Abstract
This article describes the first reported case of myasthenia gravis (MG) seropositive for both acetylcholine receptor antibody and low-density lipoprotein receptor-related protein 4 antibody, complicated by autoimmune polyglandular syndrome (APS) type 3. The patient exhibited myasthenic weakness restricted to the ocular muscles and ptosis. Severe clinical deterioration ensued with predominant bulbar symptoms. MG rapidly worsened, the patient was intubated, and agranulocytosis due to thiamazole was also present, so it was necessary to perform thyroidectomy with tracheostomy and thymectomy in two phases. Both the double-seropositive MG and the APS were involved in the patient's rapid deterioration.
Keywords: acetylcholine receptor, autoimmune polyglandular syndrome, low-density lipoprotein receptor, myasthenia gravis
Introduction
Myasthenia gravis (MG) is an organ-specific autoimmune disease that affects postsynaptic receptors at neuromuscular junctions. Approximately 80% of patients with MG have antibodies against acetylcholine receptor (AChR), and approximately 4% of patients with MG have antibodies against muscle-specific kinase (MuSK). Low-density lipoprotein receptor-associated protein 4 (LRP4) antibodies have recently been identified as another pathogenic antibody in MG and are reportedly present in approximately 2% of patients (1,2). Double-seropositive MG patients with AChR antibodies and LRP4 antibodies have rarely been reported. These patients have frequently presented with bulbar symptoms (e.g. dysphagia, aspiration of liquids, dysphonia, or difficulty chewing) (3-7), and none of them have had concomitant autoimmune polyglandular syndrome (APS).
APS, also called polyglandular autoimmune syndrome, was first described by Neufeld in 1980 (8). Based on the types of autoimmune diseases that coexist, it has been classified as APS types 1 to 4. APS type 3 is associated with autoimmune thyroid disease and other autoimmune diseases along with an absence of Addison's disease, and it is reportedly the most common type in Japan (9).
In this case study, we describe an MG patient with AChR antibodies, LRP4 antibodies, and concomitant APS.
Case Report
The patient was a 37-year-old man with type 1 diabetes who has been treated with insulin self-injection since 3 years of age. His HbA1c had been in the range of 9-10%, and he had begun retinal photocoagulation for proliferative diabetic retinopathy at 26 years of age. Two months prior to the presentation, he had no symptoms; however, he had a high alkaline phosphatase (ALP) level and measured thyroid function along with hyperthyroidism. He was subsequently diagnosed with Graves' disease. Left eye ptosis and diplopia had occurred one month prior to the current presentation and had gradually deteriorated. At the patient's first visit, he was diagnosed with diabetic oculomotor nerve palsy. After two weeks, his diplopia and ptosis had worsened and become bilateral.
On a physical examination, his blood pressure was 116/68 mmHg, and his pulse was 105 beats/min with regular slight tachycardia. A neurological examination revealed severe ptosis in the left eye and mild ptosis in the right eye. The left eye movement was fixed in the middle. The right eye exhibited limitation of adduction and abduction. There was no pupil abnormality or eye protrusion. Tendon reflexes were absent in the upper and lower limbs, but muscle strength in the limbs was normal. Mild hypesthesia was present in the fingertips and in the feet. There was no ataxia, dysarthria, or dysphagia.
The results of laboratory tests were hemoglobin A1c 11.3%, glutamic acid decarboxylase (GAD) antibody 11.0 U/mL, thyroid-stimulating hormone (TSH) 0.0 μIU/mL, free triiodothyronine 8.5 pg/mL, free thyroxine 2.5 ng/dL, thyroglobulin 81.20 ng/mL, TSH receptor antibody 27.9 IU/L, thyroid peroxidase antibody 129.0 IU/L, and thyroglobulin antibody <10.0 IU/mL, and the adrenal function was normal. Human leukocyte antigen (HLA) typing revealed the presence of the DRB1*08:02-DQB1*03:02 and DRB1*13:02-DQB1*06:04 haplotypes. On a cerebrospinal fluid test, there were 2 cells/μL, and the protein concentration was 51 mg/dL (Table 1). On a nerve conduction velocity test, median, ulnar, and tibial compound muscle action potential had decreased, and the sural sensory nerve action potential and H wave were absent.
