Graphical abstract
Keywords: Dental nanomaterials nanoparticles, Nanocomposites, Nanocoatings, Antimicrobial biomaterials
Abstract
Objectives
The number of dental nanomaterials has increased significantly over the past years. A variety of commercial dental nanomaterials are available and researched. Nevertheless, how these nanomaterials work, what makes them special and whether they are superior to traditional dental materials is not always clear to dentists and researchers. The objective of this review paper is, therefore, to give an overview of the principles of nanomaterials and basic research and applications of dental nanomaterials.
Methods
The fundamentals of materials science of nanomaterials as well as their advantages and disadvantages are elaborated. The most important dental nanomaterials are discussed. This is mainly based on a survey of the literature and a review of the most frequently cited scientific papers in the international peer reviewed journal Dental Materials over the past five years. The developments of commercial dental nanomaterials as well as aspects of their clinical use are considered in this review.
Results
Nanomaterials have unique structures and properties that distinguish them from other materials. The journal Dental Materials is the journal with the highest numbers of articles and citations on the subject of dental nanomaterials. The most frequently reported dental nanomaterials are nanocomposites, nanoparticles, antimicrobial nanomaterials and bio-mineralization systems. Hallmarks of dental nanomaterials include a set of unique properties and challenges in the preparation of these materials.
Significance
By understanding the physical principles of dental nanomaterials, their strengths, limitations and their specific benefits will be better appreciated. Dental nanomaterials have potential for the future but currently do not always exhibit superior properties, for example in clinical situations.
1. Introduction
What I cannot create I do not understand.
Richard P. Feynman
Nanotechnologie ist in aller Munde
Deutsche Apotheker Zeitung
When the first author of this paper was a faculty member at Cornell University in the mid 1990s, the spirt of Richard P. Feynman, who was a Cornell faculty member until 1951, was still lingering on campus and in the labs. After Feynman’s famous and visionary lecture “There’s Plenty of Room at the Bottom” [1] at Caltech in December 1959, many scientists saw him as the founding father of nanotechnology. In this lecture, he discussed information on a small scale, better electron microscopes, biological systems, small computers, miniaturization by evaporation, problems of lubrication, fabrication of materials and devices at the atomic/molecular scales and more. Although he did not refer to dentistry in his lecture, in a TV interview with Gell-Mann, Feynman used the example of brushing teeth to look at (common) things from a new point of view to develop new ideas [2].
Since Feynman’s days, nanotechnology has moved from the state of a “vision”, or “science fiction”, to being a “real thing”, i.e., it has become reality. Many concepts Feynman dreamt up became true. Today, nanotechnology has left a major footprint in virtually all fields of science, engineering, technology, and medicine and dentistry are no exception to this.
Why is that so? It is so because nanomaterials and nanodevices have properties that were previously unaccomplishable and they make possible solutions to problems previously unsolvable [3].
Applications of nanotechnology in dentistry are vast. They include but are not limited to dental diagnostics, preventive dentistry, dental materials, prosthodontics, endodontics, conservative and aesthetic dentistry, periodontics, implantology, and regenerative dentistry and nano-products [4]. Nanotechnology plays also a major role in equipment used by dental practitioners such as LED light curing units [5], protection of dental hard tissues against acid containing foods [6,7] and characterization of dental materials and dental hard tissues [8] and many more.
Obviously, a detailed review of all these different and diverse areas in one paper does not make sense. In addition, to the best of our knowledge, no current review exists focused on nanomaterials for dentistry. Therefore, and since the journal this current review is intended for is called Dental Materials, we will focus in this review on nanostructures and nanomaterials relevant for dental materials.
In the past five years as of end of April 2020, the top ten journals ranked by impact factor (2018) [9] in the categories Dentistry, Oral Surgery & Medicine published a total 6936 articles [10]. Of these publications 146 have the word “nano” (including the truncated word “nano*) in the title and 415 have “nano” (including the truncation) as a topic [10]. The number of citations of articles in these journals in the same time period with “nano” as a topic increased strongly from 55 in 2016 to 1461 in 2019. The journal Dental Materials published 859 articles in the past five years as of end of April 2020, of which 78 have the word “nano” in the title and 188 as a topic. The survey showed furthermore that among the top ten journals in the category Dentistry, Oral Surgery & Medicine, Dental Materials is by far the journal with the most nanotitles and nanosubjects in its articles.
Within these articles in Dental Materials, the most frequently covered topics (dental nanomaterials) are in this order: nanocomposites, nanoparticles/nanotubes/nanofibers including silver nanoparticles, antimicrobial materials, (bio-) mineralization and coatings. Therefore, we will focus on these topics in this review.
2. What is nano and nanotechnology?
The Greek word nάνoς means dwarf. In science nano refers to one billionth (American scale), denoting a factor of 10−9. Since such a small number is hard to imagine for the human brain, analogies have been found to grasp the nanoscale. For example, the quotient of a meter (m) to a nanometer (nm) is approximately equal to the quotient of the diameter of the planet earth and the diameter of a hazelnut. One nanometer is approximately the length that a fingernail grows in one second [11].
A general definition of a material being a nanomaterial is, when it is smaller than 100 nm in one dimension [12]. This definition is based on the material’s structure. A more rigorous definition considers in addition to the structure the properties of the nanomaterial, i.e. that the material possess properties that are specific for the smallness of the material [12]. This suits materials scientists, since they aim to develop structure-property relationships of materials. An important example relevant for nanomaterials in dentistry are the nanoparticles in nanofilled dental composites. Although synthetic nanomaterials are state of the art cutting edge materials, one should realize that living nature has developed and used natural nanomaterials for billions of years. For example, the proteins in our blood stream are functional nanomaterials.
Nanotechnology can be defined as a technology that deals with small structures or small sized materials [13]. Nanostructures or nanosized materials can be created by two different strategies. The top down strategy starts normally with a bulk material much larger than “nano”, and then uses externally applied forces (mechanical or other), to break the material down for example into nanoparticles. The bottom up strategy, essentially proposed by Feynman [1], assembles materials or structures atom by atom or molecule by molecule to obtain the desired result, although – given the magnitude of Avogadro’s Number - such a process must be massively parallel. A relevant example for nanostructures that may be useful in dentistry are nanostructured titanium surfaces for implants in which the nanostructures have an antimicrobial effect without utilizing antibiotics [14].
Although nanoscience and technology are among the fastest growing areas in science and technology and interesting per se, “nano” is not always “better”. Sometimes, the term nano is used to market products in dentistry without sufficient clinical evidence that the nanoversion of a material is significantly better than the conventional non-nanoversion of the product. But it seems that “nano” sells.
3. Basic physics and chemistry of nanomaterials
Materials on the nanoscale, i.e., nanomaterials have unique properties. These properties are physical and chemical in nature. Nanomaterials, often also called nanostructures, may be categorized by their dimension [13]. Zero-dimensional nanostructures are nanoparticles, and one-dimensional nanostructures are nanowires and nanorods. Thin films are two-dimensional nanostructures. All these structures fulfill the definition of a nanomaterial or nanostructure mentioned above: they are smaller than 100 nm in one dimension. As discussed below, nanoparticles in different varieties are important nanomaterials in dentistry, hence we focus on them in this work.
One of the most striking features of nanomaterials such as nanoparticles is the large ratio between their surface and their volume. The radii of atoms range approximately between 0.30 nm and 3.00 nm [15]. If one considers a nanoparticle of a few nm in diameter, this means that all atoms are either at the surface of the nanoparticle, or within a few atomic distances from the surface inside the particle, depending on the size of the atoms and the size of the nanoparticle. For example, for a cube of iron with an edge length of 1 nm, every atom constituting this cube is a surface atom [13].
The number of atoms that constitute a nanoparticle of a given size can be calculated to good accuracy using simple arithmetic, if the type of unit cell of the material and its lattice constant are known and assuming a spherical shape of the nanoparticle. The latter assumption is realistic, considering the forces resulting from the surface energy.
In a bulk material all atoms bond to their neighbors. Surface atoms, however, possess fewer nearest neighbors and thus have dangling or unsatisfied bonds [13]. The lack of binding partners for surface atoms leads to an inwardly directed force towards the center of the particle and a change of lattice constants (relaxation) or even the lattice type (reconstruction) [13]. If these surface atoms are part of a nanoparticle this affects the physical properties of the whole particle significantly.
As a consequence of the unsatisfied bonds at the surface of the nanoparticle, the surface atoms possess extra energy called surface energy or surface free energy or surface tension [13]. The surface energy is
| (1) |
where, G is the Gibbs free energy and A is the surface area. This is the energy necessary to create a unit area of “new “surface [13]. For a new surface to be created, bonds need to be broken, which requires energy.
These considerations show that not only the geometrical surface is important for understanding the unique properties of nanoparticles, but also the volume of the particle that is influenced by the surface. This is sometimes called physical surface [12].
