Abstract
Background
The pancreas as a site of metastasis of other primary tumors is a rare event. Pancreatic metastases may occur years after the start of treatment of a neoplasm of another organ or may be the initial manifestation of an unidentified primary tumor. The most commonly reported primary sites for pancreatic metastases are the kidneys, lungs, breast, bowel, and skin (melanoma). Case Summary. The authors report a case of pancreatic metastasis derived from a primary breast cancer that underwent endoscopic ultrasound fine-needle aspiration (EUS-FNA) core biopsy to obtain tissue, which made it possible to perform an immunohistochemical study.
Conclusion
We emphasize the importance of outpatient follow-up after the treatment of a neoplasia and the completion of control exams. In addition, we should always be aware of the finding of a secondary lesion in patients who have already been diagnosed with cancer, even if it is located in unusual organs, as in this case, where two metastases of a breast carcinoma to the pancreas were detected.
1. Introduction
The pancreas as a site of metastasis of other primary tumors is uncommon and may be difficult to differentiate from a neoplasm derived from the exocrine and/or endocrine pancreas itself, due to the overlap of its clinical presentation, imaging, and cytological features [1].
Pancreatic metastases may occur years after the start of treatment for a neoplasm from another site or may be the initial manifestation of an unidentified primary tumor. The most commonly reported primary sites for pancreatic metastases are the kidneys, lungs, breast, bowel, and skin (melanoma) [1]. Diagnosis before therapy should be the cornerstone of the decision to be made. Pancreatic tissue biopsies accurately detect this type of disease and thus establish the best form of treatment.
The authors report a case of pancreatic metastasis derived from a primary breast cancer that underwent needle puncture to obtain tissue, the result of which allowed the immunohistochemical study.
2. Case Presentation
A 72-year-old, asymptomatic, postmenopausal woman sought medical attention for routine exams with a personal history of systemic arterial hypertension, dyslipidemia, and previous appendectomy. She reported a right breast quadrantectomy in June 2016 by ductal breast adenocarcinoma (stage IIA) followed by radiotherapy. She had no family history of breast cancer and denied smoking or alcoholism. Her physical examination showed only a scar from previous surgery on her right breast. Abdominal ultrasonography on February 2019 detected a well-defined nodular lesion on the tail of the pancreas, approximately 2 cm in diameter and without compromising adjacent structures. Other imaging tests were not performed due to a significant allergy to radiological contrast. She underwent endoscopic ultrasound (EUS) which confirmed the finding of abdominal US but detected two more hypoechoic, homogeneous, round areas, with well-defined limits, of soft consistency to elastography, two located in the uncinate process and one in the pancreatic tail, measuring 7 × 8 mm, 11 × 10 mm, and 19 × 12 mm, respectively (Figures 1 and 2). They did not invade or compress the main pancreatic duct or vascular structures. EUS-FNA core biopsy was performed on all lesions with a ProCore 20G needle (Cook Medical) (Figure 3). An additional finding of the exam was the presence of gallbladder stones.
Figure 1.
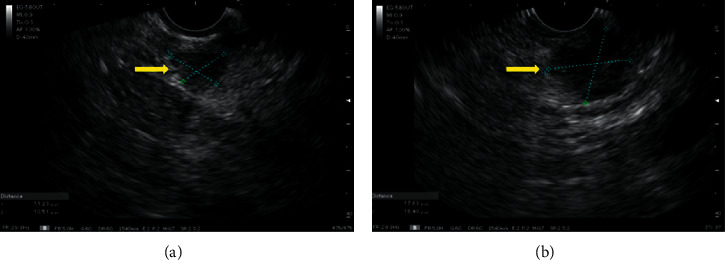
Sectorial scanning of uncinate process and tail of pancreas. (a) Hypoechoic lesion, homogeneous and well-delimited in the uncinate process and (b) another lesion with the same characteristics but larger in the pancreas tail.
Figure 2.
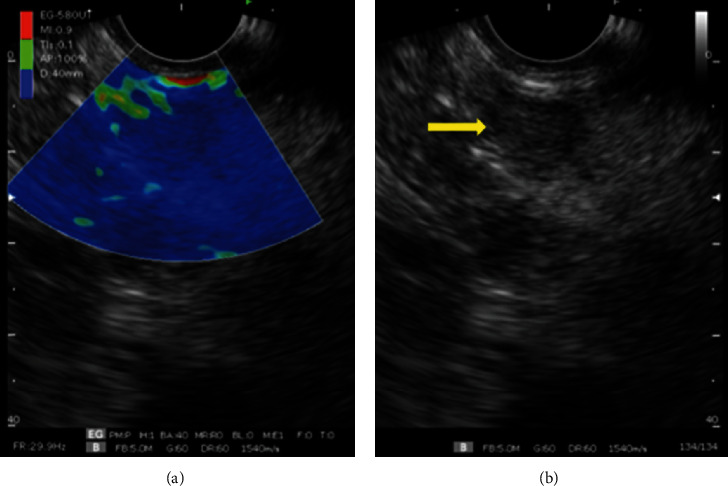
Elastography performed with a sectorial endoscopic ultrasound device. (a) Elastography of uncinate hardened process lesion (blue image). (b) Yellow arrow indicating hypoechoic lesion of uncinate process.
