Abstract
Background
There is a great deal of debate about the role of cardiovascular comorbidities and the chronic use of antihypertensive agents (such as ACE-I and ARBs) on mortality on COVID-19 patients. Of note, ACE2 is responsible for the host cell entry of the virus.
Methods
We extracted data on 575 consecutive patients with laboratory-confirmed SARS-CoV-2 infection admitted to the Emergency Department (ED) of Humanitas Center, between February 21 and April 14, 2020.
The aim of the study was to evaluate the role of chronic treatment with ACE-I or ARBs and other clinical predictors on in-hospital mortality in a cohort of COVID-19 patients.
Results
Multivariate analysis showed that a chronic intake of ACE-I was associated with a trend in reduction of mortality (OR: 0.53; 95% CI: 0.27–1.03; p = 0.06), differently from a chronic intake of ARB (OR: 1.1; 95% CI: 0.5-2.8; p=0.8). Increased age (ORs ranging from 3.4 to 25.2 and to 39.5 for 60–70, 70–80 and >80 years vs <60) and cardiovascular comorbidities (OR: 1.90; 95% CI: 1.1–3.3; p = 0.02) were confirmed as important risk factors for COVID-19 mortality. Timely treatment with low-molecular-weight heparin (LMWH) in ED was found to be protective (OR: 0.36; 95% CI: 0.21–0.62; p < 0.0001).
Conclusions
This study can contribute to understand the reasons behind the high mortality rate of patients in Lombardy, a region which accounts for >50% of total Italian deaths.
Based on our findings, we support that daily intake of antihypertensive medications in the setting of COVID-19 should not be discontinued and that a timely LMWH administration in ED has shown to decrease in-hospital mortality.
Keywords: ACE-I, ARBs, RAAS, Cardiovascular, Epidemiology, Hypertension
Highlights
-
•
This is a retrospective study on 575 consecutive patients with laboratory-confirmed SARS-CoV-2 infection from a center in Northern Italywhose whose in-hospital mortality was 20.9%.
-
•
Chronic treatment with —ACEI in COVID-19 patients is associated with a decrease and a significant delay in in-hospital mortality, differently from the chronic use of ARBs.
-
•
Increasing age and cardiovascular comorbidities are important risk factors for Covid-19 mortality, while an early introduction of LMWH in the setting of Covid-19 leads to a better prognosis.
1. Introduction
In late December 2019, a novel coronavirus emerged in Wuhan, the capital city of Hubei. The enveloped RNA betacoronavirus has been soon named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to its phylogenetic similarity to SARS-CoV [1].
On February 20, 2020, the first case of novel coronavirus disease 2019 (COVID-19) was detected in Italy [2], and 20 days later WHO declared COVID-19 outbreak a global pandemic [3].
Italy has one of the highest rates of SARS-CoV-2 infection worldwide, with 397.1 cases per 100.000 people, as well as the fourth highest mortality rate, 14.5% vs. a global average value of 5.1% (as of June 26th, 2020; https://coronavirus.jhu.edu/map.html).
The Lombardy region, with 10.6 Million people living in highly populated areas, has been the epicenter of the infection in Italy, with 39.1% of total cases and almost half of total deaths (http://www.salute.gov.it/portale/home.html).
The reason behind the high mortality rate in Italy, and mostly in Lombardy, can have several explanations: 1) the characteristics of the population (Italy has the second oldest population in Europe; https://ec.europa.eu/eurostat/data/database); 2) the organization of the health system in Lombardy where most of the medical care is provided at hospital level rather than locally with general practitioners. In addition, a low number of intensive care units is present compared to those needed for Lombardy's population; 3) the possible existence of different virus strains; 4) differences in genetic and environmental factors (e.g. air pollution); 5) overuse of antibiotics and antihypertensive agents, in Italy as compared to other countries.
A recent paper tried to assess the role of genetic variation at ACE2 gene in Italian subjects as compared to other European and East Asian cohorts in candidate genes for viral infection, namely ACE2 and TMPRSS2, as a possible explanation of the high mortality rate in Italy, with some suggestive findings that need to be further validated [4].
