Summary
Background
Late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease, characterised by rapid psychomotor decline and epilepsy, is caused by deficiency of the lysosomal enzyme tripeptidyl peptidase 1. We aimed to analyse the characteristics and rate of progression of CLN2 disease in an international cohort of patients.
Methods
We did an observational cohort study using data from two independent, international datasets of patients with untreated genotypically confirmed CLN2 disease: the DEM-CHILD dataset (n=74) and the Weill Cornell Medical College (WCMC) dataset (n=66). Both datasets included quantitative rating assessments with disease-specific clinical domain scores, and disease course was measured longitudinally in 67 patients in the DEM-CHILD cohort. We analysed these data to determine age of disease onset and diagnosis, as well as disease progression—measured by the rate of decline in motor and language summary scores (on a scale of 0–6 points)—and time from first symptom to death.
Findings
In the combined DEM-CHILD and WCMC dataset, median age was 35·0 months (IQR 24·0–38·5) at first clinical symptom, 37·0 months (IQR 35·0 –42·0) at first seizure, and 54·0 months (IQR 47·5–60·0) at diagnosis. Of 74 patients in the DEM-CHILD dataset, the most common first symptoms of disease were seizures (52 [70%]), language difficulty (42 [57%]), motor difficulty (30 [41%]), behavioural abnormality (12 [16%]), and dementia (seven [9%]). Among the 41 patients in the DEM-CHILD dataset for whom longitudinal assessments spanning the entire disease course were available, a rapid annual decline of 1·81 score units (95% CI 1·50–2·12) was seen in motor–language summary scores from normal (score of 6) to no function (score of 0), which occurred over approximately 30 months. Among 53 patients in the DEM-CHILD cohort with available data, the median time between onset of first disease symptom and death was 7·8 years (SE 0·9) years.
Interpretation
In view of its natural history, late-infantile CLN2 disease should be considered in young children with delayed language acquisition and new onset of seizures. CLN2 disease has a largely predictable time course with regard to the loss of language and motor function, and these data might serve as historical controls for the assessment of current and future therapies.
Funding
EU Seventh Framework Program, German Ministry of Education and Research, EU Horizon2020 Program, National Institutes of Health, Nathan’s Battle Foundation, Cures Within Reach Foundation, Noah’s Hope Foundation, Hope4Bridget Foundation.
Introduction
Late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease1,2 is a form of neuronal ceroid lipofuscinosis. The autosomal recessive storage disorder results in a severe, progressive neurodegenerative syndrome with onset most commonly occurring in children aged 2–4 years.3 The disease leads to seizures, loss of language, psychomotor function, blindness, and premature death.4,5 CLN2 disease occurs globally, but data on incidence and prevalence are scarce.6–8
CLN2 disease is caused by mutations in the CLN2 gene, which encodes the lysosomal serine protease tripeptidyl peptidase 1.9 Loss of tripeptidyl peptidase 1 activity leads to accumulation of autofluorescent storage material (ie, ceroid lipofuscin).5,10 Although substantial allelic heterogeneity exists in the CLN2 gene, two mutations (c.5091G→A [splicing error] and c.622C→T [nonsense mutation]) have been identified in more than half of patients with CLN2 disease in most white populations.5,11,12 Many rarer CLN2 mutations have been reported,5,12 but the effect of these mutations on phenotype and residual tripeptidyl peptidase 1 activity is not well understood; some mutations are thought to result in delayed disease onset or prolonged disease course.9,12–15
Until May, 2017, when intraventricular enzyme replacement therapy with cerliponase alfa was approved, only palliative treatment was available for patients with CLN2 disease.16 The development of therapeutics requires an understanding of the clinical course and its variability across a wide range of patients. To date, few reports have provided a quantitative description of the clinical course of patients with CLN2 disease. Therefore, the aim of this study was to update and refine the results of previous studies on the natural history of CLN2 disease17,18 by analysing onset and characterising initial symptoms and to assess disease progression using longitudinal data on motor and language function in an international cohort of patients with CLN2 disease.
Methods
Study design and participants
In this longitudinal observational cohort study, we used data from two independent datasets of patients with untreated CLN2 disease: the DEM-CHILD and Weill Cornell Medical College (WCMC) datasets. The DEM-CHILD dataset included 74 patients, and data were collected between April 22, 2002, and July 5, 2016, at University Medical Center Hamburg-Eppendorf (Hamburg, Germany; n=63) and the University of Verona (Verona, Italy; n=11) as part of the international DEM-CHILD NCL patient database project. The WCMC dataset included data for 66 patients collected at Weill Cornell Medical College (New York, NY, USA) between March 18, 2004, and Nov 20, 2015. Patients were included in the WCMC dataset if they had genetically confirmed CLN2 disease. Patients were included in the DEM-CHILD dataset if they had genetically confirmed CLN2 disease and deficient enzyme activity. The study was approved by the local ethics committees and written informed consent was obtained from the parents of all patients before inclusion. Source data were verified by independent monitors during data collection.
Procedures
The DEM-CHILD and WCMC datasets contained quantitative rating assessments with disease-specific clinical domain scores: the Hamburg CLN2 scale17 was used for the DEM-CHILD dataset and the Weill Cornell CLN2 scale18 for the WCMC dataset (table 1). Both scales have a total score ranging from 0 to 12 scoring units of disease-based clinical assessments that measure loss of function in children during the disease course. Each scale consists of four domains with comparable motor and language domains in each scale (ie, the motor domain in the Hamburg scale and gait domain in the Weill Cornell scale assess identical functions). Within each domain of both scales, a score of 3 indicates normal function, a score of 2 represents slight abnormality, a score of 1 indicates a severe abnormality, and a score of 0 represents complete loss of function (table 1). Patients were assessed in independently by three different raters in the DEM-CHILD dataset and by three to four different raters in the WCMC dataset. No difference in rating scores was identified between the raters in the DEM-CHILD dataset. Reported scores for patients in the WCMC dataset were the mean of scores from three to four independent raters scored using a predetermined set of rules, which are available by request from DS.
