Abstract
The severity of coronavirus disease 2019 (COVID-19) is linked to an increasing number of risk factors, including exogenous (environmental) stimuli such as air pollution, nicotine, and cigarette smoke. These three factors increase the expression of angiotensin I converting enzyme 2 (ACE2), a key receptor involved in the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—the etiological agent of COVID-19—into respiratory tract epithelial cells. Patients with severe COVID-19 are managed with oxygen support, as are at-risk individuals with chronic lung disease. To date, no study has examined whether an increased fraction of inspired oxygen (FiO2) may affect the expression of SARS-CoV-2 entry receptors and co-receptors, including ACE2 and the transmembrane serine proteases TMPRSS1, TMPRSS2, and TMPRSS11D. To address this, steady-state mRNA levels for genes encoding these SARS-CoV-2 receptors were assessed in the lungs of mouse pups chronically exposed to elevated FiO2, and in the lungs of preterm-born human infants chronically managed with an elevated FiO2. These two scenarios served as models of chronic elevated FiO2 exposure. Additionally, SARS-CoV-2 receptor expression was assessed in primary human nasal, tracheal, esophageal, bronchial, and alveolar epithelial cells, as well as primary mouse alveolar type II cells exposed to elevated oxygen concentrations. While gene expression of ACE2 was unaffected, gene and protein expression of TMPRSS11D was consistently upregulated by exposure to an elevated FiO2. These data highlight the need for further studies that examine the relative contribution of the various viral co-receptors on the infection cycle, and point to oxygen supplementation as a potential risk factor for COVID-19.
Keywords: COVID-19, FiO2, hyperoxia, SARS-CoV-2, TMPRSS
INTRODUCTION
The world currently finds itself in the grip of the coronavirus disease 2019 (COVID-19) pandemic caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (18). A number of risk factors for severe COVID-19 have been identified, including advanced age, as well as comorbidities including hypertension, obesity, malignancy, and chronic lung disease (11). Risk factors could also include stimuli that modify susceptibility to SARS-CoV-2 infection, for example, by modulating the expression of receptors that mediate viral entry into cells. Endogenous physiological modulators of viral entry receptors such as estrogen (16) and interferon (19) have recently been identified. However, exogenous (environmental) modulators of virus receptor expression may represent hitherto unrecognized risk factors for COVID-19. Indeed, air pollution (3) as well as cigarette smoke (8) and nicotine (7) have recently been proposed to increase the risk of severe COVID-19 by driving expression of the SARS-CoV-2 entry receptor angiotensin I converting enzyme 2 (ACE2) (5).
COVID-19 patients with acute respiratory failure are managed with supplemental oxygen (18), and elderly patients and infants born preterm with restrictive and obstructive lung disease may use home oxygen therapy. This raises the question of whether an elevated FiO2 might alter the expression of the virus entry receptor ACE2, as well as the virus co-receptors that facilitate cellular entry of SARS coronaviruses, including the serine peptidases TMPRSS1, TMPRSS2, and TMPRSS11D (4, 10). The full spectrum of SARS-CoV-2 receptors has not been definitively clarified, and a growing number of candidate receptors continue to be profiled in the epithelium of the entire respiratory tract (1, 17). To answer the question of whether an elevated FiO2 might impact the regulation of virus receptor genes, the expression of SARS-CoV-2 entry receptors and co-receptors was assessed in lung tissue from preterm-born infants with bronchopulmonary dysplasia (BPD) managed chronically with elevated FiO2, or from mouse pups exposed to elevated FiO2 for protracted periods in an animal model of BPD. It is critical to emphasize that this study is neither a BPD study, nor a study on addressing COVID-19 in infants (9). Rather, the clinical and experimental BPD models served purely as models of chronic supplemental oxygen exposure. This report should not be misunderstood as a pediatric study. Newborn mice were employed in the present study because newborn mice tolerate protracted periods of exposure to high FiO2, whereas adult mice do not and rapidly succumb to lethal acute lung injury. The expression of entry receptors and co-receptors was also assessed in adult mouse and adult human epithelial cells originating from different regions of the respiratory tract between the nose and the alveoli, as well as the esophagus, which were exposed in vitro to elevated oxygen concentrations.
MATERIALS AND METHODS
Mouse tissue.
