Abstract
Although the COVID-19 pandemic affects predominantly the respiratory function, epidemiological studies show that multiple systems can be affected. The severe complications of SARS-CoV-2 infection seem to be induced by an inflammatory dysregulation (“cytokine storm”), which can also induce an immunodepression. Several studies highlight beneficial effects of osteopathic medicine on inflammation and immune regulation. A careful review of evidence-based literature brings to the fore significant improvements of osteopathic manipulative treatment (OMT) in adjunction to conventional care. OMT can improve the condition of infected patients by decreasing symptoms and boosting the efficiency of conventional care. OMT might also benefit surviving patients by reducing the long-lasting consequences of the infection as well as improving their quality of life during convalescence. This review should constitute an argument in favor of multidisciplinary care, although further biological and clinical research is essential to better assess the potential beneficial contributions of adjunct osteopathic medicine to conventional care in the fight against pandemics such as COVID-19.
Keywords: Osteopathic medicine, COVID-19, SARS-CoV-2, ARDS, Manual therapies, Musculoskeletal manipulations, Inflammation, Immunity
Abbreviations: ACE-2, angiotensin converting enzyme-2; ACI, acute cardiac injury; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CD4+T, T helper cell; CD8+T, cytotoxic T cell; COPD, chronic obstructive pulmonary disease; CoV-2, coronavirus-2; COVID-19, coronavirus infectious disease 2019; GALT, gut-associated lymphoid tissue; G-CSF, granulocyto-colony stimulating factor; IBS, inflammatory bowel disease; IgG, immunoglobulin G; IgA, immunoglobulin A; IL, interleukin; MCP, monocyte chemoattractant protein; OMT, osteopathic manipulative treatment; RT-PCR, reverse transcription-polymerase chain reaction; SARS, severe acute respiratory syndrome; TNF-α, tumor necrosis factor α
Introduction
As of March 12th 2020, the viral infectious disease COVID-19 has been declared a pandemic by the World Health Organization after 20 000 confirmed cases and 1000 deaths in Europe.1 At the time this article is submitted, 3 544 222 cases have been reported worldwide, including 250 977 deaths (7.1%).2 The virus responsible for COVID-19 belongs to a specific type of coronaviruses (β-CoV) that cause severe acute respiratory syndrome (SARS); it is referred to as SARS-CoV-2. Coronaviruses are a large family of viruses that infect a wide range of mammals, causing pathologies of various symptoms and severities, from the common cold to SARS.3 However, SARS-CoV-2 is very similar to SARS-CoV-1 and MERS-CoV, all of them being highly pathogenic for humans.3 SARS-CoV-2 appears to spread from person to person via droplets or direct contact. Van Doremalen et al. have shown that in specific laboratory conditions, the virus can survive in aerosols for hours, and on different surfaces for up to several days,4 contributing to the very high infection rate. The diagnosis relies on the presence of COVID-19 nucleic acid in the infected tissues.5., 6., 7., 8., 9., 10. Nasal or throat swabs constitute the main screening test by real-time reverse-transcription-polymerase chain reaction (RT-PCR),7 although chest CT scans have been proven to confirm a false negative diagnosis.7 , 9 More recently, specific serologic tests show very high sensitivity and accuracy (>97%), and allow efficient screening of asymptomatic subjects.11
As emphasized by the Glossary of Osteopathic Terminology (osteopath, p.33), the status of osteopathic practitioners varies depending on their country of professional practice.12 However, osteopathic medicine is internationally defined as a holistic approach that relies on palpatory diagnosis and manipulative treatment (commonly referred to as OMT).12 The most common reason for visits to osteopaths are musculoskeletal conditions such as pain or restricted range of motion.13 However, it has been shown that mechanical tension applied to cells induces a series of metabolic reactions that lead to varied biochemical responses, either rapid (intracellular release of Ca2+, hormonal secretion, etc.) or long-term (gene expression modulation).14 OMT has been shown to induce these so-called “mechanotransduction processes” (at least on fibroblasts).15 , 16 Considering this, a wide range of symptoms and functional disorders can also be addressed by osteopathic physicians who could thus contribute to immunity and homeostasis. Historically, osteopathic medicine played an important part in 1918 against the Spanish flu in the USA17 , 18 and physicians and scientists have recommended OMT against H5N1 avian flu in 2007.18 , 19
The aim of this review is to assess the potential influence of OMT on every pertinent aspect of COVID-19, in order to determine the preventive, curative and palliative role that could be played by osteopaths in this pandemic and similar future situations.
The literature search was conducted in April 2020 in the following databases: PubMed, ScienceDirect, OSTMED.DR, Osteopathic Research Web. In order to find all relevant material, the following search terms were used regarding OMT: osteopathic medicine, osteopathic manipulative treatment, musculoskeletal manipulations, manual therapies and names of organs. Regarding COVID-19 pathologies, symptoms and complications, the following list is non-exhaustive: COVID-19, coronavirus, SARS, MERS, ARDS, flu, inflammation, cytokines, etc. All the above terms were combined as well as possible to optimize findings.
