Summary:
Aggressive treatment of status epilepticus with anesthetic drugs can provide rapid seizure control, but it might lead to serious medical complications and worse outcomes. Using a decision analysis approach, this concise review provides a framework for individualized decision making about aggressive and nonaggressive treatment in status epilepticus. The authors propose and review the most relevant parameters guiding the risk–benefit analysis of treatment aggressiveness in status epilepticus and present real-world–based case examples to illustrate how these tools could be used at the bedside and serve to guide future research in refractory status epilepticus treatment.
Keywords: EEG, Status epilepticus, Critical care, Risk–benefit
Clinicians caring for patients with status epilepticus (SE) often face a difficult decision of whether or not to pursue aggressive treatment with anesthetic drugs.1–3 While most agree that prolonged seizures lead to worse outcomes and should be stopped, the course of action to achieve seizure control after first- and second-line anti-seizure drugs have failed is controversial.1,4 Complications from use of anesthetic medications (drugs such as midazolam, pentobarbital, and propofol, when used in doses that would induce coma) are common and may worsen outcomes for patients who are already critically ill. Making such high-stake decisions at the bedside is challenging given the complexity of individual clinical scenarios, the time pressure for treatment decisions, and uncertainty about aggressive and nonaggressive treatment efficacy.
Here we propose a structured framework to support individualized decision making about SE treatment strategies. We delineate the potential consequences and tradeoffs between aggressive and nonaggressive treatment strategies by incorporating the key variables driving patient outcomes and review the use of decision analytic methods in this area.
WHAT WE KNOW
Decision making about SE treatment intensity in clinical practice is guided by several factors (Fig. 1A). Seizure burden, initial risk, treatment efficacy, harm because of delayed seizure control, and harm because of aggressive treatment are the cardinal features in the risk–benefit analysis of aggressive treatment for SE.
FIG. 1.
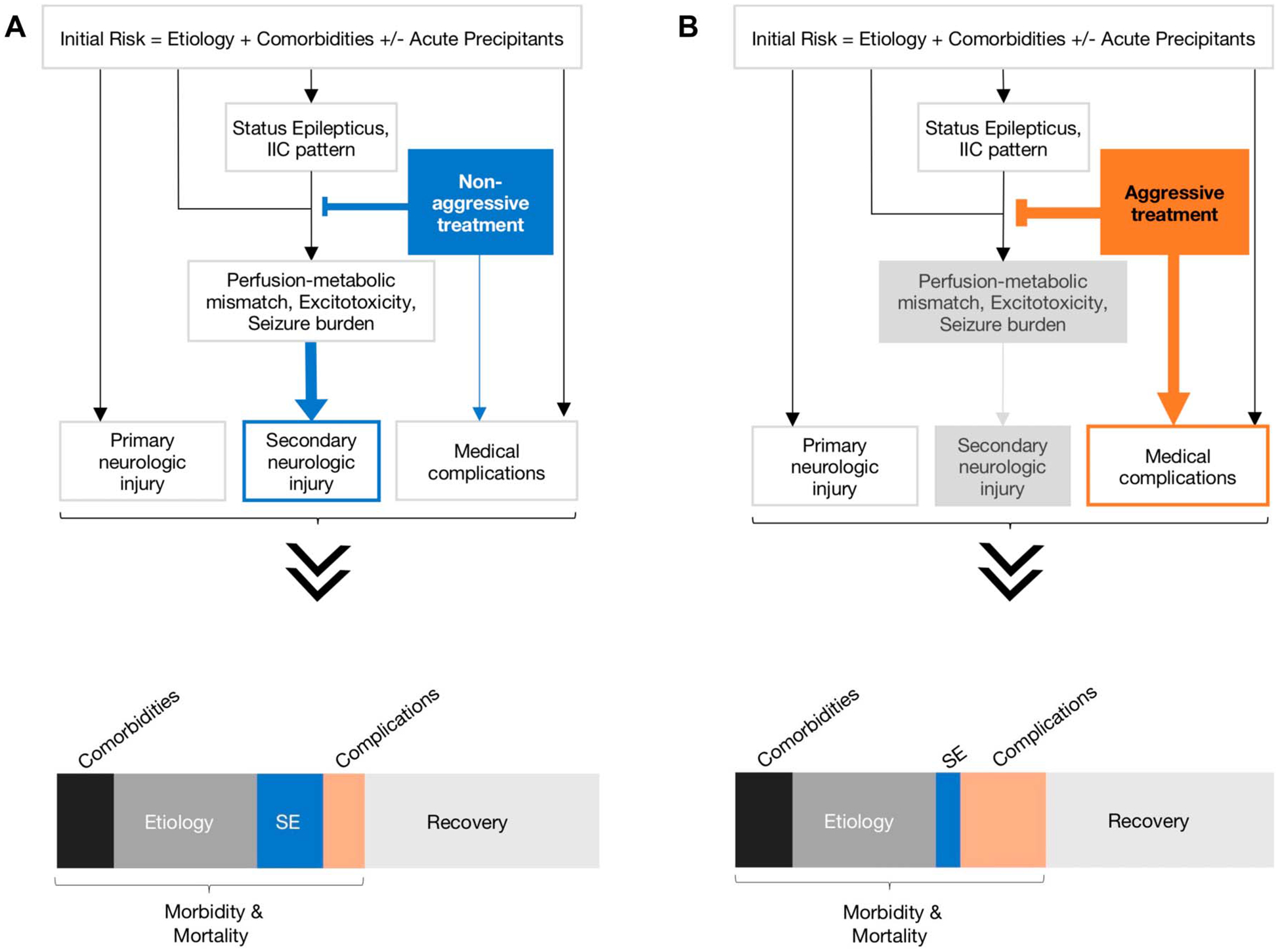
Simplified pathophysiological model for status epilepticus outcomes based on nonaggressive versus aggressive treatment strategies. This simple generic model demonstrates the relationships between an inciting event (seizure etiology and patient comorbidities) leading to status epilepticus (SE) or ictal–interictal continuum (IIC) patterns and functional outcomes and mortality. There may or may not be a new primary neurologic injury as a result of the inciting event or secondary injury from perfusion-metabolic mismatch or excitotoxicity from SE. The inciting event and its medical treatment (nonaggressive vs. aggressive) can lead to medical complications. These factors interact in a nonlinear way to produce an individual’s outcome and, across many patients, shape the distribution of functional outcomes (recovery vs. morbidity) and mortality rate. Here, we assume that the type of treatment strategy (nonaggressive vs. aggressive) does not impact the overall outcome distribution, but it does affect morbidity and mortality proportion from secondary injury and medical complications of treatment. A, Nonaggressive treatment: In this model, we assume delay in seizure control because of nonaggressive treatment, which would presumably lead to worse outcomes because of secondary injury from uncontrolled seizures, but lower rate of medical complications of treatment. B, Aggressive treatment: In this model, we assume faster seizure control with aggressive treatment, what would limit seizure burden and secondary brain injury. This approach has an increased likelihood for harm because of medical complications. The distribution of contributing factors for SE outcomes (recovery vs. morbidity and mortality) may change depending on which treatment strategy is used.