Figure 3.
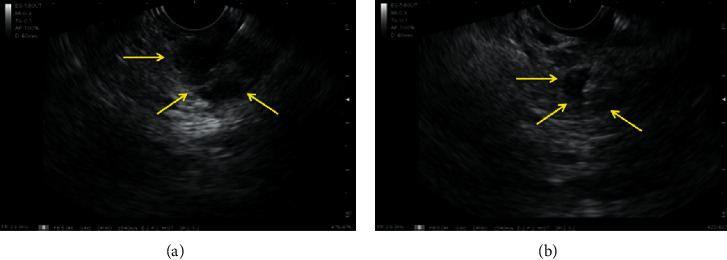
(a) and (b) EUS-FNA with Procore 20G needle.
The histological results of the punctures revealed that the lesion located on the tail of the pancreas and the largest in the uncinate process were secondary microduct or small cell-associated epithelial neoplasia isolated (metastatic ductal carcinoma). The smallest located in the uncinate process was negative for neoplasia. The immunohistochemistry of the material was positive for the GATA3 marker, which concluded that it was an invasive carcinoma of mammary origin in the form of triple negative discesa cells (estrogen negative, progesterone negative, and HER2 negative receptors) (Figure 4). The patient was diagnosed with late metastases of breast cancer to the pancreas and resumed follow-up with the oncology team.
Figure 4.
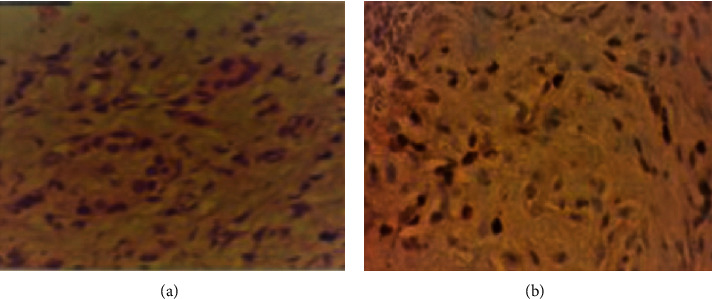
Material obtained through EUS-FNA and sent for histological and immunohistochemical study. (a) Hematoxylin-eosin revealing atypical microducts and (b) GATA3-labeled immunohistochemistry (+) -secondary breast carcinoma.
3. Final Diagnosis
Metastases of breast cancer to the pancreas.
4. Outcome and Follow-Up
The patient whose case was reported in this article resumed the follow-up with a specialized oncology team for definitive treatment of the diagnosed lesions.
5. Discussion
The pancreas as a site of metastasis is uncommon and difficult to differentiate from ductal adenocarcinoma due to its clinical presentation, imaging findings, and cytological features [1]. Pancreatic metastasis may occur years after the treatment of a primary neoplasm or may be identified even before its diagnosis, and the latter is much rarer than the first [1]. The most frequently identified primary sites for pancreatic metastasis are the kidneys, lungs, breast, bowel, and skin [1].
Few studies in the literature have evaluated the efficacy of EUS-FNA in the diagnosis of this type of lesion [2]. In a study by Fritscher-Ravens A et al. of 207 patients with focal pancreatic mass who underwent EUS-FNA, 116 were diagnosed with malignant neoplasia, of which 32 (27%) were nonmalignant primary pancreatic adenocarcinoma [3]. Recently, the use of harmonic contrast associated with endoscopic ultrasound (CH-EUS) has started to be applied as an adjuvant in the differential diagnosis of pancreatic neoplasms; this is due to the advantage of the method of being able to visualize both the parenchyma and the microvasculature without the artifacts caused by Doppler [4]. The finding of lesions with a pattern of hyperenhancement to the use of contrast, associated, in particular, with a history of hematological or renal neoplasia, are strong predictors for pancreatic metastatic lesion (lymphoma and renal carcinoma metastasis), in some selected cases avoiding, up to even, the EUS-FNA [4].
Pancreatic ductal adenocarcinomas (ADPs) are the most common solid tumors, while others such as neuroendocrine and metastases are the rarest forms [5]. Breast cancer accounts for less than 5% of metastatic pancreatic lesions [6]. Invasive lobular breast carcinoma is the type of breast cancer that most metastasizes to the digestive tract [6]. Ductal carcinoma typically exhibits solitary metastasis and is much rarer in a diffuse pattern in the digestive tract [6].