In an experimental animal model of BPD [Institutional Animal Care and Use Committee (IACUC) approval for RNA profiling of mouse lung tissues under approval references B2/277 and B2/351]. Newborn mouse pups inhaled an FiO2 of 0.21 or 0.85 from the day of birth to post-natal day (P)14 (12).
Human tissue.
Harvesting of human lung tissue from BPD patients [x̄ birth wt 706 ± 222 g; 6 male/4 female; x̄ duration FiO2 >0.5, 29 day (range 5–96 days)] and term infant controls (x̄ birth wt 2,033 ± 1,356 g; 2 male/6 female; no oxygen supplementation) without lung disease was approved by the Institutional Review Board of the University of Rochester (2). Written informed consent was obtained from guardians.
Human and mouse lung epithelial cells.
Mouse alveolar epithelial type II cells were isolated from female C57BL/6J mice (3–6 mo of age) as described previously (14). Human airways and lung epithelial cells were purchased from Cell Biologics (Planegg, Germany): tracheal (H-6033), esophageal (H-6046), bronchial (H-6033B), and alveolar (H-6053). Human nasal epithelial cells were purchased from PromoCell (C-12620). The sexes of the donors of the human respiratory tract cells were not disclosed by the vendor. Cells were not passaged, and were cultured per manufacturer’s recommendations, and maintained on air-liquid interfaces in a 21% or 85% O2 atmosphere (14).
Real-time RT-PCR profiling of gene expression.
The abundance of mRNA transcripts was screened by real-time RT-PCR (14) using the primer pairs (5′→3′): human ACE2 (ACAGTCCACACTTGCCCAAAT, TGAGAGCACTGAAGACCCATT), TMPRSS1 (CCTGCCCCTCACAGAATACAT, GACATCATTGCTGATTATGGGGA), TMPRSS2 (CAAGTGCTCCAACTCTGGGAT, AACACACCGATTCTCGTCCTC), TMPRSS11D (CACAGTTCCAGAGCTAAGGCA, ACTCCACCTTGAGGTACTCCA), with POLR2A as reference gene (GCGGAATGGAAGCACGTTAAT, CCCAGCACAAAACACTCCTC); and mouse Ace2 (GCAGATGGCTACAACTATAACCG, CCTCCTCACATAGGCATGAAGA), Tmprss1 (TACCTTCCCTTTCGAGACCCT, CCATAGAACTGTGTGTTACCCCA), Tmprss2 (GCTGTCTTGCTTTGGAGGTTC, GACAATGTGCTACCCCGTCA), Tmprss11d (GGTACAGCTCCGTAACTCGTG, GCTGGGAGACATACCCTATGGAT), with Polr2a as reference gene (CTAAGGGGCAGCCAAAGAAAC, CATTCAGCATACAACTCTAGGC). Data are presented as difference in cycle threshold (Ct), ΔCt, given by Ct(Polr2a) – Ct(gene of interest). Fold-change in mRNA abundance was given by fold-change = 2|ΔΔCt|.
Immunoblotting.
Protein extracts were prepared as described previously (15), from the neonate mouse lungs and the human tracheal epithelial cells described above. Proteins were resolved on a 12% reducing SDS-PAGE gel, electroblotted to a nitrocellulose membrane. TMPRSS11D was detected with a rabbit anti-human TMPRSS11D (ab127031, Abcam, Cambridge, UK; 1:1,500) which detected both the human and mouse antigen. Two proteins were employed to document loading equivalence: β-actin and β-tubulin. Two different loading controls were employed because β-actin has a similar molecular mass to TMPRSS11D, and loading equivalence was documented on the same membrane. Thus, the TMPRSS11D signal may have affected the β-actin signal. β-Actin was detected with a rabbit anti-human β-actin antibody (4967S, Cell Signaling Technology, Frankfurt am Main, Germany; 1:1,000), and β-tubulin was detected with a rabbit anti-human β-tubulin antibody (ab6046, Abcam, Cambridge, UK; 1:1,000). Immune complexes were detected with a horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L) (31460, ThermoFisher, Waltham, MA; 1:3,000), and visualized using SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher, Waltham, MA).
RESULTS AND DISCUSSION
Expression of SARS-CoV-2 receptors in experimental and clinical BPD.