The size of nanoparticles or more precisely their curvature affects their chemical potential μ, which is essentially the driving force of a substance to react with another, for phase changes or for diffusion. The change of surface chemical potential from atoms in a flat surface to a nanoparticle is inversely proportional to the radius of the particle:
| (2) |
where, Ω is the atomic volume and R is the particle radius [13]. Thus, nanoparticles have a large chemical potential which results in a high chemical reactivity of these particles and a high atomic diffusion from these particles. As a result, for two particles in a solvent with R1 ≫ R2 the larger particle will grow at the expense of the smaller particle through diffusion [13]. This is called Ostwald ripening.
The physical and chemical phenomena mentioned above have several important consequences for the properties of nanoscaled materials and their synthesis. Nanoparticles tend to agglomerate in order to reduce their surface energy, resulting in a loss/change of their unique properties. This effect is a challenge when producing or working with nanoparticles, for example during the production of nanoparticle filled dental composites.
During the sintering of ceramics based on nanoparticles, Ostwald ripening may lead to an undesired grain growth and inhomogeneous microstructure leading to inferior mechanical properties of the ceramic [13,16]. This is relevant to produce high strength dental ceramics. Based on their high surface energy content, nanoparticle based ceramics may have lower sintering temperatures than the macroparticle based counterparts. This is useful when dimensional stability is paramount.
The surface energy is also responsible for a drastic change of other physical properties of nanoparticles compared to bulk materials. For example, bulk gold has a melting point of 1064 °C. However, melting of gold nanoparticles is below room temperature for particle sizes less than 1.4 nm [17]. For gold nanoparticles larger than 15 nm the melting point approximates the melting point of the bulk material. Since the nanoparticles are much smaller than the wavelength of light, they do not scatter light when dispersed in transparent media [18].
4. Synthesis, stabilization and processing of nanomaterials
A major challenge in nanotechnology is the controlled and purposeful synthesis of nanomaterials and nanostructures. The methods to synthesize nanomaterials are vast and depend on several factors such as dimension of the materials created (0D, 1D, 2D, 3D) and the material class produced.
The most frequently used type of nanomaterial in dentistry and a common denominator in dental nanomaterials are 0D nanoparticles. Especially nanoscale particles and surface structures represent nanotechnology among consumers [19]. Therefore, we focus on the synthesis of nanoparticles in this section.
The Latin word “pars” means part. Nanoparticles can be created by both the top down or the bottom up approach. In addition, synthesis methods for nanoparticles depend of the material class the particle belongs to: metal, ceramic or polymer. Nanoparticles can be synthesized via the solid, liquid or gas phase. Depending on the method of synthesis, nanoparticles have an irregular or regular shape and broad or narrow particle size distributions.
The classic top-down approach for creating ceramic nanoparticles is very fine grinding or colloid milling. Ball mills use steel or other balls rotating in a hollow cylinder to crush the material by impact and attrition. A ring and ball mill consists of two types of rings separated by a series of large balls, like a thrust bearing [20], that crush the material in between two particles. The attrition mill mechanically reduces solid particle size by intense agitation of a slurry of material being milled and coarse milling media [21]. Such mills are frequently used for the creation of ceramic nanoparticles with sizes down to a few tens of nanometres.
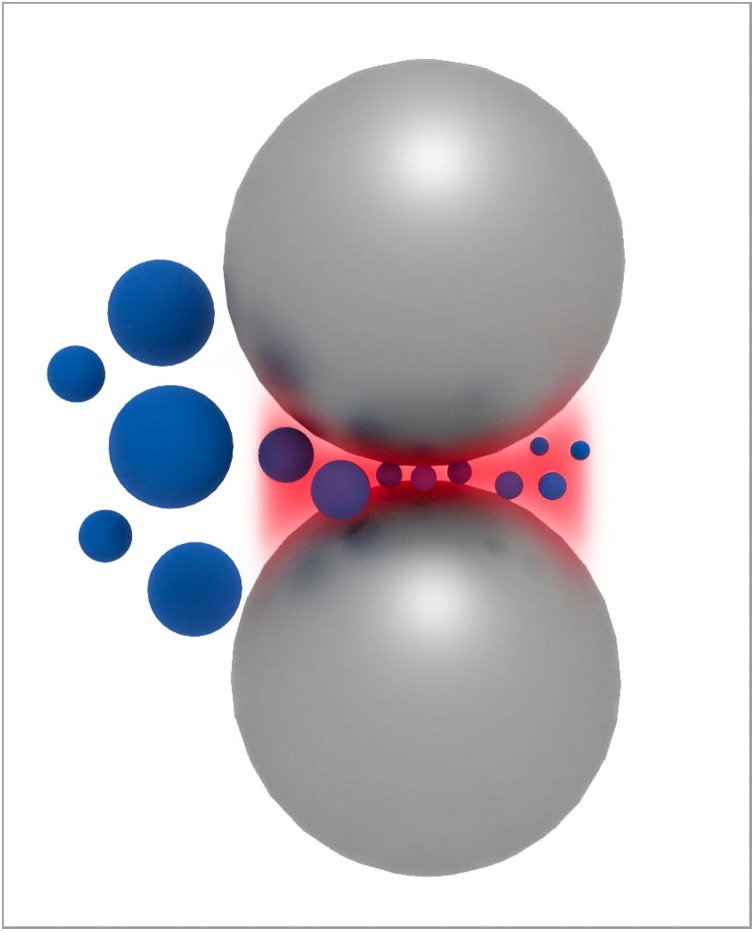
Physical and technological factors limit the smallest particle sizes that can be obtained by top-down milling of materials. First, the smaller the particles, the stronger they are. This is because smaller particles are single crystals and/or have normally fewer defects, such as grain boundaries, than larger ones. This makes it more difficult to crush particles to sizes smaller than a few tens of nanometres. A second limit of milling are the properties of the milling balls: their active (crushing) volume, Young’s modulus, and kinetic energy (Fig. 1 ). As a rule of thumb, the ratio of the diameters of the milling balls and the particles are 1000:1, i.e., to obtain 100 nm particles the milling balls should have a diameter of 100 μm.
Fig. 1.
Principle of particle crushing in a ball mill. The large milling balls crush particles within their active volume (shaded area between the milling balls). Image courtesy J. Bossert und I. Firkowska-Boden, FSU Jena.
Bottom-up approaches for the creation of nanoparticles use changes in thermodynamic equilibrium to induce phase transformations of materials. A starting point for this can be supersaturated non-equilibrium solutions (step 1) in which nuclei form (step 2) and then grow to particles (step 3). Nanoparticles can be obtained through heterogenous or homogenous nucleation [13]. Nanoparticles form if the number of nuclei is large and the growth is limited with the aim of obtaining many nanoparticles with a narrow size distribution. This can for example be accomplished by strong supersaturation, limiting diffusion, supercooling, low concentrations and increasing the viscosity.
Metallic nanoparticles in metal colloidal dispersions are typically synthesized via reduction of metal complexes in dilute solutions under controlled reduction reaction conditions [13]. Dental materials frequently used silver nanoparticles due to their antimicrobial properties. Metal cations such as silver in sufficient dose have a damaging effect to living cells. The antimicrobial action of these ions is based on cell wall and cell membrane damages, oxidation of proteins and lipids and disrupting hydrogen bond between DNA strains. Since silver has a strong tendency to oxidize and nanoparticles have a large surface to bulk ratio, silver nanoparticles may have a high silver oxide content [22]. Silver nanoparticles are typically synthesized via wet chemistry methods from AgNO3 complexes in the presence of a reducing agent [23].
The manufacturers of dental composites use different approaches to produce nanofillers for their nanofilled composites and only publish their methods to a limited extent. Microfillers in dental composites are sometimes aggregates of nanoparticles. Some dental composite manufactures produce many of their filler particles by a sol gel process [24]. The sol gel process is suitable to produce metal oxide nanoparticles such as silicon oxide, zirconium oxide or hybrid polymers from colloidal dispersions. Particles are made from small molecule liquid precursors, called sol (i.e. from solution). Nanoparticles form through hydrolysis reactions. The particles grow and aggregate in solution which leads to an increase of viscosity of the sol and then subsequently form a gel (viscoelastic solid). The gels are then dried and further treated; for example by tempering or sintering.
The sintering process may be modified to produce loosely agglomerated nanoparticles, i.e., nanoclusters [24]. Such nanoclusters behave similarly to the densified particles found in other composites in terms of providing high filler loading [24]. The resulting composite is claimed to have high strength and wear resistance with significantly improved polish retention and optical properties [24].
Other manufactures use highly dispersed and non-aggregated nanofillers, aiming for homogeneous dispersion and complete resin wetting of nano-sized filler particles, to improve the aesthetic and mechanical properties of composites. For example, organically modified ceramic nanoparticles can be produced via controlled hydrolysis and condensation reactions [25]. The nanoceramic particles have a size of 2.3 nm as investigated by X-ray diffraction [25].