Table 1:
Hamburg and Weill Cornell scales for assessing functional ability in patients with neuronal ceroid lipofuscinosis type 2 disease
| Hamburg scale |
Weill Cornell scale |
|||
|---|---|---|---|---|
| Motor | Language | Gait | Language | |
| Score 3 | Walks normally | Normal | Normal | Normal |
| Score 2 | Frequent falls, obvious clumsiness | Recognisably abnormal | Abnormal but independent | Abnormal |
| Score 1 | No unaided walking or crawling only | Hardly understandable | Abnormal, requires assistance | Barely understandable |
| Score 0 | Immobile, mostly bedridden | Unintelligible or no language | Non-ambulatory | Unintelligible or no speech |
Data on genetic diagnosis and first symptoms were available for all 74 patients in the DEM-CHILD dataset. Of these 74 patients, the disease course (assessed by rate of decline in motor and language clinical domain summary scores) was measured longitudinally in 67 patients. The entire course of the disease has been scored without interruption in 3 month intervals since onset of disease (DEM-CHILD core data) in 41 patients, whereas only isolated score measurements were available for 26 patients. 52 patients had an initial summary score of 6 for the motor and language components; 41 patients had at least one measurement in which the summary score was 6 and 0. In 24 of the remaining 26 patients, the disease had not yet progressed to the final stages of the disease by the end of the study. The lowest summary scores of these 24 patients were 1 point in five patients, 2 in five patients, 3 in nine patients, 4 in three patients, and 5 in two patients. On the basis of the timing of these assessments, we subdivided the patients for whom data on disease progression were available in the two datasets (DEM-CHILD and WCMC) into three different cohorts (table 2).
Table 2:
Overview of included cohorts
| DEM-CHILD dataset (n=67) | WCMC dataset (n=66) | |
|---|---|---|
| Core data cohort, n | 41 | 0 |
| Comparison cohort 1, n | 21 | 12 |
| Comparison cohort 2, n | 5 | 54 |
Of the 74 patients in the DEM-CHILD dataset, 67 patients had clinical scoring data available and formed the DEM-CHILD core data and comparison cohorts.
The core data cohort included 41 patients from the DEM-CHILD database for whom longitudinal quantitative disease-specific rating assessments spanning the entire disease course (ie, from onset of first symptoms to complete loss of psychomotor function) were available. Because of the variable time between clinical onset and diagnosis, the clinical course before diagnosis was constructed retrospectively from family interviews and patient records and integrated with prospectively collected data from the time of diagnosis onwards. These data were collected at the neuronal ceroid lipofuscinosis specialty clinic of the University Medical Center Hamburg-Eppendorf only, where patients were assessed prospectively every 3–6 months. Patients were scored longitudinally from diagnosis until complete loss of function or death, with 28 scoring timepoints obtained per patient. Data cutoff was July5, 2016. The DEM-CHILD core data were inspected and approved by the US Food and Drug Administration and European Medicines Agency.
Comparison cohort 1 included patients who had at least two disease-specific rating assessments as a function of time with an interval of 5–24 months between assessments. However, in contrast to the DEM-CHILD core data, these assessments did not span the entire course of disease. 21 patients in the DEM-CHILD dataset and 12 patients in the WCMC dataset met the inclusion criteria for comparison cohort 1 (table 2).
Comparison cohort 2 included patients who had cross-sectional data with only one disease-specific rating assessment or two assessments for which the interval between the assessments was too short (≤140 days) or too long (≥710 days). Five patients in the DEM-CHILD dataset and 54 patients in the WCMC dataset met the criteria for comparison cohort 2 (table 2). Detailed information regarding selection criteria for all cohorts is provided in the appendix.
Data on language acquisition were collected by structured interviews of parents and review of clinical patient charts in 36 patients from the DEM-CHILD cohorts (appendix).
The key outcomes were CLN2 disease progression, assessed by rate of decline in motor and language clinical domain summary scores (0–6 total points), onset and type of first symptoms, and time from first symptom to death.
Statistical analysis
The complete disease course was assessed using a mixed ordinal regression model, and expected means and corresponding bootstrap intervals were calculated. To compare the other cohorts with the DEM-CHILD core data cohort, bootstrap percentiles were calculated using the same model. Subsequently, each single motor–language score measurement collected in the two comparison cohorts was superimposed on the respective percentile graphs of the DEM-CHILD core data to analyse comparability, and the percentage of datapoints that fell between the 10thand 90th percentiles of the DEM-CHILD core data were calculated.
The rate of decline was estimated for patients with a complete disease course (ie, patients whose scores decreased from 6 to 0) by fitting a mixed linear regression model with random intercept and random slope per patient to all data from onset (last measurement of 6) to total loss of function (first measurement of 0). To account for the plateaus at the beginning and the end of the disease course, and to determine the stage at which most rapid decline in motor and language function occurred, rate of decline was additionally computed for the decline from score 5 to 1.
