Abstract
Purpose
The global COVID-19 pandemic has resulted in a renewed focus on the importance of personal protective equipment (PPE) and other interventions to decrease spread of infectious diseases. Although several ophthalmology organizations have released guidance on appropriate PPE for surgical procedures and ophthalmology clinics, there is limited experimental evidence that demonstrates the efficacy of various interventions that have been suggested. In this study, we evaluated high-risk aspects of the slit-lamp exam and the effect of various PPE interventions, specifically the use of a surgical mask and a slit-lamp shield.
Design
Experimental simulation study.
Methods
This was a single-center study in a patient simulation population. This study examined the presence of particles in the air near or on a slit-lamp, a simulated slit-lamp examiner, or a simulated patient using a fluorescent surrogate of respiratory droplets.
Results
Simulated coughing without a mask or slit-lamp shield resulted in widespread dispersion of fluorescent droplets during the model slit-lamp examination. Coughing with a mask resulted in the most significant decrease in droplets; however, particles still escaped from the top of the mask. Coughing with the slit-lamp shield alone blocked most of forward particle dispersion; however, significant distributions of respiratory droplets were found on the slit-lamp joystick and table. Coughing with both a mask and slit-lamp shield resulted in the least dispersion to the simulated examiner and the simulated patient. Scanning electron microscopy demonstrated particle sizes of 3-100 μm.
Conclusions
Masking had the greatest effect in limiting spread of respiratory droplets, whereas slit-lamp shields and gloves also contributed to limiting exposure to droplets from SARS-CoV-2 during slit-lamp examination.
The COVID-19 global pandemic has had a profound effect on clinical care around the world. As a result of the outbreak, many ophthalmology clinics temporarily halted elective clinical visits and surgical procedures. Furthermore, the pandemic has resulted in fervent discussion regarding best practices to limit the spread of infectious diseases in the clinical setting. This disease, like many other upper respiratory infections, is highly transmissible via respiratory droplets, with recent reports suggesting airborne transmission of the virus can also occur.1, 2, 3
Several independent ophthalmology organizations, including the American Academy of Ophthalmology (AAO), have released guidelines regarding the resumption of clinical care and recommendations on appropriate personal protective equipment (PPE) that should be used when providing patient care. AAO guidelines recommend surgical masks for patients, masks and eye protection for providers, and slit-lamp breath shields.4 However, there has been limited evidence regarding the efficacy of these interventions. As clinical activities resume, there is a need for robust data to inform use and efficacy of PPE in the clinic. Recent work compared several commercially available slit-lamp shields for degrees of respiratory droplet spread protection, which provided necessary evidence for best clinical practice.1 However, several open questions remain for the development of best-practice, evidence-based guidelines for PPE during ophthalmic examination. In this study, we developed a patient cough simulator to evaluate high-risk areas of respiratory droplet contamination during a slit-lamp examination (Figure 1 ).
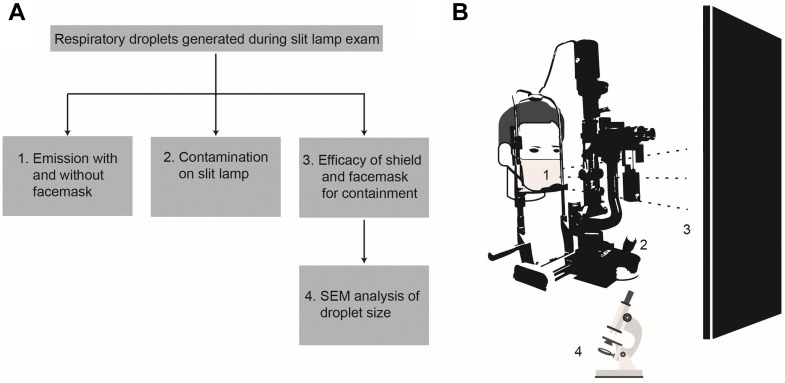
Figure 1.
Overview of experimental strategy. A. Four aspects of respiratory risk during the slit-lamp examination were investigated: 1) effect of surgical masks on droplet emission; 2) droplet contamination on the slit lamp and table; 3) droplets reaching the examiner; and 4) microscopic analysis of droplets. B. Depiction of slit-lamp experimental design. SEM = scanning electron microscope.
