Abstract
Emerging infectious diseases continue to be of a significant importance worldwide with the potential to cause major outbreaks and global pandemics. In 2002, the world had witnessed the appearance of the severe acute respiratory syndrome coronavirus in China which disappeared abruptly within 6 months. About a decade later, a new and emerging novel coronavirus named the Middle East respiratory syndrome coronavirus (MERS-CoV) was described in a patient from Saudi Arabia. These two coronaviruses shared multiple similarities in the epidemiology, clinical presentations, and posed challenges in its prevention and management. Seven years since its discovery, MERS-CoV continues to be a lethal zoonotic pathogen capable of causing severe pneumonia with high case fatality rates and the ability to cause large health care-associated outbreaks.
Keywords: MERS, Middle East respiratory syndrome coronavirus, epidemiology, control measures, transmission, Saudi Arabia
Emerging infectious diseases continue to be of a significant importance worldwide with the potential to cause major outbreaks and global pandemics. In 2002, the world had witnessed the appearance of the severe acute respiratory syndrome coronavirus (SARS-CoV) in China. 1 2 And about a decade later, a new and emerging coronavirus was described in a patient from Saudi Arabia. 3 4 The virus was identified as a novel coronavirus and later was named the Middle East respiratory syndrome coronavirus (MERS-CoV). 5 These two coronaviruses shared multiple similarities in the epidemiology, clinical presentations, and posed challenges in prevention and management. 6 7 For any new emerging zoonotic pathogen, there are five stages in the evolution to cause diseases limited to humans. In stage 1, the pathogen is confined to the animal host; in stage 2, human infections occur as a result of animal contacts; in stage 3, there is a limited human-to-human transmission; stage 4, there are multiple outbreaks with human-to-human transmission; and stage 5, infections occur within humans. 8 In this review, we describe the epidemiology, clinical features, and outcome of both SARS and MERS-CoV.
The SARS-CoV and MERS-CoV
SARS-CoV and MERS-CoV are enveloped positive strand RNA betacoronaviruses. The first coronavirus was isolated from humans in 1965 and was cultivated on human ciliated embryonal tracheal cells. 9 Coronaviruses are enveloped, and positive stranded RNA viruses classified as a family within the Nidovirales order. There are four genera: α, β, gamma, and delta, and human coronaviruses belong to the α or the β genera. 10 In 2002, SARS-CoV outbreak was described and the virus was 50 to 60% identical and distantly related to known coronaviruses. 11 The newly described virus was able to cause disease in macaques with a similar spectrum of disease. 12 While the MERS-CoV belongs to lineage C betacoronavirus and emerged in September 2012 and continuous to cause sporadic cases and clusters of disease mostly in the Arabian Peninsula. 13
SARS Outbreak Evolution and Clinical Characteristics
The initial description of the SARS outbreak was announced in November 2002 through non-official reports of the occurrence of an outbreak of respiratory illness in Guangdong Province, China, 14 and few months later, this was reported to the World Health Organization (WHO). Analysis of the virus showed a point-source outbreak. 15 The disease was recognized due to the occurrence of a cluster of atypical pneumonias occurring in Vietnam, Hong Kong, Canada, United States, and Singapore. 1 16 17 18 19 20 21 22 23 All cases were linked to a patient who stayed in hotel M in Hong Kong, and subsequently, patients traveled from Hong Kong to Ireland, Vietnam, Singapore, United States, and Canada. 24 This outbreak involved 30 countries in 6 continents and caused a total of 8,098 cases with a case fatality rate of 9.5%. 25 The clinical spectrum of the disease ranged from mild to severe disease requiring mechanical ventilation. 26 The clinical picture followed an initial febrile illness, followed by a period of improvement then a clinical deterioration. 27 28 29 The need for intensive care unit (ICU) care was described in 17 to 30% of SARS patients. 28 29 30 In another study, 15% of SARS patients required mechanical ventilation. 27 Patients also had extra-respiratory symptoms such as diarrhea. 31 It was interesting to note that health care workers (HCWs) constituted 21% of all SARS cases. 32 33 34 The disease was associated with 10% case fatality rate, 35 and the presence of diabetes mellitus and other comorbidities was associated with increased fatality rates. 30 SARS was thought to cause milder disease in children with no fatalities. 36 One reason for the rapid spread of SARS was the occurrence of superspreaders. 35 Superspreading event is described as the ability of certain individuals to infect a disproportionately large number of secondary patients relative to a typical infectious individual.
The origin of the SARS virus is thought to be animal and a similar virus was isolated from Himalayan palm civets ( Paguma larvata ), raccoon dogs ( Nyctereutes procyonoides ), and from a Chinese ferret badger ( Melogale moschata ). 37 In addition, antibodies against SARS-CoV were found among wild animal traders in Guangdong Province. 37 38 A seroprevalence of 72.7% was well known among those with trading history involving P. larvata . 38 Although most patients with SARS had symptomatic disease, there are few seroprevalence studies and one study showed that 124 (12%) of 1,030 individuals were positive by ELISA and 0.19% by the SARS-specific immunofluorescence assay (IFA). 39 In another study, seroprevalence among HCWs was 2.3% 40 and a meta-analysis showed an overall seroprevalence of 0.10%. 41 There were no approved therapeutic or preventative options for SARS, while a variety of therapeutic agents were used. 42 SARS human cases disappeared abruptly by June 2003 with no approved vaccine or therapeutic agents developed or applied.
