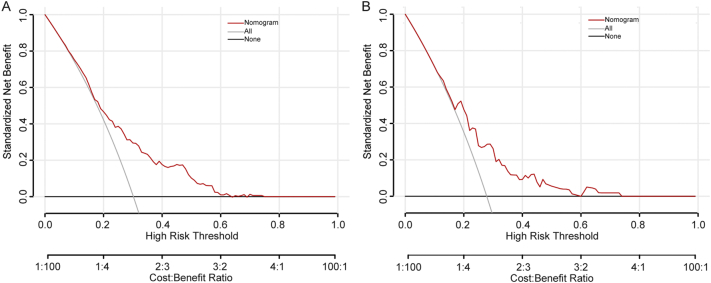
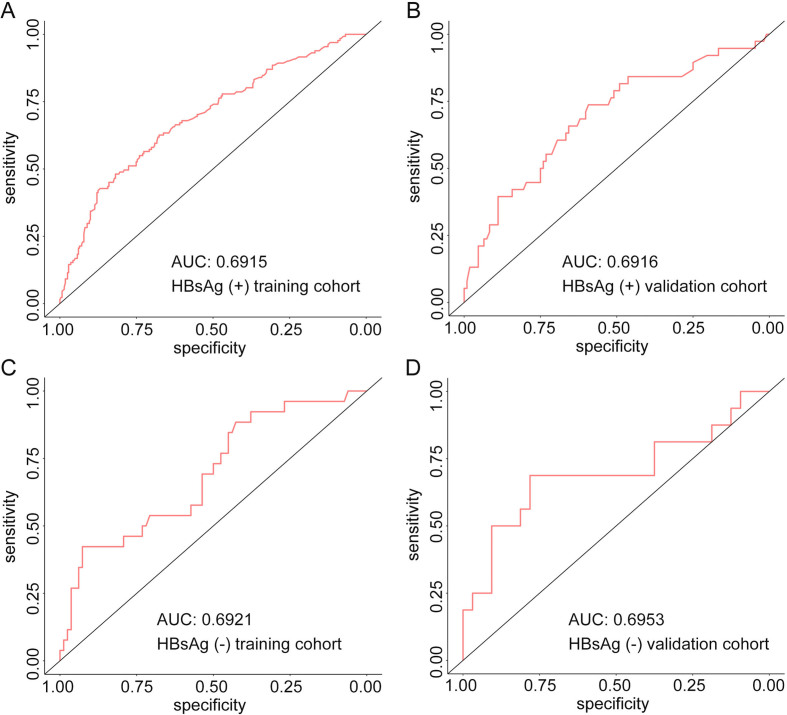
To evaluate the predictive ability of the nomogram-based prediction model for MVI risk, ROC curve was utilized. As shown in Fig. 3A and B, the AUC was 0.702 and 0.690 in the training and validation cohorts, respectively. According to the DCA results, the present nomogram model provided a greater standardized net benefit compared to “treat-all” and “treat-none” strategies when the risk threshold ranged approximately from 0.3 to 0.6 in both cohorts (Fig. 4). In consideration of the possible effects of HBV status on MVI risk, patients were further divided into HBV-positive and HBV-negative groups in both cohorts based on hepatitis B surface antigen (HBsAg) status. As shown in Supplementary Fig. 1, among HBV positive patients, the AUC was 0.6915 and 0.6916 in the training cohort and validation cohort, respectively. In patients with negative HBV infection, the AUC was 0.6921 and 0.6953 in the training cohort and validation cohort, respectively. The ROC curve indicated comparably discriminative capacity of this nomogram-based predictive model of MVI in HCC developed from viral hepatitis and non-viral hepatitis.
Fig. 3.
Receiver operating characteristic (ROC) curve and the under curve (AUCs) of ROC curves in the training cohort (A, N = 520) and the validation cohort (B, N = 194).
Fig. 4.
Decision curve analysis for the nomogram in predicting MVI presence in the training cohort (A, N = 520) and the validation cohort (B, N = 194).
Supplementary Fig. 1.
Receiver operating characteristic (ROC) curve and the area under curve (AUC) of ROC curves in the training cohort with positive HBsAg (A, N = 412), validation cohort with positive HBsAg (B, N = 146), training cohort with negative HBsAg (C, N = 108) and validation cohort with negative HBsAg (D, N = 48).