Methods
This study was done in a single-center using a patient simulation of an ophthalmologic clinic visit as the study population. The main outcomes measured were the presence of fluorescent particles in the air near or on the slit lamp, examiner, or patient.
Three-Dimensional Printing Parameters
An open-source 3-dimensional (3D) head file was modified using Blender (Blender Foundation, Amsterdam, Netherlands) first by cutting along 2 planes to reconfigure the dimensions. Then, a boolean difference was performed using an 8.0 × 8.0 × 19.7-cm cylinder to make a cylindrical hole parallel to the head. A hole orthogonal to the previous cylinder was used to provide an opening at the lips for spraying GloGerm (Glo Germ Company, Moab, Utah, USA). The head was printed on an Ultimaker s5 (Ultimaker, Waltham, Massachusetts, USA) with polylactic acid, at a 0.2 mm layer height and using 25% infill.
Coughing simulator
GloGerm MIST, a fluorescent surrogate of respiratory droplets used in previous simulation studies, was used as a simulator for patient coughing.5 GloGerm MIST was sprayed using the included pressurized spray canister, and the resulting mist was visualized using a handheld ultraviolet flashlight (Escolite 51 LED 395 nM Ultraviolet Blacklight Detector, Garden Century LLC, Rosemead, California, USA). The pressurized canister was placed inside of a 3D printed head to simulate a patient examination. Details of 3D printing are available upon request.
For some trials, a blue surgical mask (3M Standard Procedure Mask, 3M, Saint Paul, Minnesota, USA) was placed over the mouth and nose. All simulated coughs were 1 second in duration. GloGerm velocity was calculated by measuring the distance traveled by individual droplets per frame captured at 120 frames/second. We measured 0.057 m traveled over 0.0083 seconds, or 7.0 m/s (Supplemental Movie 1). To capture respiratory droplets that reached the slit-lamp examiner, a 78 × 114-cm black board was placed at the level of the oculars and imaged between trials (Figure 1, B). Some trials included a 21.5 × 20-cm, slit-lamp plastic shield hanging from the oculars of the slit lamp. This was placed 17.5 cm from the patient and 14 cm from the physician.
A second simulation of patient breathing used a total of 3 ml of GloGerm sprayed in 4-second intervals over 60 seconds through a syringe with an atomizer tip, which was widely used to simulate respiratory droplets generated via breathing in the past.6, 7, 8
Slow Motion Droplet Video Recording and Respiratory Droplet Visualization
All photographs and videos were taken using a tripod-mounted Sony A7III digital camera. The blacklight was placed in a stationary position, and video was recorded at 120 frames/second at 1080p resolution. Photographs were taken in a dark room with a 5-second exposure. All post-capture editing was completed in Adobe Photoshop (Adobe, San Jose, California, USA) (photos) or Adobe Premiere Pro 2020 (videos) with all changes in contrast and brightness identical in all trials. Two still frames were exported for representative images in Figure 2 . For visualization of differences between still images, photos were auto-aligned, the difference was calculated between images, and then the resulting difference was inverted and thresholded. Percent droplet coverage was determined by the area of respiratory droplets on the board divided by the total area of an approximate 35 × 35-cm square on the board, centered where the physician's or patient's head would be located.
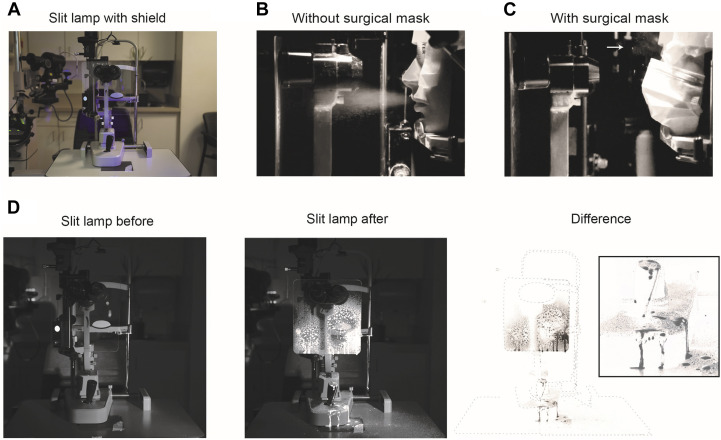
Figure 2.