MERS-CoV Evolution and Origin of the Virus
The first case of MERS-CoV was reported in a businessman who lived in Bisha, Kingdom of Saudi Arabia (KSA) who presented to health care with pneumonia in early June 2012 and on transfer to a hospital in Jeddah, he rapidly succumbed to death within 10 days of diagnosis with multiorgan failure. The virus was later isolated and reported in September 2012 as the newly emerging MERS-CoV. As of January 2020, there have been a total of 2,468 cases of human MERS-CoV cases reported to WHO from 27 countries. More than 80% of cases have been reported from the Arabian Peninsula with KSA being the most affected country. There have been 851 reported mortalities with an overall case fatality rate of MERS-CoV estimated at 35% ( Fig. 1 ). The exact origin of MERS-CoV is not known. However, MERS-CoV is likely to have originated from bats based on the isolation of other lineage C β-coronaviruses closely related to MERS-CoV and the isolation of a bat coronavirus that resembles MERS-CoV. Throat swabs, urine, feces, and serum samples were collected from wild bats in the KSA including the area where the first MERS-CoV patient had lived and worked. A 190-nucleotide fragment of the RNA-dependent RNA polymerase region of MERS-CoV genome was detected in one fecal pellet from an Egyptian tomb bat ( Taphozous perforates ). 43 The amplified sequence was identical to that of the MERS-CoV sequence from the first index human case. 43 The one-humped dromedaries ( Camelus dromedarius ) had been linked to MERS-CoV ( Fig. 2 ). Multiple studies showed high prevalence of MERS-CoV antibodies in dromedary camels in the Arabian Peninsula, North Africa, and Eastern Africa. 44 45 46 47 48 49 50 In addition, studies have shown that MERS-CoV antibodies were present in stored camel sera as early as early 1990s, suggesting the presence of MERS-CoV in dromedaries for over 20 years before its first description in humans. 50 51 52 MERS-CoV antibodies were detected more commonly among camels > 2 years of age compared with younger camels. 46 52 53 54 In addition, MERS-CoV was detected from respiratory tract samples by reverse transcriptase polymerase chain reaction (RT-PCR) in oronasal and fecal samples from dromedary camels in the Arabian Peninsula. 52 53 54 55 56 57 58 In contrast to the MERS-CoV antibodies, juvenile camels shed more MERS-CoV as detected by PCR. 52 53 54 55 In addition, viable MERS-CoV was isolated in cell cultures from nasal and fecal samples from dromedary camels. 54 57 59 60 61 There had been studies documenting the isolation of similar and near-identical MERS-CoV strains from epidemiologically linked humans and dromedary camels. 61 62 63 In addition, sequence of the MERS-CoV spike, ORF3–4a, and nucleocapsid regions were identical from asymptomatic contacts and their camels. 64 The most recent common ancestor of all human MERS-CoV was found phylogenetically to date to the end of the year 2010. 65 In addition, animal reservoir is geographically dispersed. 66 67
Fig. 1.
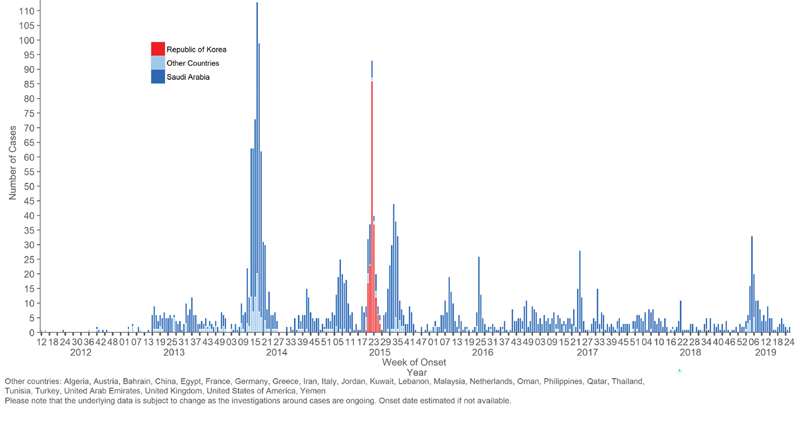
Epicurve of confirmed global cases of MERS-CoV from September 2012 to July 16, 2019. MERS-CoV, Middle East respiratory syndrome coronavirus; WHO, World Health Organization.
Fig. 2.

Camels: a possible intermediary source of Middle Eastern respiratory syndrome coronavirus.
Clinical Features and Laboratory Findings
The clinical and laboratory presentations of SARS-CoV and MERS-CoV are similar with some minor differences highlighted in Table 1 . The clinical picture of MERS-CoV cases ranges from asymptomatic to severe cases. In many cases, the presenting symptoms are respiratory and 33% of patients have gastrointestinal symptoms such as vomiting and diarrhea. 68 69 70 71 72 73 Most hospitalized MERS-CoV patients present with fever, cough, and shortness of breath with clinical and radiological evidence of pneumonia. 70 71 72 74 It seems that severe disease is a characteristic of primary cases, immunocompromised, and those with underlying comorbidities namely diabetes, kidney, and heart disease. In severe cases, there are multiple complications including respiratory and renal failure, acute liver injury, cardiac arrhythmias, and coagulopathy. 69 70 73 75 There are few studies which showed no predictive signs or symptoms to differentiate patients with community-acquired pneumonia from those with MERS-CoV infection. 72 76 The median incubation period was 5.2 days (95% confidence interval [CI], 1.9–14.7), and the serial interval was 7.6 days (95% CI, 2.5–23.1). 69 The median time to hospitalization, ICU admission, mechanical ventilation, and death were 5, 7, and 11 days, respectively. 69 77 MERS-CoV carries a high case fatality rate (28.6–63.6%) specially among elderly patients with several comorbidities, while in young healthy patients, they present with mild to no symptoms. 71 78 One study found a lower case fatality rate similar to the rate reported in patients from South Korea of 9%. 79 80 The variability of the case fatality rates may be related to host factors, associated comorbidities, care provided, and yet unidentified factors. 78 In addition, the case fatality rate is inversely related to the percentage of asymptomatic cases as the percentage of these patients increased to 29%, and the case fatality rate decreased to 30%. 70 71 77 81 82 83 In addition, the case fatality rate is higher among critically ill patients 76 78 79 80 comparing between MERS-CoV and non-MERS-CoV patients in relation to age, clinical, and laboratory features. 72 76 84 85 86 In KSA, extensive testing for MERS-CoV is being done over the past 6 years with > 50,000 patients presenting to emergency care with respiratory symptoms being screened for MERS-CoV each year with a very low yield of 0.7% being positive. 87 This excessive testing is applied in combination with a visual triage in all emergency rooms of all health care facilities (governmental and private) utilizing a clinical score cutoff of > 4 for MERS-CoV infection showing sensitivity and specificity of 74.1 and 18.6%, respectively, in predicting MERS-CoV diagnosis. 88
Table 1. Comparison of demographic, clinical, and laboratory features between MERS-CoV and SARS-CoV.
