This prognostic study uses data from patients with outpatient medical or gynecologic oncology encounters to investigate the validity of a machine learning algorithm to predict patient 180-day mortality.
Key Points
Question
Can a machine learning algorithm prospectively identify patients with cancer at risk of 180-day mortality?
Findings
In this prognostic cohort study of 24 582 patients seen in oncology practices within a large health care system, a machine learning algorithm integrated into the electronic health record accurately identified the risk of 180-day mortality with good discrimination and positive predictive value of 45.2%. When added to performance status– and comorbidity-based classifiers, the algorithm favorably reclassified patients.
Meaning
An integrated machine learning algorithm demonstrated good prospective performance compared with traditional prognostic classifiers and may inform clinician and patient decision-making in oncology.
Abstract
Importance
Machine learning (ML) algorithms can identify patients with cancer at risk of short-term mortality to inform treatment and advance care planning. However, no ML mortality risk prediction algorithm has been prospectively validated in oncology or compared with routinely used prognostic indices.
Objective
To validate an electronic health record–embedded ML algorithm that generated real-time predictions of 180-day mortality risk in a general oncology cohort.
Design, Setting, and Participants
This prognostic study comprised a prospective cohort of patients with outpatient oncology encounters between March 1, 2019, and April 30, 2019. An ML algorithm, trained on retrospective data from a subset of practices, predicted 180-day mortality risk between 4 and 8 days before a patient’s encounter. Patient encounters took place in 18 medical or gynecologic oncology practices, including 1 tertiary practice and 17 general oncology practices, within a large US academic health care system. Patients aged 18 years or older with outpatient oncology or hematology and oncology encounters were included in the analysis. Patients were excluded if their appointment was scheduled after weekly predictions were generated and if they were only evaluated in benign hematology, palliative care, or rehabilitation practices.
Exposures
Gradient-boosting ML binary classifier.
Main Outcomes and Measures
The primary outcome was the patients’ 180-day mortality from the index encounter. The primary performance metric was the area under the receiver operating characteristic curve (AUC).
Results
Among 24 582 patients, 1022 (4.2%) died within 180 days of their index encounter. Their median (interquartile range) age was 64.6 (53.6-73.2) years, 15 319 (62.3%) were women, 18 015 (76.0%) were White, and 10 658 (43.4%) were seen in the tertiary practice. The AUC was 0.89 (95% CI, 0.88-0.90) for the full cohort. The AUC varied across disease-specific groups within the tertiary practice (AUC ranging from 0.74 to 0.96) but was similar between the tertiary and general oncology practices. At a prespecified 40% mortality risk threshold used to differentiate high- vs low-risk patients, observed 180-day mortality was 45.2% (95% CI, 41.3%-49.1%) in the high-risk group vs 3.1% (95% CI, 2.9%-3.3%) in the low-risk group. Integrating the algorithm into the Eastern Cooperative Oncology Group and Elixhauser comorbidity index–based classifiers resulted in favorable reclassification (net reclassification index, 0.09 [95% CI, 0.04-0.14] and 0.23 [95% CI, 0.20-0.27], respectively).
Conclusions and Relevance
In this prognostic study, an ML algorithm was feasibly integrated into the electronic health record to generate real-time, accurate predictions of short-term mortality for patients with cancer and outperformed routinely used prognostic indices. This algorithm may be used to inform behavioral interventions and prompt earlier conversations about goals of care and end-of-life preferences among patients with cancer.
Introduction
Patients with cancer frequently receive care near the end of life that is not concordant with their wishes and worsens their quality of life.1,2,3,4,5,6 In an effort to curb unwarranted end-of-life care, national quality metrics in oncology increasingly focus on treatment intensity and hospice use near the end of life.7,8,9,10 Achieving such metrics relies on clinicians accurately predicting a patient’s risk of mortality. However, oncologists are often unable to identify patients’ short-term mortality risk, with a bias toward overestimating life expectancy.11,12,13 Underestimating mortality risk is associated with aggressive end-of-life care among patients with cancer.14,15 Better assessment of short-term mortality risk may inform advance care planning and increase goal-concordant cancer care.16,17,18,19,20,21
By using vast longitudinal data from the electronic health record (EHR), machine learning (ML) algorithms may improve mortality risk prediction compared with population-level and randomized controlled trial estimates.22,23,24 However, most EHR-based ML algorithms to predict mortality have only been validated on retrospective data from patients receiving older treatment strategies. This raises questions about the generalizability of ML mortality risk prediction algorithms in real-world practice. To ensure accuracy and clinical usefulness, ML mortality risk prediction algorithms must be prospectively validated in large, unselected cohorts of patients with cancer and compared with existing prognostic indices.
In this prognostic cohort study, we describe the prospective performance of a previously trained ML algorithm based on structured EHR data to predict 180-day mortality in outpatients seen in oncology practices within a large academic system.25 We hypothesized that this algorithm would have good discrimination and calibration and outperform routinely used prognostic indices in oncology.