As for today, there are discordant results regarding the use of either angiotensin converting enzyme inhibitors (ACE—I) or angiotensin II receptor blockers (ARBs) as for their possible impact on COVID-19 mortality. Both ACE-I and ARBs act on the renin-angiotensin-aldosterone system (RAAS) and are widely prescribed for primary hypertension [5], heart failure, diabetic nephropathy and secondary prevention of myocardial infarction (MI) [6]. ACE-I exerts its effect by inhibiting the angiotensin converting enzyme (ACE), a specific protease found primarily in the vascular endothelium of the lungs and kidneys which catalyzes the conversion of angiotensin I to angiotensin II, which in turn causes vasoconstriction [7]. ARBs act by antagonizing the effects of angiotensin II on its AT1 and AT2 receptors [8], expressed in kidney [9,10], adrenal cortex [11], arterioles [12], and brain [13,14].
The carboxypeptidase angiotensin-converting enzyme 2 (ACE2), which is highly expressed in the lower airways, degrades angiotensin II into its active form angiotensin 1–7, which in turn dilates blood vessels, reduces inflammation, and inactivates angiotensin II before it could interact with its receptors [15].
SARS-CoV-2 binds to ACE2 with its spike glycoprotein which allows it to gain entry into the host cell and by that to potentially cause an acute respiratory distress syndrome (ARDS) and respiratory failure [16].
In addition, hypertension has been found to be an important risk factor for mortality with a hazard ratio (HR) of 3.05 for in-hospital mortality in 191 COVID-19 patients and of 1.70–1.82 for death and acute respiratory distress syndrome (ARDS) in 201 COVID-19 patients [17]. Similarly, cardiovascular comorbidities are associated with greater mortality [18].
Considering the wide use of antihypertensive drugs, we conducted a single-center retrospective observational study on 575 COVID-19 patients consecutively admitted to the Humanitas Clinical and Research Center Emergency Department (ED) in order to evaluate its possible impact on in-hospital mortality in COVID-19 patients.
The secondary endpoint of our study was to investigate the role of other clinical predictors on in-hospital mortality.
2. Methods
2.1. Study design and participants
This single-center, retrospective, observational study was conducted at the Emergency Department (ED) of Humanitas Clinical and Research Center. The study was approved by the ethical committee and performed in accordance with the principles of the Helsinki declaration [19].
We analyzed 575 consecutive patients who were admitted to the ED with fever and cough complaints from February 21, 2020, to April 14, 2020, and had been diagnosed with COVID-19, according to WHO interim guidance [20]. Laboratory confirmation of SARS-CoV-2 infection was done by RT-PCR performed on a nasopharyngeal swab in all subjects.
Patients were discharged from the hospital or transferred to a health care facility if they had stable oxygen levels (in room air >95%) for more than 48 h as measured by a pulse oximeter.
2.2. Data collection
Clinical electronic medical records were reviewed for all patients tested positive for SARS-CoV-2 infection. Data were collected and stored in a dedicated database built solely for this purpose. Any missing or uncertain records were clarified through direct communication with the relevant health-care providers and family members.
We collected data on age, gender, comorbidities, chronic treatment, time interval between symptoms onset and ED admission, treatment provided during ED stay (antiviral agents, antibacterial agents, hydroxychloroquine and low molecular weight heparin (LWMH) therapy), days of hospitalization, and on outcome in terms of survival.
The data were available only to authorized personnel, stored on a local server and retrieved for this analysis.
2.3. Endpoints
The primary endpoint of the study was the occurrence of in-hospital mortality. The secondary outcome was the survival time, which was defined as the time between ED admission and either death, patient's transfer to another health care facility or hospital discharge. Analyses tested the role of ACE-I and ARBs and were adjusted for confounders with potential impact on survival, these including age, gender, comorbidities, time interval between onset of symptoms and ED admission, and treatments provided in the ED.