Discussion
Patients with early- to intermediate-stage HCC are the major subjects undergoing curative therapies. However, even with the great advance in hepatectomy and liver transplantation, the long-term prognosis of the above population is still poor, with high recurrence rates after primary resection [29]. Among the factors associated with tumor recurrence, the presence of MVI has been considered as a marker of aggressive tumor behavior, which could greatly affect patient prognosis and tumor recurrence, especially in patients undergoing curative treatment [[30], [31], [32]]. In this present study, our analysis suggested that younger age, higher AFP and GGT levels, larger tumor size and cirrhosis were independent risk factors for MVI presence.
In consideration of the roles of MVI presence on post-operative outcome and pre-operative clinical decision-making, the actual pre-operative estimation of MVI presence has been investigated in a variety of studies [[32], [33], [34], [35]]. Imaging examinations, serum biomarkers and tissue features are the major variables that have been incorporated into different prediction models of MVI risk [32,33,36]. Preoperative non-smooth tumor margin on CT has been proposed to be associated with the presence of MVI [34,36]. Elevated AFP level is the most widely-recognized serum marker for predicting MVI risk. In a meta-analysis enrolling 20 observational studies, Rodríguez-Perálvarez et al. [32] demonstrated that higher AFP level, larger tumor size, incomplete tumor capsule and multifocal lesions were associated with higher risk of MVI presence in advanced-stage HCC. Here, in our study by including 714 patients with stage I–III HCC, higher AFP level and larger tumor size were also significantly associated with MVI risk. Additionally, age, cirrhosis and GGT level have been identified as independent risk factors associated with MVI risk. We report that a younger age generally suggests a higher risk of MVI. However, age exerts a paradoxical effect on the prognosis of HCC [37]. And Shen et al. [25] has demonstrated that an elder age is associated with higher risks of MVI presence. Cirrhosis is a well-defined indicator for poor prognosis in HCC [38]. GGT is an important enzyme reflecting liver function. Elevated GGT level is associated with HCC recurrence and poor survival, possibly because GGT level is associated with worse liver function and the degree of malignancy of HCC [39,40].
Nomogram has been recognized as a user-friendly prediction tool with high accuracy and good discriminative power [23,41], which has been widely used to predict prognosis and recurrence of tumor patients [24]. In our study, the five clinicopathological variables significantly associated with MVI presence were incorporated into a preoperative estimation model of MVI risk based on nomogram. In both training and validation cohorts, the calibration curves revealed good agreement between the predicted MVI risk and actual MVI presence. The AUC values were 0.702 and 0.690 in the training cohort and validation cohort, respectively. Additionally, DCA curves showed greater net benefit of the established nomogram model, indicating its clinical usefulness.
To evaluate the performance of nomogram model, we assessed the sensitivity and specificity of the model, as manifested by ROC curve. Calibration plot showed good agreement between predicted MVI risk and actual MVI presence. For clinical use of this nomogram-based model, DCA was used to calculate the standardized net benefit, where risk threshold between 0.3 and 0.6 indicated greater net benefit. Therefore, our nomogram serves as a non-invasive preoperative predicting method to assess MVI risk in HCC patients.
Our study covers both patients with viral hepatitis and non-viral hepatitis, while the majority of previous studies focus on HBV-related HCC [33,42,43]. ROC curves in subgroup analysis shows relatively satisfactory discriminative performance of the nomogram model regardless of the HBV infection status, indicating that our findings might be comparably suitable for HCC developed from both viral hepatitis and non-viral hepatitis. However, there are several limitations in our study. To begin with, it is a retrospective study, thus selection bias could be not excluded. And prospective studies are warranted to further validate the reliability of the nomogram. Secondly, the use of CT might bring potential limitation. Although CT is commonly used for HCC evaluation, it is still inferior to MRI. Finally, because MVI status is not the only factor determining therapeutic strategies for HCC, other relevant factors should also be taken into consideration, including liver function reserve and performance status.
To sum up, five clinicopathological variables (age, AFP level, tumor size, cirrhosis and GGT level), independent risk factors for MVI presence in HCC patients, were incorporated into a nomogram model. This nomogram-based prediction model provided optimal preoperative estimation of MVI risk in non-metastatic HCC patients.
The following is the supplementary data related to this article.
CRediT authorship contribution statement
Chihao Zhang: Conceptualization, Methodology, Formal analysis, Writing - original draft. Ran Zhao: Formal analysis, Software, Visualization. Fancheng Chen: Resources, Software. Yiming Zhu: Data curation, Visualization, Supervision. Liubo Chen: Conceptualization, Methodology, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yiming Zhu, Email: zymamm@163.com.