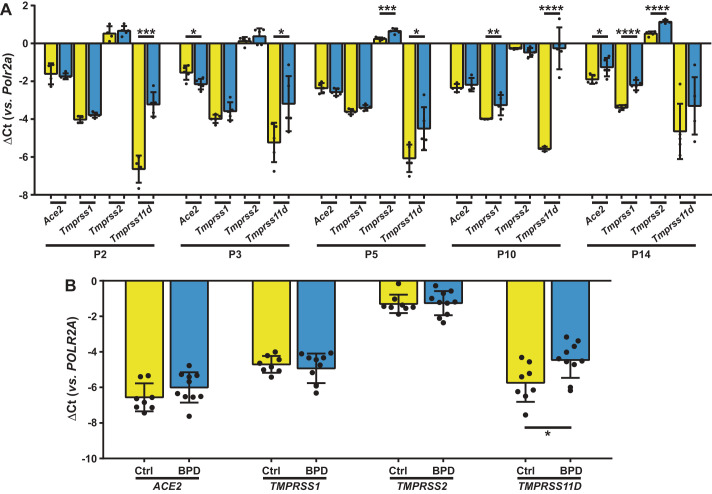
Exposure of newborn mouse pups to FiO2 0.85 increased lung expression of Tmprss11d 10.5-fold after 2 days, and Tmprss11d expression remained elevated, up to 40-fold at 10 days (Fig. 1A). By 14 days, Ace2, Tmprss1, and Tmprss2 expression was increased 1.5-, 2-, and 1.5-fold, respectively (Fig. 1A). The increased protein expression of TMPRSS11D in the lungs of mice exposed to FiO2 0.85 was validated by immunoblot (see Fig. 3A). In BPD patients that were chronically managed with FiO2 > 0.5, no changes in ACE2, TMPRSS1, and TMPRSS2 expression were noted. However, a 2.5-fold increase in TMPRSS11D expression was noted, compared with term infants without oxygen supplementation (Fig. 1B).
Fig. 1.
Steady-state levels of lung mRNA transcripts encoding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry receptors and co-receptors in experimental animals and clinical subjects chronically exposed to elevated FiO2. A: steady-state mRNA levels were determined by real-time RT-PCR for Ace2, Tmprss1, Tmprss2, and Tmprss11d in lung homogenate cDNA from C57BL/6J mouse pups (n = 5 animals per group) at post-natal day (P)2, 3, 5, 10, and 14, exposed either to room air (FiO2 0.21; yellow bars) or hyperoxia (FiO2 0.85; blue bars) for the first 14 days of post-natal life. B: steady-state mRNA levels were similarly determined for ACE2, TMPRSS1, TMPRSS2, and TMPRSS11D in lung homogenate cDNA from infants without (Ctrl, n = 8 patients; yellow bars) or with bronchopulmonary dysplasia (BPD; n = 10 patients; blue bars). Data reflect mean ΔCt ± SD. Pairwise comparisons were made between the 21% O2 and 85% O2 groups by unpaired Student’s t test (A), and between the Ctrl and BPD groups by Mann-Whitney U test (B). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ACE2, angiotensin I converting enzyme 2; POLR2A/Polr2a, RNA polymerase II subunit A; TMPRSS, transmembrane serine protease.
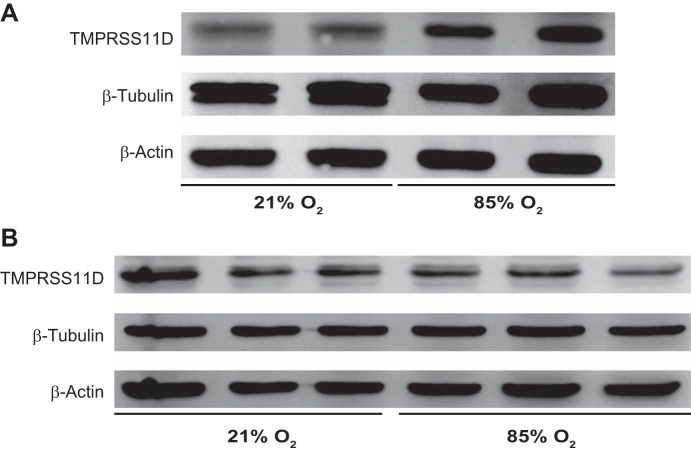
Fig. 3.