| MERS-CoV 8 36 37 38 39 | SARS-CoV 1 28 40 | |
|---|---|---|
| Date of first case report (place) | April 2012 (Jordan) | November 2002 (China) |
| June 2012 (first KSA case) | ||
| Incubation period | Mean: 5.2 d (95% CI: 1.9–14.7) | Mean: 4.6 d (95% CI: 3.8–5.8) |
| Range: 2–13 d | Range: 2–14 d | |
| Serial interval | 7.6 d | 8.4 d |
| Age group | ||
| Adults | 98% | 93% |
| Children | 2% | 5–7% |
| Age (y): range, median | Range: 1–94; median: 50 | Range: 1–91; mean: 39.9 |
| Mortality | ||
| CFR—overall | 41.8% | 9.6% |
| CFR in patients with comorbidities | 13.3% | 1–2% |
| Time from onset to death | Median 11.5 d | Mean 23.7d |
| Sex (M, F) | M: 64.5%, F: 35.5% | M: 43%, F: 57% |
| Presenting symptoms | ||
| Fever >38°C | 98% | 99–100% |
| Chills/rigors | 87% | 15–73% |
| Cough | 83% | 62–100% |
| Dry | 56% | 29–75% |
| Productive | 44% | 4–29% |
| Hemoptysis | 17% | 0–1% |
| Headache | 11% | 20–56% |
| Myalgia | 32% | 45–61% |
| Malaise | 38% | 31–45% |
| Shortness of breath | 72% | 40–42% |
| Nausea | 21% | 20–35% |
| Vomiting | 21% | 20–35% |
| Diarrhea | 26% | 20–25% |
| Sore throat | 14% | 13–25% |
| Rhinorrhea | 6% | 2–24% |
| Comorbidities | 76% | 10–30% |
| Diabetes | 10% | 24% |
| Chronic renal disease | 13% | 2–6% |
| Chronic heart disease | 7.5% | 10% |
| Malignancy | 2% | 3% |
| Hypertension | 34% | 19% |
| Obesity | 17% | N/A |
| Smoking | 23% | 17% |
| Viral hepatitis | Not known | 27% |
| Laboratory results | ||
| CXR abnormalities | 100% | 94–100% |
| Lymphopenia (<1.5 × 10 9 /L) | 32% | 68–85% |
| Leukopenia (<4.0 × 10 9 /L) | 14% | 25–35% |
| Thrombocytopenia (<140 × 10 9 /L) | 36% | 40–45% |
| Elevated LDH | 48% | 50–71% |
| Elevated ALT | 11% | 20–30% |
| Elevated AST | 14% | 20–30% |
| Ventilatory support required | 80% | 14–20% |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CFR, case fatality rate; CI, confidence interval; CXR, chest X-ray; KSA, Kingdom of Saudi Arabia; LDH, lactate dehydrogenase; MERS-CoV; Middle East respiratory syndrome coronavirus; SARS-CoV, severe acute respiratory syndrome coronavirus.
Source : Reproduced with permission from Hui et al. 161
Predictors of 30-day mortality included factors such as age > 65 years, being a non-HCW, the presence of preexisting comorbidities, presentation with severe disease, hospital-acquired infections, and corticosteroid use. 70 89 90 91 The use of continuous renal replacement therapy and extracorporeal membrane oxygenation (ECMO) were additional risk factors for increased fatality. 91 92 93 However, one study showed ECMO lowering in-hospital death. 94
Laboratory Tests
The diagnosis of MERS-CoV infection relies on the confirmation by real-time reverse transcriptase PCR of respiratory tract samples. Lower respiratory samples provide better yield and is the sample source of choice for testing. 95 96 However, a single negative test should not rule out infection and a repeat testing is indicated as some patients may have intermittent positive tests. 97 Serologic testing for MERS-CoV utilizes IFA, serum neutralization, or protein microarray assays to detect MERS-CoV antibodies. 98 The utility of serodiagnosis relies on two serum samples taken 14 days or more apart. Serodiagnosis begins with a screening ELISA or IFA and a confirmatory neutralization assay. 99 100 101 Testing for MERS-CoV by PCR detected the virus in the patient serum, urine, and feces but at a much lower level than those found in the lower respiratory tract. 102 Patients with MERS-CoV infection had abnormal laboratory findings including: leukopenia, lymphopenia, thrombocytopenia, and elevated hepatic enzymes. 68 71 72 76 103 A risk analysis showed that the following were associated with increased risk of death: presence of comorbidity (relative risk [RR] = 3), male gender (RR = 1.6), exposure to dromedary camels (RR = 1.6), and consumption of camel milk (RR = 1.5). 104 Overall, over the past 7 years, 50% of MERS-CoV cases reported to WHO were associated with human-to-human transmission in hospitals. Among 61 MERS-CoV patients presenting with MERS-CoV in 2017, 9 (15%) were associated with a hospital outbreak, 10 (16%) were household contacts, and 42 (69%) were sporadic cases. Of the 42 sporadic cases, 50% had camel contact. 105 In an outbreak investigation of a cluster of MERS cases in a nonhealth care–associated setting, 18 (2.2%) of 828 contacts were positive for MERS-CoV infections. 106 This rate was similar to household contact study of 4.3%. 101
Intrahospital Transmission
Health care–associated infection is the hallmark of the transmission of MERS-CoV between patients and from patients to HCWs. 69 70 74 81 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 Of the factors contributing to intrahospital transmission is the occurrence of superspreading events. In the outbreak in the Republic of Korea, three patients were epidemiologically connected to 73% of the transmissions and each infected 23, 28, and 85 individuals. 124 In addition, superspreader phenomena also occurred in the first reported outbreak in Al-Hasa, Saudi Arabia. 69 A recent systematic review outlined the contributing factors to health care–associated MERS-CoV transmissions and included: absent physical barriers between beds, inadequate isolation of suspected MERS patients, lack of isolation and negative pressure rooms, unfamiliarity and underrecognition of MERS infection, insufficient compliance with infection control measures, aerosol generating procedures, presence of multiple friends and family members in the patient's room, and the phenomena of “medical shopping.” 125 HCWs may act as contributors to the spread of MERS-CoV infection. In one study, MERS-CoV PCR was positive in 4.5% among exposed HCWs 126 and another study showed 15 (1.3%) of 1,169 HCWs were positive by PCR and 5 (0.68%) of 737 HCWs were positive by serology. 127 Other studies showed none of 38 HCWs was positive by serology 128 and none of 48 contacts was positive. 129 In Korea, 36 (19.9%) of 181 confirmed MERS-CoV cases were HCWs. 130 However, studies had showed that most positive HCWs were asymptomatic or had mild disease. 131 Although major hospital outbreaks were thought to be linked to intrahospital transmission of MERS-CoV, MERS-CoV genome sequence in these outbreaks showed multiple introductions of the virus with human-to-human transmissions. 66 67 69 There were three distinct MERS-CoV genotypes. 67
