This randomized phase 2 clinical trial assesses the efficacy and safety of plinabulin compared with pegfilgrastim for the prevention of chemotherapy-induced neutropenia following docetaxel chemotherapy in adult patients with non–small lung cancer.
Key Points
Question
What is the recommended phase 3 dose of the non–granulocyte colony-stimulating factor small molecule plinabulin for prevention of chemotherapy-induced neutropenia in adults with non–small lung cancer (NSCLC)?
Findings
This randomized phase 2 clinical trial of 55 patients with NSCLC compared 3 plinabulin doses (5, 10, and 20 mg/m2) with pegfilgrastim 6 mg in patients receiving intermediate febrile-neutropenia risk chemotherapy. The plinabulin 40-mg fixed dose, which is equivalent to the 20 mg/m2 dose, given on the same day as chemotherapy had the same duration of days of severe neutropenia as pegfilgrastim, the current standard of care.
Meaning
This study found that fixed-dose plinabulin was noninferior to pegfilgrastim in duration of severe neutropenia and will be compared with pegfilgrastim in a phase 3 trial of patients with NSCLC to confirm these results.
Abstract
Importance
Plinabulin is a novel, non–granulocyte colony-stimulating factor (GCSF) small molecule with both anticancer and neutropenia-prevention effects.
Objective
To assess the efficacy and safety of plinabulin compared with pegfilgrastim for the prevention of chemotherapy-induced neutropenia following docetaxel chemotherapy in patients with non–small lung cancer.
Design, Setting, and Participants
This was a randomized, open-label, phase 2 clinical trial of 4 treatment arms that was conducted in 19 cancer treatment centers in the United States, China, Russia, and Ukraine. Participants were adult patients with non–small cell lung cancer whose cancer had progressed after platinum-based chemotherapy. Data were collected from April 2017 through March 2018 and analyzed from August 2019 through February 2020.
Interventions
All patients received docetaxel 75 mg/m2 on day 1 and were randomly assigned to 1 of 3 doses of plinabulin (5, 10, or 20 mg/m2) on day 1 or to pegfilgrastim 6 mg on day 2. Patients were treated every 21 days for 4 chemotherapy cycles.
Main Outcomes and Measures
The primary end point was the determination of the recommended phase 3 dose of plinabulin based on the days of severe neutropenia during chemotherapy cycle 1. Daily complete blood cell counts and absolute neutrophil counts were drawn during times of anticipated neutropenia during cycle 1.
Results
Of the 55 patients randomized and evaluated, the mean (SD) age was 61.3 (10.2) years, and 38 (69.1%) were men. With each escalation of the plinabulin dose, the incidence of any grade of neutropenia decreased. There were no significant differences in mean (SD) days of severe neutropenia among those treated with pegfilgrastim (0.15 [0.38] days) when dosed at day 2 vs plinabulin 20 mg/m2 (0.36 [0.93] days; P = .76) when dosed at day 1, and no safety signals were detected.
Conclusions and Relevance
Single dose-per-cycle plinabulin has a similar neutropenia protection benefit as pegfilgrastim. Plinabulin 40 mg fixed dose, which is pharmacologically equivalent to 20 mg/m2, will be compared with pegfilgrastim 6 mg in the phase 3 portion of this trial. Noninferior days of severe neutropenia will be the primary end point, and bone pain reduction, thrombocytopenia reduction, and quality of life maintenance will be secondary end points.
Trial Registration
ClinicalTrials.gov Identifier: NCT03102606
Introduction
Cytotoxic anticancer chemotherapy has off-target myelosuppressive toxic effects, including neutropenia. Neutropenia predisposes patients to potentially life-threatening infections and infection-related complications. The duration and severity of chemotherapy-induced neutropenia (CIN) are associated with infection.1 Febrile neutropenia (temperature of >38.3 °C and absolute neutrophil count [ANC] <0.5 × 109 cells/L) increases the risk for the development of infection-related chemotherapy complications, including antibiotic use, hospitalization, sepsis, and death. Filgrastim, a granulocyte colony-stimulating factor (G-CSF) administered daily after chemotherapy, reduced days of severe neutropenia (DSN), febrile neutropenia (FN), hospitalization, antibiotic use, and infection in a randomized placebo-controlled clinical trial.2 Pegfilgrastim, a filgrastim modification with once-per-chemotherapy cycle dosing, is noninferior to filgrastim.3 Biosimilar agents have been approved in the United States based on noninferior DSN compared with filgrastim and pegfilgrastim.4,5,6,7
Chemotherapy regimens are classified into low, intermediate, and high FN risk. For high FN risk regimens, primary prophylactic G-CSF is recommended; for intermediate-risk regimens in patients with high FN risk, prophylactic G-CSF is also recommended in the National Comprehensive Cancer Network guidelines.8 For patients receiving intermediate FN risk (ie, 10%-20%) chemotherapy, primary or routine prophylactic G-CSF use is not recommended. Granulocyte colony-stimulating factor derivatives are associated with bone pain9,10 and patient inconvenience because they require a separate injection 24 hours after chemotherapy.