Effect of a slit-lamp shield on simulated cough. A. Photograph of slit lamp with slit-lamp shield used for all experiments. B. Still image from Supplemental Movie 2 demonstrating forward moving particles during experimental trial with unmasked simulated patient. C. Same as B, with masked simulated patient. Arrows demonstrate respiratory particles ejected upward. D. Before (left), after (middle), and subtracted (right) image of slit lamp from experimental trial with unmasked simulated patient. Inlay of slit-lamp joystick (right) demonstrating significant contamination of joystick.
Scanning Electron Microscope Quantification
Carbon tape (Nisshin EM, Tokyo, Japan) was used to capture GloGerm droplets. A scanning electron microscope (SEM; FEI Apreo, Thermo Fisher Scientific, Hillsboro, Oregon, USA) was used to examine the size and morphology of droplets at an acceleration voltage of 1 kV.
Results
Slow motion video recordings were taken of GloGerm respiratory droplets ejected from the 3D printed head to simulate a forceful cough. For reference, our simulated exit velocity of the GloGerm MIST was measured at 7.0 m/s, which was consistent with previous estimates of cough velocity between 6 and 28 m/s (Supplemental Movie 2).9 Identical recordings with a surgical mask over the patient's mouth demonstrated the drastic reduction in forward moving droplets (Figure 2, B and C; Supplemental Movie 1). Respiratory particles were seen ejected upward past the nares with the facemask present (Figure 2, C, white arrow).
The slit lamp was next imaged before and after a simulated cough without a mask but with a slit-lamp shield. When the difference between the images were calculated, we observed most of the respiratory droplets contained by the shield. However, we identified significant contamination on the joystick and the slit-lamp table (Figure 2, D).
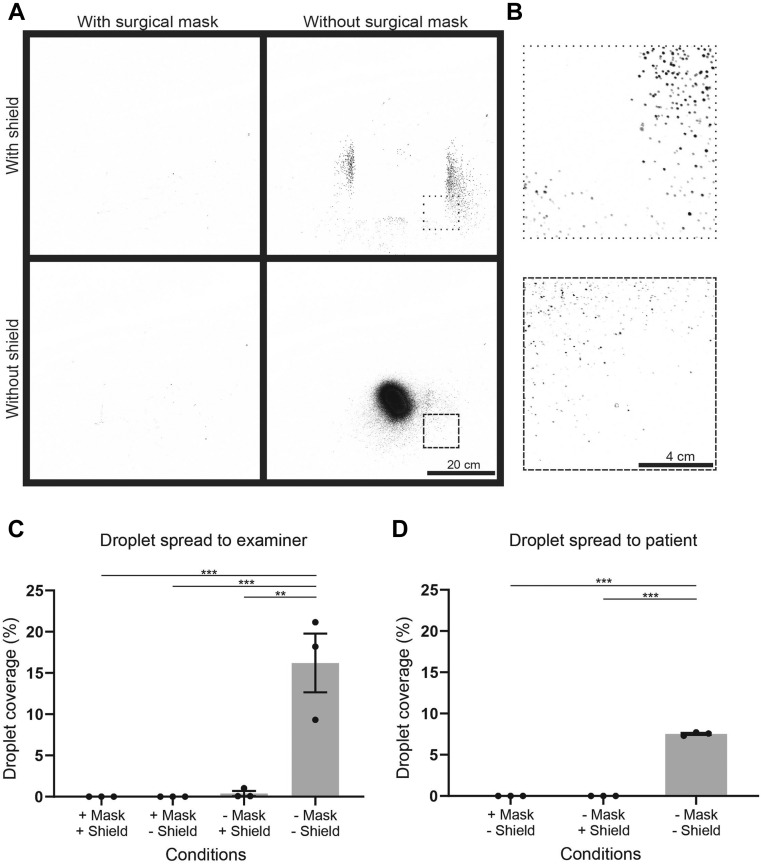
We next assessed how well the surgical mask and slit lamp shield prevented respiratory droplets from reaching the examiner. We sprayed GloGerm MIST through the simulated patient with or without a mask, and with or without a slit-lamp shield present. When we compared images of the board before and after the simulated cough, we were able to identify the pattern of droplets that reached the examiner (Figure 3 , A). When no protection was in place, a dense core of droplets could be seen. A shield alone was able to block most of the central droplets; however, peripheral particles could still be seen (Figure 3, B). Minimal droplets were identified when the face mask was present (Figure 2, A). Quantification of the droplet spread that reached the examiner showed significantly less percentage coverage when using either the slit-lamp shield or the surgical mask than in the unprotected condition (Figure 3, C; Supplemental Table 1). To further replicate a typical slit-lamp encounter, we simulated a short respiratory burst at a reduced velocity every 5 seconds for 60 seconds (Supplemental Figure 1, A and B). In this experimental paradigm, the respiratory droplet coverage with a slit-lamp shield present was not significantly different than the cough simulation. Together, these data suggested a combination of a surgical mask and a slit-lamp shield could block most forward-moving respiratory droplets.