Polymer nanoparticles including nanospheres and nanocapsules are frequently used for drug delivery purposes [26]. They often contain active pharmaceutical ingredients within each particle or have adsorbed macromolecular substances on their surface. The preparation of polymer nanoparticles can be divided into two approaches, i.e., two-step and one-step procedures.
Polymer nanoparticles can be prepared by polymerization-based methods such as emulsion polymerization, dispersion polymerization or interfacial complexation or by polymer participation methods such as single/double emulsion, solvent displacement or salting out [27].
Drugs can be incorporated either during nanoparticle preparation or after. Typical polymers used for polymer nanoparticles are chitosan, polyacrylamide, polyacrylate and polyesters [27].
In order to release drugs from the nanoparticle into the human body, often biodegradable polymer nanoparticles are used. Polymer nanoparticle based drug delivery has several advantages, compared to conventional drug application, such as targeting to specific tissues and cells via ligand specificity, absorption of polymer nanoparticles into cells, lower doses of drugs necessary, reduced toxic effects, sustained drug release at the target site and enhancement of therapeutic potential of drugs [28]. Nevertheless, several major hurdles such as particle aggregation or control of release kinetics need to be overcome to accomplish all these advantages.
Disadvantages of polymer nanoparticles for drug delivery include the relatively high costs of production, the challenge of controlling release kinetics and the tendency of polymer nanoparticles to agglomerate.
Agglomeration is the major challenge when processing and working with nanoparticles. It needs to be avoided or reduced to maintain the unique properties of nanoparticles. Two major strategies to stabilize nanoparticles in suspension are electrostatic stabilization and steric stabilization or combinations of both [13].
In electrostatic stabilization, the attractive forces between nanoparticles are counterbalanced by repulsive Coulomb forces. This is, for example, accomplished by attaching negative surface charges on the nanoparticles through ion adsorption or the creation of a Stern layer [13].
Steric stabilization is based on multiple short polymer chains adsorbed on the surface of the nanoparticles. If nanoparticles mutually approach, the surface polymer segments of the different particles also get closer and have less freedom to move. This causes a reduction of entropy (ΔS < 0) which increases the Gibbs free energy [13]. As a result of this thermodynamic penalty the particles do not agglomerate. If the interactions of the polymer chains with the surrounding medium (e.g. water) is stronger than that between the polymer molecules, enthalpy contributes to the non-agglomeration of the nanoparticles.
As mentioned above, the most common application of nanoparticles in dentistry is their use in dental composites. Dispersing nanoparticles in a polymer matrix is intrinsically challenging because of unfavorable entropic interactions between the matrix and the nanoparticles [29]. However, the simplest ways to create a resin-composite incorporating discrete (non-agglomerated) nanoparticles is to start with the unfilled monomer mixture. To the liquid monomer mixture may be added up to ca. 30% vol/vol of non-agglomerated nanofiller without an enormous increase in the viscosity of the system. This nano-incorporating monomer mixture can then be mixed with larger particles in the micron and sub-micron size range to form a nano-hybrid paste. Since highly-filled composites only contain ca. 20% vol/vol monomer, the final proportion of nano-filler in the composite may be as low as 6–8%. Nevertheless, the structural benefit of nanoparticles in a nano-hybrid is that they can fit between the spaces remaining when larger particles are in direct contact. Furthermore, they have an effect of reducing the absolute monomer content and thereby the magnitude of properties such as polymerization shrinkage that can have adverse consequences. Some manufactures add nanoparticles (aerosil SiO2) to keep the larger particles suspended in the resin and not settle out.
Further methods of dispersing nanoparticles in polymer matrix composites include chemical methods such as sol-gel routes (see above), surface treatment and functionalization such as grafting of polymer chains on the nanoparticle filler surface. Dense polymer brushes can lead to a good dispersion of nanoparticles in (uncured) polymer matrixes when the length of the grafted chains is comparable to that of the matrix molecules [29].
Nanoparticles can also be dispersed in polymer matrixes using mechanical methods such as high performance milling (see above). A three-roll mill (calender) applies high shear forces to particle agglomerates in polymer matrixes to break them up by calendaring. Furthermore ultrasonication, stirring with high shear forces [30] or attrition milling are useful for breaking up nanoparticle agglomerates in the preparation of polymer-based nanocomposites.
The resulting nanoparticles and nanoparticle clusters can be mixed in the mills with solvents and activated resins to form a fluid dispersion incorporating well dispersed particles [31]. Subsequently, the suspension is transferred to a spray granulator where small droplets form from which the solvent evaporates during heat treatment, resulting in resin-particle spheres with a narrow size distribution [31]. The spheres are then cured producing completed fillers for composites.
5. Typical nanomaterials of high relevance for dentistry
As mentioned above, our survey of articles in the journal Dental Materials showed that the most frequently covered topics in dental nanomaterials are in this sequence: nanocomposites, nanoparticles/nanotubes/nanofibers including silver nanoparticles, antimicrobial nanomaterials, (bio-)nanomineralization and nanocoatings.
Table 1 shows a ranking of the nanotopics published in the past five years up to the end of April 2020 in Dental Materials.
Table 1.
Nanotopics of articles published in the journal Dental Materials in the past five years as of end of April 2020 ranked by frequency. Note that double counting is possible, for example if an article addresses two topics e.g. nanocomposites and nanoparticles or nanoparticles and antimicrobial nanomaterials.
| Ranking # | Topic | Frequency (number of articles addressing this topic) |
|---|---|---|
| 1 | Nanocomposites | 34 |
| 2 | Nanoparticles incl. nanotubes and nanofibers | 32 |
| 3 | Antimicrobial nanomaterials | 18 |
| 4 | (Bio-) nanomineralization | 13 |
| 5 | Nanocoatings | 8 |
| 5 | Drug release / drug delivery / nanocarrier materials | 8 |
| 6 | Nanocrystalline ceramics | 7 |
| 7 | Materials’ nanomechanics | 6 |
| 8 | Tissue engineering nanomaterials | 2 |
| 8 | Nanomaterials for implantology | 2 |
| 8 | Graphene based nanomaterials | 2 |
To keep this review concise, we will focus on the top five ranked topics here. In addition, we consider nanomechanical testing of dental materials as an example of characterizing dental nanomaterials or dental tissues.
6. Dental nanocomposites and nanoparticles
The main application of nanoparticles in dentistry is their use as fillers in nanocomposites. When designing a new particle based composite material, the simple rule-of-mixtures allows us to predict Young’s modulus or the strength of the material. For example, under the condition of isostrain, the stress of a composite is
| σc = σf Vf + σm Vm | (3) |
where σ is the stress, V the volume fraction and the subscript c, f and m denote the composite, fibers (fillers) and matrix, respectively [32]. This rule essentially states that the strength of the composite is a volume-weighted average of the strengths of the filler and the matrix [32].
Rules of mixtures were developed by Voigt [33] and Reuss [34] about 130 and 90 years ago, respectively. The Young’s modulus of a composite ranges between an upper and a lower limit depending on the Young’s moduli of filler and matrix and their volume fractions [35]. The filler-matrix interfacial bond important for the stress transfer in dental composites is not considered in these rules.
It is interesting to note that the rule of mixtures equations do not contain the parameter particle size. The boundary conditions for rules of mixtures, however, are that these rules apply to composites with large filler particles. To the best of the knowledge of the authors, however, there are no straightforward rules of mixtures to predict the properties of nanocomposites. For more complex situations the theory of elasticity is used. Important factors in the calculation of the properties of nanocomposites are the size of the nanoparticles, their shape as well as the compatibility with the polymer matrix.
When considering benefits or limitations of nanocomposites, it is problematic that the actual proportional amount of nanoparticles to larger particle sizes is rarely disclosed by manufacturers. Moreover, it is difficult to quantitatively “reverse engineer” these formulations via techniques that are often adequate with larger filler particles, such as ashing experiments or thermogravimetric analysis unless great care is taken to avoid loss of the tiny nanoparticles to the atmosphere. The main dental materials that may be exclusively reinforced with nanoparticles are certain dental adhesive formulations. Virtually all restorative “nanocomposites” are actually “nano-hybrids” that contain much larger volume-fractions of non-nano sub-micron or micron-sized particles.