Inflammatory biochemical pathways
Pathophysiology of SARS viruses
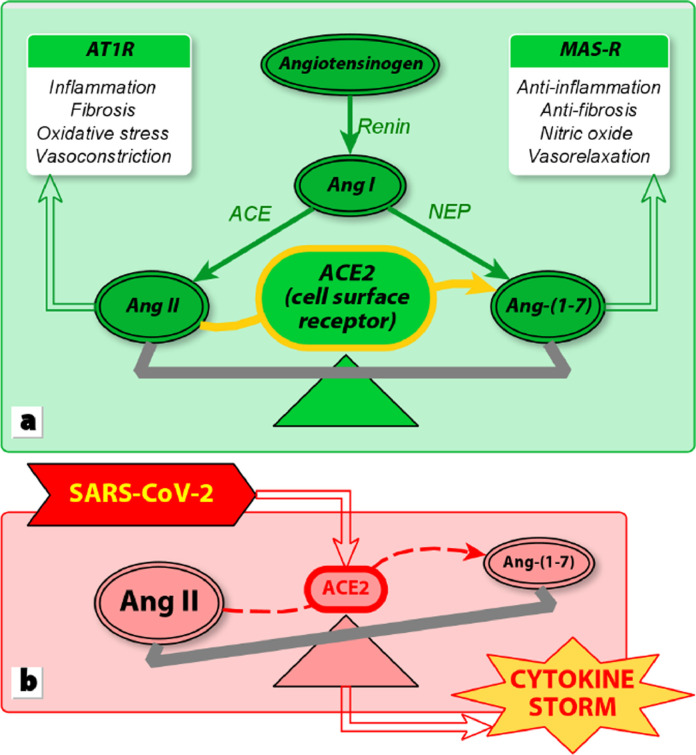
The angiotensin-converting enzyme-2 (ACE2) is a surface protein expressed in the cells of essentially all tissues; its activity has been shown to be very high in the colon, kidney, gallbladder, myocardium, breast, ovary, lung, etc.20 , 21 It hydrolyzes angiotensin II (Ang II) into Ang-(1–7). By fixating on its specific receptors (AT1R), Ang II induces cytokines production that results in local inflammation, fibrosis, oxidative stress and vasoconstriction. The fixation of Ang-(1–7) on its specific receptors (MAS-R) causes opposite effects (Fig. 1 ).
Fig. 1.
(a) Physiological regulation of the inflammatory process by ACE2. (b) Dysregulation induced by SARS-CoV-2. Based on South et al., 2020.21
SARS viruses enter host cells by fixating specifically on ACE2, inducing its downregulation.3 As shown on Fig. 1, this leads to a compensatory overproduction of Ang II by ACE, as well as a very low rate of hydrolysis of Ang II into Ang-(1–7).3 Therefore, the main process that has been identified to explain SARS (and its resulting high death rate) appears to be a viral hyper-inflammation.22 , 23
This phenomenon, well studied and documented in previous literature, is usually referred to as a “cytokine storm”.24 Tisoncik et al. emphasize on the complexity of cytokines’ classification and physiological description, mainly due to the fact that each of them has several target cells, multiple roles, and can induce various responses (essentially depending on the presence or absence of other cytokines). However complex, the cytokine storm can be summarized as a loss of balance between pro- and anti-inflammatory mechanisms. In the early minutes to hours post-infection, several pro-inflammatory cytokines appear (TNF-α, IL-1β, IL-8, MCP-1…). TNF-α and IL-1β induce an important production of IL-6 (which concentration in peripheral blood is commonly used to assess the intensity of systemic cytokine responses22 , 24). MCP-1 is a chemoattractant cytokine that recruits several leukocytes during an immune response, including basophils for which they activate degranulation. In the case of SARS-CoV-2, the infection induces a primary inflammation at a local site, mostly (but not exclusively as detailed further) in the lungs. The acute inflammation causes swelling resulting in fibrosis and persistent organ dysfunction, due to an “acute mononuclear/neutrophilic inflammatory response followed by a chronic fibroproliferative phase marked by progressive collagen deposition in the lung”.24
To balance the acute inflammatory response, anti-inflammatory cytokine IL-10 appears shortly after. However, IL-10 high concentration is associated with an increased fibrosis, as well as an immune downregulation termed “immunoparalysis”.24 This state is mainly defined by T lymphopenia – especially CD4+T cells, which are known to play an essential role in immunity responses, their decrease contributing to immunosenescence of the elderly25 – and a decrease of functional neutrophils and monocytes.26 The persistence of this immunosuppressed state 3-4 days after the initial acute sepsis ends up increasing the mortality risk instead of preventing it.24 In these contexts, the local inflammation can spread to the systemic circulation, leading to systemic sepsis (symptoms being persistent hypotension, hyper- or hypothermia, leukocytosis or leukopenia, thrombocytopenia).22 , 24 At this point, multiple organ dysfunction syndrome associated with immunosuppression results in a high mortality rate.3 , 22 , 24 , 26
OMT modulation of inflammatory and immune processes
Lymph is known to suppress inflammation, mainly through cytokines regulation; it also increases endothelial cell permeability, redistributes leukocytes, and in vitro studies have shown that lymph can inhibit macrophages activity and neutrophil apoptosis.27 A specific category of OMT techniques generally referred to as ‘pump techniques’ are promoted to influence the lymphatic circulation.19
During pump techniques, the lymph flow is increased as well as the total leukocytes count (neutrophils, monocytes, CD4+T, CD8+T, IgG, IgA1 ) in the thoracic and mesenteric duct lymph.28., 29., 30. Pump techniques also increase the mobilization of leukocytes into the lymphatic circulation from the Gut-Associated Lymphoid Tissues (GALT is “the largest mass of lymphoid tissue in the body” and produces 60% of the daily total Ig, then secreted into the gastrointestinal tract31).29 , 30
Animal studies have shown that pump techniques increase the lymph concentration of MCP-1, thus contributing in inflammatory responses.32 In vivo studies also show that specific OMT seems to rapidly modulate the concentration of several cytokines in the lymph ducts (30 min post-treatment).32 , 33 Licciardone et al. have shown a significant decrease of TNF-α after OMT in patients with chronic low back pain.34 , 35 An in vitro study confirmed the causality between increased lymph flow and TNF-α decreased production.27 OMT also induces a significant decrease in the level of monocytes, as well as that of nitric oxide (which plays a complex regulatory role in inflammation).33 It is also important to note that osteopathic pump techniques do not affect the macrophages viability.27
An in vitro study on fibroblasts cultures showed that strain from repetitive motion (similar to that of self-reeducation or physiotherapy) induces pro-inflammatory mechanisms (measured by a significant increase of IL-1α, IL-1β, IL-2, IL-3, IL-6); there was no such increase of these IL after specific OMT, in fact IL-3 and IL-6 productions significantly decreased.16 , 36 Moreover, specific OMT applied after repetitive motion strain reversed the pro-inflammatory mechanisms previously induced. Some preliminary results also show that the strain direction, frequency and orientation modifies the biochemical responses.36
There is evidence that OMT could increase antibody response to vaccination: Jackson et al. observed a faster and more important production of antibodies for patients who received specific OMT after hepatitis B vaccination.37 Animal studies also show that the efficiency of antibiotics to diminish the bacterial load in acute pneumonia is significantly improved when associated with pump techniques.38 Lastly, Walkowski et al. have shown that OMT induces an increase of specific immune cells not only in the lymphatic circulation but in the peripheral blood circulation as well, supporting the idea of a direct influence of OMT on immunity.33
All of the above suggests that mastered specific OMT could contribute to modulate immunity, inflammatory processes and fibroblast proliferation that result from an infection such as SARS. OMT could also be a beneficial adjunct to other therapies, both to enhance their efficiency and to diminish adverse side effects.