Figure 3.
Efficacy of personal protective equipment on respiratory droplet contamination for patient and examiner. A. Subtracted images of each experimental condition of patient cough. B. Zoomed-in regions from A, depicted with dashed outlines. C. Quantification of replicates of the area of droplet presence over a standard 35 × 35-cm region on the black board placed where an examiner would be, with simulated cough from a patient. ∗∗p < .01; ∗∗∗p < .001. One-way analysis of variance, post-hoc Tukey's test. D. Quantification of replicates of the area of droplet presence over a standard region on the wooden board placed where a patient would be, with simulated cough from an examiner. ∗∗∗p < .001. One-way analysis of variance, post-hoc Tukey's test.
We next assessed the reverse scenario, testing the efficacy of the surgical mask and slit-lamp shield in preventing respiratory droplets from the examiner from reaching the patient. We found significantly fewer droplets reaching the patient using either PPE than in the unprotected condition (Figure 3, D; Supplemental Table 1). No droplets were detected in 3 replicates using either the facemask or the slit-lamp shield.
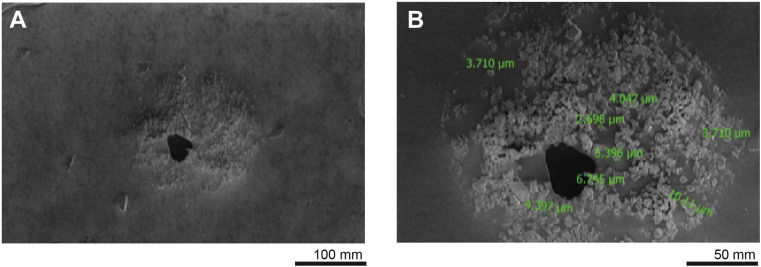
Finally, we determined the microscopic architecture of the individual droplets (Figure 4 , A) seen in the previous experiment. Using SEM, we identified 3-10 μm droplet nuclei contained within 1 approximately 150 μm respiratory droplet (Figure 4, B).
Figure 4.
Scanning electron microscopy of simulated respiratory droplets. A. Scanning electron microscopy images of simulated respiratory particles. B. Individual measurements, in green, of fluorescent GloGerm particles within a larger respiratory droplet.
Discussion
In this study, we characterized the risk to ophthalmologists from patients coughing through a simulated patient slit-lamp examination, as well as the risk to patients from ophthalmologists. Using simulated respiratory droplets, we found the most effective intervention for containing spread was masking for both patient and physician. When combined with a slit-lamp shield, most respiratory droplets were blocked from reaching either the patient or the examiner. Without any protection, we also noted fewer droplets that reached the patient than drops that reached the examiner. Because the slit-lamp shield hanging from the oculars was 3 cm closer to the examiner than the patient, these data were also consistent with previous reports that closer shield distance prevented greater spread of respiratory droplets.1 We also identified the slit-lamp joystick and examination table as high-risk areas for contamination because of their location under the shield, which suggested that gloves might prevent physician contact with droplets.
Our simulator was likely an underestimation of particle spread, because GloGerm primarily forms droplets of 100 μm, which is much larger than most respiratory (>5-10 μm) or airborne particles (<5 μm).10
Our cough simulator was similar to other cough simulators used in the literature. We used aerosolized GloGerm MIST as a fluorescent surrogate of expelled respiratory droplets, which was used in other cough simulation studies for assessing respiratory droplet spread during dental anesthesia, as well as during intubation for anesthesia.11 , 12 GloGerm speed was estimated at 7.0 m/s (Supplemental Movie 2), which was consistent with previous estimates of cough velocity between 6 and 28 m/s.9
Respiratory particles were observed escaping through the top of the mask lateral to the nose, which was consistent with previous findings.13 , 14 This posed a potential risk when providers were close to the patient's face, such as during intravitreal injections or when using a direct ophthalmoscope. Although further studies are needed to fully evaluate the risk of virus transmission during these close encounters, our data suggested that use of eye protection is prudent.