Median time from first seizure or other first symptom to death was analysed using the Kaplan-Meier method. Age of the last disease severity scale measurement was used to censor patients for whom date of death was unknown. Continuous variables are presented as mean (SD) or median (IQR), and categorical variables are presented as absolute or relative frequencies.
All statistical analyses were done using Stata (version 14.0).
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to the raw data. The corresponding author had full access to all the data and had final responsibility to submit for publication.
Results
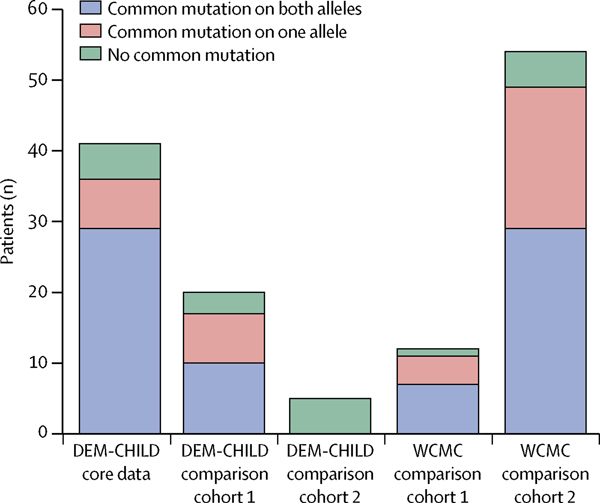
The DEM-CHILD dataset included 74 patients, of whom 35 (47%) were boys and 39 (53%) were girls. 18 nationalities were included, and most patients were from Germany (29 [39%]), Italy (11 [15%]), and Poland (eight [11%]; appendix). c.622C→T and c.509–1G→C were the two most common CLN2 mutations identified. Of the 74 patients in the DEM-CHILD dataset, 42 (57%) were either homozygous for one of these alleles (30 [41%]) or compound hetero zygous for both alleles (12 [16%]). 18 other mutations were identified in the remaining 32 (43%) of 74 DEM-CHILD patients. Only 14 (19%) of 74 patients had a genotype with no common mutations in the disease alleles (figure 1; appendix).
Figure 1: Genotype distribution in different patient cohorts.
Common mutations are c.622C→T and c.509–1G→C.
The WCMC cohort included 66 patients, of whom 28 (42%) were boys and 38 (58%) were girls. 12 nationalities were included, and most patients were from the USA (32 [48%]), Canada (seven [11%]), and England (seven [11%]; appendix). Mutational background was similar to the DEM-CHILD cohort: 36 (55%) of all patients were either homozygous (22 [33%]) or compound heterozygous (14 [21%]) for the two most common alleles. 21 other mutations were identified in the remaining 30 (45%) of 66 WCMC patients. Only six (9%) of 66 patients had a genotype with no common mutations in the disease alleles (figure 1; appendix). The sex of patients was equally balanced in both datasets.
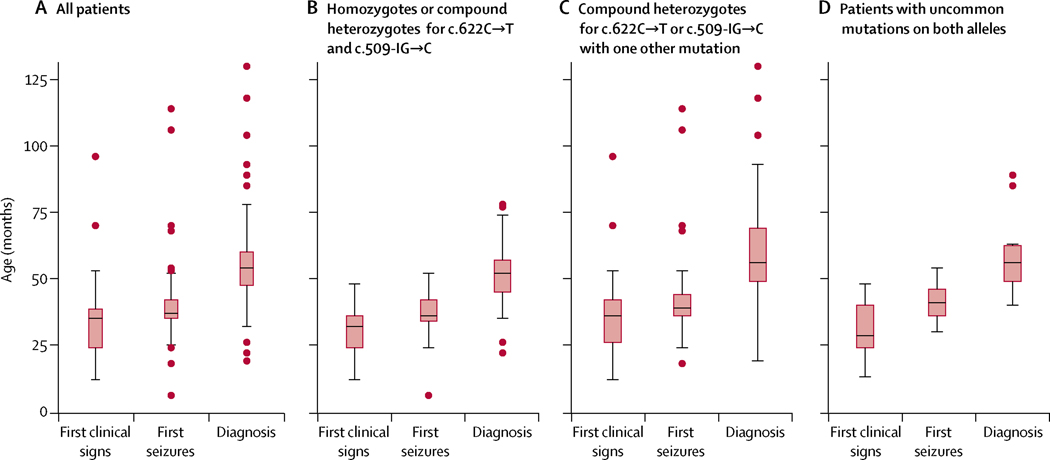
Of 74 patients in the DEM-CHILD dataset, the most common first symptoms of disease were seizures (52 [70%]), language difficulty (42 [57%]), motor difficulty (30 [41%]), behavioural abnormality (12 [16%]), and dementia (seven [9%]). For the combined DEM-CHILD and WCMC datasets (n=140), the median age at first clinical symptom was 35·0 months (IQR 24·0–38·5; figure 2A). Analysis of age at symptom onset stratified by genotype showed that disease onset is largely similar across cohorts and genotypes: the median age at first symptom was 32·0 months (IQR 24·0–36·0) in patients with the two most common mutations on both alleles, 36·0 months (IQR 26·0–42·0) in patients with the two most common mutations on one allele only, and 28·5 months (IQR 24·0–40·0) in patients without the two common mutations (figure 2B–D).
Figure 2: Age at first clinical sign, first seizure, and diagnosis.
Data are combined from both DEM-CHILD and WCMC cohorts (n=140). Boxes show median values (middle lines) with 25th and 75th percentiles; whiskers show values within two-thirds of the IQR. Circles represent data points that fall outside this range. CLN2= Late-infantile neuronal ceroid lipofuscinosis type 2.