Seizure burden is a semi-quantitative measure that summarizes seizure duration and seizure spatial involvement in the brain.5,6 Higher seizure burden is associated with a decline in neurologic function and worse long-term functional outcomes.6,7 Seizures with longer duration are associated with increased cerebral metabolic demand that can lead to transient or permanent acute neuronal injury and to long-term alterations in neural network behavior and cellular metabolism that contribute to epileptogenesis.4 Resilience of cerebral regions to these metabolic, cellular, and network changes from prolonged seizures varies across cortical regions, and the extent of cerebral cortex involvement and its accompanying clinical phenomenology are important factors in defining the type and severity of SE, beyond seizure duration.
The role of neuroimaging studies in decision making regarding treatment intensity has increased over time, as imaging allows for evaluation of both structural and metabolic changes due to SE.8–10 These studies corroborate findings from animal work demonstrating the association between increased metabolic demand from prolonged seizures and worsened short- and long-term outcomes. The metabolic, cellular, and network effects of prolonged ictal or interictal discharges (as opposed to seizures) and the consequences of aggressive versus nonaggressive treatment for this category of epileptiform discharges are even less clear.10–12 With the increased availability of EEG monitoring in the intensive care unit, treatment of ictal–interictal patterns is a frequent challenge to clinicians who have limited guidance or evidence to support individualized treatment strategies.3,13,14
The concept of seizure burden is not sufficient to determine decisions about treatment intensity decisions in SE. The initial risk for poor outcomes of the underlying disorder that caused SE in the first place, along with patient comorbidities, must also be considered. Three cardinal examples of different seizure types complicating decision making in SE are generalized convulsive, focal, and absence SE. Seizure burden in focal SE is lower than in generalized convulsive SE. This is corroborated by animal studies demonstrating that neuronal injury is seen in focal SE later than in generalized convulsive SE (60 vs. 30 minutes). These findings support the common clinical practice of treating generalized convulsive seizures more aggressively than focal seizures. However, absence SE, a generalized type of seizure with involvement of several brain regions, seems to have even less risk, and perhaps no risk, of causing permanent neuronal injury.15,16 Therefore, if we rely on the concept of seizure burden alone (seizure duration × seizure spatial involvement), we might erroneously recommend more aggressive treatment of absence SE than necessary.
These examples emphasize that treatment decisions must take into consideration the initial risk from the underlying disorder together with seizure burden. There is strong evidence supporting aggressive treatment of convulsive SE, which has a high seizure burden and high initial risk for poor outcomes.17,18 Delay in convulsive SE treatment is associated with increased morbidity and mortality, and anesthetic use is recommended in most cases after first- and second-line anti-seizure medications have failed.19,20 Absence status and focal SE have a much lower initial risk for poor outcomes, and a more conservative approach is often suggested. Advanced age and presence of comorbidities are also important factors determining baseline risk (Fig. 1B).21–25 Unfortunately, less data about outcomes from different treatment strategies is available for nonconvulsive SE (NCSE). As with other forms of SE, the clinical examination in NCSE can vary considerably, from deep coma to lethargy, staring, mutism, or the “wandering well” state. These clinical presentations also affect calculations for initial risk assessment about aggressive treatment.26,27
Another important concept is treatment efficacy. Two scenarios with very different initial risks and treatment efficacy are seizures because of inappropriate discontinuation of anti-seizure medications in patients with well-controlled epilepsy and seizures because of acute structural brain injury. The likelihood of SE control with treatment is much higher for patients who have missed anti-seizure medications because their seizures are known to respond to a specific agent. This prior knowledge is unavailable for patients with de novo SE. In addition, the initial risk for these patients is also remarkably different, as patients with stroke or hypoxic–ischemic brain injury already have a high probability of poor outcomes and complications that are independent of developing SE and may also have lower treatment efficacy likelihood.