Seasonality of MERS-CoV
The emergence of MERS-CoV had led to many speculations regarding the seasonality of this disease and initially thought to occur mostly in March–May and September–November. 81 132 133 One reason for such a significant increase in April–May 2014 was a large outbreak in Jeddah, Saudi Arabia. 107 However, seasonal variation may be the result of seasonality in the calving of dromedaries in November and March. 10 44 46 48 52 55 77 82 83 134 Such a concept was studied and it was found that the prevalence of MERS-CoV was higher in camels in the winter (71.5%) than the summer season (6.2%). 135 Looking at all MERS-CoV cases from 2012 to 2016, the mean monthly cases were higher in the winter and summer months. 136 Evaluation of cases from January 2013 and December 2017 included a total of 2,025 cases and showed a noteworthy decrease in the annual cases in 2016 to 2017. 137 Of all the 2,025 cases, 38.2% occurred in the Spring and 36.4% occurred in the Summer. 137 However, there was no variation on the number of cases per year, and either per month or per season. 137
Therapeutic Options
Currently, there is no approved therapy for MERS-CoV infection. Studies showed superiority of interferon (IFN)-β compared with other IFN types 138 and that polyethylene glycol IFN-α had excellent cytopathic inhibitory effect. 139 In addition, the combination of INF-α2b and ribavirin showed augmentation of action and lower concentrations of IFN-α2b and ribavirin were required. 140 However, the data from clinical use of these two agents in retrospective studies showed no therapeutic advantages of these on survival of patients. 42 141 142 143 144 145 A retrospective analysis showed that using INF to treat patients with positive MERS-CoV RT-PCR was associated with a case fatality rate of 90% compared with 44% in those with negative MERS-CoV RT-PCR test. 68 Another study showed survival rates of 78.3, 75, and 68.4% using IFN-β, IFN-α, and ribavirin, respectively. 146 The use of the antiretroviral therapy for MERS-CoV was tried using pegylated IFN, ribavirin, and lopinavir/ritonavir 143 and another eight patients received mycophenolate mofetil and the latter patients survived. 146 A randomized controlled trial using a combination of lopinavir–ritonavir and IFN-β1b is being conducted. 147
Seroprevalence of MERS-CoV
Although MERS-CoV PCR testing is the main methodology for the diagnosis of MERS-CoV infection, serologic tests confirmed 8 (6.4%) of 124 Jordanian contacts who were positive. 119 Seroprevalence of 356 abattoir workers and blood donors found that 8 (2.2%) were weakly positive by immunofluorescence assay (IFA), and none was had positive neutralization titers. 148 A seroprevalence study found none of 268 children with respiratory tract infections to be positive. 149 In an evaluation of 280 household contacts, 12 (4.3%) were probable cases by serology. 101 However, in a population-based survey of 10,000 samples, the seroprevalence was 0.15% and the camel shepherd and abattoir workers had 17- and 26-fold increase in seroprevalence in comparison to the general population. 150
Infection Control
MERS-CoV is stable in the environment and can survive on plastic and steel for up to 48 hours at lower temperature and humidity. However, MERS-CoV is less viable at higher temperature and humidity. 151 This finding was confirmed by another study where a temperature of 65°C had a strong negative effect on viral infectivity compared with a temperature of 25°C. 152 In the hospital setting, WHO advocates contact and droplet precautions with airborne isolation when dealing with aerosol-generating procedures. 153 154 However, both the United States and the European Centre for Disease Prevention and Control recommend the use of airborne infection isolation precautions. 155
MERS and Camel Connections
In a recent study from Egypt, Senegal, Tunisia, Uganda, Jordan, Saudi Arabia, and Iraq, MERS-CoV was detected in camels using either PCR or serology. 156 The positivity rate using PCR ranged from 0% in Uganda, Jordan, and Iraq to 3.1% in Saudi Arabia, 5.5% in Senegal, and 8.2% in Egypt. 156 It was shown that seropositivity is very high (84.5%) among tested camels compared with PCR positivity of 3.8%. 157 Studies from Saudi Arabia showed either no significant difference in seropositivity of MERS-CoV in camels in different regions 156 or had detected variable seropositivity to MERS-CoV (37–100%). 158 It is worth mentioning that Somalia and Sudan are the main source of imported camels into Saudi Arabia. 156
The seroprevalence of MERS-CoV is lower (30.3%) in juvenile camels (<2 years of age) compared with adult camels (82.6%) 156 as described in the previous studies. 53 Also, the detection rate of MERS-CoV RNA by PCR is higher in adults (16.1%) compared with juvenile camels (1.7%). 156 What is unusual is the ability of MERS-CoV to causes reinfection of camels in the presence of antibodies. 56 156 Another important finding of MERS-CoV in camels is that camels rarely show signs of infection. 156 159 Although it has been postulated that drinking camel milk is one of the key sources of infection in the Arabian peninsula, a study found no MERS-CoV in the urine of naturally infected camels. 160
Conclusion
Emerging respiratory viruses, specially MERS-CoV, continue to challenge the public health infrastructure of countries of the Arabian Peninsula with the risk of transmission and outbreaks in other countries though travel. Although it is still debated by some, bats appear to be the common natural source of both SARS and MERS. There are considerable similarities in the clinical features of both MERS-CoV and SARS-CoV, but MERS tends to progress much faster to respiratory failure than SARS. Although SARS-CoV clinical cases disappeared since mid-2003, both MERS-CoV and SARS-CoV are still listed as priority pathogens by the WHO research and development blueprint. The case fatality rate of MERS-CoV is much higher and likely related to older age and comorbid illness of the sporadic cases. Several gaps continue in our knowledge about disease prevention and treatment, and more studies are needed to understand the pathogenesis, viral kinetics, mode of disease transmission, any other intermediary source, and treatment options of MERS to guide public health infection control measures and treatment.
Footnotes
Conflict of Interest None declared.