Immunohistochemistry of the material obtained by puncture is an important tool to confirm the origin of metastatic carcinoma. Mammoglobin (MGB), GCDFP-15, estrogen receptors (ER), and progesterone (PR) act as specific immunohistochemical markers in the evaluation of metastatic breast carcinoma [7]. GATA3 is a member of the zinc-finger transcription factor family [7]. This marker plays an important role in breast development and cell differentiation of the luminal epithelium as well as in the development of other tissues and has been implicated in breast cancer growth, differentiation, progression, and metastasis [7]. GATA3 is widely expressed in estrogen receptor negative luminal breast cancer and is more sensitive than GCDFP-15 and MGB, making it a useful marker for breast carcinoma [7].
The 5-year survival of invasive breast cancer is 90%, but when there are distant metastases, a typical ductal adenocarcinoma becomes incurable and a 5-year survival of approximately 25% [6]. Those with metastasis to the pancreas have a survival range of 1 to 50 months [6].
There is controversy regarding the resection of pancreatic metastases [6]. There are reports that resection of isolated pancreatic lesions may increase the survival and therefore should be the conduct of choice, while other authors advocate systemic treatment initially due to morbidity and mortality associated with duodenopancreatectomy [6].
Among chemotherapeutic drugs, taxanes, docetaxel, and paclitaxel are the drugs of choice in the treatment of metastatic breast cancer [8]. They can be used as single agents or in combination with other chemotherapeutic drugs [8].
6. Conclusion
In conclusion, we emphasize the importance of outpatient follow-up after the treatment of a neoplasm and the completion of control exams. In addition, we should always be aware of the finding of a secondary lesion in patients who have already been diagnosed with cancer, even if it is located in unusual organs, as in this case, where two metastases of a breast carcinoma to the pancreas were detected.
Data Availability
The data used to support the article are available within the article.
Consent
Informed consent to publish was obtained from the patients.
Disclosure
This case report was presented as an e-poster during the ENDO 2020 – 2nd World Congress of GI Endoscopy, March 7–10, 2020.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Romanini S. G. designed the study, drafted the article, and analyzed and interpreted the data; Serrano J. P. R., Castro J. S. L., Torres I. T., Ingold A., Borini A. L., Zulske L. A. S., Matias M. B. F., Marchi J. S., Pulla J. A. S., and Andengh J. C. analyzed the data. Ardengh J. C. approved the final version to be published. All authors read and approved the final manuscript.
References
- 1.Pang J. C., Roh M. H. Metastases to the pancreas encountered on endoscopic ultrasound-guided, fine-needle aspiration. Archives of Pathology & Laboratory Medicine. 2015;139(10):1248–1252. doi: 10.5858/arpa.2015-0200-ra. [DOI] [PubMed] [Google Scholar]
- 2.Atiq M., Bhutani M. S., Ross W. A., et al. Role of endoscopic ultrasonography in evaluation of metastatic lesions to the pancreas. Pancreas. 2013;42(3):516–523. doi: 10.1097/mpa.0b013e31826c276d. [DOI] [PubMed] [Google Scholar]
- 3.Fritscher-Ravens A., Brand L., Knofel W. T., et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. The American Journal of Gastroenterology. 2002;97(11):2768–2775. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 4.Fusaroli P., D’Ercole M. C., De Giorgio R., Serrani M., Caletti G. Contrast harmonic endoscopic ultrasonography in the characterization of pancreatic metastases (with video) Pancreas. 2014;43(4):584–587. doi: 10.1097/mpa.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 5.Gagovic V., Spier B. J., DeLee R. J., et al. Endoscopic ultrasound fine-needle aspiration characteristics of primary adenocarcinoma versus other malignant neoplasms of the pancreas. Canadian Journal of Gastroenterology. 2012;26(10):691–696. doi: 10.1155/2012/761721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliiger J., Gorbaty M. Metastasis to the pancreas and stomach from a breast cancer primary: a case report. Journal of Community Hospital Internal Medicine Perspectives. 2017;7(4):234–237. doi: 10.1080/20009666.2017.1369379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou Y., Shen R., Chaudhary S., Tonkovich D., Li Z. Utility of different immunostains for diagnosis of metastatic breast carcinomas in both surgical and cytological specimens. Annals of Diagnostic Pathology. 2017;30:21–27. doi: 10.1016/j.anndiagpath.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 8.ÇRe T., Altintas A., Pasa S., Urakçi Z., Isikdogan A. Breast carcinoma metastasis to the tail of pancreas. The Journal of Breast Health. 2009;5(4):228–230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the article are available within the article.