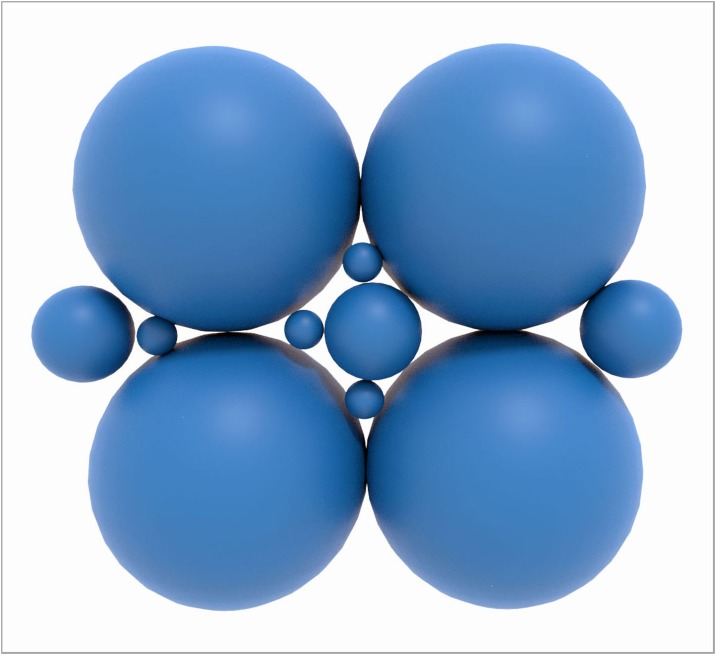
From a packing perspective, nano particles in combination with lager particles allow a higher theoretical packing density (Fig. 2 ). This is not only relevant for dental composites but also for dental ceramics where it helps to create dense and tough materials with less defects.
Fig. 2.
Packing of particles of different sizes relevant for dental composites and dental ceramics. Nanoparticles allow to fill the voids between the larger particles and, thus increase the particle packing density. Image courtesy J. Bossert und I. Firkowska-Boden, FSU Jena.
The main claimed advantages of nanocomposites over other composite materials include a high surface/volume ratio that allows small filler size and reduced inter-particle separation, enhanced mechanical properties, high ductility without strength loss, scratch resistance, improved optical properties (light transmission depends on particle size) and improved thermal properties [36]. Especially for dental nanocomposites simplified and enhanced aesthetic properties such as high gloss and gloss stability and excellent polishability and adaptability are claimed by their manufacturers. A systematic review of in vitro studies, however, found no evidence to support the choice for nanofill or submicron composites over traditional microhybrids based on better surface smoothness and/or gloss, or based upon maintenance of those superficial characteristics after surface challenges [37].
Disadvantages of nanocomposites may include reduced toughness and impact performance, insufficient understanding of the connection of formulation and structure-property relationship and difficult control of particle dispersion [36].
An important question of both scientific and clinical relevance is whether dental nanocomposites have similar or superior mechanical properties compared to conventional dental composites.
To this end, Beun et al. compared the inorganic fraction and the mechanical properties of three nanofilled composites with four universal hybrid and two microfilled composites (all commercial) [38]. Care should be taken to use the right nomenclature (microfilled or nanofilled composites). They found that the examined nanofilled resin composites showed higher elastic moduli than those of universal and microfilled composites, except for one composite. The microfilled composites showed by far the most inferior mechanical properties. Flexural strength seemed not to be a discriminating factor in this study. It was concluded that nanofilled resin composites show mechanical properties at least as good as those of universal hybrids and can thus be used for the same clinical indications as well as for anterior restorations due to their good aesthetic properties.
Other studies did not confirm the superior mechanical properties of nanocomposites [39,40]. A true comparison between nanocomposites and conventional composites, however, is not straightforward, since the filler content, filler geometry and other differences in the composition must be considered in the analysis of the results.
Karabela et al. studied the properties of dental resin composites with different nanosilica particles with average particle sizes of 40, 20, 16, 14, and 7 nm [41]. They found that the prepared composites contain different amounts of silica filler, but with the same amount of silanized silica and organic matrix showed similar flexural strength and flexural modulus, except the composite with the smallest filler particle size, which showed lower flexural modulus. If these results can be generalized, it may mean that there is a lower limit of the particle size that affects the mechanical properties of a composite. However, more research in this area is necessary to accept or reject this hypothesis.
Beyond their structural and mechanical functions as nanocomposite fillers, nanoparticles in composites may have therapeutic and/or preventive effects. Zhang et al. developed a composite containing nanoparticles of amorphous calcium phosphate that may contribute to long-term inhibition of caries [42]. These rechargeable CaP composites had long-term and sustained Ca and P ion release and have potential use as caries-inhibiting restorations. The Ca and P ion recharge and re-release approach may have further applicability to dental composites, adhesives, cements and sealants to promote long-term caries-inhibition [42].
Nanoparticles have a high surface to volume ratio which contributes to their reactivity. This, together with their smallness, which allows nanoparticles to pass through biological barriers such as cell membranes, renders nanoparticles potentially bioharzardous [43] and cytotoxic [44,45]. Nevertheless, knowledge about the complex interactions of nanoparticles with biological systems is still largely incomplete. It seems a consensus that, especially, free nanoparticles may pose potential health risks. In composites, however nanoparticles are embedded into a polymer matrix and immobilized. Therefore, the risk from these particles seems negligible. Through abrasive processes (intra oral wear, finishing/polishing procedures), however, these particles may be released and may enter the digestive system. Regarding the toxicity of such nanoparticles little is known, mainly because data and findings from the in vivo and in vitro studies are still very limited [46,47].
Composites may contain different types of micro- and nanoparticles such as glass, silica, titanium and silver [48] which influence the composite mechanical properties, radiopacity, biocompatibility or equip composite with antimicrobial properties. Silver, zinc or copper nanoparticle containing dental materials have antimicrobial effects and are, therefore, discussed below in the section on antimicrobial dental nanomaterials.
7. Antimicrobial dental nanomaterials
The oral cavity hosts several types of microorganisms which cause disorders such as caries, calculus, gingivitis or periodontitis. Invasive procedures in the oral cavity that use dental materials, such as restoration of caries decayed teeth with composites or the placement of implants are particularly affected by microbial action. It is therefore logical, to equip dental materials with antimicrobial properties.
The antimicrobial action of silver has probably been known since early recorded history [49]. Today silver is one of the most reported antimicrobial agents in the scientific literature. The antimicrobial action of silver and some other metals is based on damaging effects of metal cations to living cells. Silver leads to cell wall and cell membrane damage and to reaction with biomacromolecules within the cells. Silver-based antimicrobials are demonstratively effective against microorganisms such as bacteria, viruses, and fungi [50].
The high surface to volume ratio of silver nanoparticles promises an increased effectiveness of the silver dose against microorganisms. Combined with the advent of methods to produce silver nanoparticles with narrow size distributions and homogeneity [51] and the vast application areas in dentistry, the number of publications addressing this has boomed in the last ten years.
Silver nanoparticles have been applied in nanocomposites, implant coatings; anti-caries formulations; in the treatment of oral cancer and many more [50]. In-vitro results reveal the excellent antimicrobial activity of silver nanoparticles when associated with acrylic resins, resin co-monomers, adhesives, intracanal medication and implant coatings to fight microbial infections, especially caries [50]. The number of studies confirming these positive in vitro results in clinical situations, however, is very low.
Not all sizes of silver nanoparticles seem to have the same antimicrobial effectiveness. Ginjupalli et al. [52] showed that silver nanoparticles of a size range 80–100 nm are superior in imparting antimicrobial activity to irreversible hydrocolloid compared to smaller particle size ranges.
Despite the proven antimicrobial effects of silver and some of its compounds, this material has several disadvantages. First, silver is toxic to all body cells not only to microbes. This problem is compounded by difficulties in controlling silver dosage. Silver nanoparticles may be useful to address this dosage problem [53]. Furthermore, silver may lead to discoloration of materials or tissues [54]. If silver nanoparticle based dental materials can be effective in clinical situations regarding their antimicrobial effect, biocompatibility and aesthetics are still to be elucidated.
Silver nanopartilces show also antiviral properties. Lv et al. [55] studied the inhibitory effect of silver nanopartilces (particle size less than 20 nm), silver nanowires and silver colloids on corona virus induced host cell infection in vitro. They found that silver nanomaterials are effective in prevention of corona virus mediated cell infection as a virucidal agent or as an inhibitor of viral entry. These findings may provide new insight into antiviral therapy of corona virus infections.
Hojati et al. showed that resin composites containing zinc oxide (ZnO) nanoparticles have an antimicrobial effect [56]. A direct contact test showed that, by increasing the nanoparticle content, the microbial growth of Steptococcus mutans was significantly diminished. However, addition of ZnO nanoparticles led to a significantly reduced depth of cure of the composite while some mechanical properties remained unchanged and others increased.
Copper has been also known for a long time to have antimicrobial effects. Gutiérrez et al. investigated copper nanoparticles added in different concentrations to etch-and-rinse adhesive [57]. They found that addition of copper nanoparticles did not affect several mechanical properties tested and higher concentrations of copper nanoparticles produced adhesive–dentin interfaces that are more resistant to microleakage. The copper nanoparticles significantly increased antimicrobial activity.