Seizure burden, initial risk, and treatment efficacy should be taken into consideration in determining whether aggressive treatment with anesthetics should be used or not. These parameters will also determine another important parameter in the risk–benefit assessment for aggressive treatment: harm because of delay in seizure control. The threshold to initiate an anesthetic agent can be much higher in clinical scenarios with lower seizure burden, low initial risk, or higher treatment efficacy likelihood, so practitioners might consider it reasonable to delay introduction of an anesthetic agent and wait for a second-, third-, or even fourth-line agent to take effect as the preferred course of action. The converse is true for convulsive SE, which demands rapid control. This concept was critical in changing the definition of SE from 30 minutes to a 5-minute duration. The 30-minute definition was based in animal studies showing that permanent neuronal injury was often observed after this threshold was reached.28 The change to a 5-minute rule was driven by operational considerations noting that convulsive SE is unlikely to resolve spontaneously after that time and thus should be treated as a medical emergency.19,29 The likelihood of seizure self-termination decreases as a function of time, and several studies have shown that the longer convulsive generalized seizures last, the higher the likelihood of pharmacoresistance and need for additional anti-seizure agents or anesthetics.30,31
The pathophysiological mechanisms that contribute to the relative pharmacoresistance of prolonged SE are unclear but may involve dysfunction of physiologic modes of inhibition at the cellular and network levels.32–34 The change to the 5-minute definition of SE promoted a sense of urgency in starting treatment, but it should not necessarily become an urgency to escalate treatment. Decisions about escalation of therapy should be individualized to determine what would be a reasonable waiting period for anti-seizure medications to take effect.
The concepts of seizure burden, initial risk, treatment efficacy, and harm from delay in seizure control are cardinal parameters in the risk–benefit equation guiding decision making about using aggressive treatment in SE. This risk–benefit analysis, however, must also incorporate the risk of harm because of aggressive treatment (Fig. 1B). Anesthetic use for seizure control is associated with systemic side effects that might contribute to poor outcomes.35–39 Although our understanding of these parameters in convulsive SE is advanced, allowing us to advocate in favor of aggressive treatment in virtually all scenarios, the complexity and heterogeneity of NCSE makes estimation of these parameters for NCSE more difficult and thus the management of NCSE less clear.2 Nonconvulsive SE is associated with high morbidity and mortality, and guidance to practitioners about personalizing treatment strategies is urgently needed.21–23
WHAT THE STUDIES TELL US
Introduction to Decision Analysis
Decision analytic (DA) methods provide a theoretical framework for integrating evidence-based information in a coherent way to inform the treatment of individual patients and groups of patients.40 Most medical practitioners engage in at least an implicit risk–benefit calculus when weighing medical decisions, but the complexity of the decision-making process sometimes necessitates the use of tools derived from DA.
Decision analysis assists and improves decision making under uncertainty by breaking down complex questions into manageable parts. Decision analysis also incorporates and helps identify sources of uncertainty and presents them in a quantitative way in the form of probabilities and relates decision pathways to expected outcomes in an explicit manner. Decision analysis is powerful because it considers the potential outcomes from different courses of action, allowing it to reflect both the costs of those actions and the valuations of the outcomes after these decisions. One of the major values of a DA model is that it makes the role of each piece of information in the decision-making process explicit. This facilitates the incorporation of evidence-based practices and also allows one to construct mathematically coherent arguments about the optimal decision among different alternatives available with respect to specified outcomes. This is particularly helpful in settings in which the information available to clinicians is limited.
In neurology, DA has been applied to clarify the predicted outcomes of different decisions across a multitude of clinically relevant questions, including SE, as well as others, including statins after intracerebral hemorrhage, aspirin for acute stroke of unknown etiology in resource limited settings, weighing the value of memory loss in the surgical evaluation of left temporal lobe epilepsy, initiation of anti-seizure drug therapy after single unprovoked seizure, justifying early epilepsy surgery for temporal lobe epilepsy, and selection of first-line therapy in multiple sclerosis.2,41–47 The breadth and diversity of questions asked using this framework highlights the versatility of DA methods.
Decision analysis is not without weaknesses. It has been criticized because of its narrow definition of clinical problems and oversimplification of decision making, but this is in many ways one of its major strengths.40 These methods have also been criticized as a “black box approach,” not open to scrutiny, that replaces human judgment and dehumanizes care.40 Again, when used well, DA provides simulated numbers to corroborate what should be mathematically true if the assumptions we hold about the parameters in the decision model are accurate. It would be wrong to interpret the results of the DA as implying that one must manage any given patient in a specific way. Rather, it provides insight into which of several possible decisions would be expected to be optimal in most cases, and within specified ranges of the relevant parameters of the model. Another criticism of DA is the inadequacy of primary research available in some cases to drive DA models. The approaches of DA, however, allow one to make an informed best guess about these values, allowing rational discussion about what could be possible, pending additional data from primary research of the patient population.