Therapeutic effects of adjunct OMT
It seems important to emphasize that, due to the pressing nature of COVID-19 topicality, all the following values vary greatly depending on authors: some symptoms might be overlooked leading to undervalued rates, other studies might focus on specific affliction leading to overvalued rates.
Large epidemiological meta-analyses showed the main symptoms of COVID-19 to be fever (85–89%), cough (65–68%), fatigue (38–42%), sputum production (34%) and dyspnea (19–21%).39 A smaller study showed that appetite loss appears to be very common (>80%).40 COVID-19-induced digestive symptoms have been observed: the most common is diarrhea (3.8% to 29% depending on authors), as well as abdominal discomfort, nausea and vomiting.5 , 10 , 40 Although 42% of COVID-19 patients exhibit only respiratory symptoms on hospital admission, 45% present with respiratory and digestive symptoms, and 3% exhibit digestive symptoms exclusively.40 Some authors also describe a 36.4% rate of various neurological symptoms,41 and at least 5% of COVID-19 patients also present anosmia and taste loss.42 , 43
The main severe complications are acute respiratory distress syndrome (ARDS) (5.6–13.2%), acute cardiac infection (ACI, 5.8%), septic shock (4.7%) and acute kidney infection (AKI, 2.8%); the global mortality rate ranges from 2.0% to 4.4%.10 The risk of complications and death is higher in male patients over 50 years old and in patients with comorbidities, mainly diabetes (7.7%), high blood pressure (15.6%) and cardiovascular diseases (4.7%).39 Recent systematic reviews show that children are also infected, with similar initial symptoms. However, the rate of infected children is very low, the severity is moderate to mild, their prognosis is better than adults’. Inflammatory dysregulation is less common, and lymphocytopenia seems rare.44 , 45
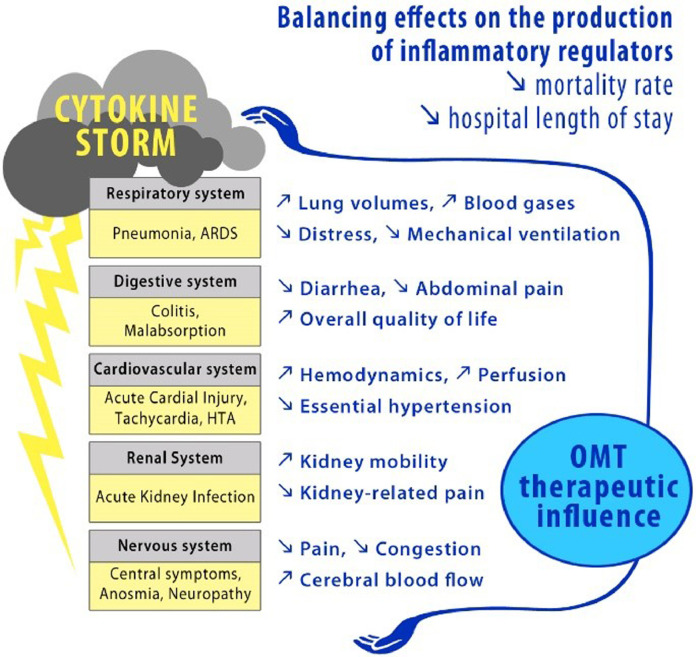
The following paragraphs are organized by system and begin with a brief presentation of the known pathophysiological aspects of the SARS-CoV-2 infection. Then, literature allowed us to identify OMT key-elements that could influence those pathophysiological processes and relieve either the symptom(s), the systemic affliction, or both (Fig. 2 ).
Fig. 2.
Effects of OMT on COVID-19 pathological process and symptoms. A systemic presentation of the main symptoms induced by the cytokine storm.