Methods
Setting
This study was performed at medical or gynecologic oncology practices in Pennsylvania and New Jersey within the University of Pennsylvania Health System (UPHS). Eligible practices included a large tertiary practice, in which clinicians subspecialize in 1 cancer type (eg, lymphoma), and 17 general oncology practices, in which clinicians usually treat multiple cancer types. The UPHS institutional review board approved this study with a waiver of informed consent, classifying the study as quality improvement. This study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline for prediction algorithm validation.26
Study Population
Patients were eligible for this study if they were aged 18 years or older and had an encounter between March 1, 2019, and April 30, 2019 (the baseline period), that was listed in Clarity, an Epic reporting database that contains structured data elements of individual EHRs for patients within the UPHS.27 As this was a pragmatic study, the sample size was determined by the number of patient encounters during the baseline period and not by a priori power calculations. For patients with multiple encounters during the baseline period, the last encounter in the baseline period was defined as the index encounter. Patients were followed up until death. Patients who were living at 180 days from the index encounter were censored in analyses. Of 25 588 patients who had any eligible encounter during the baseline period, 24 582 (96.1%) were eligible for this study. Excluded patients included 867 (3.4%) whose appointment was scheduled after weekly predictions were generated and 139 (0.5%) who were only seen in benign hematology, palliative care, or rehabilitation practices.
Study Design
This study prospectively evaluated a gradient-boosted ML algorithm used to predict 180-day mortality among outpatients with cancer. We previously trained this algorithm, which uses 559 structured EHR features as inputs, using historic data from 2016.25 Of note, certain variables, including Eastern Cooperative Oncology Group (ECOG) performance status, stage, and genetic variants, were not included as features in the algorithm because they were not reliably coded in structured format at the time of algorithm training. Algorithm specifications, features, handling of missing data, cross-validation, and source code are described in this previous publication. The algorithm was integrated into the EHR at UPHS in early 2019. Of the 18 eligible practices in this study, 7 medical oncology practices were part of the original algorithm training data set, and 11 medical and gynecologic oncology practices were not part of the original algorithm training data set. The algorithm was not recalibrated after training; thus, all features and weights were locked before implementation into the UPHS EHR as part of this prospective cohort study. Importantly, no patient data used in this study were used in the original algorithm training.
In this study, patients who had an encounter in 1 of 18 practices during the baseline period were followed up for 180 days after the index encounter. We selected a 60-day baseline period during which the algorithm ran silently for all encounters and clinicians were not exposed to algorithm predictions; thus, algorithm output could not immediately affect care patterns (eg, early stoppage of chemotherapy, early referral to hospice) that could have influenced the outcome of 180-day mortality. Beginning shortly after this 60-day period, select clinicians began being exposed to predictions as part of a randomized controlled trial.28 During the baseline period, a database of all required structured EHR data was updated nightly. The algorithm engine ran automatically once a week on Thursdays at 7:00 am and used EHR data updated on the previous night to generate risk predictions of 180-day mortality for each patient whose encounter was scheduled for the coming Monday through Friday during the baseline period. Patient encounters that were scheduled after the prior Thursday at 7:00 am did not have an associated prediction and were not included in the cohort.
Outcome
The primary outcome of this study was 180-day mortality, defined as any death within the 180 days after the index encounter. Cause of death was not available for this analysis. Date of death was derived from the first date of death recorded in either the EHR or the Social Security Administration Death Master File and matched to patients within the UPHS by social security number and date of birth.29 All dates of death were ascertained on January 20, 2020.
Statistical Analysis
We used descriptive statistics to compare characteristics of the cohort, stratified by site of care (tertiary academic vs general oncology and practices that were included in the algorithm training data set vs practices that were not included in the algorithm training data set). To assess algorithm discrimination, we calculated the area under the receiver operating characteristic curve (AUC) as our primary performance metric. To account for class imbalance due to low overall rates of 180-day mortality, we also calculated the area under the precision-recall curve (AUPRC).30 Algorithm calibration was assessed by plotting the predicted vs observed rate of 180-day mortality and by calculating the scaled Brier score, which is a measure of calibration ranging from 0 (poor calibration) to 1 (perfect calibration).31 To assess other performance metrics of operational importance, we calculated positive predictive value (PPV), negative predictive value (NPV), sensitivity, and alert rate (the percentage of all encounters deemed as high risk) at various risk thresholds. All threshold-dependent performance metrics in the primary analysis are presented at a prespecified 40% risk of 180-day mortality unless otherwise specified, because this was the threshold used to report the performance of the algorithm after training and was determined after consultation with select oncology clinicians.25 To assess for heterogeneity of algorithm performance, we calculated performance metrics across important prespecified subgroups: disease site, practice type (tertiary vs general), self-reported race, sex, insurance type, and stage (American Joint Committee on Cancer 8th edition I-III vs IV).