Decision Analysis in SE Treatment
When Kaplan et al.26 lamented that “the equation that has not yet been calculated is whether the small incidence of permanent cognitive side effects (after SE) constitutes a greater morbidity than the small incidence of respiratory suppression, hypotension, cardiac dysrhythmia, or even deaths which have followed the use of intravenous antiseizure medications,” in a sense, they were requesting that the management of SE be examined with DA methods. This quote perfectly captures the calculus at hand: what we would like to know is whether the risks of harm because of delay in control of epileptic discharges outweigh the risks of treating them aggressively (Fig. 1). Decision analysis allows one to construct this equation, integrating multiple pieces of information with varying degrees of uncertainty to produce a recommendation that is optimal under a set of assumptions that are explicitly defined and based on expected utility.
To date, there is only one report of DA applied to the domain of SE, and it is worth reviewing in detail.2 In this report, the authors constructed a risk–benefit equation for the management of NCSE and considered the question of aggressive versus nonaggressive management of NCSE with respect to functional outcome and mortality based on five clinical parameters: (1) baseline mortality rate of the inciting etiology, (2) efficacy of nonaggressive treatment in gaining control of seizures, (3) relative contribution of seizures to overall mortality, (4) the degree of excess disability expected in the case of delayed seizure control, and (5) the mortality risk of aggressive treatment. Of note, some of these clinical parameters can be obtained directly from data in the literature (e.g., baseline mortality rate, mortality risk of aggressive treat), whereas others are less easily defined for specific etiologies (relative contribution of seizures to overall mortality, degree of excess disability because of delayed seizure control). Nevertheless, a strength of DA methods is that users can suggest clinically plausible values to fill these gaps and assess the sensitivity of model predictions to variations in these parameters.
Outcomes in this model follow the Glasgow Outcome Scale (GOS), and each of these is assigned a corresponding quality-of-life (QOL) score using health state values from previously published DAs. For this analysis, QOL is defined from 0 to 100, with QOL = 100 equivalent to best functional outcome with no disability, QOL = 0 equivalent to death, and intermediate values of QOL reflect varying levels of functional disability (Fig. 2A).
FIG. 2.
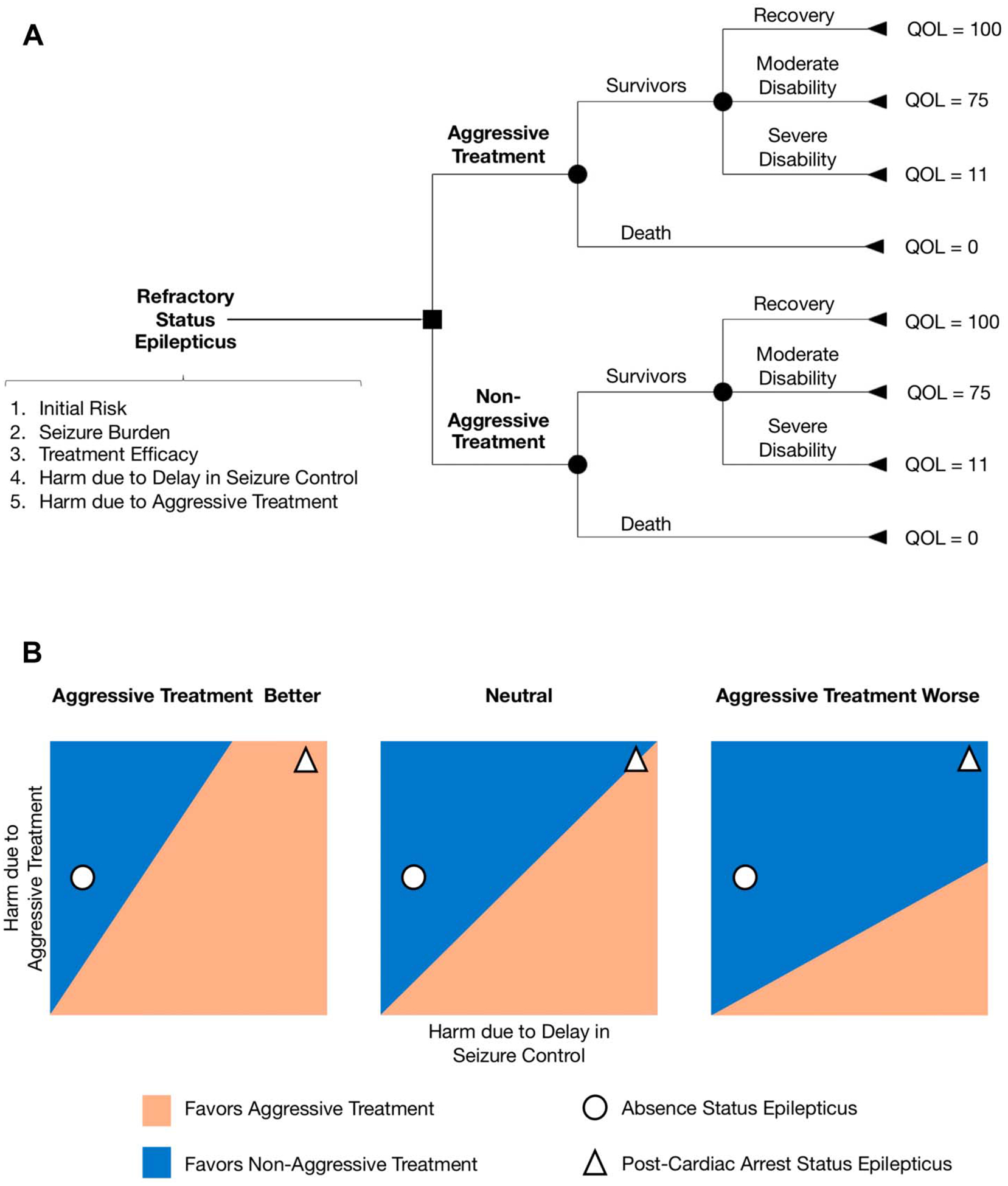
Decision analysis in status epilepticus (SE). A, A decision tree for management of SE with aggressive versus nonaggressive treatment. Outcomes are allocated among survivors or nonsurvivors. Survivors can have three types of functional status: fully recovered, moderate disability, or severe disability. B, Hypothetical two-way sensitivity analysis for harm because of aggressive treatment versus harm because of delay in seizure control. We use two base-case examples to illustrate how two-way sensitivity analysis might help decision making about SE treatment strategies (nonaggressive vs. aggressive). For absence SE, the nonaggressive treatment strategy is always favored, as the harm because of delay in seizure control is small. However, the optimal treatment strategy for post-cardiac arrest SE might be quite different if we assume that the harm because of delay in seizure control is more likely in post-cardiac arrest SE. Treatment efficacy for absence SE is higher than for post-cardiac arrest SE, so the harm because of aggressive treatment is likely to be greater in post-cardiac arrest SE. QOL, quality of life.
