Abstract
This quality improvement study examines a patient-oriented intervention to support decision-making about low-risk thyroid cancer treatment.
The treatment paradigm for low-risk thyroid cancer increased in complexity following release of the 2015 American Thyroid Association Guidelines for Adults with Differentiated Thyroid Cancer.1,2 The guidelines were intended to “complement informed, shared patient-healthcare provider deliberation” when making treatment decisions.1 However, patient-clinician deliberation can fail to meet patients’ needs and may exclude available treatments.3 Patients with low-risk thyroid cancer report not knowing what questions to ask, while physicians report not knowing how to elicit and incorporate patients’ preferences.3,4 Our objective was to design a patient-oriented intervention to support decision-making about low-risk thyroid cancer treatment.
Methods
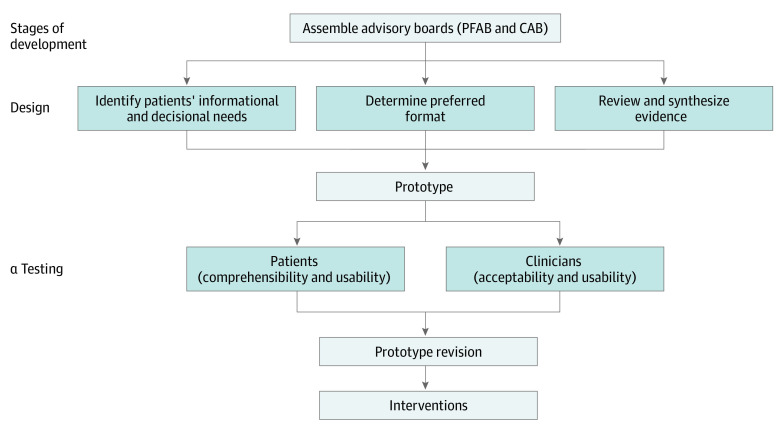
We followed an iterative process outlined by the International Patient Decision Aid Standards (Figure) and engaged stakeholders by forming a patient-family advisory board (PFAB) with survivors of thyroid cancer and family members (n = 6) and a clinician advisory board (CAB) with clinical experts (thyroid surgeons, endocrinologists, and a radiologist from community and academic practices [n = 10]).5 Stakeholder boards met individually and together at each stage of the development process. While we initially sought to design 1 intervention, stakeholders believed that 2 complementary formats supported a wider range of users.
Figure. Iterative, Stakeholder-Driven Process Used to Design and Test the Decision Support Interventions.
The patient-family advisory board (PFAB) and clinician advisory board (CAB) met individually and together at each stage of the development process listed to review and revise the interventions.
To identify the format and content, we (1) reviewed validated surgical decision support tools, (2) performed a systematic review of the literature and websites to generate a comprehensive list of questions (n = 302) patients and family members should ask their physician, and (3) elicited questions and content from the stakeholder boards. We used consensus processes, including ranking, voting, and discussion, to define the information and questions necessary for making a treatment decision about low-risk thyroid cancer. Patient α testing was performed with the PFAB and with the Community Advisors on Research Design and Strategies group from the Wisconsin Network for Research Support. This group includes individuals with diverse racial, socioeconomic, and educational backgrounds. Physician α testing was performed with the CAB and with thyroid surgeons and endocrinologists at the 87th Annual American Thyroid Association meeting in Victoria, British Columbia, Canada (October 18-22, 2017). This project was exempt from research status by the University of Wisconsin institutional review board. It does not consider stakeholder engagement research unless the stakeholders are participants in a study or are reviewing information containing protected health information from a study. The stakeholders were invited via writing to participate in a year-long process to develop research ideas and patient-oriented materials. Written consent was not required. The stakeholders signed an engagement contract that was similar to a consent form.
Results
We held 16 stakeholder meetings (8 PFAB, 5 CAB, and 3 joint) between July 2016 and December 2017 to develop a 1-page treatment comparison chart and a trifold pamphlet containing a question prompt list (available at https://www.hipxchange.org/ThyroidCancerTreatmentChoice). Both interventions considered active surveillance, lobectomy, and total thyroidectomy as treatment options.
Treatment Comparison Chart
Stakeholders identified 29 discrete informational elements critical for decision-making about low-risk thyroid cancer treatment.6 This information covered several domains, including treatment, guideline recommendations, outcomes (short-term and long-term), follow-up, and quality of life. The Table summarizes information needed for decision-making within these domains.
Table. Summary of the Content of the Treatment Comparison Chart and Question Prompt List.
| Domain | Treatment comparison chart | Abbreviated questions from prompt list |
|---|---|---|
| Nature of disease |
|
|
| Treatment |
|
|
| Guideline recommendations |
|
NA |
| Outcomes | ||
| Short-term and quality-of-life effects |
|
|
| Long-term |
|
|
| Follow-up |
|
NA |
| Surgeon experience and costs | NA |
|
Abbreviation: NA, not applicable.
Question Prompt List
The prompt list included 20 questions that stakeholders determined patients should definitely ask or may not know to ask their physician. Questions covered treatment options, short- and long-term outcomes, complications, thyroid hormone levels, lymph node metastasis, surgeon volume, costs, and unanticipated issues (Table).
Discussion
This project developed 2 decision support interventions about low-risk thyroid cancer treatment. To our knowledge, these are the first stakeholder-designed interventions to help patients, surgeons, and endocrinologists navigate this complex decisional process.2 These interventions are designed to enhance rather than replace patient-physician communication, empower patients and families, and fill existing gaps in online information.6 Patients and their families can prepare by reviewing the interventions before their consultation or physicians can introduce the materials during the visit. The interventions can be delivered online or by mail, email, or medical record–based electronic messaging. Before broad scale implementation through patient and physician organizations, further testing is needed to determine the clinical efficacy of these interventions on patient-centered outcomes, such as treatment choice, decision satisfaction, and regret.
References
- 1.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitt SC, Lubitz CC. Editorial: complex decision making in thyroid cancer: costs and consequences-is less more? Surgery. 2017;161(1):134-136. doi: 10.1016/j.surg.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Doubleday AR, Saucke MC, Bates MF, Pitt SC. Patient-surgeon decision-making about treatment for very low-risk thyroid cancer. Trends Cancer Res. 2019;14:79-89. http://www.researchtrends.net/tia/article_pdf.asp?in=0&vn=14&tid=57&aid=6498 [Google Scholar]
- 4.Jensen CB, Saucke MC, Francis DO, Voils CI, Pitt SC. From overdiagnosis to overtreatment of low-risk thyroid cancer: a thematic analysis of attitudes and beliefs of endocrinologists, surgeons, and patients. Thyroid. 2020;30(5):696-703. doi: 10.1089/thy.2019.0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13(suppl 2):S2. doi: 10.1186/1472-6947-13-S2-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doubleday AR, Novin S, Long KL, Schneider DF, Sippel RS, Pitt SC. Online information for treatment for low-risk thyroid cancer: assessment of timeliness, content, quality, and readability. J Cancer Educ. 2020;27:1-8. doi: 10.1007/s13187-020-01713-5 [DOI] [PMC free article] [PubMed] [Google Scholar]