Respiratory system
The respiratory symptoms are common to numerous and often benign pathologies. But the main outcome of COVID-19 is viral pneumonia, which induces abnormal CO2 rejection in the alveolar region, leading to the inflammation of the pulmonary parenchyma.46 CT scans of COVID-19 patients show that most lesions are bilateral, multilobar and peripheral; vascular hypertrophy of the lesions are often observed47 as well as a retraction and thickening of the pleura.7 , 48 Complications such as pleural effusion or lymphadenitis have been observed. All of this concurs with an acute inflammatory response consistent with the cytokine storm description. This is called acute lung injury, or ARDS in its more severe form, which is common in SARS-CoV and influenza infections.3 , 24 By the Berlin definition, ARDS is characterized by a type of acute diffuse inflammatory lung injury leading to increased pulmonary vascular permeability, increased lung weight and loss of aerated lung tissue.49 Paradoxically, it is facilitated by the downregulation of ACE2 induced by SARS-CoV infection, since ACE2 has been shown to play a protective part against it.3
Chronic obstructive pulmonary disease (COPD) is a common affliction of which the main symptoms are similar to those of COVID-19 (shortness of breath, cough, sputum production).50 The literature shows that OMT applied to COPD patients seems to improve lung functionality, with significant improvements of blood gases, increase of total lung capacity and decrease of residual volume.50 , 51 A randomized controlled trial also showed a significant and lasting increase of the forced vital capacity.52 In the end, OMT contributed to an improvement of COPD symptoms and exercise capacity, and might induce similar beneficial effects to COVID-19 patients. Moreover, Herridge et al. observed that survivors of ARDS suffered from spirometric and lung volumes impairments up to 6 months after discharge, and blood gases impairments up to over a year after discharge; global quality of life is impaired, especially exercise capacity.53 Adjunct OMT might reduce the recovery period and could improve global condition for patients recovering from ARDS.
Considering the pathophysiological process, SARS-CoV-2 infection induces similar pulmonary complications than other and better known viral pneumonias.47 , 54 This allows us to speculate on the potential effects of OMT in COVID-19 patients, based on observation from previous research. A Cochrane systematic review shows that adjunction of OMT to conventional care for pneumonia allows a two days’ average diminution of hospital length of stay.55 , 56 A multicenter double-blind controlled trial showed that adjunct OMT reduces the rate of respiratory distress by 8%, equally reducing the necessity for mechanical ventilators; this study also showed that OMT reduces the hospital mortality rate by 6% in adults and up to 11% in the elderly (≥ 75 years old).56
Digestive system
SARS-CoV-2 nucleic acid has been identified in fecal matter, confirming that the digestive tract is very likely to be another entry point for it.5 , 6 , 20 ACE2 is highly expressed in the epithelial cells of the entire digestive tract as well as in enterocytes; the infection leads to a destruction or a dysfunction of those cells, inducing an inflammatory response contributing to malabsorption, unbalanced intestinal secretion, perturbation of the enteric nervous system and a loss of coordination of the immune responses by the GALT.6 A disturbed intestinal microbiota is also strongly suspected even though the processes are still to be fully understood.40 , 57 All of these alterations explain the observed digestive symptoms which, when they exist, appear in most cases earlier than the respiratory symptoms and thus could help in early detection of the infection.3 , 40
Irritable bowel syndrome (IBS) is characterized by episodes of abdominal pain, nausea and diarrhea, all of which are similar symptoms to those induced by COVID-19. Causes of IBS include local micro-inflammations fostered by increased cytokine production, which result in a disruption of the enteric nervous system, an altered bowel motility, a modification of the gut microbiota and a perturbation of the immune processes.58 , 59 A systematic review by Müller et al. shows that OMT has proven significantly efficient to relieve the symptoms of IBS, and to improve the quality of life of IBS patients.60 Therefore, it is likely that OMT could relieve COVID-19 patients with similar digestive symptoms.
Furthermore, Mao et al. pointed out that patients suffering from chronic inflammatory diseases such as IBS are at higher risk of opportunistic infections and recommend specific attention in regard to the COVID-19 pandemic.61 OMT could thus play a preventive part for healthy patients with medical history such as chronic inflammatory diseases of the gut or surgical scarring, by restoring local mobility and regulating local micro-inflammatory processes. As discussed above, OMT also stimulates the GALT resulting in leukocytes secretion which could contribute to rebalance the local dysfunctions.29 , 30
Cardiovascular system
In a sample of 416 hospitalized patients, Shi et al. observed a frequent association of acute cardiac injury (ACI) and ARDS (58.5% of cases), and a 51.2% mortality rate in patients with ACI.8 Chronic cardiovascular diseases increase the risk of ACI.8 , 39 Moreover, their treatments’ implications in COVID-19 are being studied as well, since many of them target the renin-angiotensin-aldosterone system in which ACE2 is a key enzymatic component.21
Current knowledge tends to show that there is no direct viral myocardial infection, but signs of interstitial inflammatory infiltration consistent with acute pericarditis which leads to myocardial inflammation, fibrosis, necrosis and eventually ventricular dysfunction.8 , 62 Like in other systems, the cytokine storm would be a rational explanation to this pathophysiological process; furthermore, it is consistent with the biological findings in ACI patients (leukocytosis, elevated C-reactive protein and procalcitonin).8
A prospective study showed that immediate OMT following coronary artery bypass graft induced physiologic hemodynamic improvements in the cardiac and perfusion functions (through significant increased mixed venous oxygen saturation, cardiac index and thoracic impedance).63 Another study with patients suffering from essential hypertension showed that regular OMT for a year induced a significant improvement (reduced blood pressure and reduced intima-media thickness).64 Thus, OMT has proven beneficial in both acute and chronic heart afflictions and could play a part reducing the incidence of COVID-19 comorbidities (cardiovascular diseases, high blood pressure). OMT may also minimize the symptoms of ACI and its potential consequences for surviving patients.