The authors consider four different neurologic conditions commonly associated with NCSE whose outcomes and management differ widely, including (1) absence SE, (2) SE because of subtherapeutic anti-seizure drugs, (3) post-cardiac arrest SE, and (4) SE related to intraparenchymal hemorrhage. For example, in the case of absence SE, this condition tends to be managed conservatively because functional outcomes are reportedly very good without aggressive treatment.48 In fact, any disability and mortality related to this condition would be predicted to occur as a result of overly aggressive therapy with intravenous anesthetics, although limited data are available to confirm. By contrast, post-cardiac arrest SE has poor baseline mortality and functional outcomes whether seizures are treated aggressively or not, but it is believed that poorly controlled seizures in this setting contribute substantially more to morbidity and mortality than in absence SE.
Models were built for each of these conditions by defining the five parameters and the expected mortality and QOL distribution in the baseline state (without either treatment), based on data from the literature where available. The risk–benefit equation then computed the expected mortality and QOL under the assumption of aggressive treatment with intravenous anesthetics (which carries the risks of medical complications affecting mortality) and under the assumption of nonaggressive treatment (which carries the risk of additional seizure-related mortality and morbidity because of delay in seizure control).
The authors demonstrated how the equation predicts QOL for the two different treatment strategies, illustrating through sensitivity analyses the conditions under which one strategy is superior to the other (i.e., higher expected QOL) when the value of one clinical parameter is varied (one-way sensitivity analysis) or two clinical parameters are varied simultaneously (two-way sensitivity analysis) while holding the remaining parameters constant. Importantly, this analysis identifies the conditions in which clinical equipoise could occur (corresponding to the intersection of the estimated QOL curves for strategy 1 vs. strategy 2) if ever. Another important point is that the risk–benefit equation by itself does not recommend a specific treatment strategy—it highlights which of the two strategies is optimal under specified ranges of values for the five parameters. A preferred management, however, can be determined by calculating the net QOL difference between strategies over a range of typical values for the five parameters, with the magnitude of this difference reflecting the strength of that preference.
For absence SE, the analysis predicts that the optimal treatment strategy is nonaggressive treatment across a wide range of values for the five parameters, with a 20% difference in estimated QOL between the strategies (100 vs. 80, for nonaggressive vs. aggressive treatment, respectively). The only exceptions to this result (referred to as “crossover points”) occur when “disability because of delay in seizure control” is allowed to increase up to 36% (reflecting the percentage of patients with a 1-point reduction in GOS because of seizure-related injury). This result highlights the fact that if absence status occurs in the setting of another neurologic process that has the potential to enhance the injuriousness of generalized discharges, there may be circumstances in which the aggressive treatment of absence status would be preferred. Meanwhile, for post-cardiac arrest SE, the preferred management is aggressive treatment, but the strength of this preference is weak (<1% difference between estimated QOL between strategies, 4.7 vs. 5.1 for nonaggressive vs. aggressive, respectively), and there are a number of crossover points where this recommendation changes, e.g., if “baseline mortality” or “mortality related to aggressive treatment” is increased or if the efficacy of nonaggressive treatment is improved, there are points where nonaggressive treatment becomes preferred. We recreated this DA framework for SE treatment incorporating the two base-case examples of absence SE and post-cardiac arrest (Fig. 2B). These examples serve to highlight how decision making about treatment strategies must be individualized to specific clinical scenarios. Even if we assume that one strategy is overall better than the other, nonaggressive or aggressive treatment might still be more beneficial or harmful based on the five parameters guiding SE risk–benefit analysis.
Taken together, these analyses emphasize that even for specific diagnoses, there is no one-size-fits-all management strategy, and careful consideration of the five clinical parameters in each individual patient case is necessary to determine the optimal risk–benefit balance.