Steady-state TMPRSS11D protein expression levels in human tracheal epithelial cells and in the lungs of mice or exposed elevated oxygen levels. A: newborn mouse pups were exposed to either an FiO2 of 0.21 or 0.85 for the first 14 days of postnatal life, after which lungs were harvested for protein isolation. B: primary human tracheal epithelial cells were cultured on an air-liquid interface and exposed to a 21% O2 or an 85% O2 incubator headspace environment, and processed for protein isolation. Protein extracts were resolved on a 12% reducing SDS-PAGE gel, electroblotted to nitrocellulose, and TMPRSS11D was detected by immunoblot. Blots were reprobed for both β-actin (which has the same formula mass as TMPRSS11D), and β-tubulin, which served as controls for loading equivalence. The uncropped blots are provided in Supplemental Fig. S1 (https://doi.org/10.6084/m9.figshare.12855341.v1). TMPRSS, transmembrane serine protease.
Impact of elevated oxygen levels on SARS-CoV-2 receptor expression.
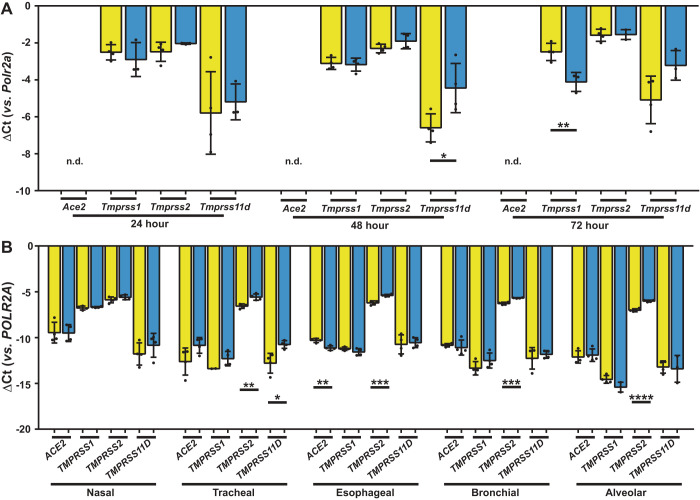
A pilot range-finding study was used to identify the minimum duration of oxygen exposure that was required to induce perturbations to co-receptor gene expression (Fig. 2A). Exposure of mouse alveolar epithelial type II cells to 85% O2 in vitro increased Tmprss11d expression 4.4-fold by 48 h (Fig. 2A) without affecting Ace2, Tmprss1, or Tmprss2 expression. In human airways, lung and gut epithelial cells, TMPRSS2 expression was consistently increased in tracheal (2-fold), esophageal (1.7-fold), bronchial (1.5-fold), and alveolar (2-fold) epithelial cells after 48 h exposure to 85% O2 (Fig. 2B). TMPRSS11D expression was increased (4-fold) in tracheal epithelial cells. However, no increase in the protein expression of TMPRSS11D was detected in tracheal epithelial cells after a 48-h 85% O2 exposure (Fig. 3B see also Supplemental Fig. S1; https://doi.org/10.6084/m9.figshare.12855341.v1). These data suggest that while a 48-h hyperoxia-exposure period is sufficient to increase TMPRSS11D mRNA transcript levels, an impact of hyperoxia on steady-state protein expression levels may require a longer oxygen-exposure period. There was no impact of 85% O2 exposure on the expression of ACE2 or TMPRSS1 (Fig. 2B).
Fig. 2.
Steady-state levels of mRNA transcripts encoding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry receptors and co-receptors in airways and lung epithelial cells. A: steady-state mRNA levels were determined by real-time RT-PCR for Ace2, Tmprss1, Tmprss2, and Tmprss11d in cDNA prepared from adult C57BL/6J mouse alveolar epithelial type II cells (n = 4 independent cell-cultures per group) incubated on an air-liquid interface under room air (21% O2: yellow bars) or hyperoxia (85% O2: blue bars) conditions for 24, 48, or 72 h. B: steady-state mRNA levels were similarly determined for ACE2, TMPRSS1, TMPRSS2, and TMPRSS11D in adult human nasal, tracheal, esophageal, bronchial, and alveolar epithelial cells (n = 4 independent cell-cultures per group) incubated on an air/liquid interface under room air (21% O2: yellow bars) or hyperoxia (85% O2: blue bars) conditions for 48 h. Data reflect mean ΔCt ± SD. Pairwise comparisons were made between the 21% O2 and 85% O2 groups by unpaired Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. n.d., not detected. ACE2, angiotensin I converting enzyme 2; POLR2A/Polr2a, RNA polymerase II subunit A; TMPRSS, transmembrane serine protease.