There is no criterion standard prognostic index for patients with cancer. To compare the ML algorithm with other commonly used prognostic references in general oncology, we used logistic regression to create 2 univariable classifiers based on ECOG (ECOG ≥2 vs <2) and number of Elixhauser comorbidities (Elixhauser ≥3 vs <3).32,33 We calculated the aforementioned performance metrics for these univariable classifiers based on ECOG values that were coded less than or equal to 30 days before the index encounter and Elixhauser comorbidities coded before the index encounter, respectively. Patients without coded ECOG values were not used to calculate the performance of the ECOG classifier. The Elixhauser comorbidity index was available for all patients. To compare the ML algorithm against these 2 indices, we calculated 3 metrics: (1) the difference in AUC (∆AUC), (2) the difference in PPV (∆PPV), and (3) the additive net reclassification index (NRI).34 The NRI measures the improvement in predictive performance gained by adding the ML algorithm to existing prognostic indices; positive values of the NRI indicate overall correct reclassification of true positives and true negatives, whereas negative values indicate incorrect reclassification. To calculate the NRI, we first developed a logistic regression algorithm with mortality (dead vs alive) as the outcome and baseline classifier (ECOG or Elixhauser) as the only covariate. We then developed another logistic regression algorithm (enhanced classifier) that added the ML classifier (prediction score from ML algorithm fourth quartile vs first to third quartile) as a covariate to the baseline classifiers. We used the fourth quartile of the prediction score from the ML algorithm as the threshold to classify patients into high- and low-risk groups and calculated the NRI based on the number of patients who were reclassified.
All analyses were conducted using the Sklearn, version 0.21.3 package in Python (Python Software Foundation); all hypothesis testing used 2-sided P < .05 to determine statistical significance. Statistical analysis was performed between January 1, 2020, and March 15, 2020.
Sensitivity Analysis
For patients with multiple encounters during the baseline period, we repeated analyses using the first and a random encounter as the index encounter. To assess threshold-dependent performance metrics, we used 10%, 20%, and 30% risk of 180-day mortality as the risk threshold and recalculated performance metrics.
Results
The cohort consisted of 24 582 patients, of whom 1022 (4.2%) died within 180 days of their index encounter. In the full cohort, the median (interquartile range) age was 64.6 (53.6-73.2) years, 15 319 (62.3%) were women, 18 015 (76.0%) were White, and 10 658 (43.4%) were seen in the tertiary practice. A total of 15 059 patients (61.3%) were married, and 6754 (27.5%) had an Elixhauser count of greater than or equal to 3. There were large differences between the patients who were alive and those who were deceased within 180 days of the index encounter (Table 1). Compared with living patients, deceased patients were more likely to have documented stage IV cancer (136 [59.1%] vs 835 [22.2%]), ECOG greater than or equal to 2 (261 [56.5%] vs 702 [12.2%]), and Elixhauser count greater than or equal to 3 (653 [63.9%] vs 6101 [25.9%]). Full characteristics of patients, stratified by practice site, inclusion in original algorithm training data, and ECOG documentation status, are available in eTables 1, 2, and 3 in the Supplement, respectively.
Table 1. Study Population Characteristics.
| Characteristic | No. (%)a | ||
|---|---|---|---|
| Total (n = 24 582) | Alive (n = 23 560) | Dead (n = 1022) | |
| Age, median (IQR), y | 64.6 (53.6-73.2) | 64.4 (53.3-73.0) | 69.7 (60.5-78.0) |
| Women | 15 319 (62.3) | 14 803 (62.8) | 516 (50.5) |
| Race/ethnicity | |||
| Non-Hispanic White | 18 015 (76.0) | 17 263 (76.0) | 752 (75.8) |
| Black | 3951 (16.7) | 3769 (16.6) | 182 (18.3) |
| Other | 1734 (7.3) | 1676 (7.4) | 58 (5.8) |
| Marital status | |||
| Married | 15 059 (61.3) | 14 451 (61.4) | 608 (59.6) |
| Unmarried | 9493 (38.7) | 9081 (38.6) | 412 (40.4) |
| Insurance | |||
| Medicare + managed Medicare | 12 244 (50.8) | 2409 (10.4) | 77 (7.8) |
| Medicaid + managed Medicaid | 1247 (5.2) | 7917 (34.2) | 201 (20.4) |
| Managed care | 8118 (33.7) | 1178 (5.1) | 69 (7.0) |
| Commercial insurance | 2486 (10.3) | 11 608 (50.2) | 636 (64.7) |
| ECOG performance status | |||
| 0 | 2822 (45.3) | 2785 (48.3) | 37 (8.0) |
| 1 | 2440 (39.2) | 2276 (39.5) | 164 (35.5) |
| ≥2 | 963 (15.5) | 702 (12.2) | 261 (56.5) |
| Elixhauser comorbidity score | |||
| 0-1 | 12 866 (52.3) | 12 695 (53.9) | 171 (16.7) |
| 2 | 4962 (20.2) | 4764 (20.2) | 198 (19.4) |
| ≥3 | 6754 (27.5) | 6101 (25.9) | 653 (63.9) |
| Cancer stage | |||
| I-III | 3028 (75.7) | 2934 (77.8) | 94 (40.9) |
| IV | 971 (24.3) | 835 (22.2) | 136 (59.1) |
| Practice site | |||
| Tertiary academic | 10 658 (43.4) | 10 136 (43.0) | 522 (51.1) |
| Breast | 1862 (11.0) | 1811 (11.3) | 51 (6.3) |
| Gastrointestinal tract | 1193 (7.1) | 1074 (6.7) | 119 (14.7) |
| Thoracic | 1060 (6.3) | 961 (6.0) | 99 (12.3) |
| Genitourinary | 1048 (6.2) | 1009 (6.3) | 39 (4.8) |
| Gynecologic | 1049 (6.2) | 1029 (6.4) | 20 (2.5) |
| Leukemia | 1062 (6.3) | 993 (6.2) | 69 (8.6) |
| Lymphoma | 1259 (7.5) | 1214 (7.6) | 45 (5.6) |
| Melanoma | 403 (2.4) | 381 (2.4) | 22 (2.7) |
| Myeloma | 1179 (7.0) | 1138 (7.1) | 41 (5.1) |
| Neuro-oncology | 355 (2.1) | 333 (2.1) | 22 (2.7) |
| General oncology | 13 924 (56.6) | 13 424 (57.0) | 500 (48.9) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
Values are presented as No. (%) unless otherwise specified.