Copper nano particles are effective against viruses as shown for example by Fujimori et al. [58]. They observed inactivation of influenza A H1N1 pandemic 2009 strain by Cu(I) iodide nanoparticles. This involved hydroxyl radicals and resulted in the degradation of hemagglutinin and viral proteins. Copper has been shown also to be effective against human coronavirus on materials surfaces [59].
To avoid the problems of metal ion-releasing antimicrobial dental materials, alternatives are researched intensively. These include nanocarriers or nanocapsules, dental nanocomposites containing a combination of antimicrobial monomers and calcium phosphates and others.
Lee et al. loaded PMMA with mesoporous silica nanoparticles [60]. While this changed the mechanical properties, depending on the incorporated concentration, an anti-adherent effect against Candida albicans and Streptococcus oralis was observed without cytotoxicity in nanoparticle-incorporated PMMA. The anti-microbial effect was observed over 2 weeks due to the slow release of amphotericin B after loading it into nanoparticle-incorporated PMMA.
Triclosan and indomethacin-loaded nanocapsules were successfully incorporated into an adhesive system by Genari et al. Such fillers for adhesive systems have a potential to act as antimicrobial and anti-inflammatory systems with continuous action [61]. Although triclosan has clear antimicrobial effects, its use is controversially discussed due to potentially negative effects on human health [62]. Due to the limited number of studies and therefore limited weight of evidence, however, it seems that a conclusive statement about triclosan toxicity for humans cannot be made currently [63].
Wang et al. developed a bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens [64]. This nanocomposite contained a combination of antimicrobial monomers (dimethylaminohexadecyl methacrylate) and amorphous calcium phosphate nanoparticles. This nanocomposite showed a strong inhibiting effect against all six species of periodontitis-related pathogens, i.e., Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum and Enterococcus faecalis. This composite may have potential in Class V restorations to restore root caries and combat periodontitis.
Graphene and graphene oxide are two dimensional carbon nanomaterials with unique properties. Lee et al. created PMMA incorporating nano-sized graphene oxide (nGO) which led to improved mechanical properties [65]. PMMA incorporating nGO showed an anti-adhesive effect against microbial species (C. albicans, E. coli, S. aureus, and S. mutans) in artificial saliva. The authors suggest an increase in hydrophilicity as possible mechanism of the antimicrobial-adhesive effects of nGO-PMMA. They observed sustained antimicrobial-adhesive effects for up to 28 days. The advantage of this approach is that no metal ions or antibiotics were necessary for the antimicrobial effect. Graphene may also have an effect on the differentiation of osteogenic and other cells [66]. More research, however, is necessary to consolidate these findings.
Antimicrobial effects on materials can be accomplished by surface nanostructures. Narendrakumar et al. compared adherence of oral Streptococci to titanium nanotubules [67]. The samples included TiO2 nanotubes formed by anodization of titanium foil of 100, 50 and 15 nm diameter, a nanoporous (15 nm pore diameter) surface and compact TiO2 control. They showed that adherence increased with increasing nanotubule diameter. There was also a correlation between adherence and surface fluoride content. They showed that the adherence of oral streptococci can be successfully modified by titanium anodization.
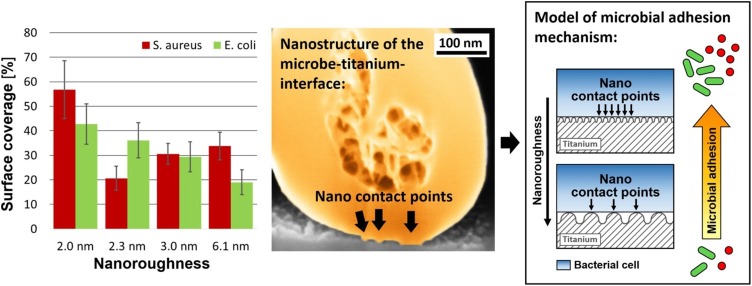
Lüdecke et al. investigated the adhesion of Escherichia coli and Staphylococcus aureus to nano-rough titanium created by PVD [14]. Microbial adhesion was reduced by up to 55.6% on the nano-rough titanium. By high resolution SEM-FIB preparation, they showed that the initial microbial adhesion on nano-rough surfaces is controlled via nanoadhesion points (Fig. 3 ). The late microbial adhesion on nano-rough surfaces was controlled by the attachment area between surface peaks and microbes.
Fig. 3.
Microbial adhesion of S. Aureus and E. Coli on nanorough titanium (2D nanomaterial). The microbial surface coverage depends on the nanoroughness and the type of microbe (shown left). The adhesion of the microbes is mediated by nano contact points between the titanium and the microbes (shown in the center, SEM-FIB micrograph). In addition to the nanoroughness, the surface peak density of the titanium plays an important role in the microbial adhesion.
These last two works are particularly interesting since they show antimicrobial effects of nanostructured titanium based on physical effects, but not by release of toxic silver or copper metal ions which is highly relevant for dental implants.
8. Nano-biomineralization
Nanotechnology has contributed to multiple aspects of the scientific study of mineralised hard tissues. In the case of dental tissues, enamel and dentin are equally important and there are also issues relating to the root canal, where demineralization is also a problem.
Enamel demineralization within the outermost 1 μ layer, apart from bacterial involvement, by dietary acids in the pH range 2–4 is a clinical problem widespread in the population. This erosive dissolution process results initially in surface softening. Since, under normal physiological conditions, teeth are bathed in saliva there might be the expectation that these high concentrations of calcium and phosphate ions would suffice to induce remineralization and re-hardening. Lippert et al. [68] investigated this question via tapping mode AFM and AFM nanoindentation on enamel specimens exposed to intra-oral conditions for potential remineralization following prior demineralizing challenges. These definitive experiments confirmed some previous work concluding that surface softened enamel was not capable of re-hardening under the chosen experimental conditions.
Studies such as this have stimulated the quest for nano-scale agents to promote enamel repair/regeneration [69,70]. The problem addressed by Krishnan et al. [69] was enamel damage associated with orthodontic treatment and bracket debonding, such as white spot lesions that can be precursors to carious lesions. They synthesized strontium-substituted nano-HAp via a co-precipitation method, which was less cytotoxic than pure n-HAp by MTT assay. The needle-shaped nano particles tended to cluster and were characterized by TEM and XRD. Treated surfaces were investigated by SEM, EDAX, AFM and microhardness. Statistically significant enhancements in microhardness were apparent.
The study by Xiao et al. [70] addressed the enamel remineralization challenge via chimaeric peptide-mediated nanocomplexes of carboxymethyl chitosan guiding amorphous nano-CaPO4 particles. This aimed to biomimetically replicate the orientated assembly of ACP guided by amelogenin in the biomineralization of enamel. XRD/SEM/CLSM and nano-indentation methods were applied to check the remineralization effects. NaOCl was used as an oxidizing agent to depolymerize chitosan and aid the transition of ACP nanoparticles into stable arrays and then crystals. This sophisticated approach, employing combinatorial biology protocols and nanotechnology, evidently succeeded in forming enamel-like crystals that were mostly perpendicular to the acid-etched enamel surface and accompanied by a recovered level of mechanical property performance.
Recent studies of the corresponding dentin remineralization challenge have deployed a variety of approaches [[71], [72], [73], [74]]. Weir et al. [71] developed nanocomposites containing nanoparticles of amorphous calcium phosphate (nACP) or nACP and tetracalcium phosphate (TTCP). The resin phase of these composites was ethoxylated bisphenol A dimethacrylate (EBPADMA) and pyromellitic glyceroldimethacrylate (PMGDM). After restoring the dentin lesions with nanocomposites as well as a non-releasing commercial composite control, the specimens were treated with cyclic demineralization (pH 4, 1 h per day) and remineralization (pH 7, 23 h per day) for 4 or 8 weeks. Calcium (Ca) and phosphate (P) ion releases from composites were measured. Dentin lesion remineralization was measured at 4 and 8 weeks by transverse microradiography (TMR). These novel nACP-based nanocomposites were able to achieve dentin lesion remineralization coupled with acid-neutralization and good mechanical properties. The same research group have also investigated a novel dentin remineralization method that aims to be effective even in an acidic solution without any initial calcium phosphate ions [72]. They also studied the effects of combining poly(amido amine) or PAMAM with nACP nanocomposite on dentin remineralization, acid neutralization and dentin hardness. The novel PAMAM + nACP was the most effective method in the short-term to induce dentin remineralization in this challenging environment. This could be useful to inhibit caries in patients with dry mouth where the local pH is often acidic and lacking saliva with Ca and P ions.
As a further development [73], this group investigated long-term fluid challenges to dentin demineralization with the PAMAM/nACP system. nACP composite was immersed at pH 4 to exhaust its calcium (Ca) and phosphate (P) ions, and then recharged with Ca and P ions, to test the remineralization of the exhausted and recharged nACP composite. After fluid challenges, the PAMAM plus nACP strategy still achieved full dentin remineralization.