Changes in the expression of the SARS-CoV-2 entry receptor ACE2 and the virus co-receptors TMPRSS1, TMPRSS2, and TMPRSS11D are believed to influence the risk of severe COVID-19. This has led to speculation that air pollution and smoking, which upregulate ACE2 expression in the respiratory epithelium, may exacerbate the course of COVID-19 in affected patients. Consistent with other reports that analyzed adult human respiratory tract tissue (6), the analyses reported here reveal that in cultured epithelial cells from trachea, bronchi, and alveoli, TMPRSS2 was more abundant at baseline than was ACE2; and that ACE2 was most abundant in the nasal epithelium compared with more distal respiratory tract epithelium. Furthermore, baseline abundance of TMPRSS2 and ACE2 were comparable between cultured tracheal, bronchial, and alveolar epithelial cells.
The key question posed in this study: “does increased FiO2 drive SARS-CoV-2 entry receptor or co-receptor expression?” was addressed using lung tissue from patients with BPD, as well as an experimental animal model of BPD. This was neither a BPD nor a pediatric study. Rather, clinical or experimental BPD was employed as a model of supplemental oxygen support with lengthy use of an FiO2 of >0.5. The data presented here document that elevated FiO2 increased expression of TMPRSS2 and TMPRSS11D, which are required for efficient viral entry of SARS-CoV-2 (13). TMPRSS11D in particular has emerged in this study as being oxygen-responsive in both mouse and human airways and lungs. These data are potentially important, given that COVID-19 patients with acute respiratory failure are managed with oxygen support, which might increase viral co-receptor expression in the airways and lung epithelium of affected patients, thus accelerating the infection cycle. High-risk patients such as elderly (or young) patients with chronic lung disease in which home oxygen therapy forms part of routine disease management, or preterm born survivors of BPD discharged on home oxygen, may be similarly affected. These data highlight the need for further studies that examine the relative contribution of the various viral co-receptors on the infection cycle, and point to oxygen supplementation as a potential risk factor for COVID-19.
GRANTS
W.S. and R.E.M. were supported by the Max Planck Society; I.V., S.H., W.S., and R.E.M. were supported by the German Center for Lung Research (Deutsches Zentrum für Lungenforschung; DZL) and the German Research Foundation (Deutsche Forschungsgemeinschaft; DFG) through EXC2026 (390649896) and KFO309 (284237345). R.E.M. and W.S. were supported by the DFG through SFB1213 (268555672). R.E.M. was supported by the DFG through Mo 1789/1-1 (160966624) and Mo 1789/4-1 (420759458). S.H. and W.S. were supported by the DFG through SFB-TR84 (114933180).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M., M.G., F.C., S.K.R., C.N., I.V., S.H., G.P., W.S., and R.E.M. conceived and designed research; D.M., M.G., F.C., S.K.R., and C.N. performed experiments; D.M., M.G., F.C., S.K.R., C.N., I.V., S.H., G.P., W.S., and R.E.M. analyzed data; D.M., M.G., F.C., S.K.R., C.N., I.V., S.H., G.P., W.S., and R.E.M. interpreted results of experiments; D.M., M.G., F.C., S.K.R., and C.N. prepared figures; D.M., M.G., F.C., S.K.R., C.N., and R.E.M. drafted manuscript; D.M., C.N., and R.E.M. edited and revised manuscript; D.M., M.G., F.C., S.K.R., C.N., I.V., S.H., G.P., W.S., and R.E.M. approved final version of manuscript.