Renal system
The mortality rate is strongly increased for patients who develop acute kidney injury (AKI); Ruan et al. report a 91.7% mortality rate, Li et al. report a 5.3 higher mortality risk than patients without AKI.10 , 23 Two main hypothesis have been formulated to explain AKI in COVID-19: first, SARS-CoV-2 could enter kidney cells through an ACE2-dependant pathway (ACE2 expression in the kidneys appear to be 100-fold higher than in the lungs); second, the cytokine storm could induce secondary inflammatory effects on the kidneys, causing hypoxia, shock and rhabdomyolysis.65 This is consistent with the inflammatory processes previously described, and seems to be confirmed by clinical observation of increased creatine kinase levels.10 , 23 , 65
Our research has not lead us to studies evaluating the influence of OMT on kidney functionality. However, a systematic review by Bovo et al. shows the benefit of complementary and alternative medicine (not exclusively OMT) in various nephrology symptoms.66 Kaufman investigates OMT protocols (albeit untried) for renal and urinary symptoms.67 Tozzi et al. have shown that a restricted mobility of the kidneys (during breathing, evaluated through real-time ultrasound) is associated with non-specific low back pain, and that specific OMT improves the kidneys mobility, thus reducing pain perception.68 Since inflammation induces fibrosis and alters tissue texture and mobility, we hypothesize that a similar OMT approach might improve kidney function in AKI, and is likely to minimize the consequences of AKI for surviving patients.
Nervous system
COVID-19 manifests both in the central and peripheral nervous systems with various symptoms: headaches, dizziness, impaired consciousness, ataxia, epilepsy, acute, cerebrovascular disease, anosmia, taste loss, neuralgia.69 Wu et al. point out that neurological symptoms are more likely to occur in severely affected patients.41 In deceased patients, SARS-CoV-2 nucleic acid was reported in the cerebrospinal fluid, associated with brain tissue edema; a case of viral encephalitis was also reported.41 Similarly to the processes described in the other systems, Wu et al. discuss direct infectious pathways, as well as indirect degeneration resulting from inflammatory-induced hypoxia and/or immune dysfunction (possibly leading to secondary bacterial infections).41
Anosmia and taste loss are more prevalent in COVID-19 than in previous SARS and other coronaviruses infections.42 It seems important to note than even though those symptoms are quite common in other infections (common cold, flu, etc.), they occur in COVID-19 without nasal obstruction or any other rhinitis symptoms.42 The human pathophysiological processes have yet to be precisely explained, but they have been extensively studied in rodents. It appears that olfactory sensory neurons do not express ACE2, which does not support a primary nervous infection by SARS-CoV-2. However, the olfactory epithelial support cells and stem cells, the nasal epithelial cells and oral mucosal cells all highly express ACE2.20 , 70 Smell and taste functionality could be either directly affected, or secondary to the local inflammatory processes (edema, fibrosis, etc.) induced by the cytokine deregulation previously described.70 The fact that stem cells are affected could also explain the occasional persistence of the symptoms.
A systematic review by Schmid et al. strongly supports the theory that specific OMT could stimulate areas of the central nervous systems and activate descending inhibitory pathways, resulting in significant combined effects of hypoalgesia, sympathetic nervous system excitation and motor activity.71 These observations signify that OMT could play a part in the management of some of the COVID-19 symptoms such as headaches and neuralgia. A recent randomized controlled trial using magnetic resonance imagery has shown evidence that OMT can also significantly modify the cerebral blood flow.72 Moreover, the recently explored “glympathic system” (MeSH was introduced in 2019) emphasize the continuity between the body lymphatic system and cerebrospinal and interstitial fluids.73 Though further research is needed, these observations support the idea that OMT could, like in other systems, directly influence pathophysiological and immunity processes in the central nervous system.73
To our knowledge, there has not yet been specific research about the influence of OMT on anosmia and taste loss. But several OMT protocols have been described to address mechanical or inflammatory issues around the nasal bones and the upper respiratory tract.74 , 75 Also, Apoznanski et al. showed in a single-case report that one OMT session cured a baby with dacryostenosis, stopping the necessity for antibiotics and avoiding surgical procedure;76 and Lee-Wong et al. showed that self-reported pain, pressure and congestion was significantly improved by OMT in patients with chronic sinusitis.77
Conclusions
The aim of this review was to determine the potential field of action of osteopathic medicine in regards to SARS-CoV-2 pathophysiological processes and its resulting symptoms. The scientific literature showed that all severe complications of SARS infections seem consecutive to a dysregulation of inflammation and immunity caused by a cytokine storm. OMT has proven to be an important therapeutic adjunction to conventional care against infections, especially when the use of anti-inflammatory medication is limited. Biological and clinical evidence show its benefices against several pathologies with symptoms analog to those of COVID-19. This is especially true for pneumonia, with a substantial decrease in the necessity for a mechanical ventilation support, a shortening of the hospital length of stay and a significantly lower mortality rate. Since OMT has been shown to modify significantly the immunological profile of some circulating cytokines and leukocytes, it could also prove beneficial for prevention in non-infected patients. This, by stimulating the immune system and reducing the harmful effects of previous comorbidities, such as fibrosis and local inflammatory deregulation. Finally, future studies should lead to prove a positive impact of OMT on post-infection tissue sequelae (fibrosis, oxidative stress…), which could prevent the installation of chronic conditions.