The median age at first seizure in the combined DEM-CHILD and WCMC dataset was 37·0 months (IQR 35·0–42·0; figure 2A). Patients with uncommon alleles generally had later onset of first seizures: the median age at first seizure was 36·0 months (IQR 34·0–42·0) in patients who were either homozygous or compound heterozygous for the two common alleles, 39·0 months (IQR 36·0–44·0) for individuals who were compound heterozygous and had one uncommon allele, and 41·0 months (IQR 36·0–46·0) for individuals with uncommon mutations on both alleles (figure 2B–D).
The median age at diagnosis in the combined DEM-CHILD and WCMC dataset was 54·0 months (IQR 47·5–60·0; figure 2A), and patients were diagnosed after a mean of 22·7 months (SD 9·8)from the onset of first symptoms. Patients homozygous or compound heterozygous for the two common alleles were diagnosed at a median age of 52·0 months (IQR 45·0–57·0; figure 2B). Median age at diagnosis was 56·0 months (IQR 49·0–69·0) for patients who were compound heterozygous and had one uncommon allele (figure 2C), and 56 months (IQR 49·0–62·5) for patients with two uncommon alleles (figure 2D).
Data derived from detailed parent interviews in the DEM-CHILD cohort showed that 30 (83%) of 36 patients had a delay in early language development, of whom six (17%) acquired their first single word after age 18 months, seven (19%) spoke two word sentences after age 24 months, and 19 (53%) spoke whole sentences after age 36 months (data not shown).
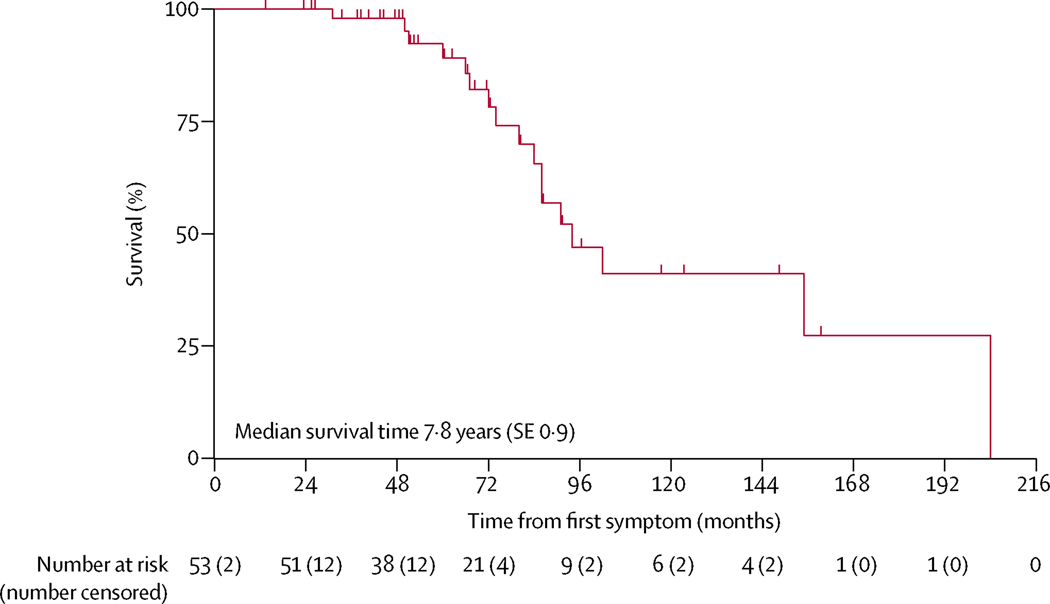
Of the 74 patients in the entire DEM-CHILD cohort, both vital status (ie, whether the patient was alive or dead)and age at first symptom were known for 53 (72%) patients. Date of death was known for 20 (27%) of 74 patients and median age at death was 10·0 years (SE 3·2). The median time between onset of first disease symptom and death was 7·8 years (SE 0·9;figure 3).
Figure 3: Kaplan-Meier analysis of survival from onset of first symptom in the DEM-CHILD cohort.
Data for age at first symptom onset was available for 53 patients in the DEM-CHILD cohort.