Other researchers [74] have studied demineralization and remineralization of dentin and its collagen matrix at the nanoscale by amorphous, microcrystalline, and in situ formed HAp.
The relative affinity of the collagen and hydroxyapatite components of dentin toward HAp depends on these three types. The techniques used were concurrent resonance-enhanced atomic force microscopy coupled with infrared probe (AFM-IR) chemical mapping, nano-indentation, and scanning electron microscopy (SEM). A mineralization technique, via ion diffusion through a phosphate-linked chitosan gel, preferably deposited on the collagen bundles and thus allowed for an even and controlled growth of HAp guided by the completely demineralized collagen matrix of dentin.
Where bioactive nanoparticles have been incorporated into restorative materials, mostly they have been added as a co-dispersed phase in resin-composites. However, Porter et al. [75] have added silver nanoparticles to glass-ionomer cements (GIC) as an anti-biofilm agent. Several commercial GICs were modified by addition of 6, 10 and 24 μg per GIC capsule of α-lipoic acid-capped AgNPs. AgNP-modified GICs showed significant antibiofilm activity and retained mechanical properties equivalent or superior to non-modified GICs.
9. Nano coatings and nano mechanical testing of dental materials
Nano-mechanical measurements can and have been made on a wide range of dental materials, including surface coatings [76]. Nano-indentation involves a ramped indentation force applied via an indentor geometry (typically Berkovitch) into the test surface followed by ramped unloading, thus giving an indentation cycle. Both the area and the depth of indentation are very small, which is both an advantage and a disadvantage. The advantage is that thin coatings may be probed, as well as the outermost layers of a substance such as enamel. Moreover, different phase regions within the prepared surface area may be probed separately. The disadvantage is that sometimes it is desired to know the averaged-out response of a surface comprising several phases. In that case, meso- or micro- hardness indentation is preferable.
Alsayed et al. [76] investigated several types of barrier coating for enamel using loads up to 2 mN and depths up to 200 μm. Nanoindentation and scanning electron microscopy suggested that all the studied materials effectively prevented demineralization in coated areas.
Shimomura et al. [77] used spherical indenter tips with radii of 243 and 1041 nm to determine stress–strain curves of enamel. Force–displacement curves were recorded using quasi-static loading strain rates of 0.031, 0.041, and 0.061 s−1. The storage moduli, from a superimposed signal amplitude (dynamic strain at 220 Hz) during primary quasi-static loading and calculated via quasi-static elastic theory, were simultaneously measured. Modulus mapping was considered to be an extremely low quasi-static loading indentation test. The results suggested that the increase of the elastic limit during high-loading strain was associated with exceptional contact elasticity at the nanoscale of the enamel structure and the consequent extension of the contact area - i.e., a temporary pile-up response, was dependent on the enamel nanocrystals and surrounding protein. This highlights the capability of nanomechanical measurements, coupled with appropriate theory, to gain deep insight into structure/property characteristics.
In earlier studies, Lippert at al. [68,78] used AFM and AFM nanoindentation to study the clinically important topic of toothbrush abrasion of surface softened enamel. They showed that toothbrushing of surface softened enamel led to minor changes in the surface morphology and nanomechanical properties. The amount of enamel lost due to toothbrushing was independent of the demineralisation time and was lower compared to the mineral loss caused by the demineralization treatments.
El-Safty et al. [79] determined by nanoindentation the hardness and elastic modulus of 10 resin composites, including a series with systematically varied filler loading, plus other representative materials that fall into the categories of flowable, bulk-fill and conventional nano-hybrid types. For a specific resin matrix, both elastic moduli and nanohardness correlated positively with filler loading. For the resin-composites investigated, the group-average elastic moduli and nanohardnesses for bulk-fill and flowable materials were lower than those for conventional nano-hybrid composites. Bulk-fill composites are now a rapidly developing field, so the specific trends identified are liable to change, whereas the experimental methodology remains of great value.
10. Future perspectives and conclusions
Throughout history new technologies have been abruptly influenced and changed the life of societies, some of the more recent examples being the smart phone or the blockchain. Characteristics of disruptive technologies are that they create new markets and value networks and eventually disrupt existing market and value networks, displacing established market-leading firms, products, and alliances [80,81].
As such, nanotechnology may be considered a disruptive technology since it can have many different applications and replaces older technologies such as large particles in dental composites. For example, nanoparticle technology can influence many products and services [82] such as dental restorative or antimicrobial materials. Green ways to synthesize nanomaterials are needed to minimize the impact of nanomaterials production on the environment.
This review has shown that nanotechnology and especially nanomaterials have not only entered dentistry but may become a disruptive technology in it. Many scientific activities and more and more dental materials products contain nanomaterials of which nanoparticles are the most prominent.
This review has also shown that few of the new research results and products reported in this area seem to have a real break-through character, but rather represent improvements of different extent. In some cases, the buzz word nano seems to be used to market products rather than depicting superior materials properties.
The large majority of studies dealing with dental nanomaterials are in vitro studies and the (positive) effect of the nanomaterials in-vivo was in most cases not investigated or shown. In some cases, the nano improvement effect of the reported material is minimal or controversial.
From this review several perspectives for future developments in the field of dental nanomaterials may be deduced:
First, research and product development in this field of dental nanomaterials should focus on the challenges, i.e. real and significant improvements of dental materials supported by hard scientific evidence. Changes in the population and related changes in the materials needed may be an important factor to be considered for this.
Secondly, improved or new dental nanomaterials need to be put to the test in real clinical situations. Only if they show superior performance in these settings “nano” convinces.
Some future perspectives of resin based dental materials considering nanotechnology have been presented by the first author of this review a decade ago [83]. They included introducing dental materials with antimicrobial properties, introducing stimuli responsive smart or self-repairing materials and developing materials for dental hard tissue regeneration. At that time, these topics were mostly in a finding phase. This list shows clearly that the challenges have not changed and also that this requires much more complex dental materials and sophisticated technologies than the traditional ones still used. As this current review shows, these topics are now addressed by innovative dental nanomaterials through a strong increase in research activities to create a new quality of dental materials based on nanotechnology. The topics mentioned above moved in the last decade from the finding phase to the validation and application phases, respectively and in some cases have been introduced to the market.
From these topics, perhaps materials with antimicrobial properties is the most flourishing and the one with one of the highest potential clinical impact.
As discussed in this review, the topic of materials for dental hard tissue regeneration has been greatly influenced by nanobiomineralization approaches and is expected to growth further in the future. The reason for this is not only the strongly growing research interest in this area, but also the appeal of more natural tooth repair approaches with more sustainable materials using principles found in nature.
Although the topic list above is likely to be relevant also for future developments in dental nanomaterials science, the authors would like to expand the list in this current review. First, dental implants and especially their nano surface structure are likely to play a greater role in the future [84]. This prognosis is based on aging populations and still unsolved problems such as loosening or infection in connection with dental implants. Antimicrobial concepts utilizing dental nanomaterials and/or novel nanocoatings may be the key to address these challenges. Second, and also in relation to dental implants but also relevant to other areas of aesthetic dentistry as well, are advanced dental nanoceramics. Significantly enhanced structural reliability through flaw control, damage tolerance [85] and additive manufacturing [86] using novel mix ceramic nanocompositions may lead to significant progress in these materials. Third nano-biosensing may play a greater role in the future of dental materials [87]. This may be systems that measure important physical, chemical and biological data and in connection with digital systems processing these data [88] and imitate appropriate actions such as drug release for example to reduce microbes or counteract implant loosening.
There is much room for improvement and further development of dental materials. A new quality of dental materials may be created if nanotechnology is used and other new areas of its application in dentistry are explored. The benefit for the patient and the quality of dental treatment, if such new nano materials are developed and introduced, however, are self-evident [83]. Let us boldly move in this direction.
Acknowledgements
KDJ gratefully acknowledges the partial financial support of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), project number 316213987, SFB 1278 PolyTarget (project A06).
References
- 1.Feynmann R.P. There’s plenty of room at the bottom. Eng Sci. 1960;23:22–36. [Google Scholar]
- 2.www.youtube.com: Feynman: Take the world from another point of view (1/4).
- 3.Sudha P.N., Kirubanandam S., Vijayalakshmi K., Barhoum A. Emerging applications of nanoparticles and architecture nanostructures. Current prospects and future trends Micro and nano technologies. Elsevier; Amsterdam: 2018. Nanomaterials history, classification, unique properties, production and market; pp. 341–384. Chapter 12. [Google Scholar]
- 4.AlKahtani R.N. The implications and applications of nanotechnology in dentistry: a review. Saudi Dent J. 2018;30:107–116. doi: 10.1016/j.sdentj.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jandt K.D., Mills R.W. A brief history of LED photopolymerization. Dent Mater. 2013;29:605–617. doi: 10.1016/j.dental.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Beyer M., Reichert J., Heurich E., Jandt K.D., Sigusch B.W. Pectin, alginate and gum arabic polymers reduce citric acid erosion effects on human enamel. Dent Mater. 2010;26:831–839. doi: 10.1016/j.dental.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Jandt K.D. Probing the future in functional soft drinks on the nanometre scale—towards tooth friendly soft drinks. Trends Food Sci Technol. 2006;17:263–271. [Google Scholar]
- 8.Lippert F., Parker D.M., Jandt K.D. In situ remineralisation of surface softened human enamel studied with AFM nanoindentation. Surf Sci. 2004;553(1–3):105–114. [Google Scholar]
- 9.InCites Journal Citation Reports https://jcr.clarivate.com/on 26.04.2020.