Osteopathic medicine is already considered by many as an efficient tool to relieving pain by “resetting joints”. However, the evidence-based effects of OMT highlighted in this review show that its therapeutic effects extend far beyond that. In a context of global ageing and an increase of emerging pathogens, this review provides researchers with many ways to develop solid research hypotheses, and encourages further research to better comprehend OMT biological pathways. More clinical evidence – through rigorous controlled trials – is also necessary, especially regarding a new and highly-infectious disease such as COVID-19; at this point, very little is known of the long term evolution of this pandemic, and of the consequences that will ensue. This review is intended to offer all healthcare practitioners a better understanding of osteopathic medicine, as the authors look forward to more inclusive recommendations for OMT in multidisciplinary care. Osteopathic medicine is holistic, inclusive and accessible: it could be a substantial support for patients and a strong adjunction to preventive medical care.
Acknowledgments
The authors wish to thank the founder of Ecole Supérieure d'Ostéopathie Roger Caporossi and the president of Ecole Supérieure d'Ostéopathie Christophe Caporossi for their support, and Serge Pin for all his valuable guidance.
Footnotes
CD4+T = T helper cell, CD8+T = cytotoxic T cell, IgG = Immunoglobulin G, IgA = Immunoglobulin A
References
- 1.World Health Organization. Coronavirus disease (COVID-19) outbreak – WHO announces COVID-19 outbreak a pandemic [Internet]. 2020[cited 2020 Mar 28]. Available from:http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic
- 2.European Centre for Disease Prevention and Control. Situation update worldwide, as of 13 April 2020. Epidemiological update. [Internet].2020[cited 2020 Apr 13]. Available from:https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
- 3.Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Rev GeroSci. 2020 doi: 10.1007/s11357-020-00186-0. http://www.ncbi.nlm.nih.gov/pubmed/32274617 [cited 2020 Apr 13]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. [cited 2020 Apr 13]; Available from: https://www.nejm.org/doi/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020:118–119. doi: 10.1053/j.gastro.2020.02.054. AprilAvailable from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Kang Z, Gong H, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020;2020.01.30.927806.
- 7.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. Am J Roentgenol. 2020:1–8. doi: 10.2214/AJR.20.22975. June. [DOI] [PubMed] [Google Scholar]
- 8.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020:1–8. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Wu M, Yao J, et al. Caution on kidney dysfunctions of COVID-19 patients. SSRN Electron J. 2020:1–25. [Google Scholar]
- 11.Zhao R, Li M, Song H, et al. Early detection of SARS-CoV-2 antibodies in COVID-19 patients as a serologic marker of infection. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa523/5827750. Available from: https://academic.oup.com/cid/advance-article/doi/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Association of Colleges of Osteopathic Medicine . third ed. AACOM; 2017. Glossary of osteopathic terminology.www.aacom.org/resources/bookstore/Pages/glossary.aspx editorAvailable from: [Google Scholar]
- 13.Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2005;6:43. doi: 10.1186/1471-2474-6-43. http://www.ncbi.nlm.nih.gov/pubmed/16080794 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Z, Guo SS, Fässler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215(4):445–456. doi: 10.1083/jcb.201609037. https://rupress.org/jcb/article/215/4/445/38805/Integrinmediated Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bordoni B, Zanier E. Understanding fibroblasts in order to comprehend the osteopathic treatment of the fascia. Evid-Based Complement Altern Med. 2015;2015:1–7. doi: 10.1155/2015/860934. [cited 2020 Apr 19]Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meltzer KR, Standley PR. Modeled repetitive motion strain and indirect osteopathic manipulative techniques in regulation of human fibroblast proliferation and interleukin secretion. J Am Osteopath Assoc. 2007;107(12):527–536. [PubMed] [Google Scholar]
- 17.Ward EA. Influenza and its osteopathic management. 1937. J Am Osteopath Assoc. 2000;100(5):325–328. http://www.ncbi.nlm.nih.gov/pubmed/10850020 Available from: [PubMed] [Google Scholar]
- 18.Patterson MM. The coming influenza pandemic: lessons from the past for the future. J Am Osteopath Assoc. 2005;105(11):498–500. [PubMed] [Google Scholar]
- 19.Hruby RJ, Hoffman KN. Avian influenza: an osteopathic component to treatment. Osteopath Med Prim Care. 2007;1:1–19. doi: 10.1186/1750-4732-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):1–5. doi: 10.1038/s41368-020-0074-x. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Circ Physiol. 2020;318(5):1084–1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [cited 2020 Apr 18]Available from: http://link.springer.com/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan Q, Yang K, Wang W, Jiang L, Song J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med. 2020:1. doi: 10.1007/s00134-020-06028-z. [cited 2020 Apr 18]Available from: http://link.springer.com/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moro-García MA, Alonso-Arias R, López-Larrea C. When aging reaches CD4+ T-cells: phenotypic and functional changes. Front Immunol. 2013;4(MAY):1–12. doi: 10.3389/fimmu.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;53(9):1689–1699. doi: 10.1093/cid/ciaa248/5803306. Available from: https://academic.oup.com/cid/advance-article/doi/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo R, Schander A, Hodge LM. Lymphatic pump treatment mobilizes bioactive lymph that suppresses macrophage activity in vitro. J Am Osteopath Assoc. 2018;118(7):455–461. doi: 10.7556/jaoa.2018.099. [DOI] [PubMed] [Google Scholar]