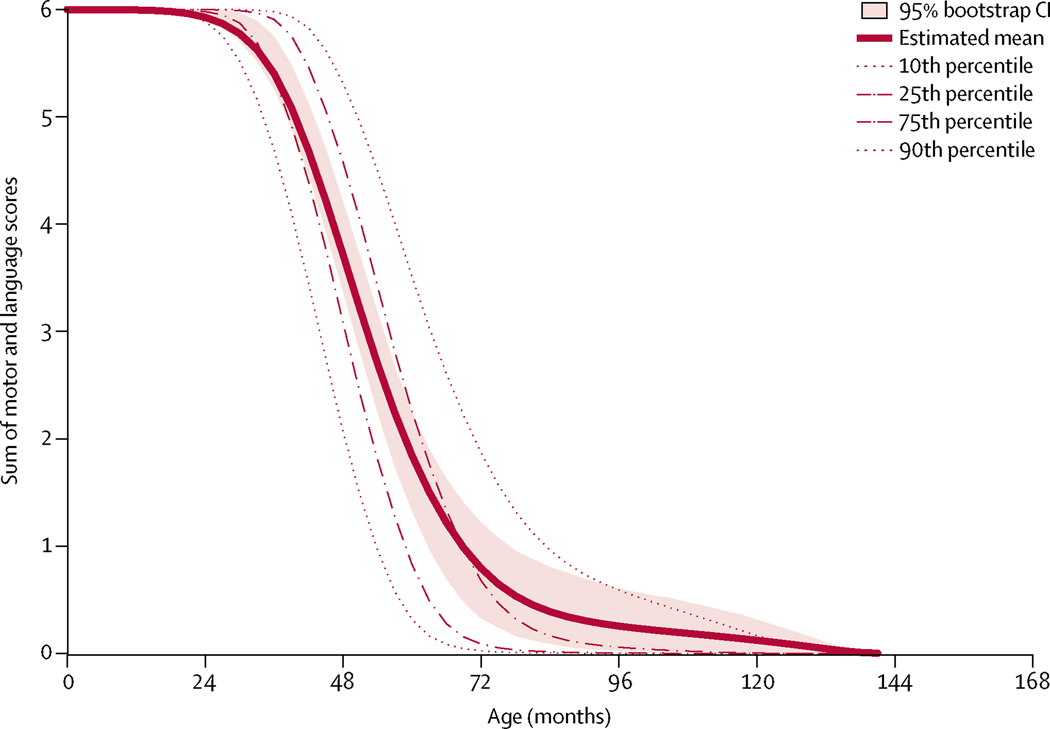
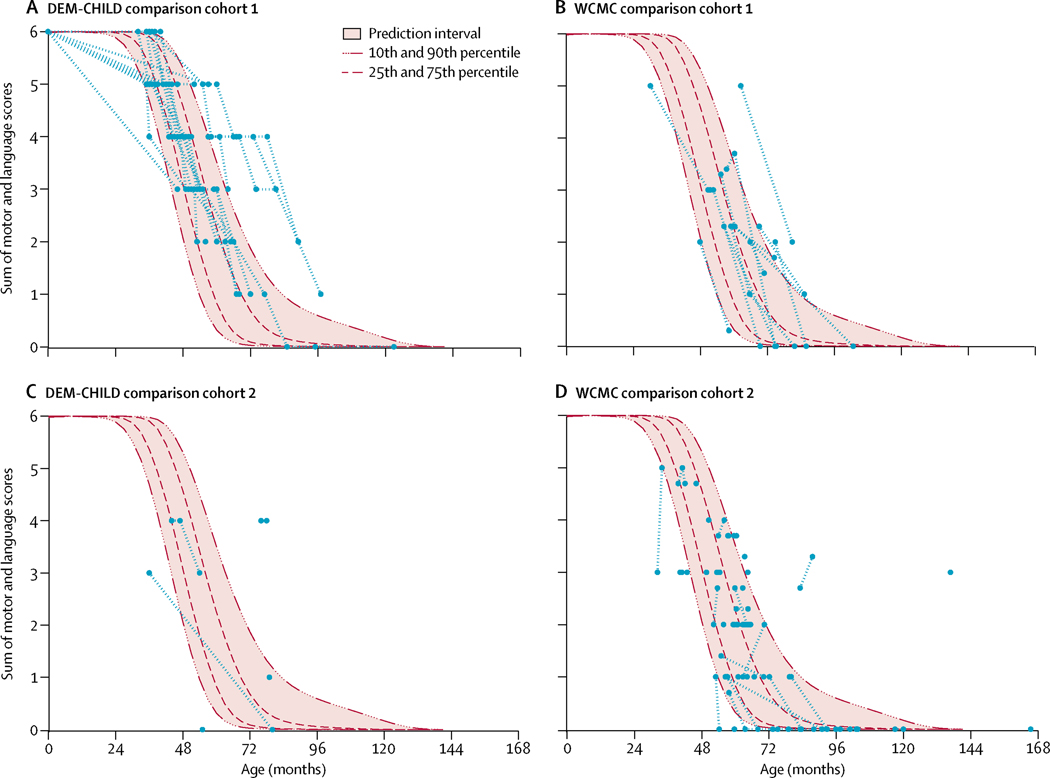
Longitudinal data for the DEM-CHILD core data cohort showed a rapid decline in motor–language summary scores from normal (score of 6) to no function (score of 0), which occurred over approximately 30 months (figure 4). Similarly, data derived from the DEM-CHILD and WCMC comparison cohorts showed a rapidly progressive decline from normal to no motor and language function over approximately 30 months. When superimposed on the DEM-CHILD core data, 81% of DEM-CHILD comparison cohort 1 datapoints, 56%of DEM-CHILD comparison cohort 2 datapoints, 71% of WCMC comparison cohort 1 datapoints, and 80% of WCMC comparison cohort 2 datapoints fell between the 10th and 90th percentiles of the DEM-CHILD data (figure 5A–D). Patients outside the 10th and 90th bootstrap percentiles had uncommon genotypes. For example, a Japanese patient in the DEM-CHILD comparison cohort 2 was compound heterozygous for two uncommon mutations, c.1015C→T and c.731T→C, with a motor–language score of 4 at age 78 months. Additionally, a Spanish patient in the WCMC comparison cohort 2 who had a mild phenotype and was compound heterozygous for the common c.622C→T and the uncommon c.887–10A→G mutation had a motor–language score of 3 at age 136 months.
Figure 4: Longitudinal motor–language scores for the DEM-CHILD core cohort.
Data are cross-sectional summaries of disease severity for all patients in the DEM-CHILD core data cohort for whom longitudinal scoring data were available (n=41).
Figure 5: Motor–language scores of comparison cohorts superimposed on DEM-CHILD core data.