2.4. Statistical analysis
Categorical variables were represented in terms of frequency distributions, while quantitative variables were described as means and standard deviations (SD) or median and interquartile range (IQR). We performed univariate analyses using chi-square test for independence for categorical variables and unpaired t-test (or, if assumptions were not met, Mann-Whitney's test) for quantitative variables. Multivariate analysis was performed with in-hospital mortality as dependent variable and as predictors a priori selected variables (age, gender and chronic use of ACE-I and ARBs) and other variables which were significant with p < 0.1 at univariate testing with a backward selection based on likelihood ratio to find the most parsimonious model.
Proportionality of hazards was tested by means of Schoenfeld's test. Cox regression model for proportional hazard was used to estimate hazard ratios for those taking ACE—I, ARBs or none of those medications, while controlling for other relevant clinical factors. In inferential testing, p < 0.05 was considered statistically significant. Forrest plots have been performed using a logarithmic scale for Odds Ratios (OR) and 95% upper and lower confidence intervals (CI).
Analyses were performed with IBM SPSS software version 26.0 and StataCorp STATA software version 13.1.
3. Results
As of April 14, 2020, 1247 consecutive patients presented to our ED were isolated as suspected COVID-19 cases, of whom 672 tested negatives for SARS-CoV-2 on RT-PCR performed on a nasopharyngeal swab the later not included in this study. The remaining 575 adult patients who were found positive for COVID-19 (46.1% of total) represented the study cohort. Among those found positive for SARS-CoV-2 infection, the male to female ratio was 1.94 with a mean age of 64.8 years (SD 14.6, range 27–93 years), 361 subjects (62.7%) were older than 60 years (Table 1 ).
Table 1.
Baseline clinical features of patients stratified by anti-hypertensive agents (ACE-I and ARB).
| Total |
ACEi |
ARB |
|||||
|---|---|---|---|---|---|---|---|
| n = 575 | Treated (n = 83) | Not treated (n = 492) | p-value | Treated (n = 71) | Not treated n = 504 | p-value | |
| Age, mean (SD), min-max | 64.8 (14.5) 27–93 |
74.2 (11.4) 37–93 | 63.2 (14.4) 27–93 |
p < 0.0001 | 68.3 (12.0) 37–93 |
64.4 (14.8) 27–93 |
p = 0.03 |
| Female, n (%) | 195 (33.9%) | 26 (31.3%) | 169 (34.4%) | p = ns | 23 (32.4%) | 172 (34.1%) | p = ns |
| Comorbidities, median (IQR), range | 2 (2) 0–1 |
2 (2) 1–7 |
1 (2) 0–10 |
p < 0.0001 | 2 (2) 0–6 |
1 (2) 0–10 |
p < 0.0001 |
| Antivirals, n (%) | 391 (69.1%) |
62 (74.7%) |
329 (68.1%) |
p = ns | 48 (67.6%) |
343 (69.3%) |
p = ns |
| Antibiotics, n (%) | 515 (90.8%) |
81 (97.6%) |
434 (89.7%) |
p = 0.02 | 66 (93.0%) |
449 (90.5%) |
p = ns |
| Hydroxychloroquine, n (%) | 442 (78.2%) | 71 (85.5%) | 371 (77.0%) | p = ns | 56 (78.9%) | 386 (78.1%) | p = ns |
| LMWH, n (%) | 240 (42.6%) | 42 (51.2%) | 198 (41.1%) | p = ns | 34 (47.9%) | 206 (41.8%) | p = ns |
| Time to ED (days), median (IQR), min-max | 7 (4) 0–351 |
5 (4) 0–21 |
7 (7) 0–35 |
p = ns | 7 (6) 0–35 |
7 (7) 0–30 |
p = 0.03 |
| LOS in days, median (IQR), min-max | 9 (3) 0–57 |
11 (8) 0–38 |
9 (7) 0–57 |
p = 0.03 | 11 (12) 0–36 |
9 (9) 0–57 |
p = ns |
| In-hospital death (n, %) | 120 (20.9%) |
23 (27.7%) |
97 (19.7%) |
p = ns | 17 (23.9%) |
103 (20.4%) |
p = ns |
Time to ED: time from symptoms onset to ED access. LOS: length of hospitalization. LMWH: low-molecular weight heparin; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker.