REFERENCES
- 1.Aguiar JA, Tremblay BJ, Mansfield MJ, Woody O, Lobb B, Banerjee A, Chandiramohan A, Tiessen N, Cao Q, Dvorkin-Gheva A, Revill S, Miller MS, Carlsten C, Organ L, Joseph C, John A, Hanson P, Austin RC, McManus BM, Jenkins G, Mossman K, Ask K, Doxey AC, Hirota JA. Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. Eur Respir J 56: 2001123, 2020. doi: 10.1183/13993003.01123-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, Pryhuber GS. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med 186: 349–358, 2012. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frontera A, Cianfanelli L, Vlachos K, Landoni G, Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J Infect 81: 255–259, 2020. doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 88: 1293–1307, 2014. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH III, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Nakano S, Sun L, Fulcher ML, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O’Neal WK, Randell SH, Boucher RC, Baric RS. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182: 429–446.e14, 2020. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung JM, Yang CX, Sin DD. COVID-19 and nicotine as a mediator of ACE-2. Eur Respir J 55: 2001261, 2020. doi: 10.1183/13993003.01261-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, Dorscheid DR, Sin DD. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 55: 2000688, 2020. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lingappan K, Karmouty-Quintana H, Davies J, Akkanti B, Harting MT. Understanding the age divide in COVID-19: why are children overwhelmingly spared? Am J Physiol Lung Cell Mol Physiol 319: L39–L44, 2020. doi: 10.1152/ajplung.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menou A, Duitman J, Flajolet P, Sallenave JM, Mailleux AA, Crestani B. Human airway trypsin-like protease, a serine protease involved in respiratory diseases. Am J Physiol Lung Cell Mol Physiol 312: L657–L668, 2017. doi: 10.1152/ajplung.00509.2016. [DOI] [PubMed] [Google Scholar]
- 11.Miller R, Englund K. Transmission and risk factors of OF COVID-19. Cleve Clin J Med. In press. 10.3949/ccjm.87a.ccc029. [DOI] [PubMed] [Google Scholar]
- 12.Nardiello C, Mižíková I, Silva DM, Ruiz-Camp J, Mayer K, Vadász I, Herold S, Seeger W, Morty RE. Standardisation of oxygen exposure in the development of mouse models for bronchopulmonary dysplasia. Dis Model Mech 10: 185–196, 2017. doi: 10.1242/dmm.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11: 1620, 2020. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rath P, Nardiello C, Surate Solaligue DE, Agius R, Mižíková I, Hühn S, Mayer K, Vadász I, Herold S, Runkel F, Seeger W, Morty RE. Caffeine administration modulates TGF-β signaling but does not attenuate blunted alveolarization in a hyperoxia-based mouse model of bronchopulmonary dysplasia. Pediatr Res 81: 795–805, 2017. doi: 10.1038/pr.2017.21. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Camp J, Quantius J, Lignelli E, Arndt PF, Palumbo F, Nardiello C, Surate Solaligue DE, Sakkas E, Mižíková I, Rodríguez-Castillo JA, Vadász I, Richardson WD, Ahlbrecht K, Herold S, Seeger W, Morty RE. Targeting miR-34a/Pdgfra interactions partially corrects alveologenesis in experimental bronchopulmonary dysplasia. EMBO Mol Med 11: e9448, 2019. doi: 10.15252/emmm.201809448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stelzig KE, Canepa-Escaro F, Schiliro M, Berdnikovs S, Prakash YS, Chiarella SE. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 318: L1280–L1281, 2020. doi: 10.1152/ajplung.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Rostami MR, Leopold PL, Mezey JG, O’Beirne SL, Strulovici-Barel Y, Crystal RG. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med 202: 219–229, 2020. doi: 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH II, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J, Banovich N, Barbry P, Brazma A, Desai T, Duong TE, Eickelberg O, Falk C, Farzan M, Glass I, Haniffa M, Horvath P, Hung D, Kaminski N, Krasnow M, Kropski JA, Kuhnemund M, Lafyatis R, Lee H, Leroy S, Linnarson S, Lundeberg J, Meyer K, Misharin A, Nawijn M, Nikolic MZ, Ordovas-Montanes J, Pe’er D, Powell J, Quake S, Rajagopal J, Tata PR, Rawlins EL, Regev A, Reyfman PA, Rojas M, Rosen O, Saeb-Parsy K, Samakovlis C, Schiller H, Schultze JL, Seibold MA, Shalek AK, Shepherd D, Spence J, Spira A, Sun X, Teichmann S, Theis F, Tsankov A, van den Berge M, von Papen M, Whitsett J, Xavier R, Xu Y, Zaragosi LE, Zhang K; HCA Lung Biological Network . SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035.e19, 2020. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]