WHAT WE WANT TO KNOW
The work reviewed above highlights how DA methods have been used to inform the decision to manage SE with aggressive versus nonaggressive treatment, but the approach also has applications for decisions related to the management of convulsive SE and other treatment-warranting activity on the ictal–interictal continuum (IIC). The reliance of DA models on key clinical parameters and published outcomes data emphasizes areas where new, more accurate, or detailed information about each of these parameters could inform better DA models. Overall, we need to know about the functional outcomes after seizure-related processes during hospitalization in more detailed and quantitative ways, including (1) more detailed information related to the quantification and classification of seizure-related activity from continuous video EEG monitoring and (2) standardized methods for reporting functional outcomes after hospitalization and at long-term follow-up (months and years later).
Reviewing each parameter of the risk–benefit equation for “what we want to know” includes the following: first, the efficacy of nonaggressive treatment in gaining control of seizures needs further study, as more effective nonaggressive treatment could change the risk–benefit calculations, particularly for conditions in which mortality is already high because of the baseline risks, including the causes of SE and NCSE. Efforts to evaluate the efficacy of nonanesthetic medications in the management of SE are informing this parameter.49,50
Second, the relative contribution of seizures to overall mortality and the degree of excess disability expected in the case of delay in seizure control are still unclear. The mortality of NCSE interacts with comorbidities, but the degree to which NCSE independently contributes to morbidity and mortality (after controlling for other factors) is controversial.51–54 How seizure-related activity affects functional outcomes requires additional investigation. Although clearly differing by inciting etiology, evidence suggests that seizure-related activity has effects on functional outcomes that correlate with the quantity of seizure activity over time and spatial extent, i.e., the seizure burden.5,6 In other words, seizure activity is not an all-or-none process, with more frequent seizure activity involving greater spatial distribution over the brain causing more potential injury than less frequent, spatially restricted ictal activity—a concept that deserves further application in future investigations. In addition, our understanding of how activity on the IIC relates to neuronal injury and, in turn, to changes in functional outcome requires deeper investigation. Further investigations with multimodal data incorporating abnormalities in cerebral perfusion, metabolism, and radiographic changes corresponding to IIC-related activity may demonstrate which such activity has a greater potential for neuronal injury, independent of etiology.1 A severity score, the Treatment-Warranting Injurious IIC Spectral Severity Score, has been developed to define IIC patterns that may warrant greater need for treatment, incorporating these considerations.1 Use of the Treatment-Warranting Injurious IIC Spectral Severity Score scoring criteria both retrospectively (and ideally, prospectively) could provide better insight into functional outcomes of subgroups of patients based on standardized electrographic scoring criteria.
Finally, the mortality risk of aggressive treatment is still debated and may differ by center.38,39 No randomized clinical trial to date has compared aggressive treatment with intravenous anesthetics to nonaggressive management in SE. One ongoing study is evaluating aggressive versus nonaggressive treatment in post-cardiac arrest SE exclusively.55 Several observational cohort or retrospective studies have evaluated the risk of aggressive treatment strategy. These studies were mainly from single centers, and most compared outcomes between patients who received anesthetics and those who did not receive them.38,56–58 This approach is inevitably biased by patient selection and local practices, and few studies used propensity matching in an attempt to minimize this bias and account for group differences. Propensity matching can balance some factors related to baseline risk, but it cannot fully characterize the patient condition that led to the decision of starting anesthetics in the first place. Seizure burden, treatment efficacy likelihood, treatment effect, and additional baseline risk factors were not incorporated in an attempt to balance groups with aggressive and nonaggressive treatment strategies. More importantly, those comparisons were made at the group level for nearly all patients and therefore cannot capture the complexity and heterogeneity of SE treatment. As those studies generally come from single centers, it is not surprising that there is remarkable variability in morbidity, mortality, and complications. Although not directly answering whether outcomes are worsened by aggressive treatment or not, these studies are very important in highlighting the high morbidity of refractory NCSE and its treatment.
The “aggressive treatment” label also has several shades of gray. Some regard the decision to pursue aggressive treatment in a patient who is already intubated as moot, but the harmful effects of aggressive treatment go beyond the effects of intubation alone. Aggressive treatment ranges from a single anesthetic agent for a short period to the use of multiple anesthetic agents and barbiturates for weeks or months. Depth and duration of anesthesia can vary from sedatives sufficient to achieve seizure control to the level of a “light burst suppression” or to deep or near-complete background suppression for 24 to 48 hours.56,57,59 Although it is not known whether a treatment goal of a burst suppression EEG is superior to simple seizure suppression, it is clear that deeper and more prolonged anesthesia and use of multiple anesthetics or barbiturates can cause serious complications and adverse outcomes.35 Patient comorbidities also play a significant role in outcomes and can affect determination of the baseline risk for aggressive treatment decision making and increase the likelihood for systemic complications from such therapy.21–23,25 One of the main limitations to evaluating aggressive treatment tradeoffs in SE is the fact that is not possible to easily separate which complications are caused by SE from those caused by anesthetics or baseline risks, as well as not comparing the weight of each type of complication in the final outcome.