Algorithm Performance
Algorithm performance metrics in the full cohort and stratified by practice site are described in Table 2. In the full cohort, the AUC was 0.89 (95% CI, 0.88-0.90), the AUPRC was 0.34, and the scaled Brier score was 0.29. Algorithm performance did not differ between patients seen at the tertiary academic vs general oncology practices. Among practice sites that were not part of the original algorithm training, the AUC was 0.86. Performance characteristics varied across disease-specific groups within the tertiary practice, ranging from an AUC of 0.74 (neuro-oncology) to 0.96 (breast oncology). Algorithm performance was slightly better for women compared with men (AUC, 0.91 vs 0.86; AUPRC, 0.37 vs 0.31; PPV, 0.47 vs 0.43; sensitivity, 0.30 vs 0.25) and for patients with stage IV compared with stage I to III cancer (AUPRC, 0.47 vs 0.24; PPV, 0.49 vs 0.31; sensitivity, 0.39 vs 0.25) (eTable 4 in the Supplement). There were no significant differences in performance across race/ethnicity and insurance status.
Table 2. Algorithm Performance.
| Variable | AUC (95% CI) | AUPRC | Scaled Brier score | PPV | NPV | Sensitivity | Specificity | No. | Rate | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Alert | |||||||||
| Overall | 0.89 (0.88-0.90) | 0.34 | 0.29 | 0.45 | 0.97 | 0.27 | 0.99 | 24 582 | 0.04 | 0.025 |
| Practice sitea | ||||||||||
| Included in algorithm training | ||||||||||
| Yes | 0.89 (0.88-0.90) | 0.37 | 0.29 | 0.47 | 0.97 | 0.29 | 0.98 | 18 927 | 0.05 | 0.03 |
| No | 0.86 (0.83-0.88) | 0.22 | 0.26 | 0.36 | 0.98 | 0.17 | 0.99 | 5655 | 0.03 | 0.01 |
| Practice site | ||||||||||
| Tertiary academic | 0.89 (0.87-0.90) | 0.37 | 0.31 | 0.45 | 0.97 | 0.31 | 0.98 | 10 658 | 0.05 | 0.03 |
| Breast | 0.96 (0.94-0.98) | 0.49 | 0.38 | 0.57 | 0.99 | 0.41 | 0.99 | 1862 | 0.03 | 0.02 |
| Gastrointestinal | 0.85 (0.81-0.88) | 0.38 | 0.32 | 0.39 | 0.92 | 0.29 | 0.95 | 1193 | 0.10 | 0.08 |
| Thoracic | 0.82 (0.77-0.86) | 0.38 | 0.32 | 0.42 | 0.93 | 0.34 | 0.95 | 1060 | 0.09 | 0.08 |
| Genitourinary | 0.88 (0.80-0.94) | 0.33 | 0.43 | 0.39 | 0.98 | 0.36 | 0.98 | 1048 | 0.04 | 0.03 |
| Gynecologic | 0.91 (0.87-0.95) | 0.24 | 0.25 | 0.50 | 0.99 | 0.25 | 1.00 | 1049 | 0.02 | 0.01 |
| Leukemia | 0.85 (0.79-0.90) | 0.39 | 0.27 | 0.50 | 0.96 | 0.33 | 0.98 | 1062 | 0.07 | 0.04 |
| Lymphoma | 0.91 (0.85-0.95) | 0.29 | 0.14 | 0.50 | 0.97 | 0.16 | 1.00 | 1259 | 0.04 | 0.01 |
| Melanoma | 0.90 (0.84-0.95) | 0.35 | 0.20 | 0.25 | 0.95 | 0.09 | 0.98 | 403 | 0.06 | 0.02 |
| Myeloma | 0.91 (0.85-0.96) | 0.44 | 0.26 | 0.62 | 0.98 | 0.32 | 0.99 | 1179 | 0.04 | 0.02 |
| Neuro-oncology | 0.74 (0.64-0.83) | 0.14 | 0.21 | 0.11 | 0.94 | 0.05 | 0.98 | 355 | 0.06 | 0.03 |
| General oncology | 0.89 (0.87-0.90) | 0.31 | 0.27 | 0.45 | 0.97 | 0.24 | 0.99 | 13 924 | 0.04 | 0.02 |
Abbreviations: AUC, area under the receiver operating characteristic curve; AUPRC, area under the precision-recall curve; PPV, positive predictive value; NPV, negative predictive value.
These data only refer to the site’s inclusion in the original algorithm training (based on 2016 data). No individual patient data used in the original algorithm training were reused in the prospective validation.