While a limited number of patients (7.1%) had mild symptoms and were discharged within few hours, the majority required hospitalization and 10.9% were transferred to an Intensive Care Unit (ICU). Chronic diseases were reported in 329 patients (57.1%), these most commonly included: hypertension in 43.1%, cardiovascular diseases in 27.1%, diabetes in 20%, neoplastic diseases in 11.9% and respiratory diseases in 10.1% of patients. The median time from onset of symptoms to hospitalization was 7 days (range: 0–35). On ED admission, 391 patients (69.1%) were administered with antiviral agents (lopinavir/ritonavir (250 mg/50 mg) or darunavir/cobicistat (800 mg/150 mg), 90.8% with antibacterial agents (mainly ceftriaxone 2 g. once a day or piperacillin/tazobactam), 42.6% with low molecular weight heparin (LMWH), and 72.8% with hydroxychloroquine 200 mg twice a day. In the study cohort, 14.4% of patients were chronically treated with ACE—I, 12.3% with ARBs, 14% with statins and 9.4% with anti-coagulants. Most of patients used ACE-I (84.3%) or ARBs (88.4%) for hypertension. When we stratified for ACE-I and ARBs treatment (Table 1), we found that ACE-I users were older (74.2 vs 63.2 years; p < 0.00001) and had more comorbidities (2 vs 1, p < 0.0001) compared to non-users, with results replicated in the comparison between ARBs treated and untreated subjects. A difference, albeit not significant, was found in the mortality between ACE-I users and non-users (32.4% vs 24.9%) and between ARBs-users vs non-users (30.9% vs 25.4%). Multivariate analyses showed that the main reason of these findings was due to the age difference between the two groups. Moreover, a longer hospital stay was observed in ARBs-users as compared to non-users (11 vs 9 days; p = 0.02).
In hospital mortality was 20.9%, while 55.5% of patients were discharged, 3.8% moved to a long-term care facility and 19.8% still hospitalized at time of data analysis. Mean age at time of death was 77.1 years (SD 10.3, range: 38–93).
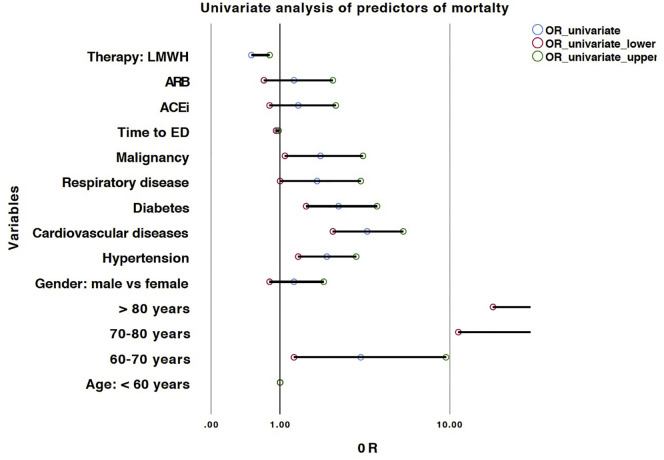
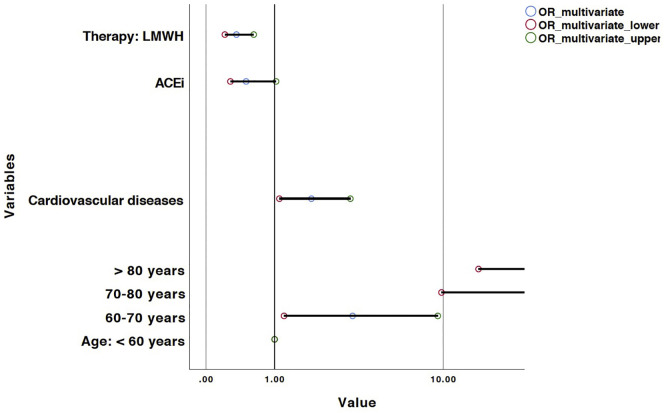
Univariate and multivariate logistic regression models are represented in Table 2 , and related forest plots in Fig. 1, Fig. 2 respectively.