Liubo Chen, Email: liubochen@zju.edu.cn.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bosch F.X., Ribes J., Diaz M., Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5–s16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Yuen M.F., Hou J.L., Chutaputti A. Hepatocellular carcinoma in the Asia Pacific Region. J. Gastroenterol. Hepatol. 2009;24(3):346–353. doi: 10.1111/j.1440-1746.2009.05784.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhong J.H., Ke Y., Gong W.F., Xiang B.D., Ma L., Ye X.P. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann. Surg. 2014;260(2):329–340. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 5.Miura J.T., Johnston F.M., Tsai S., Eastwood D., Banerjee A., Christians K.K. Surgical resection versus ablation for hepatocellular carcinoma </=3 cm: a population-based analysis. HPB. 2015;17(10):896–901. doi: 10.1111/hpb.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon R.T., Fan S.T., Lo C.M., Liu C.L., Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann. Surg. 2002;235(3):373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim K.C., Chow P.K., Allen J.C., Chia G.S., Lim M., Cheow P.C. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann. Surg. 2011;254(1):108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Li J., Shen F., Lau W.Y. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2018;33(2):347–354. doi: 10.1111/jgh.13843. [DOI] [PubMed] [Google Scholar]
- 9.Hirokawa F., Hayashi M., Asakuma M., Shimizu T., Inoue Y., Uchiyama K. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg. Oncol. 2016;25(1):24–29. doi: 10.1016/j.suronc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Roayaie S., Blume I.N., Thung S.N., Guido M., Fiel M.I., Hiotis S. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Zhu X.D., Ji Y., Ding G.Y., Shi G.M., Shen Y.H. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B. BMC Cancer. 2017;17(1):58. doi: 10.1186/s12885-017-3050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J., Chakraborty J., Chapman W.C., Gerst S., Gonen M., Pak L.M. Preoperative prediction of microvascular invasion in hepatocellular carcinoma using quantitative image analysis. J. Am. Coll. Surg. 2017;225(6) doi: 10.1016/j.jamcollsurg.2017.09.003. 778-88.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhir M., Melin A.A., Douaiher J., Lin C., Zhen W.K., Hussain S.M. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann. Surg. 2016;263(6):1112–1125. doi: 10.1097/SLA.0000000000001556. [DOI] [PubMed] [Google Scholar]
- 14.Zaydfudim V.M., Vachharajani N., Klintmalm G.B., Jarnagin W.R., Hemming A.W., Doyle M.B. Liver resection and transplantation for patients with hepatocellular carcinoma beyond Milan criteria. Ann. Surg. 2016;264(4):650–658. doi: 10.1097/SLA.0000000000001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzaferro V., Llovet J.M., Miceli R., Bhoori S., Schiavo M., Mariani L. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 16.Renzulli M., Brocchi S., Cucchetti A., Mazzotti F., Mosconi C., Sportoletti C. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology. 2016;279(2):432–442. doi: 10.1148/radiol.2015150998. [DOI] [PubMed] [Google Scholar]
- 17.Gouw A.S., Balabaud C., Kusano H., Todo S., Ichida T., Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transplant. 2011;17(Suppl. 2):S72–S80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J., Seier K., Gonen M., Balachandran V.P., Kingham T.P., D'Angelica M.I. Utility of serum inflammatory markers for predicting microvascular invasion and survival for patients with hepatocellular carcinoma. Ann. Surg. Oncol. 2017;24(12):3706–3714. doi: 10.1245/s10434-017-6060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterling R.K., Wright E.C., Morgan T.R., Seeff L.B., Hoefs J.C., Di Bisceglie A.M. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am. J. Gastroenterol. 2012;107(1):64–74. doi: 10.1038/ajg.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambin P., Rios-Velazquez E., Leijenaar R., Carvalho S., van Stiphout R.G., Granton P. Radiomics: extracting more information from medical images using advanced feature analysis. Eur. J. Cancer (Oxford, England: 1990) 2012;48(4):441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y., He L., Huang Y., Chen S., Wu P., Ye W. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom. Radiol. (New York) 2017;42(6):1695–1704. doi: 10.1007/s00261-017-1072-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee S., Kim S.H., Lee J.E., Sinn D.H., Park C.K. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J. Hepatol. 2017;67(3):526–534. doi: 10.1016/j.jhep.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Iasonos A., Schrag D., Raj G.V., Panageas K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008;26(8):1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 24.Balachandran V.P., Gonen M., Smith J.J., DeMatteo R.P. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J., Wen T., Chen W., Lu C., Yan L., Yang J. Model predicting the microvascular invasion and satellite lesions of hepatocellular carcinoma after hepatectomy. ANZ J. Surg. 2018;88(11):E761–e6. doi: 10.1111/ans.14473. [DOI] [PubMed] [Google Scholar]