Table 1.
Laboratory Findings.
| WBC | 6,820 | /μL | HbA1c | 11.3 | % | GAD ab | 11.0 | U/mL | |||||
| RBC | 540×104 | /μL | Free T3 | 8.5 | pg/mL | TSH-R ab | 27.9 | ||||||
| Hb | 15.7 | g/dL | Free T4 | 2.5 | ng/dL | TPO ab | 129.0 | IU/mL | |||||
| Plt | 17.3×104 | /μL | TSH | 0.0 | μIU/mL | Tg Ab | <10.0 | ||||||
| CRP | 0.1 | mg/dL | Thyroglobulin | 81.20 | ng/mL | AChR ab | 0.9 | nmol/L | |||||
| ALP | 739 | IU/mL | Cortisol | 6.3 | μg/dL | Musk ab | <0.02 | nmol/L | |||||
| AST | 20 | U/L | ACTH | 15.9 | pg/mL | LRP4 ab | 1.81 | AI | |||||
| ALT | 26 | U/L | anti-ganglioside abs | Negative | |||||||||
| BUN | 12.6 | mg/dL | |||||||||||
| Cre | 0.55 | mg/dL | |||||||||||
| Na | 139 | mEq/L | CSF | ||||||||||
| K | 4.5 | mEq/L | Cell count | 2 | /μL (Lym 100%) | HLA | |||||||
| Cl | 105 | mEq/L | Protein | 51 | mg/dL | DRB1*08:02-DQB1*03:02 | |||||||
| Ca | 9.3 | mEq/L | Glucose | 126 | mg/dL | DRB1*13:02-DQB1*06:04 | |||||||
AChR: acetylcholine receptor, ACTH: adrenocorticotropic hormone, ALT: alanine aminotransferase, AST: aspartate transaminase, BUN: blood urea nitrogen, Cre: Creatinine, CSF: cerebrospinal fluid, eGFR: estimated glomerular filtration rate, GAD: glutamic acid decarboxylase, Hb: hemoglobin, HLA: human leukocyte antigen, LRP4: low-density lipoprotein receptor-associated protein 4, Musk: muscle-specific kinase, Tg: thyroglobulin, TPO: thyroid peroxidase, TSH: thyroid-stimulating hormone
The patient did not exhibit ataxia, but Miller Fisher syndrome was suspected due to subacute eye movement disorder, loss of tendon reflexes, and H wave. In addition, the cerebrospinal fluid protein level was slightly elevated. Treatment with intravenous immunoglobulin (IVIg at 400 mg/kg/day for 5 days) was started. Although thiamazole was added for Graves' disease, he developed a fever and agranulocytosis, and thiamazole was discontinued. He was subsequently determined to be weakly positive for AChR antibody (0.9 nmol/L; cut-off <0.3) and negative for all ganglioside GQ1b antibodies. His ptosis responded markedly on a tensilon test, and MG was diagnosed. On a repetitive stimulation test, the mitral muscle and abductor digiti minimi were normal, but there was waning in the musculus orbicularis oculi.
The patient's initial ocular symptoms were treated with orally administered ambenonium and naphazoline eye drops. Within approximately two weeks, bulbar symptoms, such as dysphagia and shortness of breath, developed. At that time, AChR antibody significantly increased to 10.8 nmol/L, and the patient was also found to be LRP4 antibody-positive. MuSK antibodies were negative. He was treated with plasma exchange of two double-filtration plasmapheresis (DFPP) treatments. Each plasma processing volume was 3,500 mL. He then received 3 plasma adsorption (PA) treatments of 2,000 mL each. He received IVIg (400 mg/kg/day for 5 days), prednisolone (5 mg was gradually increased to 20 mg), and tacrolimus (3 mg), but his symptoms progressed. He developed myasthenic crisis, necessitating intubation. His condition was classified as Myasthenia Gravis Foundation of America V.