Motor-language score data for DEM-CHILD comparison cohort 1 (A), WCMC comparison cohort 1 (B), DEM-CHILD comparison cohort 2 (C), and WCMC comparison cohort 2 (D). Each assessment is shown as single blue point. Longitudinal assessments for each patient are linked by dashed red lines. Mean scores and 95% CIs, and 10th, 25th, 75th, and 90th percentiles for the DEM-CHILD core data are shown for comparison.
The estimated mean annual rate of decline in combined motor and language score was 1·81 score units per year (95% CI 1·50–2·12) in the DEM-CHILD core data cohort. Once a significant loss in motor and language function had occurred (ie, loss of one scoring point in either motor or language domain), the estimated annual rate of decline increased to 2·43 score units per year (95% CI 2·07–2·79) between the motor–language domain summary scores of 5 to 1.
Discussion
To our knowledge, this is the largest international study of quantitative natural history data for CLN2 disease. We characterised important disease features and the earliest disease signs and symptoms with the aim of aiding early diagnosis and establishing the associations between disease progression, age, and genotype using a motor–language clinical rating scale that can be easily administered in the clinic. The resulting international cooperative analyses provide a homo geneous quantitative description of the clinical course of CLN2 disease.
The two independent cohorts of patients with CLN2 disease had a highly homogeneous course of disease progression. Genotype distribution was consistent with previously reported data5,11,12,17–19 for individuals with CLN2 disease. Overall, 56%of patients were homozygous or compound heterozygous for the two most common mutations (c.622C→T and c.509–1G→C).11
Combined data from the two datasets showed that first clinical signs were observed at a median age of 35 months, and seizures or delays in language development were the most common first symptoms reported. Since the seizure phenotype in CLN2 disease is highly variable—with multiple seizure types observed over the course of the disease, including myoclonic, tonic, atonic, absence, and tonic-clonic—it is difficult to assess this using a reliable scoring system. Therefore, we assessed the age at first seizure only. Myoclonic seizures can predominate as the disease progresses.20 Previous reports9,12–14 indicate that uncommon alleles might be associated with late onset of first clinical signs. However, considering the results for our large international cohort of patients, we conclude that disease onset is largely predictable across cohorts and genotypes in these patients.
Consistent with other reports,18,19 latency to diagnosis is considerable. CLN2 disease was diagnosed after a mean duration of 22·7 months from the onset of first symptoms, and after substantial decline in function. Early diagnosis of CLN2 disease remains a major challenge despite the development of clinical diagnostic algorithms, and the availability of CLN2 genetic testing and tripeptidyl peptidase 1 enzyme assays.16 The delay between symptom onset and diagnosis represents a considerable burden in patients with CLN2 and their families because it delays genetic counselling, increases the risk of having younger siblings who are affected, and delays the initiation of clinical and psychosocial management strategies. Until May 2017, when intraventricular enzyme replacement therapy with cerliponase alfa was approved, only palliative treatment was available for patients with CLN2.21 Moreover, with the approval of intraventricular enzyme replacement therapy as first-line treatment by the US Food and Drug Administration and European Medicines Agency in May, 2017,22,23 delayed diagnosis means that disease progression can be substantial before treatment is initiated. The diagnostic delay results in a mean functional decline of more than 3 score units, which represents an almost complete loss of previously attained motor–language function. Early diagnosis is therefore beneficial for all patients with CLN2 disease since an approved therapy is now available and additional experimental therapies are being developed.20,22,23
Abnormal language development in patients with CLN2 has occasionally been reported.17,19 We reviewed the early development of language with the aim of shortening time to diagnosis by aiding recognition of first clinical signs of disease before onset of regression. Our data showed that abnormal or delayed development of language abilities in toddlers might precede classic disease symptoms, such as seizures and ataxia. Although there are no clear definitions of delayed language development in the literature and the prevalence in the general population is 13–20%,24–26 a high proportion of patients with CLN2 disease had delayed language acquisition in our study, which suggests that an unexplained onset of seizures in children who have delayed language acquisition should lead to the clinical suspicion of CLN2 disease. The availability of a simple enzymatic dry blood spot test could help to diagnose this severe genetic disease27 before the onset of loss of function. Early diagnosis is of considerable importance for potential therapy, family counselling, and patient management.
For the quantitative delineation of the clinical course of CLN2 disease in this study, we used two domains of disease-specific clinical rating scales, the Hamburg17 and the WCMC18 motor and language subscales. These subscales are almost identical.17,18 We selected the motor and language subscales because motor and language function are clinical hallmarks of CLN2 disease and the domains define a large dynamic range of disease progression (from normal function to complete loss of function).
The course of disease of all DEM-CHILD patients for whom uninterrupted longitudinal motor and language scoring data were available was strongly predictable with a narrow confidence interval. Results of this study are consistent with previous reports17,18 that patients with CLN2 disease have a rapid decline in clinical function (during a 2–3 year time period), from onset of first symptoms to later stages with almost complete functional loss. Specifically, motor–language scores decreased rapidly from 3 years of age, progressively declining from normal function to no remaining function after approximately 2·5 years.
The mean estimated rate of clinical decline was 1·81 score units per year in the DEM-CHILD core data cohort. Once a significant loss in function had occurred, the rate of clinical decline was more rapid from a score of 5 to 1 (2·43 score units each year), in which the rate of decline is almost linear. This finding highlights the importance of early diagnosis and treatment because the disease progresses most rapidly immediately after the onset of first symptoms.