Plinabulin (BPI-2358, formerly NPI-2358) is a small non–G-CSF molecule that stabilizes intracellular microtubule formation in vitro,11 has human anticancer activity,12 and when combined with docetaxel reduces docetaxel-induced severe neutropenia.13 In a murine model, use of plinabulin alleviated neutropenia induced by microtubule-stabilizing (docetaxel), DNA cross-linking (cyclophosphamide), and DNA intercalating (doxorubicin) chemotherapies, yet did not affect bone marrow or blood G-CSF levels.14 In this study (PROTECTIVE-1), we investigated the efficacy and safety of plinabulin compared with pegfilgrastim in the intermediate FN risk docetaxel dose of 75 mg/m2 in a phase II dose-escalation trial for which CIN prevention was an unmet need.
Methods
Study Design and Patient Eligibility
This multicenter, open-label, randomized phase 2 study enrolled adult patients with advanced or metastatic non–small cell lung cancer (NSCLC) who had disease progression after platinum-based therapy and had an adequate hematopoietic, hepatic, and renal function; an Eastern Cooperative Oncology Group performance status of 0 or 1, and 1 or more risk factors requiring primary neutropenia prophylaxis.15 Exclusion criteria included concurrent administration of chemotherapy or radiation therapy, active infection, or the use of strong cytochrome P450 3A4 inhibitors. Full eligibility criteria are listed in the trial protocol (Supplement 1). Patients were randomized 1:1:1:1 on study entry into 1 of the 4 treatment arms through use of Suvoda, an interactive web response system. The phase 2 portion of this study was initially blinded but was amended after 6 patients were enrolled to an open label to facilitate pharmacokinetic and pharmocodynamic (PK/PD) sampling. No interim analysis of this phase 2 portion of the trial was planned. All patients gave informed consent, and appropriate treatment site institutional review boards oversaw and approved the study. The Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines were followed.
Procedures
Patients received docetaxel 75 mg/m2 on day 1 and were randomly assigned to either pegfilgrastim 6 mg on day 2 or to plinabulin 5, 10, or 20 mg/m2 given over 30 minutes, 30 minutes after docetaxel on day 1, and no day 2 treatment. Patients were to be treated every 3 weeks for 4 treatment cycles. Docetaxel premedication with corticosteroids was specified for all cycles, and dose reductions were specified for cycles 2 through 4. Complete blood counts and ANC were drawn at the same time each day and measured at a central laboratory at a pretreatment screening visit; on days 1, 2, 6 through 10, and 15 of cycle 1; on days 1 and 8 of cycles 2 through 4; at the end of treatment; and at a 30-day end of treatment follow-up. Cycle 1, day 1 preinfusion and postinfusion blood pressure (BP) was measured every 15 minutes for approximately 4 hours with an automated device, and pretreatment BP on day 1 and at day 8 was recorded for all cycles, as well as frequently during cycle 1 and at plinabulin preinfusion and postinfusion in each cycle. Bone pain was evaluated with the Brief Pain Inventory Short Form questionnaire prior to study drug infusion on day 1 and on days 2, 3, 5, 7, 9, and 21 of cycle 1.16,17 Health-related quality of life was evaluated by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 and EuroQol Group, 5-level questionnaire collected before docetaxel infusion on day 1 of each cycle.18,19,20
End Points and Assessment
The primary efficacy objective of this study was DSN in cycle 1. Days of severe neutropenia was defined as the number of days with an ANC of less than 0.5 × 109 cells/L, equivalent to days of grade 4 neutropenia. The primary safety objectives were adverse events and BP on day 1 within 4 hours after docetaxel infusion.
Pharmacokinetics and Pharmacodynamics
For pharmacokinetic modeling, blood was taken at predose, at end of infusion, and at 60 minutes, 4.5 hours, and 24 hours postinfusion for plasma plinabulin concentration. For pharmacodynamic relationship modeling, exposure–ambulatory BP measurement (ABPM), exposure–corrected QT, and exposure–neutropenia were characterized using a sequential PK/PD modeling approach using NONMEM software (ICON Development). The cosine model was used for the plinabulin exposure–ABPM association to account for the circadian rhythm of ABPM. A semiphysiological model characterized the time course of neutropenia in the exposure–neutropenia model. The effect of plinabulin and docetaxel were best described with maximum effect of drug concentration models.21 Simulations summarized DSN and severity of neutropenia, computed as the area below the threshold of 0.5 × 109/L and above the ANC–time response curve in the first chemotherapy cycle (area over the curve) to determine the most efficacious dose of plinabulin in reducing docetaxel-induced neutropenia.