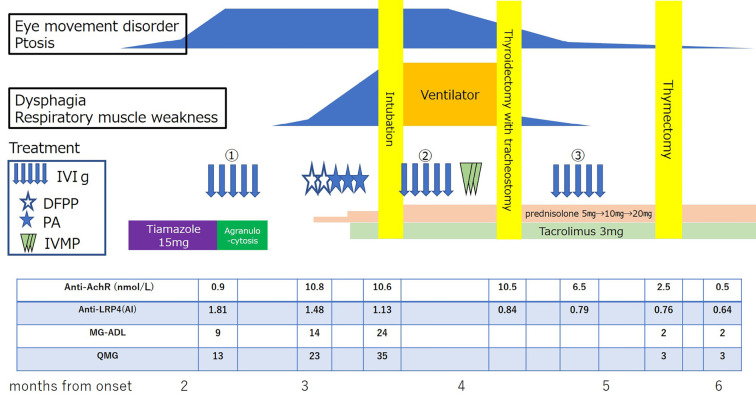
Intravenous methylprednisolone pulse (1,000 mg for 3 days) and additional IVIg were administered, and his symptoms gradually improved. Thymic hyperplasia was noted on computed tomography, and thymectomy was planned. Because agranulocytosis was caused by thiamazole, however, total thyroidectomy was also necessary. First, the patient underwent thyroidectomy and tracheostomy. One month later, thoracoscopic extended thymectomy was performed. There was no exacerbation during the perioperative period, and we performed tracheostomy closure. Regarding the thymic pathology, thymic tissue with Hassall bodies was observed in islands. There was no thymoma. The AChR antibody titer increased from 0.9 to 10.8 nmol/L and then decreased to 0.5 nmol/L, consistent with the clinical course. The LRP4 antibody titer was highest at the time of the onset of eye movement disorder alone (antibody index 1.81) and then decreased over time (Figure).
Figure.
The clinical course after the onset. AChR: acetylcholine receptor, DFPP: double filtration plasmapheresis, IVIg: intravenous immunoglobulin, IVMP: intravenous methylprednisolone, MG-ADL: myasthenia gravis-activities of daily living, PA: plasma adsorption, QMG: quantitative myasthenia gravis score
Discussion
Double-seropositivity for AChR and LRP4 antibodies is rare. Furthermore, it has been reported that only 0.2% of AChR-positive MG cases in China (3) and 7.5% of those in Europe (4) were also positive for LRP4. It is possible that there are differences between races, or that there are more hidden cases that have not actually been measured. There have been a few case reports of AChR/LRP4-positive MG (5-7) but none with APS complications. MG patients with LRP4 antibodies who are negative for AChR and MuSK antibodies usually have mild ocular myasthenic symptoms. Clinical data derived from previously reported patients with AChR/LRP4-positive MG indicate that most are Myasthenia Gravis Foundation of America III or worse, initially exhibiting bulbar symptoms or ocular symptoms followed by bulbar symptoms (3-7).
The weakness observed in the present patient was restricted to ocular muscles in the early period, and then severe bulbar weakness occurred later. The changes in clinical symptoms were relatively acute. We thought that the change in clinical course might have been associated with double-seropositivity for relevant antibodies. The LRP4 titer was high and gradually decreased from the onset. The AChR titer, by contrast, was initially low and then increased as the symptoms worsened. The initial levels of LRP4 antibody mainly caused ocular symptoms, but as the AChR titer rapidly increased, the symptoms rapidly worsened, and the patient developed myasthenic crisis. In MG patients, who are known to be positive for AChR, especially those with sudden changes in symptoms or concomitant APS, as in this case, testing for LRP4 antibody should be considered. The presence of both antibodies is rare, but it is important to also test for LRP4 antibodies even in patients who are known to be positive for AChR antibodies, as their condition may become more severe.
Factors affecting conversion from ocular MG to generalized MG are still debatable (10). The presence of an additional autoimmune disease was reportedly associated with a markedly higher risk of exacerbation and emergency treatment (11). The current patient had several additional auto-antibodies related to APS, and this may have contributed to the aggravation of his condition.
MG is well known to be associated with various autoimmune diseases. Cases of MG associated with thyroid diseases (Hashimoto's disease and Graves' disease), rheumatoid arthritis, systemic lupus erythematosus, and insulin-dependent diabetes mellitus have also been described (12). There have been several reports of MG and APS (13-19). Six of eight patients had bulbar symptoms that tended to be severe, and three were intubated. Patients are often young to middle-aged, and thymoma has never been reported, but thymic hyperplasia tends to be slightly more common (Table 2). To our knowledge, the current report is the first to describe a case of double-seropositive MG in conjunction with APS. Since it is not common to report LRP4 titers, double-positive patients may have gone undiagnosed in MG cases with APS.