References
- 1.Lee N, Hui D, Wu A et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 2.Shaw K. The 2003 SARS outbreak and its impact on infection control practices. Public Health. 2006;120(01):8–14. doi: 10.1016/j.puhe.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki A M, van Boheemen S, Bestebroer T M, Osterhaus A D, Fouchier R A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Corman V M, Eckerle I, Bleicker T et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17(39):17. doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- 5.de Groot R J, Baker S C, Baric R S et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Tawfiq J A, Zumla A, Memish Z A. Coronaviruses: severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus in travelers. Curr Opin Infect Dis. 2014;27(05):411–417. doi: 10.1097/QCO.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 7.Al-Tawfiq J A, Zumla A, Gautret P et al. Surveillance for emerging respiratory viruses. Lancet Infect Dis. 2014;14(10):992–1000. doi: 10.1016/S1473-3099(14)70840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfe N D, Dunavan C P, Diamond J.Origins of major human infectious diseases Nature 2007447(7142):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyrrell D A, Bynoe M L.Cultivation of a novel type of common-cold virus in organ cultures BMJ 19651(5448):1467–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan J F, Lau S K, To K K, Cheng V C, Woo P C, Yuen K-Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28(02):465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drosten C, Günther S, Preiser W et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 12.Kuiken T, Fouchier R A, Schutten Met al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome Lancet 2003362(9380):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Boheemen S, de Graaf M, Lauber C et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3(06):e00473–e12. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng V C, Lau S K, Woo P C, Yuen K Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(04):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ksiazek T G, Erdman D, Goldsmith C S et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 16.Leung G M, Hedley A J, Ho L-M et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141(09):662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- 17.Tsang K W, Ho P L, Ooi G C et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 18.Poutanen S M, Low D E, Henry B et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348(20):1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC).Outbreak of severe acute respiratory syndrome–worldwide, 2003 MMWR Morb Mortal Wkly Rep 20035211226–228. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC).Preliminary clinical description of severe acute respiratory syndrome MMWR Morb Mortal Wkly Rep 20035212255–256. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC).Update: severe acute respiratory syndrome–United States, June 4, 2003 MMWR Morb Mortal Wkly Rep 20035222525–526. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC).Severe acute respiratory syndrome (SARS) and coronavirus testing–United States, 2003 MMWR Morb Mortal Wkly Rep 20035214297–302. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC).Severe acute respiratory syndrome–Singapore, 2003 MMWR Morb Mortal Wkly Rep 20035218405–411. [PubMed] [Google Scholar]
- 24.Parashar U D, Anderson L J. Severe acute respiratory syndrome: review and lessons of the 2003 outbreak. Int J Epidemiol. 2004;33(04):628–634. doi: 10.1093/ije/dyh198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization (WHO).Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003 WHO; 2015 [Google Scholar]
- 26.Chiang C-H, Shih J-F, Su W-J, Perng R-P. Eight-month prospective study of 14 patients with hospital-acquired severe acute respiratory syndrome. Mayo Clin Proc. 2004;79(11):1372–1379. doi: 10.4065/79.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung J J, Wu A, Joynt G M et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59(05):414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu L-Y, Lee C-C, Green J A et al. Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg Infect Dis. 2003;9(06):713–717. doi: 10.3201/eid0906.030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peiris J S, Chu C M, Cheng V Cet al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study Lancet 2003361(9371):1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth C M, Matukas L M, Tomlinson G Aet al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area [see comment] [erratum appears in JAMA 2003 Jul 16;290(3):334] JAMA 2003289212801–2809. [DOI] [PubMed] [Google Scholar]
- 31.Kwan Y W, Leung C W, Chiu M C. Diarrhoea as the presenting sign in an adolescent suffering from severe acute respiratory syndrome. Eur J Pediatr. 2005;164(04):227–230. doi: 10.1007/s00431-004-1618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuttall I, Dye C.Epidemiology. The SARS wake-up call Science 2013339(6125):1287–1288. [DOI] [PubMed] [Google Scholar]
- 33.Nuttall I, Dye C.Epidemiology. The SARS wake-up call Science 2013339(6125):1287–1288. [DOI] [PubMed] [Google Scholar]
- 34.Cameron P A, Rainer T H.SARS: a wake up call for a health care system under stress Emerg Med (Fremantle) 200315(5-6):409–412. [DOI] [PubMed] [Google Scholar]
- 35.Lapinsky S E, Granton J T. Critical care lessons from severe acute respiratory syndrome. Curr Opin Crit Care. 2004;10(01):53–58. doi: 10.1097/00075198-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Leung C W, Chiu W K. Clinical picture, diagnosis, treatment and outcome of severe acute respiratory syndrome (SARS) in children. Paediatr Respir Rev. 2004;5(04):275–288. doi: 10.1016/j.prrv.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guan Y, Zheng B J, He Y Qet al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China Science 2003302(5643):276–278. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC).Prevalence of IgG antibody to SARS-associated coronavirus in animal traders–Guangdong Province, China, 2003 MMWR Morb Mortal Wkly Rep 20035241986–987. [PubMed] [Google Scholar]
- 39.Tsai M-H, Lin T-Y, Chiu C-H et al. Seroprevalence of SARS coronavirus among residents near a hospital with a nosocomial outbreak. J Formos Med Assoc. 2008;107(11):885–891. doi: 10.1016/S0929-6646(08)60205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ip M, Chan P K, Lee N et al. Seroprevalence of antibody to severe acute respiratory syndrome (SARS)-associated coronavirus among health care workers in SARS and non-SARS medical wards. Clin Infect Dis. 2004;38(12):e116–e118. doi: 10.1086/421019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung G M, Lim W W, Ho L M et al. Seroprevalence of IgG antibodies to SARS-coronavirus in asymptomatic or subclinical population groups. Epidemiol Infect. 2006;134(02):211–221. doi: 10.1017/S0950268805004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Momattin H, Mohammed K, Zumla A, Memish Z A, Al-Tawfiq J A. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV)–possible lessons from a systematic review of SARS-CoV therapy. Int J Infect Dis. 2013;17(10):e792–e798. doi: 10.1016/j.ijid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Memish Z A, Mishra N, Olival K J et al. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19(11):1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reusken C B, Haagmans B L, Müller M A et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reusken C B, Ababneh M, Raj V Set al. Middle East respiratory syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013 Euro Surveill 2013185020662. Available at:https://www.omicsonline.org/open-access/the-most-effective-therapeutic-regimen-for-patients-with-severe-middle-eastrespiratory-syndrome-coronavirus-merscov-infection-2332-0877-1000223.php?aid=59207 [DOI] [PubMed] [Google Scholar]
- 46.Hemida M G, Perera R A, Wang P et al. Middle East respiratory syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013;18(50):20659. doi: 10.2807/1560-7917.es2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- 47.Alexandersen S, Kobinger G P, Soule G, Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transbound Emerg Dis. 2014;61(02):105–108. doi: 10.1111/tbed.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reusken C B, Messadi L, Feyisa A et al. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis. 2014;20(08):1370–1374. doi: 10.3201/eid2008.140590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nowotny N, Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveill. 2014;19(16):20781. doi: 10.2807/1560-7917.es2014.19.16.20781. [DOI] [PubMed] [Google Scholar]