Table 2.
Predictors of in-hospital mortality at univariate and multivariate analysis.
| Predictors of mortality | ||||
|---|---|---|---|---|
| Predictors | Univariate |
Multivariate (n = 372) |
||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age: < 60 years | 1.0 (Ref.) | 1.0 (Ref.) | ||
| 60–70 years | 3.5 (1.3–9.6) | 0.02 | 3.4 (1.2–9.4) | 0.02 |
| 70–80 years | 27.0 (11–66.2) | <0.0001 | 25.2 (9.8–64.4) | <0.0001 |
| > 80 years | 40.5 (16–102.6) | <0.0001 | 39.5 (14.7–106.5) | <0.0001 |
| Gender: male vs female | 1.3 (0.8–2.1) | p = 0.2 | 1.2 (0.6–2.2) | 0.6 |
| Hypertension | 2.2 (1.4–3.3) | <0.0001 | 0.9 (0.5–1.7) | 0.7 |
| Cardiovascular diseases | 3.8 (2.4–5.9) | <0.0001 | 1.9 (1.1–3.3) | 0.02 |
| Diabetes | 2.6 (1.6–4.3) | <0.0001 | 1.1 (0.5–2.4) | 0.7 |
| Respiratory disease | 1.9 (1–3.5) | 0.04 | 0.8 (0.3–2.0) | 0.7 |
| Malignancy | 2 (1.1–3.6) | 0.02 | 1.0 (0.4–2.3) | 0.9 |
| Time to ED | 0.95 (0.92–0.97) | <0.0001 | 0.98 (0.92–1-04) | 0.5 |
| ACEi | 1.4 (0.8–2.5) | 0.19 | 0.5 (0.3–1.0) | 0.06 |
| ARB | 1.3 (0.7–2.4) | 0.38 | 1.1 (0.5–2.8) | 0.8 |
| Therapy: LMWH | 0.5 (0.3–0.8) | <0.001 | 0.4 (0.2–0.6) | <0.0001 |
Time to ED: time from symptoms onset to ED access. LOS: length of hospitalization. LMWH: low-molecular weight heparin; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker.
Fig. 1.
Forest plot of predictors of in-hospital mortality at univariate logistic regression analysis. X axis was treated in a logarithmic scale.
Fig. 2.
Forest plot of predictors of in-hospital mortality at multivariate logistic regression analysis. X axis was treated in a logarithmic scale.
Multivariate logistic regression model was performed to assess the role of ACE-I and ARBs as well as of other parameters on mortality risk. As shown in Table 2 and Fig. 2, the most parsimonious model (n = 447, R2 = 0.43) showed that age is a risk factor with OR ranging from 3.4 (95% CI: 1.2–9.4; p = 0.02) for patients between 60 and 70 years-old, 25.2 (95% CI: 9.8–64.4; p < 0.001) for 70–80 year-old patient group and 39.5 (95% CI: 14.7–106.5; p < 0.001) in those >80 years as compared to those <60 years of age. ACE-I use was protective with a border-line significance (OR: 0.5; 95% CI: 0.3–1.0; p = 0.06), whereas comorbid cardiovascular condition was a risk factor (OR: 1.9; 95% CI: 1.1–3.3; p = 0.02). As of ED admission, a LMWH use in ED was associated with an important reduction in mortality (OR: 0.4; 95% CI: 0.2–0.6; p < 0.001).
We used survival analyses to further explore significant findings identified in the logistic regression. Results confirmed that the use of ACE-I was a significant protective factor for COVID-19 in-hospital mortality (Hazard Ratio: 0.61; 95% CI: 0.38–0.98; p = 0.04) with a highly significant model (p = 9.99E-22; n = 444) (Table 3 ).
Table 3.