Development of the two-domain motor–language clinical rating scale also enabled comparison of disease courses between the independently collected and rated WCMC and DEM-CHILD cohorts. Superimposing the clinical scores for motor–language domains of the comparison cohorts on the longitudinal DEM-CHILD core data analysis showed a similar association between age and disease severity: more than 70% of datapoints for the three comparison cohorts (DEM-CHILD comparison cohort 1, WCMC comparison cohort 1, and WCMC comparison cohort 2) fell within the 10th and 90th percentiles of the longitudinal DEM-CHILD core data. 56% of DEM-CHILD comparison cohort 2 cross-sectional datapoints overlapped with the longitudinal DEM-CHILD core data, which might be due to the different genotype distribution among these patients (all five patients were homozygous or compound heterozygous for uncommon alleles only). This finding is consistent with other publications,20 which found a correlation between late disease onset and a milder phenotype. In our study, a Spanish patient in the WCMC comparison cohort 2 had a motor–language score of 3 at age 136 months. This patient also had a late disease onset (96 months) and was compound heterozygous for the common c.622C→T and the uncommon c.887–10A→G mutations.
Our study had limitations, as we were unable to study the correlation between residual tripeptidyl peptidase 1 activity and disease progression, especially in some patients who were atypical with regard to age of onset and disease progression. However, since this large patient cohort was derived from multiple centres and diagnosis was done at various metabolic laboratories, it is difficult to compare results from enzyme analyses. Different laboratories use different patient material for testing (eg, fibroblasts, leucocytes, dry blood spots) and different diagnostic methods (eg, fluorometric analysis, mass spectrometry) with variable sensitivity to assess residual enzyme activity. Therefore, enzyme activity results were not used for correlation analyses with clinical progression.
Taken together, these findings suggest that the rate of disease progression was similar for the DEM-CHILD and WCMC datasets and that, despite patients residing in different countries and being independently rated, the results are highly reproducible across the two cohorts (ie, the results are not cohort-dependent and rater bias is not apparent). Data presented in this study have been used as natural history control data for completed and ongoing experimental therapy trials of intraventricular enzyme replacement therapy (NCT01907087, NCT02485899, NCT02678689) and intraparenchymal adeno-associated gene therapies (NCT00151216, NCT01161576, NCT01414985)21 in CLN2 disease.
In conclusion, this large quantitative longitudinal analysis of patients with late-infantile CLN2 disease confirms that the course of disease is highly predictable. Data from this study can be used as valid controls for the investigation of experimental therapies for CLN2 disease.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for studies published in English from database inception to March 1, 2018, using the search terms “neuronal ceroid lipofuscinosis”, “CLN2 disease”, “late infantile NCL”, “natural history”, “Batten disease”, “first symptoms”, and “rate of disease progression”. We also searched the bibliographies of retrieved articles. Before this study, knowledge about the natural history of neuronal ceroid lipofuscinosis type 2 (CLN2) disease was limited. Previous studies used only cross-sectional or retrospectively collected data, had small patient cohorts (<30 patients), and were from single centres, thus limiting generalisability. No longitudinal studies have been done; therefore, prospective natural history data from an international cohort are urgently needed to assess the efficacy of new therapies.
Added value of this study
To our knowledge, this is the first longitudinal, multicentre study of patients with CLN2 disease to provide detailed data about the natural history of the disease. Our data have already been and will be used as natural history control data for completed and ongoing trials of intraventricular enzyme replacement therapy (NCT01907087, NCT02485899, NCT02678689) and intraparenchymal adeno-associated gene therapies (NCT00151216, NCT01161576, NCT01414985). These data will also be important for future clinical trials investigating treatment options for CLN2 disease, such as gene therapy and small-molecule therapies.
Implications of all the available evidence
Independently collected natural history data can be used as controls in clinical trials for rare disease in which accrual of placebo controls might be difficult. Additionally, knowledge about clinical presentation and first symptoms will support early diagnosis and treatment.
Acknowledgments
We thank the patients and their families for contributing important data supporting this study. The DEM-CHILD project was funded by the EU Seventh Framework Program (281234), the German Federal Ministry of Education and Research (NCL2Treat 01GM1516C), and the EU’s Horizon 2020 research and innovation programme (66691). Many data from patients with CLN2 were obtained through generous support from Freunde der Kinderklinik at the Clinic for Degenerative Brain Diseases in Children (Hamburg, Germany). These studies were also partly funded by the National Institutes of Health (NIH; 1R01NS061848 and U54NS065768) and supported by fundraising by Nathan’s Battle Foundation, Cures Within Reach, Noah’s Hope, and Hope4Bridget. None of the authors are employed by the NIH.
MN reports grants from the European Commission and the German Federal Ministry of Education and Research and personal fees from BioMarin during the conduct of the study, and personal fees from BioMarin outside of the submitted work. ASi reports consulting fees from BioMarin. DJ, PS, and TA are employees of BioMarin. DS reports licensing fees from BioMarin Pharmaceuticals. MD reports grants and consulting fees from BioMarin during the conduct of the study, and grants from BioMarin outside of the study. RGC reports licensing fees from, and is a consultant for, BioMarin Pharmaceuticals. AK reports consulting fees and travel expenses from BioMarin. ASc reports grants from the European Commission and the German Federal Ministry of Education and Research and personal fees from BioMarin during the conduct of the study; and personal fees from BioMarin outside of the study.
Footnotes
Declaration of interests
All other authors report no competing interests.