- 50.Corman V M, Jores J, Meyer B et al. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992-2013. Emerg Infect Dis. 2014;20(08):1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hemida M G, Perera R A, Al Jassim R A et al. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. 2014;19(23):19. doi: 10.2807/1560-7917.es2014.19.23.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alagaili A N, Briese T, Mishra N et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5(02):e00884–e14. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hemida M G, Alnaeem A, Chu D K et al. Longitudinal study of Middle East respiratory syndrome coronavirus infection in dromedary camel herds in Saudi Arabia, 2014-2015. Emerg Microbes Infect. 2017;6(06):e56. doi: 10.1038/emi.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wernery U, Corman V M, Wong E Y et al. Acute Middle East respiratory syndrome coronavirus infection in livestock Dromedaries, Dubai, 2014. Emerg Infect Dis. 2015;21(06):1019–1022. doi: 10.3201/eid2106.150038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khalafalla A I, Lu X, Al-Mubarak A I, Dalab A H, Al-Busadah K A, Erdman D D. MERS-CoV in upper respiratory tract and lungs of dromedary camels, Saudi Arabia, 2013-2014. Emerg Infect Dis. 2015;21(07):1153–1158. doi: 10.3201/eid2107.150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farag E A, Reusken C B, Haagmans B L et al. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect Ecol Epidemiol. 2015;5:28305. doi: 10.3402/iee.v5.28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raj V S, Farag E A, Reusken C B et al. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg Infect Dis. 2014;20(08):1339–1342. doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yusof M F, Eltahir Y M, Serhan W S et al. Prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Abu Dhabi Emirate, United Arab Emirates. Virus Genes. 2015;50(03):509–513. doi: 10.1007/s11262-015-1174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hemida M G, Chu D K, Poon L L et al. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. 2014;20(07):1231–1234. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Briese T, Mishra N, Jain K et al. Middle East respiratory syndrome coronavirus quasispecies that include homologues of human isolates revealed through whole-genome analysis and virus cultured from dromedary camels in Saudi Arabia. MBio. 2014;5(03):e01146–e14. doi: 10.1128/mBio.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haagmans B L, Al Dhahiry S H, Reusken C B et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14(02):140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Memish Z A, Cotten M, Meyer B et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20(06):1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azhar E I, El-Kafrawy S A, Farraj S A et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 64.Al Hammadi Z M, Chu D K, Eltahir Y M et al. Asymptomatic MERS-CoV infection in humans possibly linked to infected dromedaries imported from Oman to United Arab Emirates, May 2015. Emerg Infect Dis. 2015;21(12):2197–2200. doi: 10.3201/eid2112.151132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lau S KP, Wong A CP, Lau T CK, Woo P CY. Molecular evolution of MERS coronavirus: dromedaries as a recent intermediate host or long-time animal reservoir? Int J Mol Sci. 2017;18(10):E2138. doi: 10.3390/ijms18102138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cotten M, Watson S J, Zumla A I et al. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. MBio. 2014;5(01):e01062–e13. doi: 10.1128/mBio.01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cotten M, Watson S J, Kellam Pet al. Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study Lancet 2013382(9909):1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shalhoub S, Farahat F, Al-Jiffri A et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70(07):2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Assiri A, McGeer A, Perl T M et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(05):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saad M, Omrani A S, Baig K et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Assiri A, Al-Tawfiq J A, Al-Rabeeah A A et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(09):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Tawfiq J A, Hinedi K, Ghandour J et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis. 2014;59(02):160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arabi Y M, Arifi A A, Balkhy H H et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(06):389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 74.Fagbo S F, Skakni L, Chu D K et al. Molecular epidemiology of hospital outbreak of Middle East respiratory syndrome, Riyadh, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21(11):1981–1988. doi: 10.3201/eid2111.150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Hameed F, Wahla A S, Siddiqui S et al. Characteristics and outcomes of Middle East respiratory syndrome coronavirus patients admitted to an intensive care unit in Jeddah, Saudi Arabia. J Intensive Care Med. 2016;31(05):344–348. doi: 10.1177/0885066615579858. [DOI] [PubMed] [Google Scholar]
- 76.Al-Tawfiq J A, Alfaraj S H, Altuwaijri T A, Memish Z A. A cohort-study of patients suspected for MERS-CoV in a referral hospital in Saudi Arabia. J Infect. 2017;75(04):378–379. doi: 10.1016/j.jinf.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.The WHO Mers-Cov Research Group.State of Knowledge and Data Gaps of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Humans PLoS Curr 20135pii: ecurrents.outbreaks.0bf719e352e7478f8ad85fa30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nam H-S, Park J W, Ki M, Yeon M-Y, Kim J, Kim S W. High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int J Infect Dis. 2017;58:37–42. doi: 10.1016/j.ijid.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim K H, Tandi T E, Choi J W, Moon J M, Kim M S. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: epidemiology, characteristics and public health implications. J Hosp Infect. 2017;95(02):207–213. doi: 10.1016/j.jhin.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi W S, Kang C-I, Kim Y et al. Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infect Chemother. 2016;48(02):118–126. doi: 10.3947/ic.2016.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Tawfiq J A, Memish Z A. Drivers of MERS-CoV transmission: what do we know? Expert Rev Respir Med. 2016;10(03):331–338. doi: 10.1586/17476348.2016.1150784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Tawfiq J A, Memish Z A. Middle East respiratory syndrome coronavirus: epidemiology and disease control measures. Infect Drug Resist. 2014;7:281–287. doi: 10.2147/IDR.S51283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Penttinen P M, Kaasik-Aaslav K, Friaux A et al. Taking stock of the first 133 MERS coronavirus cases globally–is the epidemic changing? Euro Surveill. 2013;18(39):18. doi: 10.2807/1560-7917.es2013.18.39.20596. [DOI] [PubMed] [Google Scholar]
- 84.Mohd H A, Memish Z A, Alfaraj S H et al. Predictors of MERS-CoV infection: a large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia. Travel Med Infect Dis. 2016;14(05):464–470. doi: 10.1016/j.tmaid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garbati M A, Fagbo S F, Fang V J et al. A comparative study of clinical presentation and risk factors for adverse outcome in patients hospitalised with acute respiratory disease due to MERS coronavirus or other causes. PLoS One. 2016;11(11):e0165978. doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Command and Control Center Ministry of Health Kingdom of Saudi Arabia Scientific Advisory Board.Infection Prevention and Control Guidelines for the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection, 4th Edition 2017. Available at:http://www.moh.gov.sa/endepts/Infection/Documents/Guidelines-for-MERS-CoV.PDF. Accessed May 12, 2017