- 10.Web of Science Core Collection http://apps.webofknowledge.com/ on 26.04.2020.
- 11.https://en.wikipedia.org/wiki/Nano-.
- 12.Vollath D. Wiley-VCH; Weinheim: 2013. Nanomaterials für einsteiger. Wiley-VCH, Weinheim 2014 and Nanoparticles – nano composites – nanomaterials. [Google Scholar]
- 13.Cao G., Wang Y. 2nd ed. World Scientific; New Jersey: 2011. Nanostructures and nanomaterials. [Google Scholar]
- 14.Lüdecke C., Roth M., Yu W., Horn U., Bossert J., Jandt K.D. Nanorough titanium surfaces reduce adhesion of Escherichia coli and Staphylococcus aureus via nano adhesion points. Colloids Surf B Biointerfaces. 2016;145:617–625. doi: 10.1016/j.colsurfb.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 15.Slater J.C. Atomic radii in crystals. J Chem Phys. 1964;41:3199–3205. [Google Scholar]
- 16.Zhang D., Weng G., Gong S., Zhou D. Computer simulation of grain growth of intermediate—and final-stage sintering and Ostwald ripening of BaTiO3-based PTCR ceramics. Mater Sci Eng B. 2003;99:428–432. [Google Scholar]
- 17.Schmid G., Corain B. Nanoparticulated gold: syntheses, structures, electronics, and reactivities. J Biol Inorg Chem. 2003;17:3081–3098. [Google Scholar]
- 18.Chae S.Y., Park M.K., Lee S.K., Kim T.Y., Kim S.K., Lee W.I. Preparation of size-controlled TiO2 nanoparticles and derivation of optically transparent photocatalytic films. Chem Mater. 2003;15:3326–3331. [Google Scholar]
- 19.Roco M.C., Hersam M.C., Mirkin C.A. 1st ed. Springer; 2011. Nanotechnology research directions for societal needs in 2020, retrospective and outlook. [Google Scholar]
- 20.https://en.wikipedia.org/wiki/Pulverizer#Attrition_mill.
- 21.Sadler L.Y., Stanley D.A., Brooks D.R. Attrition mill operating characteristics. Powder Technol. 1975;12:19–28. [Google Scholar]
- 22.Lok C.N., Ho C.M., Chen R., He Q.Y., Yu W.Y., Sun H., et al. Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;12:527–534. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 23.Beyene H.D., Werkneh A.A., Bezabh H.K., Ambaye T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustainable Mater Technol. 2017;13:18–23. [Google Scholar]
- 24.3M ESPE . 2010. Technical prodcut profile Filtech. 3M Center, St. Paul, USA. www.3m.com. [Google Scholar]
- 25.Densply . Dentsply Caulk; USA: 2013. Ceram X nano ceramic restorative. Scientific compendium. [Google Scholar]
- 26.Dong F., Firkowska-Boden I., Arras M.M.L., Jandt K.D. Responsive copolymer–graphene oxide hybrid microspheres with enhanced drug release properties. RSC Adv. 2017;7:3720–3726. [Google Scholar]
- 27.Nagavarma B.V.N., Yadav H.K.S., Ayaz A., Vasudha L.S., Shivakumar H.G. Different techniques for preparation of polymeric nanoparticles – a review. Asian J Pharm Clin Res. 2012;5:16–23. [Google Scholar]
- 28.Reischl D., Zimmer A. Drug delivery of siRNA therapeutics: potentials and limits of nanosystems. Nanomed Nanotechnol Biol Med. 2009;5:8–20. doi: 10.1016/j.nano.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Smith G.D., Bedrov D. Dispersing nanoparticles in a polymer matrix: Are long, dense polymer tethers really necessary? Langmuir. 2009;25:1239–11243. doi: 10.1021/la902329v. [DOI] [PubMed] [Google Scholar]
- 30.Fiedler B., Gojny F.H., Wichmann M.H.G., Nolte M.C.M., Schulte K. Fundamental aspects of nano-reinforced composites. Compo Sci Technol. 2006;66:3115–3125. [Google Scholar]
- 31.https://youtu.be/RmvbTBcshIA.
- 32.Chawla K.K. 3rd ed. Springer New York Heidelberg Dordrecht; London: 2012. Composite materials; p. 227. [Google Scholar]
- 33.Voigt W. Ueber die Beziehung zwischen den beiden Elasticitätsconstanten isotroper Körper. Ann Phys. 1889;274:573–587. [Google Scholar]
- 34.Reuss A. Berechnung Der Fließgrenze Von Mischkristallen Auf Grund Der Plastizitätsbedingung Für Einkristalle. Z. Angewand Mathe Mechan. 1929;9:49. [Google Scholar]
- 35.Callister W.D. John Wiley & Sons; Hoboken: 2005. Fundamentals of materials science and engineering. An integrated approach. [Google Scholar]
- 36.Omanović-Mikličanin E., Badnjević A., Kazlagić A., Hajlovac M. Nanocomposites: a brief review. Health Technol. 2020;10:51–59. [Google Scholar]
- 37.Kaizera M.R., Oliveira-Ogliaria A., Cencia M.S., Opdam N.J.M., Moraesa R.R., Kaizera M.R., et al. Do nanofill or submicron composites show improved smoothness and gloss? A systematic review of in vitro studies. Dent Mater. 2014 doi: 10.1016/j.dental.2014.01.001. e41-e78. [DOI] [PubMed] [Google Scholar]
- 38.Beun S., Glorieux T., Devaux J., Vreven J., Leloup G. Characterization of nanofilled compared to universal and microfilled composites. Dent Mater. 2007;23:51–59. doi: 10.1016/j.dental.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues S.A., Ferracane J.L., Della Bona A. Flexural strength and Weibull analysis of a microhybrid and a nanofill composite evaluated by 3-and 4-point bending tests. Dent Mater. 2008;24:426–431. doi: 10.1016/j.dental.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues S.A., Scherrer S.S., Ferracane J.L., Della Bona A. Microstructural characterization and fracture behavior of a microhybrid and a nanofill composite. Dent Mat. 2008;24 doi: 10.1016/j.dental.2008.02.006. 1281-128. [DOI] [PubMed] [Google Scholar]
- 41.Karabela M.M., Sideridou I.D. Synthesis and study of properties of dental resin composites with different nanosilica particles size. Dent Mater. 2011;27:825–835. doi: 10.1016/j.dental.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L., Weir M.D., Chow L.C., Antonucci J.M., Chen J., Xu H.H.K. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater. 2016;32:2285–2293. doi: 10.1016/j.dental.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radad K., Al-Shraim M., Moldzio R., Rausch W.D. Recent advances in benefits and hazards of engineered nanoparticles. Tox Pharm. 2012;34:661–672. doi: 10.1016/j.etap.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Hanley C., Thurber A., Hanna C., Punnoose A., Zhang J., Wingett D.G. The Influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res Lett. 2009;4:1409–1420. doi: 10.1007/s11671-009-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmalz G., Hickel R., van Landuyt K.L., Reichl F.-X. Nanoparticles in dentistry. Dent Mater. 2017;33:1298–1314. doi: 10.1016/j.dental.2017.08.193. [DOI] [PubMed] [Google Scholar]
- 46.Feng X., Chen A., Zhnag Y., Wang J., Shao L., Wei L. Application of dental nanomaterials: potential toxicity to the central nervous system. Int J Nanomed Nanosurg. 2015;10:3547–3565. doi: 10.2147/IJN.S79892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cokic S.M., Ghosh M., Hoet P., Godderis L., Van Meerbeek B., Van Landuyt K.L. Cytotoxic and genotoxic potential of respirable fraction of composite dust on human bronchial cells. Dent Mater. 2020;36:270–283. doi: 10.1016/j.dental.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Jandt K.D., Al-Jasser A.M.O., Al-Ateeq K., Vowles R.W., Allen G.C. Mechanical properties and radiopacity of experimental glass-silica-metal hybrid composites. Dent Mater. 2002;18:429–435. doi: 10.1016/s0109-5641(01)00064-1. [DOI] [PubMed] [Google Scholar]
- 49.Clement J.L., Jarrett P.S. Antibacterial silver. Metal-Based-Drugs. 1994;1:467–482. doi: 10.1155/MBD.1994.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noronhaa V.T., Paula A.J., Durán G., Galembeck A., Cogo-Müller K., Franz-Montan M., et al. Silver nanoparticles in dentistry. Dent Mater. 2017;33:1110–1126. doi: 10.1016/j.dental.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y. Controlled synthesis of colloidal silver nanoparticles in organic solutions: empirical rules for nucleation engineering. Chem Soc Rev. 2013;42:2497–2511. doi: 10.1039/c2cs35289c. [DOI] [PubMed] [Google Scholar]
- 52.Ginjupalli K., Shaw T., Tellapraga C., Alla R., Gupta L., Perampalli N.U. Does the size matter? Evaluation of effect of incorporation of silver nanoparticles of varying particle size on the antimicrobial activity and properties of irreversible hydrocolloid impression material. Dent Mater. 2018;34:e158–e165. doi: 10.1016/j.dental.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Tiwari D.K., Behari J., Sen P. Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Current Sci. 2008;95:647–655. [Google Scholar]