In all cases, DA cannot replace data from primary research related to the effectiveness of interventions for specific groups of patients using randomized controlled trials. For example, the results of TELSTAR (ClinicalTrials.gov Identifier: NCT02056236), which is investigating the efficacy of treating electrographic SE after cardiac arrest, will directly inform the risk–benefit calculus for this subgroup of patients.55
WHERE WE SHOULD GO FROM HERE
As clinicians, we should continue to balance the use of DA methods with direct investigations using randomized controlled trials to understand the distribution of functional outcomes after therapeutic interventions for seizure-related activity, whether convulsive SE, NCSE, or other ictal–interictal activity. We should look forward to the results of pending randomized controlled trials that affect the management of patients with these conditions.50,55 Finally, a major area on which to focus the efforts of DA methods in the future will be toward the development of better clinician-facing tools to provide personalized risk assessment for individual patients based on the discussed parameters of the DA models and patient-specific parameters. Whereas prior methods in DA have largely focused on computing optimal decisions based on aggregate utility across all patients, newer methods using Bayesian networks may be able to provide a framework to compute the probability that a specific patient has a net benefit from treatment despite possible treatment-related harms.60
Acknowledgments
E. Amorim has received support from the Society of Critical Care Medicine (SCCM-Weil Research Grant), the Neurocritical Care Society (NCS research training fellowship 17POST33330001), American Heart Association (postdoctoral fellowship), and MIT-Philips Clinician Award. M. B. Westover has received support from NIH-NINDS (1K23NS090900, 1R01NS102190, 1R01NS102574, 1R01NS107291), the Andrew David Heitman Neuroendovascular Research Fund, and the Rappaport Foundation. The remaining author has no conflicts of interest to disclose.
This study was supported by the NIH (1R01NS102190, 1R01NS102574, 1R01NS107291, 1RF1AG064312).
REFERENCES
- 1.Kapinos G, Trinka E, Kaplan PW. Multimodal approach to decision to treat critically ill patients with periodic or rhythmic patterns using an ictal-interictal continuum spectral severity score. J Clin Neurophysiol 2018;35:314–324. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson M, Bianchi MT, Sutter R, et al. Calculating the risk benefit equation for aggressive treatment of non-convulsive status epilepticus. Neurocrit Care 2013;18:216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hocker S. Anesthetic drugs for the treatment of status epilepticus. Epilepsia 2018;59(suppl 2):188–192. [DOI] [PubMed] [Google Scholar]
- 4.Betjemann JP, Josephson SA, Lowenstein DH, Burke JF. Trends in status epilepticus-related hospitalizations and mortality: redefined in US practice over time. JAMA Neurol 2015;72:650–655. [DOI] [PubMed] [Google Scholar]
- 5.Clancy RR. The contribution of EEG to the understanding of neonatal seizures. Epilepsia 1996;37(suppl 1):S52–S59. [DOI] [PubMed] [Google Scholar]
- 6.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology 2014;82:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer RE, Luders H, Lesser RP, et al. Transient focal abnormalities of neuroimaging studies during focal status epilepticus. Epilepsia 1987;28:528–532. [DOI] [PubMed] [Google Scholar]
- 9.Lansberg MG, O’Brien MW, Norbash AM, Moseley ME, Morrell M, Albers GW. MRI abnormalities associated with partial status epilepticus. Neurology 1999;52:1021–1027. [DOI] [PubMed] [Google Scholar]
- 10.Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES. Metabolic correlates of the ictal-interictal continuum: FDG-PET during continuous EEG. Neurocrit Care 2016;24:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol 2017;74:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol 2016;79:579–590. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez V, Rodden MF, LaRoche SM. Ictal-interictal continuum: a proposed treatment algorithm. Clin Neurophysiol 2016;127:2056–2064. [DOI] [PubMed] [Google Scholar]
- 14.Bauerschmidt A, Koshkelashvili N, Ezeani CC, et al. Prospective assessment of ictal behavior using the revised Responsiveness in Epilepsy Scale (RES-II). Epilepsy Behav 2013;26:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andermann F, Robb JP. Absence status. A reappraisal following review of thirty-eight patients. Epilepsia 1972;13:177–187. [DOI] [PubMed] [Google Scholar]
- 16.Shirasaka Y. Lack of neuronal damage in atypical absence status epilepticus. Epilepsia 2002;43:1498–1501. [DOI] [PubMed] [Google Scholar]
- 17.DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent non-convulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998;39:833–840. [DOI] [PubMed] [Google Scholar]
- 18.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol 1998;44:908–912. [DOI] [PubMed] [Google Scholar]
- 19.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 20.Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutter R, Semmlack S, Opic P, et al. Untangling operational failures of the Status Epilepticus Severity Score (STESS). Neurology 2019;92:e1948–e1956. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty T, Hocker S. The clinical spectrum of new-onset status epilepticus. Crit Care Med 2019;47:970–974. [DOI] [PubMed] [Google Scholar]
- 23.Sünter G, Ağan K, Midi İ, Bingöl CA. Etiology, treatment, and outcomes of status epilepticus episodes in the elderly. Neurol Sci Neurophysiol 2019;36:22–27. [Google Scholar]
- 24.Belluzzo M, Furlanis G, Stragapede L. Predictors of functional disability at hospital discharge after status epilepticus. Epilepsy Res 2015;110:179–182. [DOI] [PubMed] [Google Scholar]