- 26.Vickers A.J., Elkin E.B. Decision curve analysis: a novel method for evaluating prediction models. Med. Decis. Making. 2006;26(6):565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z., Rousson V., Lee W.C., Ferdynus C., Chen M., Qian X. Decision curve analysis: a technical note. Ann. Transl. Med. 2018;6(15):308. doi: 10.21037/atm.2018.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Wang Y, Feng X, Wang R, Wang Y, Zeng H, et al. Contribution of Hepatitis B Virus and Hepatitis C Virus to Liver Cancer in China North Areas: Experience of the Chinese National Cancer Center. (1878–3511 (Electronic)). [DOI] [PubMed]
- 29.Tabrizian P., Jibara G., Shrager B., Schwartz M., Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann. Surg. 2015;261(5):947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 30.Sumie S., Nakashima O., Okuda K., Kuromatsu R., Kawaguchi A., Nakano M. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann. Surg. Oncol. 2014;21(3):1002–1009. doi: 10.1245/s10434-013-3376-9. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh C.H., Wei C.K., Yin W.Y., Chang C.M., Tsai S.J., Wang L.Y. Vascular invasion affects survival in early hepatocellular carcinoma. Mol. Clin. Oncol. 2015;3(1):252–256. doi: 10.3892/mco.2014.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Peralvarez M., Luong T.V., Andreana L., Meyer T., Dhillon A.P., Burroughs A.K. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann. Surg. Oncol. 2013;20(1):325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 33.Lei Z., Li J., Wu D., Xia Y., Wang Q., Si A. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151(4):356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 34.Chou C.T., Chen R.C., Lin W.C., Ko C.J., Chen C.B., Chen Y.L. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am. J. Roentgenol. 2014;203(3):W253–W259. doi: 10.2214/AJR.13.10595. [DOI] [PubMed] [Google Scholar]
- 35.Xu X.F., Yu J.J., Xing H., Shen F., Yang T. How to better predict microvascular invasion and recurrence of hepatocellular carcinoma. J. Hepatol. 2017;67(5):1119–1120. doi: 10.1016/j.jhep.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 36.Peng J., Zhang J., Zhang Q., Xu Y., Zhou J., Liu L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn. Interv. Radiol. (Ankara, Turkey) 2018;24(3):121–127. doi: 10.5152/dir.2018.17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C.H., Chang T.T., Cheng K.S., Su W.W., Yang S.S., Lin H.H. Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients. Liver Int. 2006;26(7):766–773. doi: 10.1111/j.1478-3231.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhao W.C., Fan L.F., Yang N., Zhang H.B., Chen B.D., Yang G.S. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur. J. Surg. Oncol. 2013;39(8):858–864. doi: 10.1016/j.ejso.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z., Song P., Xia J., Inagaki Y., Tang W., Kokudo N. Can gamma-glutamyl transferase levels contribute to a better prognosis for patients with hepatocellular carcinoma? Drug Discov. Ther. 2014;8(3):134–138. doi: 10.5582/ddt.2014.01025. [DOI] [PubMed] [Google Scholar]
- 40.Song P., Inagaki Y., Wang Z., Hasegawa K., Sakamoto Y., Arita J. High levels of gamma-glutamyl transferase and indocyanine green retention rate at 15 min as preoperative predictors of tumor recurrence in patients with hepatocellular carcinoma. Medicine. 2015;94(21) doi: 10.1097/MD.0000000000000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shariat S.F., Capitanio U., Jeldres C., Karakiewicz P.I. Can nomograms be superior to other prediction tools? BJU Int. 2009;103(4):492–495. doi: 10.1111/j.1464-410X.2008.08073.x. discussion 5-7. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L., Rui J.A., Wang S.B., Chen S.G., Qu Q. Risk factors of microvascular invasion, portal vein tumor thrombosis and poor post-resectional survival in HBV-related hepatocellular carcinoma. Hepato-gastroenterology. 2014;61(134):1696–1703. [PubMed] [Google Scholar]
- 43.Li Z., Lei Z., Xia Y., Li J., Wang K., Zhang H. Association of preoperative antiviral treatment with incidences of microvascular invasion and early tumor recurrence in hepatitis B virus-related hepatocellular carcinoma. JAMA Surg. 2018;153(10) doi: 10.1001/jamasurg.2018.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]