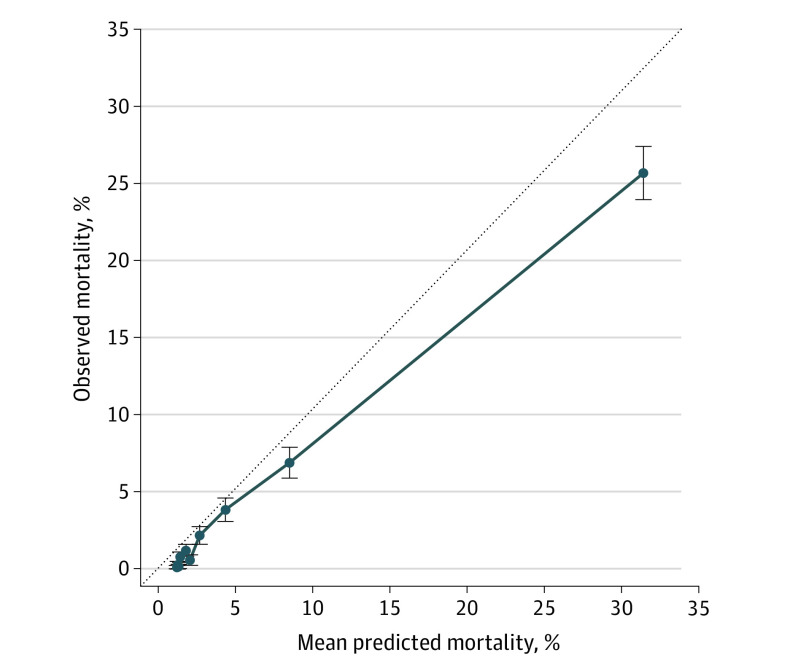
At a prespecified mortality risk threshold of 40%, 2.5% of encounters were flagged as high risk. At this threshold, the PPV was 45.2%, NPV was 96.9%, and sensitivity was 27.4%. Observed 180-day mortality was 45.2% (95% CI, 41.3%-49.1%) in the high-risk group vs 3.1% (95% CI, 2.9%-3.3%) in the low-risk group (Figure 1); high-risk patients had higher 180-day mortality across varying thresholds of predicted risk (eFigure 1 in the Supplement). The algorithm was well calibrated for patients with mortality risk less than or equal to 10% and overestimated mortality risk for patients with mortality risk greater than 10% (Figure 2). Observed mortality was 100-fold higher among patients in the highest vs lowest quartiles of predicted risk (13.2% vs 0.13%) (eFigure 2 in the Supplement).
Figure 1. Overall 180-Day Mortality of Patients Considered High vs Low Risk as Identified by a Machine Learning Algorithm.
High-risk patients defined as having greater than 40% risk of 180-day mortality.
Figure 2. Algorithm Calibration.
Points are binned deciles of predicted mortality. Mean predicted mortality refers to predicted risk of 180-day mortality from the machine learning algorithm. Observed mortality refers to the percentage of patients who died within 180 days of the index encounter. Bars refer to 95% confidence intervals of the prediction. The dotted line refers to a reference model where mean predicted mortality equals observed mortality; a perfectly calibrated algorithm would fall along the dotted line. Points falling below the dotted line overestimate risk of mortality, whereas points falling above the dotted line underestimate risk of mortality.
Algorithm Comparison
Of patients who had a coded ECOG (6225 [25.3%]), 963 (15.5%) had an ECOG greater than or equal to 2. Compared with the baseline ECOG-only classifier, the enhanced classifier integrating the ECOG and ML classifiers had a significantly higher AUC (∆AUC, 0.17; 95% CI, 0.14-0.19) and higher PPV (∆PPV, 0.18). The enhanced classifier resulted in favorable reclassification compared with the baseline classifier (NRI, 0.09; 95% CI, 0.04-0.14) (Table 3).
Table 3. Comparison of ML Algorithm Against Standard Prognostic Indices in Oncology.
| Variable | Prognostic index | |
|---|---|---|
| ECOG (n = 6225) | Elixhauser (n = 24 582) | |
| Baseline classifier AUC (95% CI) | 0.72 (0.70-0.75) | 0.69 (0.68-0.71) |
| ∆AUC (95% CI) between ML algorithm and baseline classifier | 0.17 (0.14-0.19) | 0.20 (0.18-0.21) |
| Baseline classifier PPV, No. | 0.27 | 0.10 |
| ∆PPV between ML algorithm and baseline classifier, No. | 0.18 | 0.36 |
| NRI (95% CI) from enhanced vs baseline classifier | 0.09 (0.04-0.14) | 0.23 (0.20-0.27) |
Abbreviations: AUC, area under the receiver operating characteristic curve; ECOG, Eastern Cooperative Oncology Group; ML, machine learning; NRI, net reclassification index; PPV, positive predictive value; ∆AUC, difference in AUC; ∆PPV, difference in PPV.
Compared with the baseline Elixhauser-only classifier, the enhanced classifier integrating the Elixhauser and ML classifiers had a significantly higher AUC (∆AUC, 0.20; 95% CI, 0.18-0.21) and higher PPV (∆PPV, 0.36). The enhanced classifier resulted in favorable reclassification compared with the baseline classifier (NRI, 0.23; 95% CI, 0.20-0.27) (Table 3).