Recent work suggested that surgical masks might be sufficient for reducing emission of viral particles >5 μm, but masks ae less effective below that range.15 Our SEM evaluation of respiratory droplets reaching the provider identified droplets that ranged from 3 to 100 μm, which suggested that a patient face mask alone is not sufficient to prevent all risk of respiratory or airborne contamination.
Study Limitations
There were many limitations to this study. First GloGerm MIST might not be an exact representation of respiratory secretions during a cough. Although we were able to capture larger particles, many of the droplet nuclei of <1 μm were not detected by our fluorescent methodology. In addition, we only tested 1 size slit-lamp shield and 1 type of mask as a proof-of-concept. Further work characterizing the efficacy of different commercially available masks and shields is necessary for a complete understanding of the risks associated with clinic visits.
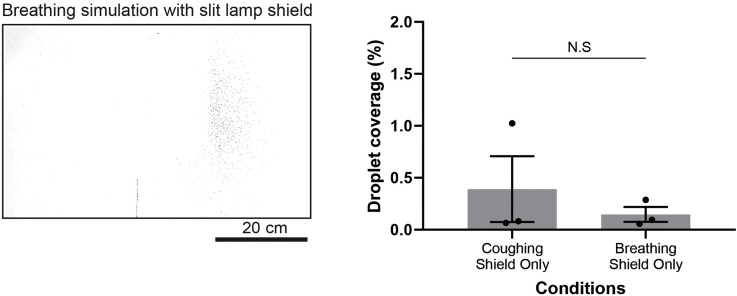
In addition, many slit-lamp encounters do not involve coughing by the patient or the examiner. In these cases, the risks posed by regular breathing are likely much lower than the data presented here. However, because we aim to provide data to assist with development of PPE guidelines for use in the clinic, it is appropriate to simulate commonly encountered, higher risk scenarios that will undoubtedly occur. Nonetheless, we replicated a breathing simulation of >60 seconds. Although we possibly overestimated droplet spread, we found similar droplet containment as with the cough simulation, which suggested our findings of appropriate PPE coverage were applicable to a wide range of scenarios. This conclusion was also seen in other studies of slit-lamp shield efficacy.16
Conclusions
As ophthalmologic clinic appointments start to resume, additional data are needed to provide best evidence-based guidelines for appropriate PPE in the clinic. Our cough simulation experiments lend support for universal masking for both patient and physician, as well as use of slit-lamp shields and gloves to limit exposure to potential SARS-CoV-2 during the ophthalmic examination.
CRediT authorship contribution statement
Sahil H. Shah: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Visualization. Anupam K. Garg: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Visualization. Shiv Patel: Conceptualization, Methodology, Investigation, Writing - review & editing, Visualization. Wonjun Yim: Methodology, Investigation, Visualization. Jesse V. Jokerst: Methodology, Resources, Supervision, Project administration, Funding acquisition. Daniel L. Chao: Conceptualization, Methodology, Writing - review & editing, Resources, Supervision, Project administration, Funding acquisition.
Acknowledgments
Funding/support: D.L.C. was supported by NIH/NEI K08EY030510 and an unrestricted department grant for Research to Prevent Blindness. J.V.J. was supported by the University of California Tobacco Related-Disease Research Program (TRDRP) under Emergency COVID-19 Research Seed Funding award number R00RG2515, the NIH under grants DP2 HL137187, R21 AI157957, and 3R21 AG065776-01S, and the NSF under grant #1845683.
Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors report no financial disclosures.
Footnotes
Supplemental Material available at AJO.com.
S.H.S. and A.K.G. contributed equally as co-first authors.
Supplemental Data
High-speed capture of simulated cough with and without surgical mask. Three trials of GloGerm cough simulation with and without a surgical mask were recorded at 120 fps and slowed to 0.1× speed. With a mask, a cloud of respiratory droplets is visible escaping upwards lateral to the nose (arrow).