- 87.Saeed A A, Abedi G R, Alzahrani A G et al. Surveillance and testing for Middle East respiratory syndrome coronavirus, Saudi Arabia, April 2015-February 2016. Emerg Infect Dis. 2017;23(04):682–685. doi: 10.3201/eid2304.161793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alfaraj S H, Al-Tawfiq J A, Gautret P, Alenazi M G, Asiri A Y, Memish Z A. Evaluation of visual triage for screening of Middle East respiratory syndrome coronavirus patients. New Microbes New Infect. 2018;26:49–52. doi: 10.1016/j.nmni.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmed A E. The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infect Dis. 2017;17(01):615. doi: 10.1186/s12879-017-2712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arabi Y M, Mandourah Y, Al-Hameed F et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(06):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 91.Alfaraj S H, Al-Tawfiq J A, Assiri A Y, Alzahrani N A, Alanazi A A, Memish Z A. Clinical predictors of mortality of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: a cohort study. Travel Med Infect Dis. 2019;29:48–50. doi: 10.1016/j.tmaid.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cha R-H, Joh J-S, Jeong I et al. Renal complications and their prognosis in Korean patients with Middle East respiratory syndrome-coronavirus from the Central MERS-CoV Designated Hospital. J Korean Med Sci. 2015;30(12):1807–1814. doi: 10.3346/jkms.2015.30.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arabi Y M, Al-Omari A, Mandourah Y et al. Critically ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45(10):1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 94.Alshahrani M S, Sindi A, Alshamsi F et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8(01):3. doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Memish Z A, Al-Tawfiq J A, Makhdoom H Q et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210(10):1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al-Tawfiq J A, Hinedi K. The calm before the storm: clinical observations of Middle East respiratory syndrome (MERS) patients. J Chemother. 2018;30(03):179–182. doi: 10.1080/1120009X.2018.1429236. [DOI] [PubMed] [Google Scholar]
- 97.Alfaraj S H, Al-Tawfiq J A, Memish Z A. Middle East respiratory syndrome coronavirus intermittent positive cases: implications for infection control. Am J Infect Control. 2019;47(03):290–293. doi: 10.1016/j.ajic.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corman V M, Müller M A, Costabel U et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17(49):49. doi: 10.2807/ese.17.49.20334-en. [DOI] [PubMed] [Google Scholar]
- 99.World Health Organization (WHO).Middle East respiratory syndrome coronavirus: case definition for reporting to WHO WHO; 2017 [Google Scholar]
- 100.Buchholz U, Müller M A, Nitsche A et al. Contact investigation of a case of human novel coronavirus infection treated in a German hospital, October-November 2012. Euro Surveill. 2013;18(08):18. [PubMed] [Google Scholar]
- 101.Drosten C, Meyer B, Müller M A et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371(09):828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 102.Drosten C, Seilmaier M, Corman V M et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13(09):745–751. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Omrani A S, Matin M A, Haddad Q, Al-Nakhli D, Memish Z A, Albarrak A M. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17(09):e668–e672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rahman A, Sarkar A. Risk factors for fatal Middle East respiratory syndrome coronavirus infections in Saudi Arabia: analysis of the WHO line list, 2013-2018. Am J Public Health. 2019;109(09):1288–1293. doi: 10.2105/AJPH.2019.305186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hakawi A, Rose E B, Biggs H M et al. Middle East respiratory syndrome coronavirus, Saudi Arabia, 2017-2018. Emerg Infect Dis. 2019;25(11):2149–2151. doi: 10.3201/eid2511.190726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Kerkhove M D, Alaswad S, Assiri A et al. Transmissibility of MERS-CoV infection in closed setting, Riyadh, Saudi Arabia, 2015. Emerg Infect Dis. 2019;25(10):1802–1809. doi: 10.3201/eid2510.190130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drosten C, Muth D, Corman V M et al. An observational, laboratory-based study of outbreaks of Middle East respiratory syndrome coronavirus in Jeddah and Riyadh, kingdom of Saudi Arabia, 2014. Clin Infect Dis. 2015;60(03):369–377. doi: 10.1093/cid/ciu812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Memish Z A, Al-Tawfiq J A, Alhakeem R F et al. Middle East respiratory syndrome coronavirus (MERS-CoV): a cluster analysis with implications for global management of suspected cases. Travel Med Infect Dis. 2015;13(04):311–314. doi: 10.1016/j.tmaid.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El Bushra H E, Abdalla M N, Al Arbash H et al. An outbreak of Middle East respiratory syndrome (MERS) due to coronavirus in Al-Ahssa Region, Saudi Arabia, 2015. East Mediterr Health J. 2016;22(07):468–475. [PubMed] [Google Scholar]
- 110.Balkhy H H, Alenazi T H, Alshamrani M M et al. Notes from the field: nosocomial outbreak of Middle East respiratory syndrome in a large tertiary care hospital–Riyadh, Saudi Arabia, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(06):163–164. doi: 10.15585/mmwr.mm6506a5. [DOI] [PubMed] [Google Scholar]
- 111.Balkhy H H, Alenazi T H, Alshamrani M M et al. Description of a hospital outbreak of Middle East respiratory syndrome in a large tertiary care hospital in Saudi Arabia. Infect Control Hosp Epidemiol. 2016;37(10):1147–1155. doi: 10.1017/ice.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Assiri A M, Biggs H M, Abedi G R et al. Increase in Middle East respiratory syndrome-coronavirus cases in Saudi Arabia linked to hospital outbreak with continued circulation of recombinant virus, July 1-August 31, 2015. Open Forum Infect Dis. 2016;3:ofw165. doi: 10.1093/ofid/ofw165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nazer R I. Outbreak of Middle East respiratory syndrome-coronavirus causes high fatality after cardiac operations. Ann Thorac Surg. 2017;104(02):e127–e129. doi: 10.1016/j.athoracsur.2017.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Assiri A, Abedi G R, Bin Saeed A A et al. Multifacility outbreak of Middle East respiratory syndrome in Taif, Saudi Arabia. Emerg Infect Dis. 2016;22(01):32–40. doi: 10.3201/eid2201.151370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hunter J C, Nguyen D, Aden B et al. Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg Infect Dis. 2016;22(04):647–656. doi: 10.3201/eid2204.151615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cauchemez S, Van Kerkhove M D, Riley S, Donnelly C A, Fraser C, Ferguson N M. Transmission scenarios for Middle East respiratory syndrome coronavirus (MERS-CoV) and how to tell them apart. Euro Surveill. 2013;18(24):20503. [PMC free article] [PubMed] [Google Scholar]
- 117.Cauchemez S, Fraser C, Van Kerkhove M D et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis. 2014;14(01):50–56. doi: 10.1016/S1473-3099(13)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chowell G, Abdirizak F, Lee S et al. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Al-Abdallat M M, Payne D C, Alqasrawi S et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59(09):1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hijawi B, Abdallat M, Sayaydeh A et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19 01:S12–S18. [PubMed] [Google Scholar]