Subgroup and Sensitivity Analyses
Overall algorithm performance characteristics were similar when, for patients with multiple encounters in the database, either the first or a random encounter was used as the index encounter (eTable 5 in the Supplement). Threshold-dependent performance characteristics varied when alternative mortality risk thresholds were applied (eTable 6 in the Supplement).
Discussion
In this prognostic cohort study, our results suggest that an ML algorithm can be feasibly integrated into the EHR to generate real-time, accurate predictions of short-term mortality risk for patients with cancer that outperform traditional prognostic indices. This study represents, to our knowledge, one of the first prospective applications of an ML mortality risk prediction algorithm in clinical oncology and provides 3 important insights into the potential clinical applicability of real-time prognostic algorithms in clinical practice.
First, this study is a true prospective validation of a previously described ML algorithm. The ML algorithm was trained using patient data from 2016. In contrast, this prospective cohort was enrolled in 2019. Thus, no patient data in this prospective validation was used as part of algorithm training. Despite many therapeutic advances since 2016, including the widespread use of immunotherapy and biomarker-based therapies, our algorithm demonstrated good performance on several clinically relevant metrics even when applied to a prospective cohort 3 years later.35 Additionally, the AUC, AUPRC, and PPV from our prospective validation were equivalent to or better than what has been reported in retrospective validations of ML mortality risk prediction algorithms in oncology.23,25,36 This demonstrates that an ML mortality risk prediction algorithm trained on retrospective data may still have good performance and clinical utility when applied in a recent cohort of patients with cancer. Importantly, the algorithm still had a clinically applicable AUC (0.86) for practices that were not included in the algorithm training.
Second, the ML algorithm demonstrated better AUC and PPV compared with the ECOG and Elixhauser classifiers, and enhanced classifiers that integrated the ML algorithm into existing prognostic indices resulted in better reclassification of patients. Published ML prognostic algorithms often either do not have a comparator or have been compared with regression-based algorithms that are not used in clinical practice.25,37 In contrast, ECOG and Elixhauser comorbidity scores are validated prognostic indices that are commonly used in decision-making around treatment selection and clinical trial enrollment.38 Although Elixhauser comorbidities are included in the ML algorithm inputs, ECOG is not included in the algorithm because it was not available as a structured data element in the EHR at the time of algorithm training.25 As ECOG and other variables of prognostic significance (eg, stage, line of treatment, genetic variants) are increasingly coded in the EHR, the performance of this algorithm could improve.
Third, the differences in prospective performance across cancer types and stages give important information regarding for which disease settings an automated ML prognostic algorithm may be most useful in general oncology. For malignancies with poorer performance (eg, melanoma, neuro-oncology), several factors that were not included in our algorithm may have greater prognostic importance; these include performance and cognitive status, molecular and genetic biomarker expression, and imaging results. When considering the implementation of a tool such as this, clinicians must consider the need to account for prognostic factors that are unique to specific cancers. Relatedly, clinicians may use this point-of-care tool differently with different threshold preferences for patients with stage I to III cancer—who are generally treated with curative intent—compared with patients with stage IV cancer who are generally treated with palliative intent. Finally, despite recent concerns over algorithm bias against underrepresented subgroups,39,40 there were no major differences in performance across site of practice, race/ethnicity, and insurance status.
Although ML algorithms may improve mortality risk prediction, it is important to demonstrate that improved accuracy can translate to better clinician and patient decision-making.41 ML mortality risk prediction tools may help oncology clinicians better identify patients who are at high risk of short-term mortality, engage in early goals of care discussions, and meet quality metrics related to end-of-life care. Indeed, in a subsequent randomized clinical trial, we applied this algorithm in clinical practice to identify oncology patients with the highest predicted mortality and used behavioral nudges to encourage clinicians to have end-of-life conversations with those patients, resulting in significant increases in such conversations.28,42 Notably, for a cancer with a high baseline rate of short-term mortality (eg, pancreatic cancer), the mortality risk threshold for intervention may be higher to avoid a high alert rate; the opposite may be true for a cancer with a low rate of short-term mortality (eg, prostate cancer, myeloma). Our algorithm retained good performance across several mortality risk thresholds, indicating that such thresholds could be customized for clinical use (eTable 6 in the Supplement).
Limitations
There are several limitations to this study. First, using a risk threshold of 40%, the sensitivity of the algorithm was only 27%. This may reflect the inability to include important features, such as stage and ECOG values, in the original algorithm training, in addition to the high mortality risk threshold used. Indeed, when using a lower but still clinically meaningful risk threshold of 10%, sensitivity improved to nearly 70% (eTable 6 in the Supplement). Third, in the comparison of the ML algorithm against the ECOG classifier, we were limited to only using coded ECOG values. Patients whose ECOG was coded were systematically different than patients whose ECOG was not coded, as shown in eTable 3 in the Supplement. However, the purpose of this analysis was not to develop a new classifier that includes ECOG but rather to assess whether the ML algorithm improves classification above and beyond the ECOG for those with a coded ECOG. Indeed, the positive NRI indicates that even within the subgroup of those with coded ECOG, the ML algorithm led to favorable reclassification of patients compared with the ECOG classifier alone. Using imputation strategies would not have been appropriate given the high degree of missingness of ECOG.