- 54.Baker C.D., Federico M.J., Accurso F.J. Case report: skin discoloration following administration of colloidal silver in cystic fibrosis. Curr Opinion Pedi. 2007;19:733–735. doi: 10.1097/MOP.0b013e3282f11fee. [DOI] [PubMed] [Google Scholar]
- 55.Lv X., Wang P., Bai R., Cong Y., Suo S., Ren X., et al. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomater. 2014;35:4195–4203. doi: 10.1016/j.biomaterials.2014.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hojati S.T., Alaghemand H., Hamze F., Babaki F.A., Rajab-Nia R., Rezvani M.B., et al. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent Mater. 2013;29:495–505. doi: 10.1016/j.dental.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Gutiérrezab M.F., Malaquiasa P., Matos T.P., Szesz A., Souza S., Bermudez J., et al. Mechanical and microbiological properties and drug release modeling of an etch-and-rinse adhesive containing copper nanoparticles. Dent Mater. 2017;33:309–320. doi: 10.1016/j.dental.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Fujimori Y., Sato T., Hayata T., Nagao T., Nakayama M., Nakayama T., et al. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol. 2012;78:951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6 doi: 10.1128/mBio.01697-15. e01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J.H., El-Fiqi A., Jo J.K., Kim D.A., Kim S.C., Jun S.K., et al. Development of long-term antimicrobial poly(methyl methacrylate) by incorporating mesoporous silica nanocarriers. Dent Mate. 2016;32:1564–1574. doi: 10.1016/j.dental.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Genari B., Leitune V.C.B., Jornada D.S., Camassola M., Arthur R.A., Pohlmann A.R., et al. Antimicrobial effect and physicochemical properties of an adhesive system containing nanocapsules. Dent Mater. 2017;33:735–742. doi: 10.1016/j.dental.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Weatherly L.M., Gosse J.A. Triclosan exposure, transformation, and human health effects. J Toxicol Environment Health. 2017;20:447–469. doi: 10.1080/10937404.2017.1399306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodman Michael, Naiman Daniel Q., Judy S. LaKind Systematic review of the literature on triclosan and health outcomes in humans. Crit Rev Toxicol. 2018;48(1):1–51. doi: 10.1080/10408444.2017.1350138. [DOI] [PubMed] [Google Scholar]
- 64.Wang L., Melo M.A.S., Weir M.D., Xie X., Reynolds M.A., Xu H.H.K. Novel bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Dent Mater. 2016;32:e351–e361. doi: 10.1016/j.dental.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 65.Lee J.H., Jo J.K., Kim D.A., Patel K.D., Kim H.W., Lee J.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent Mater. 2018;34:e63–e72. doi: 10.1016/j.dental.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 66.Xie H., Chia M., Islam I., Bentini R., Cao T., Viana-Gomesc J.C., et al. CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent Mater. 2017;33:e13–e21. doi: 10.1016/j.dental.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 67.Narendrakumara K., Kulkarni M., Addisona O., Mazare A., Junkar I., Schmuki P., et al. Adherence of oral streptococci to nanostructured titanium surfaces. Dent Mater. 2015;31:1460–1468. doi: 10.1016/j.dental.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Lippert F., Parker D.M., Jandt K.D. In situ remineralisation of surface softened human enamel studied with AFM nanoindentation. Surf Sci. 2004;553:105–114. [Google Scholar]
- 69.Krishnan V., Bhatia A., Varma H. Development, characterization and comparison of two strontium doped nano hydroxyapatite molecules for enamel repair/regeneration. Dent Mater. 2016;32:646–659. doi: 10.1016/j.dental.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Xiao Z., Que K., Wang H., An R., Chen Z., Qiu Z., et al. Rapid biomimetic remineralization of the demineralized enamel surface using nano-particles of amorphous calcium phosphate guided by chimaeric peptides. Dent Mater. 2017;33:1217–1228. doi: 10.1016/j.dental.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 71.Weir M.D., Ruan J., Zhang N., Chow L.C., Zhang K., Chang X., et al. Effect of calcium phosphate nanocomposite on in vitro remineralization of human dentin lesions. Dent Mater. 2017;33:1033–1044. doi: 10.1016/j.dental.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Liang K., Zhou H., Weir M.D., Bao C., Reynolds M.A., Zhou X., et al. Poly(amido amine) and calcium phosphate nanocomposite remineralization of dentin in acidic solution without calcium phosphate ions. Dent Mater. 2017;33:818–829. doi: 10.1016/j.dental.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 73.Liang K., Xiao S., Wu J., Weir M.D., Cheng L., Reynolds M.A., et al. Long-term dentin remineralization by poly(amido amine) and rechargeable calcium phosphate nanocomposite after fluid challenges. Dent Mater. 2018;34:607–618. doi: 10.1016/j.dental.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Sereda G., VanLaecken A., Turner J.A. Monitoring demineralization and remineralization of human dentin by characterization of its structure with resonance-enhanced AFM-IR chemical mapping, nanoindentation, and SEM. Dent Mater. 2019;35:617–626. doi: 10.1016/j.dental.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 75.Porter G.C., Tomkins G.R., Schwass D.R., Li K.C., Waddell J.N., Melandri C.J. Anti-biofilm activity of silver nanoparticle-containing glass ionomer cements. Dent Mater. 2020;36:1096–1107. doi: 10.1016/j.dental.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Alsayed E.Z., Hariri I., Nakashima S., Shimada Y., Baksh T.A., Tagami J., et al. Effects of coating materials on nanoindentation hardness of enamel and adjacent areas. Dent Mater. 2016;32:807–816. doi: 10.1016/j.dental.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 77.Shimomura N., Tanaka R., Shibata Y., Zhang Z., Li Q., Zhou J., et al. Exceptional contact elasticity of human enamel in nanoindentation test. Dent Mater. 2019;35:87–97. doi: 10.1016/j.dental.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Lippert F., Parker D.M., Jandt K.D. Toothbrush abrasion of surface softened enamel studied with tapping mode AFM and AFM nanoindentation. Caries Res. 2004;38:464–472. doi: 10.1159/000079628. [DOI] [PubMed] [Google Scholar]
- 79.El-Safty S., Akhtar R., Silikas N., Watts D.C. Nanomechanical properties of dental resin-composites. Dent Mater. 2012;28:1292–1300. doi: 10.1016/j.dental.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 80.https://en.wikipedia.org/wiki/Disruptive_innovation.
- 81.Ab Rahman A., Hamid U.Z.A., Ai Chin T. Emerging technologies with disruptive effects: a review. PERINTIS eJournal. 2017;7:111–128. [Google Scholar]
- 82.https://www.azonano.com/article.aspx?ArticleID=1246.
- 83.Jandt K.D., Sigusch B.W. Future perspectives of resin-based dental materials. Dent Mater. 2009;25:1001–1006. doi: 10.1016/j.dental.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Ruppa F., Lianga L., Geis-Gerstorfer J., Scheideler L., Hüttig F. Surface characteristics of dental implants: a review. Dent Mater. 2018;34:40–57. doi: 10.1016/j.dental.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Rekow E.D., Silva N.R.F.A., Coelho P.G., Zhang Y., Guess P., Thompson V.P. Performance of dental ceramics challenges for improvements. J Dent Res. 2011;90:937–952. doi: 10.1177/0022034510391795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galante R., Figueiredo-Pinaacd C.G., Serro A.P. Additive manufacturing of ceramics for dental applications: a review. Dent Mater. 2019;35:825–846. doi: 10.1016/j.dental.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 87.Sagadevan S., Periasamy M. Recent trends in nanobiosensors and their applications – a review. Rev Adv Mater Sci. 2014;36(2014):62–69. [Google Scholar]
- 88.Van Noort R. The future of dental devices is digital. Dent Mater. 2012;28:3–12. doi: 10.1016/j.dental.2011.10.014. [DOI] [PubMed] [Google Scholar]