- 28.Hodge LM, King HH, Williams AG, et al. Abdominal lymphatic pump treatment increases leukocyte count and flux in thoracic duct lymph. Lymphat Res Biol. 2007;5(2):127–133. doi: 10.1089/lrb.2007.1001. [cited 2020 Apr 19]Available from: http://www.liebertpub.com/doi/ [DOI] [PubMed] [Google Scholar]
- 29.Hodge LM, Bearden MK, Schander A, et al. Lymphatic pump treatment mobilizes leukocytes from the gut associated lymphoid tissue into lymph. Lymphat Res Biol. 2010;8(2):103–110. doi: 10.1089/lrb.2009.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodge LM, Downey HF. Lymphatic pump treatment enhances the lymphatic and immune systems. Exp Biol Med. 2011;236:1109–1115. doi: 10.1258/ebm.2011.011057. [cited 2020 Apr 19]Available from: http://journals.sagepub.com/doi/ [DOI] [PubMed] [Google Scholar]
- 31.Salminen S, Bouley C, Boutron M-C, et al. Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998;80(S1):S147–S171. doi: 10.1079/bjn19980108. [DOI] [PubMed] [Google Scholar]
- 32.Schander A, Fred Downey H, Hodge LM. Lymphatic pump manipulation mobilizes inflammatory mediators into lymphatic circulation. Exp Biol Med. 2012;237(1):58–63. doi: 10.1258/ebm.2011.011220. [DOI] [PubMed] [Google Scholar]
- 33.Walkowski S, Singh M, Puertas J, Pate M, Goodrum K, Benencia F. Osteopathic manipulative therapy induces early plasma cytokine release and mobilization of a population of blood dendritic cells. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Licciardone JC, Kearns CM, Hodge LM, Bergamini MVW. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: results from the osteopathic trial. J Am Osteopath Assoc. 2012;112(9):596–605. doi: 10.7556/jaoa.2012.112.9.596. [DOI] [PubMed] [Google Scholar]
- 35.Licciardone JC, Kearns CM, Hodge LM, Minotti DE. Osteopathic manual treatment in patients with diabetes mellitus and comorbid chronic low back pain: subgroup results from the OSTEOPATHIC trial. J Am Osteopath Assoc. 2013;113 http://jaoa.org/ [cited 2020 Apr 19]. Available from: [PubMed] [Google Scholar]
- 36.Standley PR, Meltzer K. In vitro modeling of repetitive motion strain and manual medicine treatments: potential roles for pro- and anti-inflammatory cytokines. J Bodyw Mov Ther. 2008;12(3):201–203. doi: 10.1016/j.jbmt.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson KM, Steele TF, Dugan EP, Kukulka G, Blue W, Roberts A. Effect of lymphatic and splenic pump techniques on the antibody response to hepatitis B vaccine: a pilot study. J Am Osteopath Assoc. 1998;98(3):155–160. [PubMed] [Google Scholar]
- 38.Hodge LM, Creasy C, Carter KR, Orlowski A, Schander A, King HH. Lymphatic pump treatment as an adjunct to antibiotics for pneumonia in a rat model. J Am Osteopath Assoc. 2015;115(5):306–316. doi: 10.7556/jaoa.2015.061. [cited 2020 Apr 19]Available from: http://jaoa.org/article.aspx?doi= [DOI] [PubMed] [Google Scholar]
- 39.Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;301 doi: 10.1016/j.jcv.2020.104371. https://linkinghub.elsevier.com/retrieve/pii/S138665322030113X Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China. Am J Gastroenterol. 2020:1. doi: 10.14309/ajg.0000000000000620. https://www.practiceupdate.com/content/clinical-characteristics-of-covid-19-patients-with-digestive-symptoms-in-hubei-china/98000 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. https://linkinghub.elsevier.com/retrieve/pii/S0889159120303573 JanuaryAvailable from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and Ageusia: common findings in COVID-19 patients. The Laryngoscope. 2020;130(7):1787. doi: 10.1002/lary.28692. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020 doi: 10.4193/Rhin20.116. http://www.ncbi.nlm.nih.gov/pubmed/32277751 AprilAvailable from: [DOI] [PubMed] [Google Scholar]
- 44.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr Int J Paediatr. 2020:1–8. doi: 10.1111/apa.15270. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liguoro I, Ferrari ME, Vidal E. SARS-CoV-2 infection in children and newborns : a systematic review. Prepr (Version 1) available Res Sq [Internet]. 2020;1–33. Available from:https://www.researchsquare.com/article/rs-24629/v1
- 46.Figueiredo LTM. Viral pneumonia: epidemiological, clinical, pathophysiological and therapeutic aspects. Review article. J Bras Pneumol. 2009;35(9):899–906. doi: 10.1590/s1806-37132009000900012. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1806-37132009000900012&lng=pt&tlng=pt Available from: [DOI] [PubMed] [Google Scholar]
- 47.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22976. May. [DOI] [PubMed] [Google Scholar]
- 48.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. July. [DOI] [PubMed] [Google Scholar]
- 49.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. Available from: http://jama.jamanetwork.com/article.aspx?doi= [DOI] [PubMed] [Google Scholar]
- 50.Zanotti E, Berardinelli P, Bizzarri C, et al. Osteopathic manipulative treatment effectiveness in severe chronic obstructive pulmonary disease: a pilot study. Complement Ther Med. 2012;20(1–2):16–22. doi: 10.1016/j.ctim.2011.10.008. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 51.Howell RK, Allen TW, Kappler RE. The influence of osteopathic manipulative therapy in the management of patients with chronic obstructive lung disease. J Am Osteopath Assoc. 1974;74(April):757–760. https://www.researchgate.net/publication/22116362_The_influence_of_osteopathic_manipulative_ therapy_in_the_management_of_patients_with_chronic_obstructive_lung_disease Available from: [PubMed] [Google Scholar]