Conclusions
The results of this prognostic study suggest that an ML algorithm to predict short-term mortality has good prospective validity and compares favorably with existing prognostic indices. Such an automated tool may complement clinician intuition and lead to improved targeting of supportive care interventions for high-risk patients with cancer.
eFigure 1. Overall 180-Day Mortality of High- vs Low-Risk Patients as Identified by Machine Learning Algorithm at Alternative Thresholds of Mortality Risk
eFigure 2. Observed Mortality Across Quartiles of 180-day Predicted Mortality Risk
eTable 1. Study Population Characteristics, Stratified by Practice Site
eTable 2. Study Population Characteristics, Stratified by Inclusion in Original Algorithm Training
eTable 3. Study Population Characteristics Stratified by ECOG Status
eTable 4. Algorithm Performance in Subgroups of Interest
eTable 5. Sensitivity Analyses Using Alternative Index Encounter Definitions
eTable 6. Sensitivity Analyses Using Alternative Thresholds of Mortality Risk
References
- 1.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321. doi: 10.1200/JCO.2004.08.136 [DOI] [PubMed] [Google Scholar]
- 2.Chastek B, Harley C, Kallich J, Newcomer L, Paoli CJ, Teitelbaum AH. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8(6):75s-80s. doi: 10.1200/JOP.2011.000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright AA, Mack JW, Kritek PA, et al. Influence of patients’ preferences and treatment site on cancer patients’ end-of-life care. Cancer. 2010;116(19):4656-4663. doi: 10.1002/cncr.25217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. doi: 10.1001/jama.300.14.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21(6):1133-1138. doi: 10.1200/JCO.2003.03.059 [DOI] [PubMed] [Google Scholar]
- 7.D’Amico TA, Bandini LAM, Balch A, et al. Quality measurement in cancer care: a review and endorsement of high-impact measures and concepts. J Natl Compr Canc Netw. 2020;18(3):250-259. [DOI] [PubMed]
- 8.Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31(4):786-796. doi: 10.1377/hlthaff.2011.0650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman DC, Fisher ES, Chang CH, et al. Quality of End-of-Life Cancer Care for Medicare Beneficiaries Regional and Hospital-Specific Analyses. The Dartmouth Atlas;2010. [PubMed] [Google Scholar]
- 10.American Society of Clinical Oncology QOPI-related measures. Published 2020. Accessed March 20, 2020. https://practice.asco.org/quality-improvement/quality-programs/quality-oncology-practice-initiative/qopi-related-measures
- 11.Krishnan M, Temel JS, Wright AA, Bernacki R, Selvaggi K, Balboni T. Predicting life expectancy in patients with advanced incurable cancer: a review. J Support Oncol. 2013;11(2):68-74. doi: 10.12788/j.suponc.0004 [DOI] [PubMed] [Google Scholar]
- 12.Chow E, Harth T, Hruby G, Finkelstein J, Wu J, Danjoux C. How accurate are physicians’ clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? a systematic review. Clin Oncol (R Coll Radiol). 2001;13(3):209-218. doi: 10.1007/s001740170078 [DOI] [PubMed] [Google Scholar]
- 13.White N, Reid F, Harris A, Harries P, Stone P. A systematic review of predictions of survival in palliative care: how accurate are clinicians and who are the experts? PLoS One. 2016;11(8):e0161407. doi: 10.1371/journal.pone.0161407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709-1714. doi: 10.1001/jama.279.21.1709 [DOI] [PubMed] [Google Scholar]
- 15.Rose JH, O’Toole EE, Dawson NV, et al. Perspectives, preferences, care practices, and outcomes among older and middle-aged patients with late-stage cancer. J Clin Oncol. 2004;22(24):4907-4917. doi: 10.1200/JCO.2004.06.050 [DOI] [PubMed] [Google Scholar]
- 16.Tang ST, Chen CH, Wen FH, et al. Accurate prognostic awareness facilitates, whereas better quality of life and more anxiety symptoms hinder end-of-life care discussions: a longitudinal survey study in terminally ill cancer patients’ last six months of life. J Pain Symptom Manage. 2018;55(4):1068-1076. doi: 10.1016/j.jpainsymman.2017.12.485 [DOI] [PubMed] [Google Scholar]
- 17.Nipp RD, Greer JA, El-Jawahri A, et al. Coping and prognostic awareness in patients with advanced cancer. J Clin Oncol. 2017;35(22):2551-2557. doi: 10.1200/JCO.2016.71.3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundquist G, Rasmussen BH, Axelsson B. Information of imminent death or not: does it make a difference? J Clin Oncol. 2011;29(29):3927-3931. doi: 10.1200/JCO.2011.34.6247 [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480-488. doi: 10.1001/archinternmed.2008.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Jawahri A, Traeger L, Park ER, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278-285. doi: 10.1002/cncr.28369 [DOI] [PubMed] [Google Scholar]
- 21.Finlay E, Casarett D. Making difficult discussions easier: using prognosis to facilitate transitions to hospice. CA Cancer J Clin. 2009;59(4):250-263. doi: 10.3322/caac.20022 [DOI] [PubMed] [Google Scholar]