Predictors of in-hospital mortality using Cox-regression analysis for survival.
| Survival analysis (n = 444) | ||
|---|---|---|
| Predictors | HR (95% CI) | p |
| Age: <60 years | 1.0 (Ref.) | |
| 60–70 years | 1.96 (0.73–5.22) | P = 0.18 |
| 70–80 years | 7.56 (3.19–17.91) | p < 0.001 |
| >80 years | 12.51 (5.22–29.96) | p < 0.001 |
| Cardiovascular comorbidity | 1.78 (1.21–2.61) | p = 0.003 |
| ACEi | 0.61 (0.38–0.99) | p = 0.04 |
| Therapy: LMWH | 0.51 (0.34–0.76) | p < 0.001 |
4. Discussion
The study describes predictors of mortality in COVID-19 patients in one of the largest single-center cohort of Italian patients reported so far, admitted to one of the biggest hospitals in the city of Milan, which is located in the epicenter of the COVID-19 emergency with the highest mortality in Italy.
In order to understand the reasons behind the high mortality rate in this metropolitan area, we performed a multivariate statistical model to assess the role of clinical predictors evaluated at ED admission and of ACE-I and ARBs chronic use. In-hospital mortality was found to be 20.9%.
ACE-I and ARBs act on the RAAS system, and they are widely used to treat primary hypertension [5], heart failure, diabetic nephropathy and secondary prevention of myocardial infarction (MI) [6]. The two drugs have indeed a different mechanism of action on the RAAS cascade. While ACE-I inhibits the conversion from angiotensin I to angiotensin II, ARBs antagonize the effects of angiotensin II on its ATI and ATII receptors [8,9]. There are different hypotheses on the effect of these two drugs on ACE2, which is the protein involved in the host cell entry of the SARS-CoV-2. Whether they are detrimental or protective for disease severity of COVID-19 is still a matter of discussion.
Ferrario and co-workers proved that after a treatment with lisinopril (ACE—I) alone, ACE2 mRNA expression levels were increased without a consensual increase in ACE2 activity [21,22]. In this regard, increased levels of ACE2 may possibly lead to an increase in the number of binding sites for SARS-CoV-2, raising the risk of COVID-19 [21,23]. On the other end, other authors hypothesize that ACE-I and ARBs may interfere with the integrity of the ACE2/angiotensin (1–7)/MAS pathway resulting in a decreased expression of ACE2 and host cell entry of the virus [24].
We found that ACE—I, which acts by inhibiting the conversion from angiotensin I to angiotensin II, showed a trend in protecting from mortality from COVID-19 and was significant in delaying mortality as shown by multivariate Cox regression analysis unlike ARBs, which antagonize the effects of angiotensin II on its receptors [2,3]. The mechanisms underlying this phenomenon are still unclear and further investigations are required in order to shed light on the subject. As demonstrated in a recent paper, another hypothesis is that soluble human ACE2 inhibitors can inhibit SARS-CoV-2 infections, and in this regard it will be useful to investigate whether ACE-I or ARB are able to interfere and increase ACE2 levels [25]. Our data partly confirmed the results of a larger retrospective, multi-centric study performed on 1128 COVID-19 adults from the Hubei province in China, which showed that an inpatient use of either ACE-I or ARBs was associated with a lower risk of all-cause mortality when compared with ACE-I/ARBs non-users [26]. The previous study showed a protective effect given by the use of either drug, with no difference between the two, unlike our cohort in which only ACE-I were found to have a protective role. The reason underling the difference between the two studies could lie in the different proportion of use of these two drugs [ACE-I (14.4% vs 2.7%) and ARBs (12.3% vs. 13.9%) in the Italian as compared to the Chinese sample].
In addition, an age difference was identified between ACE-I as compared to ARBs users (74.2 vs 68.3) in our sample. A different role of the two drug categories, ACE-I and ARBs has been suggested by a more recent meta-analysis, which showed that the use of ARBs, as opposed to ACE-I, increases the risk of SARS-CoV-2 infection in subjects <60 years [27]
Of note, two recent papers with a large sample size were performed in Lombardy and in the New York State using data from electronic health records, both of which did not confirm the protective role of ACE-I towards disease risk but not towards disease severity [[28], [29]].