Statistical Analysis
The intent-to-treat and safety analysis data sets included all patients randomized and receiving at least 1 dose of study medication. To estimate DSN, we assumed that the shape of the ANC recovery curve in patients treated with plinabulin is indistinguishable from filgrastim and its biosimilars.4 Mean values and standard deviations of ANC were available and used to generate random ANC data that asymptotically have the same means and standard deviations, and also generate the projected number of DSN. Deming regression was used to calculate the linear association between simulated nadir and DSN.22 Calculated mean DSN was 0.065 days for the plinabulin and docetaxel arm, and 1.076 days for docetaxel alone. Based on published data with filgrastim in patients receiving docetaxel, we assumed that grade 4 neutropenia in cycle 1 would occur at twice the frequency with G-CSF and docetaxel vs plinabulin and docetaxel, resulting in a presumed mean DSN of 0.13 days for the G-CSF and docetaxel combination.23
Data were presented using descriptive statistics (eg, mean, median, standard deviation, and range for continuous variables, and as integers and percentages for categorical variables). For continuous variables, methods of longitudinal assessments using mixed models were applied. Overall treatment effects were estimated (over the course of the treatment period), as were pairwise effects at individual time points. For categorical variables, χ2 tests or other appropriate statistics were applied. The trial was powered to accept a noninferiority margin for plinabulin to pegfilgrastim with 0.65 DSN in cycle 1. Reported P values were 1-sided and considered significant P = .025. SAS, version 9.4 or higher (SAS Institute), and Stata, version 15.1 (StataCorp LLC), were used for the analysis.
Results
Patients and Treatment
From April 2017 to March 2018, 55 patients were enrolled from 19 sites (Figure 1). The demographic and baseline characteristics were well balanced. No differences in medical history were apparent (eTable 1 in Supplement 2). Relative chemotherapy dose intensity delivered for all treatment arms across the 4 cycles is shown in eTable 2 in Supplement 2.
Figure 1. CONSORT Diagram.
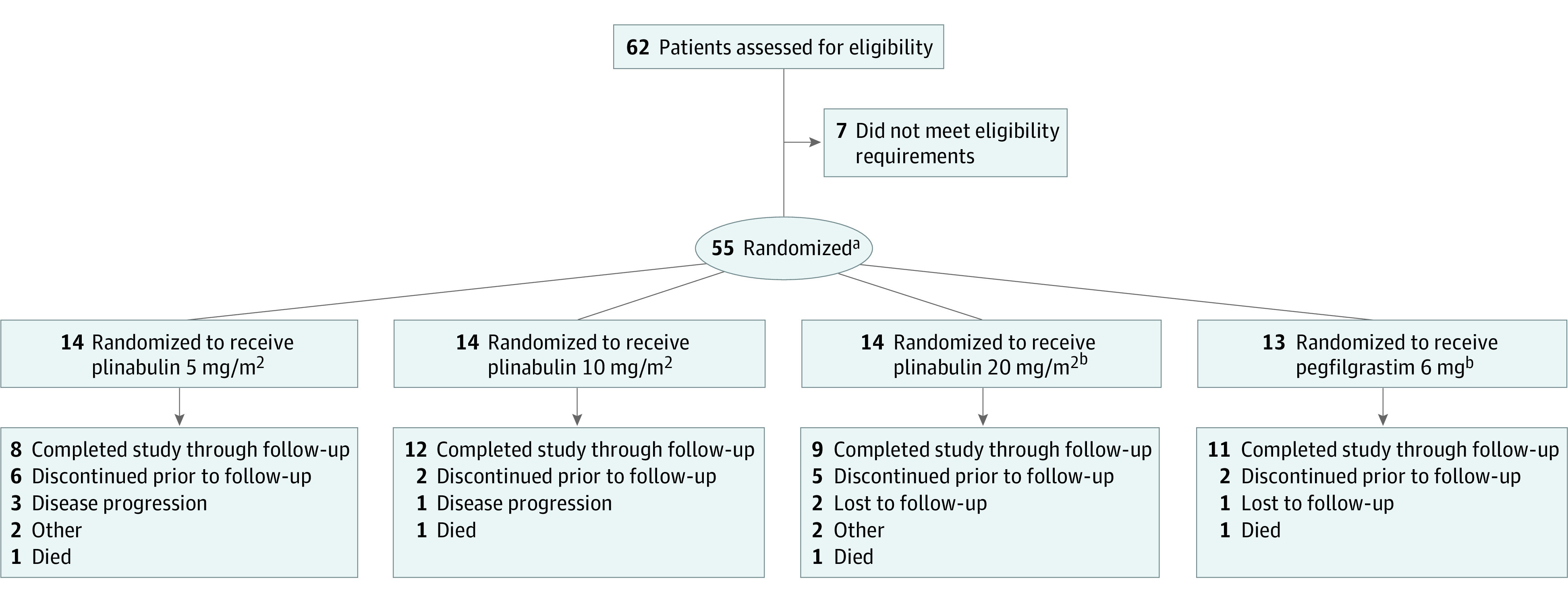
Disposition of the 62 patients screened for the study.