- 22.Gensheimer MF, Henry AS, Wood DJ, et al. Automated survival prediction in metastatic cancer patients using high-dimensional electronic medical record data. J Natl Cancer Inst. 2019;111(6):568-574. doi: 10.1093/jnci/djy178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elfiky AA, Pany MJ, Parikh RB, Obermeyer Z. Development and application of a machine learning approach to assess short-term mortality risk among patients with cancer starting chemotherapy. JAMA Netw Open. 2018;1(3):e180926-e180926. doi: 10.1001/jamanetworkopen.2018.0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brajer N, Cozzi B, Gao M, et al. Prospective and external evaluation of a machine learning model to predict in-hospital mortality of adults at time of admission. JAMA Netw Open. 2020;3(2):e1920733-e1920733. doi: 10.1001/jamanetworkopen.2019.20733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh RB, Manz C, Chivers C, et al. Machine learning approaches to predict 6-month mortality among patients with cancer. JAMA Netw Open. 2019;2(10):e1915997-e1915997. doi: 10.1001/jamanetworkopen.2019.15997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moons KGM, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1-W73. doi: 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- 27.Penn Medicine. Epic clarity. Published 2020. Accessed March 20, 2020.https://www.med.upenn.edu/dac/epic-clarity-data-warehousing.html
- 28.Machine-generated mortality estimates and nudges to promote advance care planning discussion among cancer patients. Clinicaltrials.gov identifier: NCT03984773. Updated April 24, 2020. Accessed June 1, 2020. https://clinicaltrials.gov/ct2/show/NCT03984773
- 29.National Technical Information Service Limited access death master file: final rule establishing certification program for access to death master file in effect. Published 2020. Accessed March 20, 2020. https://classic.ntis.gov/products/ssa-dmf/#
- 30.Davis J, Goadrich M The relationship between precision-recall and ROC curves. Paper presented at: Proceedings of the 23rd International Conference on Machine Learning; June 25, 2006;. Pittsburgh, PA. [Google Scholar]
- 31.Wu YC, Lee WC. Alternative performance measures for prediction models. PLoS One. 2014;9(3):e91249. doi: 10.1371/journal.pone.0091249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ECOG-ACRIN Research Group ECOG performance status. Published 2020. Accessed March 20, 2020. https://ecog-acrin.org/resources/ecog-performance-status
- 33.Lieffers JR, Baracos VE, Winget M, Fassbender K. A comparison of Charlson and Elixhauser comorbidity measures to predict colorectal cancer survival using administrative health data. Cancer. 2011;117(9):1957-1965. doi: 10.1002/cncr.25653 [DOI] [PubMed] [Google Scholar]
- 34.Leening MJG, Vedder MM, Witteman JCM, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med. 2014;160(2):122-131. doi: 10.7326/M13-1522 [DOI] [PubMed] [Google Scholar]
- 35.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535-e192535. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertsimas D, Dunn J, Pawlowski C, et al. Applied informatics decision support tool for mortality predictions in patients with cancer. JCO Clin Cancer Inform. 2018;2:1-11. doi: 10.1200/CCI.18.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahni N, Simon G, Arora R. Development and validation of machine learning models for prediction of 1-year mortality utilizing electronic medical record data available at the end of hospitalization in multicondition patients: a proof-of-concept study. J Gen Intern Med. 2018;33(6):921-928. doi: 10.1007/s11606-018-4316-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Datta SS, Ghosal N, Daruvala R, et al. How do clinicians rate patient’s performance status using the ECOG performance scale? a mixed-methods exploration of variability in decision-making in oncology. Ecancermedicalscience. 2019;13:913. doi: 10.3332/ecancer.2019.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh RB, Teeple S, Navathe AS. Addressing bias in artificial intelligence in health care. JAMA. 2019. doi: 10.1001/jama.2019.18058 [DOI] [PubMed] [Google Scholar]
- 40.Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi: 10.1126/science.aax2342 [DOI] [PubMed] [Google Scholar]
- 41.Emanuel EJ, Wachter RM. Artificial intelligence in health care: will the value match the hype? JAMA. 2019;321(23):2281-2282. doi: 10.1001/jama.2019.4914 [DOI] [PubMed] [Google Scholar]
- 42.Manz CR, Parikh RB, Evans CN, et al. Integrating machine-generated mortality estimates and behavioral nudges to promote serious illness conversations for cancer patients: Design and methods for a stepped-wedge cluster randomized controlled trial. Contemp Clin Trials. 2020;90:105951. doi: 10.1016/j.cct.2020.105951 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Overall 180-Day Mortality of High- vs Low-Risk Patients as Identified by Machine Learning Algorithm at Alternative Thresholds of Mortality Risk
eFigure 2. Observed Mortality Across Quartiles of 180-day Predicted Mortality Risk
eTable 1. Study Population Characteristics, Stratified by Practice Site
eTable 2. Study Population Characteristics, Stratified by Inclusion in Original Algorithm Training
eTable 3. Study Population Characteristics Stratified by ECOG Status
eTable 4. Algorithm Performance in Subgroups of Interest
eTable 5. Sensitivity Analyses Using Alternative Index Encounter Definitions
eTable 6. Sensitivity Analyses Using Alternative Thresholds of Mortality Risk