Advantages of single-center studies as compared to multicentric studies or to studies using health registries lie on the availability of complete data on patients and on the possibility to follow-up affected individuals despite it is usually associated with a smaller sample size..
As for today we can only say that a daily intake of antihypertensive medications (ACE-I and ARBs) in the setting of COVID-19 should not be discontinued. The replacement of a RAAS inhibitor with an antihypertensive agent of a different class may require careful monitoring to avoid the rebound effect of blood pressure, as even short periods of blood pressure instability after a therapeutic change have been associated with an increase in cardiovascular risk [[30], [31]]
As of the role of other potential predictors, we confirmed the significant role of age and cardiovascular comorbidities on mortality rate, as shown by other papers, while males were not confirmed to have a higher mortality differently from other studies [32]. On the contrary, the early administration of LMWH in ED was shown to have a protective effect on mortality in the same patients. A prophylactic dose of LMWH was administered at ED admission starting with 0.4 UI/24 h. The dose was adjusted accordingly to renal function and body weight
Recent best practices have been published to aid health-care professionals to determine the proper anti-coagulant and anti-platelet therapy in COVID-19 patients [33]. Based on our findings, it appears that timely administration of LMWH is of greater importance as compared to initiation of treatment with antivirals, antibiotics and hydroxychloroquine in order to prevent potential thromboembolic events which, according to a previous paper performed in our hospital on a series of COVID-19 patients which partially overlap with ours, occurred in 7.7% of them [34].
The paper underlies the importance of timely treatment in particular among patients with non-modifiable risk factors such as age and cardiovascular comorbidities as it can potentially improve survival rates in these patients. It is worthwhile to consider developing tools for early identification of such patients, to maximize potential outcomes.
Nonetheless, rigorous adherence to state based recommendations are also advisable (e.g. quarantine, social distancing), especially among high-risk patients.
In conclusion, despite the limited nature of the study, identification of predictors of mortality will allow a better management of patients in the face of future disease recurrence.
Funding source
None.
Declaration of Competing Interest
None.
Acknowledgements
We would like to thank and dedicate this paper to all the professional caregivers who gave time, energy and passion in this emergency in Lombardy and Italy, as well as to the many deaths and families who lost their beloved.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
References
- 1.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 3.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asselta R., Paraboschi E., Mantovani A., Duga S. Aging (Albany NY); 2020. ACE2 and TMPRSS2 Variants and Expression as Candidates to Sex and Country Differences in COVID-19 Severity in Italy. 2(11):10087-10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li E.C., Heran B.S., Wright J.M. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst. Rev. 2014;8 doi: 10.1002/14651858.CD009096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmoudpour S.H., Baranova E.V., Souverein P.C. Determinants of angiotensin-converting enzyme inhibitor (ACEI) intolerance and angioedema in the UK clinical practice research Datalink. Br. J. Clin. Pharmacol. 2016;82(6):1647–1659. doi: 10.1111/bcp.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis G.K., Roberts D.H. Molecular genetics of the renin-angiotensin system: implications for angiotensin II receptor blockade. Pharmacol. Ther. 1997;75(1):43–50. doi: 10.1016/s0163-7258(97)00021-1. [DOI] [PubMed] [Google Scholar]
- 8.Hill R.D. StatPearls. StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC; Treasure Island (FL): 2020. Vaidya PN. Angiotensin II Receptor Blockers (ARB, ARb) [PubMed] [Google Scholar]
- 9.Barreras A., Gurk-Turner C. Angiotensin II receptor blockers. Proc. (Baylor Univ. Med. Cent.) 2003;16(1):123–126. doi: 10.1080/08998280.2003.11927893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thekkumkara T.J., Cookson R., Linas S.L. Angiotensin (AT1A) receptor-mediated increases in transcellular sodium transport in proximal tubule cells. Am. J. Phys. 1998;274(5):F897–F905. doi: 10.1152/ajprenal.1998.274.5.F897. [DOI] [PubMed] [Google Scholar]