- 121.Oboho I K, Tomczyk S M, Al-Asmari A M et al. 2014 MERS-CoV outbreak in Jeddah–a link to health care facilities. N Engl J Med. 2015;372(09):846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alraddadi B, Bawareth N, Omar H et al. Patient characteristics infected with Middle East respiratory syndrome coronavirus infection in a tertiary hospital. Ann Thorac Med. 2016;11(02):128–131. doi: 10.4103/1817-1737.180027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Almekhlafi G A, Albarrak M M, Mandourah Y et al. Presentation and outcome of Middle East respiratory syndrome in Saudi intensive care unit patients. Crit Care. 2016;20(01):123. doi: 10.1186/s13054-016-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim Y S, Aigerim A, Park U et al. Sequential emergence and wide spread of neutralization escape Middle East respiratory syndrome coronavirus mutants, South Korea, 2015. Emerg Infect Dis. 2019;25(06):1161–1168. doi: 10.3201/eid2506.181722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Al-Tawfiq J A, Auwaerter P G. Healthcare-associated Infections: the hallmark of the Middle East respiratory syndrome coronavirus (MERS-CoV) with review of the literature. J Hosp Infect. 2019;101(01):20–29. doi: 10.1016/j.jhin.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alfaraj S H, Al-Tawfiq J A, Altuwaijri T A, Alanazi M, Alzahrani N, Memish Z A. Middle East respiratory syndrome coronavirus transmission among health care workers: implication for infection control. Am J Infect Control. 2018;46(02):165–168. doi: 10.1016/j.ajic.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim C-J, Choi W S, Jung Y et al. Surveillance of the Middle East respiratory syndrome (MERS) coronavirus (CoV) infection in healthcare workers after contact with confirmed MERS patients: incidence and risk factors of MERS-CoV seropositivity. Clin Microbiol Infect. 2016;22(10):880–886. doi: 10.1016/j.cmi.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wiboonchutikul S, Manosuthi W, Sangsajja C. Zero transmission of Middle East respiratory syndrome: lessons learned from Thailand. Clin Infect Dis. 2017;64 02:S167–S170. doi: 10.1093/cid/cix074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hall A J, Tokars J I, Badreddine S A et al. Health care worker contact with MERS patient, Saudi Arabia. Emerg Infect Dis. 2014;20(12):2148–2151. doi: 10.3201/eid2012.141211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim S G. Healthcare workers infected with Middle East respiratory syndrome coronavirus and infection control. J Korean Med Assoc. 2015;58:647–654. [Google Scholar]
- 131.Alraddadi B M, Al-Salmi H S, Jacobs-Slifka K et al. Risk factors for Middle East respiratory syndrome coronavirus infection among healthcare personnel. Emerg Infect Dis. 2016;22(11):1915–1920. doi: 10.3201/eid2211.160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Noorwali A A, Turkistani A M, Asiri S I et al. Descriptive epidemiology and characteristics of confirmed cases of Middle East respiratory syndrome coronavirus infection in the Makkah Region of Saudi Arabia, March to June 2014. Ann Saudi Med. 2015;35(03):203–209. doi: 10.5144/0256-4947.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zumla A, Azhar E I, Arabi Y et al. Host-directed therapies for improving poor treatment outcomes associated with the Middle East respiratory syndrome coronavirus infections. Int J Infect Dis. 2015;40:71–74. doi: 10.1016/j.ijid.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gossner C, Danielson N, Gervelmeyer A et al. Human-dromedary camel interactions and the risk of acquiring zoonotic Middle East respiratory syndrome coronavirus infection. Zoonoses Public Health. 2016;63(01):1–9. doi: 10.1111/zph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kasem S, Qasim I, Al-Doweriej A et al. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in livestock and temporal relation to locations and seasons. J Infect Public Health. 2018;11(06):884–888. doi: 10.1016/j.jiph.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aly M, Elrobh M, Alzayer M, Aljuhani S, Balkhy H. Occurrence of the Middle East respiratory syndrome coronavirus (MERS-CoV) across the Gulf Corporation Council countries: four years update. PLoS One. 2017;12(10):e0183850. doi: 10.1371/journal.pone.0183850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Al-Tawfiq J A, Memish Z A. Lack of seasonal variation of Middle East respiratory syndrome coronavirus (MERS-CoV) Travel Med Infect Dis. 2019;27:125–126. doi: 10.1016/j.tmaid.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hart B J, Dyall J, Postnikova Eet al. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays J Gen Virol 201495(Pt 3):571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Wilde A H, Raj V S, Oudshoorn Det al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment J Gen Virol 201394(Pt 8):1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Falzarano D, de Wit E, Martellaro C, Callison J, Munster V J, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Al-Tawfiq J A, Momattin H, Dib J, Memish Z A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Omrani A S, Saad M M, Baig K et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spanakis N, Tsiodras S, Haagmans B L et al. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents. 2014;44(06):528–532. doi: 10.1016/j.ijantimicag.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt T S, Al Mutairy E. Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir Ther. 2015;20(01):87–91. doi: 10.3851/IMP2792. [DOI] [PubMed] [Google Scholar]
- 145.Tawalah H, Al-Qabandi S, Sadiq M, Chehadeh C, Al-Hujailan G, Al-Qaseer M. The most effective therapeutic regimen for patients with severe Middle East respiratory syndrome coronavirus (MERS-CoV) infection. J Infect Dis Ther. 2015;03:1–5. [Google Scholar]
- 146.Al Ghamdi M, Alghamdi K M, Ghandoora Y et al. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis. 2016;16:174. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Arabi Y M, Alothman A, Balkhy H H et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(01):81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aburizaiza A S, Mattes F M, Azhar E I et al. Investigation of anti-middle East respiratory syndrome antibodies in blood donors and slaughterhouse workers in Jeddah and Makkah, Saudi Arabia, fall 2012. J Infect Dis. 2014;209(02):243–246. doi: 10.1093/infdis/jit589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gierer S, Hofmann-Winkler H, Albuali W H et al. Lack of MERS coronavirus neutralizing antibodies in humans, eastern province, Saudi Arabia. Emerg Infect Dis. 2013;19(12):2034–2036. doi: 10.3201/eid1912.130701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Müller M A, Meyer B, Corman V M et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15(05):559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van Doremalen N, Bushmaker T, Munster V J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18(38):20590. doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- 152.Leclercq I, Batéjat C, Burguière A M, Manuguerra J C. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir Viruses. 2014;8(05):585–586. doi: 10.1111/irv.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.WHO.WHO|Infection prevention and control of epidemic-and pandemic prone acute respiratory infections in health care WHO; 2015 [PubMed]
- 154.World Health Organization (WHO).Infection prevention and control of epidemic-and pandemic prone acute respiratory infections in health care WHO; 2015 [PubMed]
- 155.CDC.Interim Infection Prevention and Control Recommendations for Hospitalized Patients with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) 2015. Available at:https://www.cdc.gov/coronavirus/mers/infection-prevention-control.html. Accessed March 9, 2017
- 156.Kandeil A, Gomaa M, Nageh A et al. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Africa and Middle East. Viruses. 2019;11(08):717. doi: 10.3390/v11080717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ali M A, Shehata M M, Gomaa M R et al. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg Microbes Infect. 2017;6(01):e1. doi: 10.1038/emi.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kasem S, Qasim I, Al-Hufofi A et al. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J Infect Public Health. 2018;11(03):331–338. doi: 10.1016/j.jiph.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chu D K, Poon L L, Gomaa M M et al. MERS coronaviruses in dromedary camels, Egypt. Emerg Infect Dis. 2014;20(06):1049–1053. doi: 10.3201/eid2006.140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Farag E A, Haagmans B L, Al-Romaihi H et al. Failure to detect MERS-CoV RNA in urine of naturally infected dromedary camels. Zoonoses Public Health. 2019;66(05):437–438. doi: 10.1111/zph.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hui D S, Memish Z A, Zumla A. Severe acute respiratory syndrome versus the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]