References
- 1.Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat 2012; 33: 42–63. [DOI] [PubMed] [Google Scholar]
- 2.Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology 2012; 79: 183–91. [DOI] [PubMed] [Google Scholar]
- 3.Chang M, Cooper JD, Davidson BL, et al. CLN2 In: Mole SE, Williams RE, Goebel HH, eds. The neuronal ceroid lipofuscinoses (Batten Disease). Oxford: Oxford University Press, 2011: 80–109. [Google Scholar]
- 4.Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain Pathol 2004; 14: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics 2005; 6: 107–26. [DOI] [PubMed] [Google Scholar]
- 6.Claussen M, Heim P, Knispel J, Goebel HH, Kohlschütter A. Incidence of neuronal ceroid-lipofuscinoses in West Germany: variation of a method for studying autosomal recessive disorders. Am J Med Genet 1992; 42: 536–38. [DOI] [PubMed] [Google Scholar]
- 7.Uvebrant P, Hagberg B. Neuronal ceroid lipofuscinoses in Scandinavia. Epidemiology and clinical pictures. Neuropediatrics 1997; 28: 6–8. [DOI] [PubMed] [Google Scholar]
- 8.Moore SJ, Buckley DJ, MacMillan A, et al. The clinical and genetic epidemiology of neuronal ceroid lipofuscinosis in Newfoundland. Clin Genet 2008; 74: 213–22. [DOI] [PubMed] [Google Scholar]
- 9.Sleat DE, Donnelly RJ, Lackland H, et al. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science 1997; 277: 1802–05. [DOI] [PubMed] [Google Scholar]
- 10.Haltia M. The neuronal ceroid-lipofuscinoses. J Neuropathol Exp Neurol 2003; 62: 1–13. [DOI] [PubMed] [Google Scholar]
- 11.Zhong N, Wisniewski KE, Hartikainen J, et al. Two common mutations in the CLN2 gene underlie late infantile neuronal ceroid lipoluscinosis. Clin Genet 1998; 54: 234–38. [DOI] [PubMed] [Google Scholar]
- 12.Sleat DE, Gin RM, Sohar I, et al. Mutational analysis of the defective protease in classic late-infantile neuronal ceroid lipofuscinosis, a neurodegenerative lysosomal storage disorder. Am J Hum Genet 1999; 64: 1511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elleder M, Dvoráková L, Stolnaja L, et al. Atypical CLN2 with later onset and prolonged course: a neuropathologic study showing different sensitivity of neuronal subpopulations to TPP1 deficiency, Acta Neuropathol 2008; 116: 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Giacopo R, Cianetti L, Caputo V, et al. Protracted late infantile ceroid lipofuscinosis due to TPP1 mutations: clinical, molecular and biochemical characterization in three sibs. J Neurol Sci 2015; 356: 65–71. [DOI] [PubMed] [Google Scholar]
- 15.Mole SE, Williams RE. Neuronal ceroid-lipofuscinoses In: Pagon RA, Adam MP, Ardinger HH, et al. , eds. GeneReviews [Internet]. Seattle, WA: University of Washington, 2001. http://www.ncbi.nlm.nih.gov/books/NBK1428 (accessed July 10, 2015). [Google Scholar]
- 16.Schulz A, Kohlschütter A, Mink J, et al. NCL diseases–clinical perspectives. Biochim Biophys Acta 2013; 1832: 1801–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinfeld R, Heim P, von Gregory GH, et al. Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations. Am J Med Genet 2002; 112: 347–54. [DOI] [PubMed] [Google Scholar]
- 18.Worgall S, Kekatp ure MV, Heier L, et al. Neurological deterioration in late infantile neuronal ceroid lipofuscinosis. Neurology 2007; 69: 521–35. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Poyato MS, Marfa MP, Abizanda IF, et al. Late infantile neuronal ceroid lipofuscinosis: mutations in the CLN2 gene and clinical course in Spanish patients. J Child Neurol 2013; 28: 470–78. [DOI] [PubMed] [Google Scholar]
- 20.Kohan R, Carabelos MN, Xin W, et al. Neuronal ceroid lipofuscinosis type CLN2: a new rationale for the construction of phenotypic subgroups based on a survey of 25 cases in South America. Gene 2013; 516: 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams RE, Adams HR, Blohm M, et al. Management strategies for CLN2 disease. Pediatr Neurol 2017; 69: 102–12. [DOI] [PubMed] [Google Scholar]
- 22.European Medicines Agency. New medicine for rare neurodegenerative disorder in children. 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm555613.htm (accessed June 21, 2018).
- 23.Schulz A, Ajayi T, Specchio N, et al. Study of intraventricular cerliponase alfa for treatment of CLN2 disease. New Engl J Med 2018; 378: 1898–907. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz SM, Irwin JR, Briggs-Gowan MJ, et al. Language delay in a community cohort of young children. J Am Acad Child Adolesc Psychiatry 2003; 42: 932–40. [DOI] [PubMed] [Google Scholar]
- 25.Desmarais C, Sylvestre A, Meyer F, et al. Systematic review of the literature on characteristics of late-talking toddlers. Int J Lang Commun Disord 2008; 43: 473–75. [DOI] [PubMed] [Google Scholar]
- 26.Reilly S, Wake M, Bavin E, Eadie P, Bretherton L, Prior MR. Letter regarding ‘A systematic review of the literature on characteristics of late-talking toddlers’ by Desmarais et al et al. Int J Lang Commun Disord 2008; 43: 473–75. [DOI] [PubMed] [Google Scholar]
- 27.Lukacs Z, Santavuori P, Keil A, et al. Rapid and simple assay for the determination of tripeptidyl peptidase and palmitoyl protein thioesterase activities in dried blood spots. Clin Chem 2003; 49: 509–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.