- 11.Aguilera G. Role of angiotensin II receptor subtypes on the regulation of aldosterone secretion in the adrenal glomerulosa zone in the rat. Mol. Cell. Endocrinol. 1992;90(1):53–60. doi: 10.1016/0303-7207(92)90101-b. [DOI] [PubMed] [Google Scholar]
- 12.Schulman I.H., Raij L. The angiotensin II type 2 receptor: what is its clinical significance? Curr. Hypertens. Rep. 2008;10(3):188–193. doi: 10.1007/s11906-008-0036-8. [DOI] [PubMed] [Google Scholar]
- 13.Daniels D. Frontiers in neuroscience. diverse roles of angiotensin receptor intracellular signaling pathways in the control of water and salt intake. In: De Luca L.A. Jr., Menani J.V., Johnson A.K., editors. Neurobiology of Body Fluid Homeostasis: Transduction and Integration. CRC Press/Taylor & Francis. © 2014 by Taylor & Francis Group, LLC; Boca Raton (FL): 2014. [PubMed] [Google Scholar]
- 14.Fountain J.H., Lappin S.L. StatPearls. StatPearls Publishing. Copyright © 2020, StatPearls Publishing LLC; Treasure Island (FL): 2020. Physiology, renin angiotensin system. [Google Scholar]
- 15.Vickers C., Hales P., Kaushik V. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277(17):14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 16.Rossi G.P., Sanga V., Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9:e57278 doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323(18):1769-1770 doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 18.Stein R.A. The 2019 coronavirus: learning curves, lessons, and the weakest link. Int. J. Clin. Pract. 2020;74(4) doi: 10.1111/ijcp.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Medical A World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 20.World Health O . World Health Organization; 2020; Geneva: 2020. Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease is Suspected: Interim Guidance, 13 March 2020. [Google Scholar]
- 21.Ferrario C.M., Jessup J., Chappell M.C. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 22.Li G., Hu R., Zhang X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens. Res. 2020:1–3. doi: 10.1038/s41440-020-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rico-Mesa J.S., White A., Anderson A.S. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr. Cardiol. Rep. 2020;22(5):31. doi: 10.1007/s11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun M.L., Yang J.M., Sun Y.P., Su G.H. Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):219-222(0) doi: 10.3760/cma.j.issn.1001-0939.2020.03.016. (E014) [DOI] [PubMed] [Google Scholar]
- 25.Monteil V., Kwon H., Prado P. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;905-913.e7 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P., Zhu L., Cai J. Association of Inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;126:1671–1681 doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan CK, Huang YS, Liao HW. Renin-angiotensin-aldosterone System Inhibitors and Risks of SARS-CoV-2 Infection: A Systematic Review and Meta-analysis. Hypertension. 2020;120 doi: 10.1161/HYPERTENSIONAHA.120.15989. [DOI] [Google Scholar]
- 28.Jarcho J.A., Ingelfinger J.R., Hamel M.B., D’Agostino R.B., Sr., Harrington D.P. Inhibitors of the renin-angiotensin-aldosterone system and covid-19. N. Engl. J. Med. 2020 May;382:2462-2464 doi: 10.1056/NEJMe2012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julius S., Kjeldsen S.E., Weber M. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 30.Luzzi S., Elia A., Del Maestro M. Indication, timing, and surgical treatment of spontaneous intracerebral hemorrhage: systematic review and proposal of a management algorithm. World Neurosurg. 2019;S1878-8750(19)30105-6 doi: 10.1016/j.wneu.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Savioli G., Ceresa I.F., Macedonio S. Trauma coagulopathy and its outcomes. Med. (Kaunas) 2020;56(4): 205 doi: 10.3390/medicina56040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grasselli G., Greco M., Zanella A. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020 Jul;15 doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson R.A., Johnson D.M., Dharia R.N., Merli G.J. Anti-coagulant and anti-platelet therapy in the COVID-19 patient: a best practices quality initiative across a large health system. Hosp. Pract. 2020 doi: 10.1080/21548331.2020.1772639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020 Jul;191:9–14. doi: 10.1016/j.thromres.2020.04.024. Published online 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]