Table 2.
Characteristics of the Present and Previously Reported Patients with MG with APS.
| APS type | Sex | Age | MG related antibody | Symptoms | Thymus gland | |||
|---|---|---|---|---|---|---|---|---|
| AChR | MuSK | LRP4 | ||||||
| 13 | APS3 | Female | 15 | + | NS | NS | dysphagia, dysarthria, fatigue | Thymectomy Follicular hyperplasia |
| 14 | APS3 | Female | 30s | + | NS | NS | Ptosis diplopia | Thymectomy Hyperplasia |
| 15 | APS3 | Female | 51 | + | NS | NS | muscle weakness, fatigue, diplopia, dysarthria, dysphagia → hypercapnic respiratory failure →Intubation |
Thymectomy Lymphoid follicular hyperplasia |
| 16 | APS3 | Female | 51 | - | NS | NS | generalized muscle weakness rhinolalia, dysphagia | Treated with radiotherapy because of Thymus hyperplasia at 2 years old. |
| 17 | APS3 | Male | 14 months | - | + | NS | ptosis generalized muscle weakness nasal speech, dysphagia | NS |
| 18 | APS2 | Female | 74 | + | NS | NS | Limb weakness and dyspnea → respiratory failure → Intubation |
Normal thymus gland (CT) |
| 19 | APS3 | Male | 37 | - | Not evaluated | NS | ptosis diplopia | Normal thymus gland (CT) |
| Our case | APS3 | Male | 37 | + | - | + | ptosis, diplopia dysarthria, dysphagia → hypercapnic respiratory failure →Intubation | Tymectomy Hyperplasia |
AChR: acetylcholine receptor, APS: autoimmune polyglandular syndrome, CT: computerized tomography, LRP4: low-density lipoprotein receptor-associated protein 4, MG: myasthenia gravis, MuSK: muscle-specific kinase
NS: not stated
HLA typing revealed the presence of the DRB1*08:02-DQB1*03:02 haplotype, which is associated with susceptibility to type 1 diabetes mellitus in the Japanese population (20). DRB1*13:02-DQB1*06:04 is also associated with susceptibility to “childhood” MG in the Japanese population (21). That same haplotype was present in a case report of an adult patient with APS type 3 and MG (14). Although the associations between LRP4 antibody and HLA are unclear, these HLA haplotypes may have been involved in the onset of symptoms in the present case.
Three considerations are pertinent with regard to surgical treatment. First, near-emergent thyroidectomy is required for Graves' disease in cases involving the development of agranulocytosis with thiamazole (22). Second, if tracheostomy is performed, it should be done at the same time as thyroidectomy. Finally, thymectomy is preferred for thymic hyperplasia of early-onset MG (23). It was initially considered for this patient but decided against because the risks of wound infection and fatal mediastinitis due to simultaneous tracheostomy, thyroidectomy, and thymectomy were extremely high. Therefore, thyroidectomy with tracheostomy was later followed by thymectomy.
In the present case, the treatment of MG was complicated by APS. It was initially very difficult to distinguish MG from diabetic ophthalmoplegia or Miller Fisher syndrome because the patient had had type 1 diabetes for more than 30 years. In addition, the combination of Graves' disease and agranulocytosis due to thiamazole required thyroidectomy, which rendered the treatment of MG crisis more complex. Furthermore, the presence of another autoimmune disease may have aggravated MG.
Conclusion
The current case is the first reported one of APS with MG with both AChR and LRP4 antibodies. We surmised that AChR and LRP4 double-seropositivity along with APS both contributed to the aggravation of the patient's condition. It is necessary to test for LRP4 antibody even in patients who are known to be positive for AChR antibody, as double antibody positivity may cause their condition to become severe.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Dr. Susumu Kusunoki, Department of Neurology, Kindai University Faculty of Medicine, for the measurement of the anti-ganglioside antibodies.