Simulated respiratory droplet velocity. A simulated cough was recorded at 120fps and interpolated at 600fps next to a measuring tape. White lines were placed at the frontline of respiratory droplets in 4 consecutive frames to estimate average velocity. The video was then slowed down for visualization. The droplet front moved at approximately 2.25 inches or 0.057m per frame (white line). At 120 fps, this is equivalent to 7.0 m/s.
Supplemental Figure 1.
Efficacy of slit lamp shield for respiratory droplet spread with breathing simulation. A. Subtracted image of breathing simulation with a slit lamp shield. Scale bar = 29cm. B. Quantification of replicates of breathing simulation compared to cough simulation. Two-tailed unpaired T-test.
References
- 1.Liu J., Wang A.Y., Ing E.B. Efficacy of slit lamp breath shields. Am J Ophthalmol. 2020;218:120–127. doi: 10.1016/j.ajo.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368(6498):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- 3.Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci U S A. 2020;117(22):11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chodosh J., Holland G.N., Yeh S. Important coronavirus updates for ophthalmologists. American Academy of Ophthalmology. 2020. https://www.aao.org/headline/alert-important-coronavirus-context Available at.
- 5.Gardiner C., Veall J., Lockhart S. The use of UV fluorescent powder for COVID-19 airway management simulation training. Anaesthesia. 2020;75(7):964–965. doi: 10.1111/anae.15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman O., Meir M., Shavit D., Idelman R., Shavit I. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323(20):2091–2093. doi: 10.1001/jama.2020.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart S.L., Naidu J.J., Badh C.S., Duggan L.V. Simulation as a tool for assessing and evolving your current personal protective equipment: lessons learned during the coronavirus disease (COVID-19) pandemic. Can J Anaesth. 2020;67(7):895–896. doi: 10.1007/s12630-020-01638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Workman A.D., Welling D.B., Carter B.S., et al. Endonasal instrumentation and aerosolization risk in the era of COVID-19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020;10(7):798–805. doi: 10.1002/alr.22577. [DOI] [PubMed] [Google Scholar]
- 9.Wei J., Li Y. Human cough as a two-stage jet and its role in particle transport. PLoS One. 2017;12(1):e0169235. doi: 10.1371/journal.pone.0169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization, Pandemic and Epidemic Diseases, World Health Organization Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care: WHO guidelines. 2014. http://apps.who.int/iris/bitstream/10665/112656/1/9789241507134_eng.pdf?ua=1 Available at. [PubMed]
- 11.Chua H., Lim W.Y., Mok M., Wong P. “Closed” supraglottic airway guided intubation during the COVID-19 pandemic: a Glo Germ follow up. Anesth Analg. 2020;131(3):e168–e169. doi: 10.1213/ANE.0000000000005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanpong B., Tang M., Rosenczweig A., Lok P., Tang R. Aerosol-generating procedures and simulated cough in dental anesthesia. Anesth Prog. 2020;67(3):127–134. doi: 10.2344/anpr-67-03-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui D.S., Chow B.K., Chu L., et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. 2012;7(12):e50845. doi: 10.1371/journal.pone.0050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang J.W., Liebner T.J., Craven B.A., Settles G.S. A Schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6(Suppl 6):S727–S736. doi: 10.1098/rsif.2009.0295.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poostchi A., Kuet M.-L., Pegg K., Wilde C., Richardson P.S., Patel M.K. Efficacy of slit lamp breath shields. Eye. 2020;34(7):1185–1186. doi: 10.1038/s41433-020-0940-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High-speed capture of simulated cough with and without surgical mask. Three trials of GloGerm cough simulation with and without a surgical mask were recorded at 120 fps and slowed to 0.1× speed. With a mask, a cloud of respiratory droplets is visible escaping upwards lateral to the nose (arrow).
Simulated respiratory droplet velocity. A simulated cough was recorded at 120fps and interpolated at 600fps next to a measuring tape. White lines were placed at the frontline of respiratory droplets in 4 consecutive frames to estimate average velocity. The video was then slowed down for visualization. The droplet front moved at approximately 2.25 inches or 0.057m per frame (white line). At 120 fps, this is equivalent to 7.0 m/s.