- 25.Szklener S, Korchut A, Godek M, et al. Systemic inflammatory response syndrome in the course of status epilepticus: 7-year, two-center observational study. Epilepsy Res 2017;137:53–55. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan PW. Assessing the outcomes in patients with nonconvulsive status epilepticus: nonconvulsive status epilepticus is underdiagnosed, potentially overtreated, and confounded by comorbidity. J Clin Neurophysiol 1999;16:341–352; discussion 353. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996;37:643–650. [DOI] [PubMed] [Google Scholar]
- 28.Treatment of convulsive status epilepticus. Recommendations of the Epilepsy Foundation of America’s Working Group on Status Epilepticus. JAMA 1993;270:854–859. [PubMed] [Google Scholar]
- 29.Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia 1999;40:120–122. [DOI] [PubMed] [Google Scholar]
- 30.Gainza-Lein M, Sanchez Fernandez I, Jackson M, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol 2018;75:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill CE, Parikh AO, Ellis C, Myers JS, Litt B. Timing is everything: where status epilepticus treatment fails. Ann Neurol 2017;82:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005;25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones DM, Esmaeil N, Maren S, Macdonald RL. Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res 2002;50:301–312. [DOI] [PubMed] [Google Scholar]
- 34.Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 2006;129:18–35. [DOI] [PubMed] [Google Scholar]
- 35.Hawkes MA, English SW, Mandrekar JN, Rabinstein AA, Hocker S. Causes of death in status epilepticus. Crit Care Med 2019;47:1226–1231. [DOI] [PubMed] [Google Scholar]
- 36.Marchi NA, Novy J, Faouzi M, Stahli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med 2015;43:1003–1009. [DOI] [PubMed] [Google Scholar]
- 37.Kowalski RG, Ziai WC, Rees RN, et al. Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med 2012;40:2677–2684. [DOI] [PubMed] [Google Scholar]
- 38.Sutter R, Marsch S, Fuhr P, Kaplan PW, Ruegg S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology 2014;82:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez V, Lee JW, Westover MB, et al. Therapeutic coma for status epilepticus: differing practices in a prospective multicenter study. Neurology 2016;87:1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavakoli M, Davies HT, Thomson R. Decision analysis in evidence-based decision making. J Eval Clin Pract 2000;6:111–120. [DOI] [PubMed] [Google Scholar]
- 41.Berkowitz AL, Westover MB, Bianchi MT, Chou SH. Aspirin for acute stroke of unknown etiology in resource-limited settings: a decision analysis. Neurology 2014;83:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akama-Garren EH, Bianchi MT, Leveroni C, Cole AJ, Cash SS, Westover MB. Weighing the value of memory loss in the surgical evaluation of left temporal lobe epilepsy: a decision analysis. Epilepsia 2014;55:1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao EL, Chao LY, Ni P, et al. Antiepileptic drug treatment after an unprovoked first seizure: a decision analysis. Neurology 2018;91:e1429–e1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westover MB, Bianchi MT, Eckman MH, Greenberg SM. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol 2011;68:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bargiela D, Bianchi MT, Westover MB, et al. Selection of first-line therapy in multiple sclerosis using risk-benefit decision analysis. Neurology 2017;88:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hotan GC, Struck AF, Bianchi MT, Eskandar EN, Cole AJ, Westover MB. Decision analysis of intracranial monitoring in non-lesional epilepsy. Seizure 2016;40:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi H, Sell RL, Lenert L, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA 2008;300:2497–2505. [DOI] [PubMed] [Google Scholar]
- 48.Scholtes FB, Renier WO, Meinardi H. Non-convulsive status epilepticus: causes, treatment, and outcome in 65 patients. J Neurol Neurosurg Psychiatry 1996;61:93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Husain AM, Lee JW, Kolls BJ, et al. Randomized trial of lacosamide versus fosphenytoin for nonconvulsive seizures. Ann Neurol 2018;83:1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cock HR, Coles LD, Elm J, et al. Lessons from the Established Status Epilepticus Treatment Trial. Epilepsy Behav 2019;101:106296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol 2008;255:1561–1566. [DOI] [PubMed] [Google Scholar]
- 52.Jordan KG, Hirsch LJ. In nonconvulsive status epilepticus (NCSE), treat to burst-suppression: pro and con. Epilepsia 2006;47(suppl 1):41–45. [DOI] [PubMed] [Google Scholar]
- 53.Aminoff MJ. Management of status epilepticus. Can J Neurol Sci 1998;25:S4–S6. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez V, Januel JM, Burnand B, Rossetti AO. Role of comorbidities in outcome prediction after status epilepticus. Epilepsia 2012;53:e89–92. [DOI] [PubMed] [Google Scholar]
- 55.Ruijter BJ, van Putten MJ, Horn J, et al. Treatment of electroencephalographic status epilepticus after cardiopulmonary resuscitation (TELSTAR): study protocol for a randomized controlled trial. Trials 2014;15:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muhlhofer WG, Layfield S, Lowenstein D, et al. Duration of therapeutic coma and outcome of refractory status epilepticus. Epilepsia 2019;60:921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin JJ, Chou CC, Lan SY, et al. Therapeutic burst-suppression coma in pediatric febrile refractory status epilepticus. Brain Dev 2017;39:693–702. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez A, Lantigua H, Lesch C, et al. High-dose midazolam infusion for refractory status epilepticus. Neurology 2014;82:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenthal ES, Claassen J, Wainwright MS, et al. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Ann Neurol 2017;82:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bradley A, Van der Meer R, McKay CJ. A prognostic Bayesian network that makes personalized predictions of poor prognostic outcome post resection of pancreatic ductal adenocarcinoma. PLoS One 2019;14:e0222270. [DOI] [PMC free article] [PubMed] [Google Scholar]