References
- 1.Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 69: 418-422, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 14: 1023-1036, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Han J, Zhang Y, et al. Clinical analysis of Chinese anti-low-density-lipoprotein-receptor-associated protein 4 antibodies in patients with myasthenia gravis. Eur J Neurol 26: 1296-e84, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Zisimopoulou P, Evangelakou P, Tzartos J, et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun 52: 139-145, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa H, Taniguchi A, Ii Y, et al. Double-seropositive myasthenia gravis with acetylcholine receptor and low-density lipoprotein receptor-related protein 4 antibodies associated with invasive thymoma. Neuromuscul Disord 27: 914-917, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Tsivgoulis G, Dervenoulas G, Tzartos SJ, et al. Double-seropositive myasthenia gravis with acetylcholine receptor and lipoprotein receptor-related protein 4 antibodies. Muscle Nerve 49: 930-931, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Bokoliya SC, Kumar VP, Nashi S, et al. Anti-AChR, MuSK, and LRP4 antibodies coexistence: a rare and distinct subtype of myasthenia gravis from Indian subcontinent. Clin Chim Acta 486: 34-35, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann 9: 154-162, 1980. [PubMed] [Google Scholar]
- 9.Shimizu C, Chiba H, Koike C. Autoimmuno Polyglandular syndrome. Nihon Rinsho Suppl 3: 565-570, 2006(in Japanese). [PubMed] [Google Scholar]
- 10.Binks S, Vincent A, Palace J. Myasthenia gravis: a clinical-immunological update. J Neurol 263: 826-834, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Meel RH, Lipka AF, van Zwet EW, et al. Prognostic factors for exacerbations and emergency treatments in myasthenia gravis. J Neuroimmunol 282: 123-125, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Drachman DB. Myasthenia gravis. N Engl J Med 330: 1797-1810, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Jamiołkowska M, Bossowski A. 15-year old girl with APS type IIIc, 12 months post-thymectomy remission of myasthenia. Pediatr Endocrinol Diabetes Metab. 23: 49-55, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima S, Matsunaga M, Shibata M, et al. A case of autoimmune type 1 diabetes mellitus complicated by Myasthenia Gravis and Graves' Disease. J Japan Diab Soc 58: 198-204, 2015(in Japanese, Abstract in English). [Google Scholar]
- 15.De Marchi SU, Cecchin E, De Marchi S. Autoimmune spontaneous chronic urticaria and generalized myasthenia gravis in a patient with polyglandular autoimmune syndrome type 3. Muscle Nerve 52: 440-444, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Innico G, Frassetti N, Coppola B, Mariotti A, Lai S. Autoimmune polyglandular syndrome in a woman of 51 years. Eur Rev Med Pharmacol Sci 18: 1717-1719, 2014. [PubMed] [Google Scholar]
- 17.Duman O, Koken R, Baran RT, Haspolat S, Topaloglu H. Infantile anti-MuSK positive myasthenia gravis in a patient with autoimmune polyendocrinopathy type 3. Eur J Paediatr Neurol 18: 526-528, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Konno S, Ichijo T, Murata M, et al. Autoimmune polyglandular syndrome type 2 with myasthenia gravis crisis. Neurologist 15: 361-363, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Briscoe NK, Mezei MM. Polyglandular autoimmune syndrome type 3 in a patient with ocular myasthenia gravis. Muscle Nerve. 40: 1064-1065, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Kawabata Y, Ikegami H, Awata T, et al. Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia 52: 2513-2521, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Matsuki K, Juji T, Tokunaga K, et al. HLA antigens in Japanese patients with myasthenia gravis. J Clin Invest 86: 392-399, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight CL, Cooray SD, Kulkarni J, Borschmann M, Kotowicz M. Thyroidectomy for the treatment of Graves' thyrotoxicosis in thioamide-induced agranulocytosis and sepsis. Endocrinol Diabetes Metab Case Rep. Forthcomig. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romi F, Gilhus NE, Varhaug JE, Myking A, Aarli JA. Thymectomy in nonthymoma early-onset myasthenia gravis in correlation with disease severity and muscle autoantibodies. Eur Neurol 49: 210-217, 2003. [DOI] [PubMed] [Google Scholar]