- 52.Engel RM, Gonski P, Beath K, Vemulpad S. Medium term effects of including manual therapy in a pulmonary rehabilitation program for chronic obstructive pulmonary disease (COPD): a randomized controlled pilot trial. J Man Manip Ther. 2016;24(2):80–89. doi: 10.1179/2042618614Y.0000000074. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. Available from: http://www.nejm.org/doi/abs/ [DOI] [PubMed] [Google Scholar]
- 54.Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. 2020;71(15):756–761. doi: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang M, Yan Y, Yin X, et al. Chest physiotherapy for pneumonia in adults. Cochrane Datab Syst Rev. 2013;2013(2) doi: 10.1002/14651858.CD006338.pub3. [DOI] [PubMed] [Google Scholar]
- 56.Noll DR, Degenhardt BF, Johnson JC. Multicenter osteopathic pneumonia study in the elderly: Subgroup analysis on hospital length of stay, ventilator-dependent respiratory failure rate, and in-hospital mortality rate. J Am Osteopath Assoc. 2016;116(9):574–587. doi: 10.7556/jaoa.2016.117. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133(1):24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132(3):913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 60.Müller A, Franke H, Resch KL, Fryer G. Effectiveness of osteopathic manipulative therapy for managing symptoms of irritable bowel syndrome: a systematic review. J Am Osteopath Assoc. 2014;114(6):470–479. doi: 10.7556/jaoa.2014.098. [DOI] [PubMed] [Google Scholar]
- 61.Mao R, Liang J, Shen J, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5(5):426–428. doi: 10.1016/S2468-1253(20)30076-5. https://linkinghub.elsevier.com/retrieve/pii/S2468125320300765 [cited 2020 May 6]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inciardi RM, Lupi L, Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;2019:4–9. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O-Yurvati AH, Carnes MS, Clearfield MB, Stoll ST, McConathy WJ. Hemodynamic effects of osteopathic manipulative treatment immediately after coronary artery bypass graft surgery. J Am Osteopath Assoc. 2005;105(10):475–481. [PubMed] [Google Scholar]
- 64.Cerritelli F, Carinci F, Pizzolorusso G, et al. Osteopathic manipulation as a complementary treatment for the prevention of cardiac complications: 12-months follow-up of intima media and blood pressure on a cohort affected by hypertension. J Bodyw Mov Ther. 2011;15(1):68–74. doi: 10.1016/j.jbmt.2010.03.005. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 65.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [cited 2020 Apr 22]Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bovo L, Burrai F, Canossa F. Use of complementary and alternative medicine in nephrology: a review of RCTs. G Tec Nefrol Dial. 2017;29(3):185–192. [Google Scholar]
- 67.Kaufman BE. An osteopathic approach to the renal and urinary system. Osteopath Fam Phys. 2012;4(4):101–109. doi: 10.1016/j.osfp.2012.03.001. Available from: http://dx.doi.org/ [DOI] [Google Scholar]
- 68.Tozzi P, Bongiorno D, Vitturini C. Low back pain and kidney mobility: local osteopathic fascial manipulation decreases pain perception and improves renal mobility. J Bodyw Mov Ther. 2012;16(3):381–391. doi: 10.1016/j.jbmt.2012.02.001. http://www.ncbi.nlm.nih.gov/pubmed/22703751 Available from: [DOI] [PubMed] [Google Scholar]
- 69.Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. JAMA Neurology. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brann D, Tsukahara T, Weinreb C, Logan DW, Datta SR. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. bioRxiv [Internet]. 2020; Available from: https://doi.org/10.1101/2020.03.25.009084
- 71.Schmid A, Brunner F, Wright A, Bachmann LM. Paradigm shift in manual therapy? Evidence for a central nervous system component in the response to passive cervical joint mobilisation. Man Ther. 2008;13(5):387–396. doi: 10.1016/j.math.2007.12.007. https://linkinghub.elsevier.com/retrieve/pii/S1356689X08000209 Available from: [DOI] [PubMed] [Google Scholar]
- 72.Tamburella F, Piras F, Piras F, Spanò B, Tramontano M, Gili T. Cerebral perfusion changes after osteopathic manipulative treatment: a randomized manual placebo-controlled trial. Front Physiol. 2019;10:403. doi: 10.3389/fphys.2019.00403/full. [cited 2020 May 6]Available from: https://www.frontiersin.org/article/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hitscherich K, Smith K, Cuoco JA, et al. The glymphatic-lymphatic continuum: opportunities for osteopathic manipulative medicine. J Am Osteopath Assoc. 2016;116(3):170–177. doi: 10.7556/jaoa.2016.033. [DOI] [PubMed] [Google Scholar]
- 74.Sherman T, Qureshi Y, Bach A. Osteopathic manipulative treatment to manage ophthalmic conditions. J Am Osteopath Assoc. 2017;117(9):568–575. doi: 10.7556/jaoa.2017.111. [DOI] [PubMed] [Google Scholar]
- 75.Yao S, Mikhail N, Koutsouras G, Coombs A, Terzella MJ. Osteopathic considerations in the infections of the respiratory tract. Osteopath Fam Phys. 2017;9(1):17–25. https://ofpjournal.com/index.php/ofp/article/view/484 Available from. [Google Scholar]
- 76.Apoznanski TE, Abu-Sbaih R, Terzella MJ, Yao S. Resolution of dacryostenosis after osteopathic manipulative treatment. J Am Osteopath Assoc. 2015;115(2):110–114. doi: 10.7556/jaoa.2015.022. [DOI] [PubMed] [Google Scholar]
- 77.Lee-Wong M, Karagic M, Doshi A, Gomez S, Resnick D. An osteopathic approach to chronic sinusitis. J Allergy Ther. 2011;02(02):2–4. https://www.omicsonline.org/an-osteopathic-approach-to-chronic-sinusitis-2155-6121.1000109.php?aid=58 Available from: [Google Scholar]