aAll randomized patients received at least 1 dose of the study drug and were included in the intent-to-treat and safety sets.
bOne patient randomized to the pegfilgrastim treatment arm was erroneously given plinabulin 20 mg/m2 throughout the study.
Efficacy
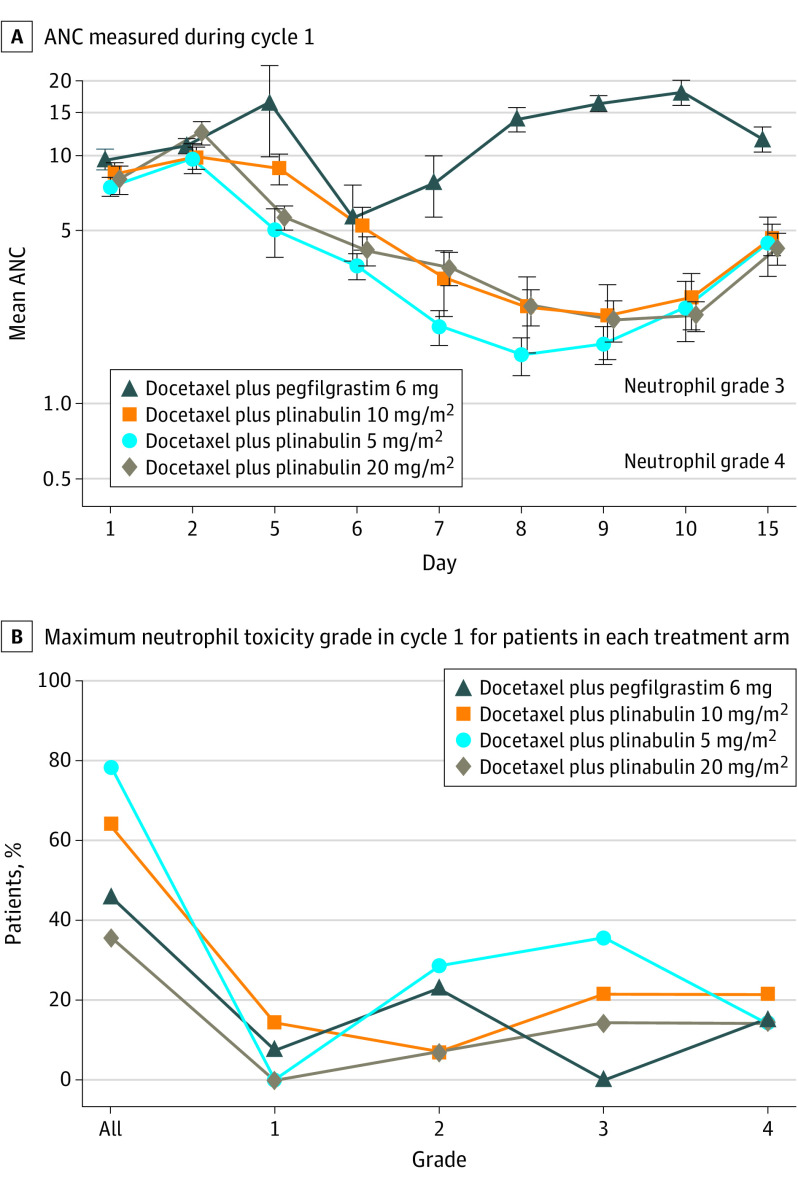
The ANC recovery curve for each treatment arm is shown in Figure 2A. With pegfilgrastim, DSN was 0.15, and with plinabulin 20 mg/m2, DSN was 0.36, which is within the prespecified noninferiority margin of 0.65. There was no difference in DSN between plinabulin 20 mg/m2 and pegfilgrastim (P = .755; Table). The plinabulin dose-response effect on neutropenia is shown in Figure 2B. With each escalation of the plinabulin dose, the incidence of any grade of neutropenia decreased. The highest plinabulin dose tested (20 mg/m2) had a numerically lower frequency of grade 4 neutropenia compared with pegfilgrastim (P = .460; eTable 3 in Supplement 2).
Figure 2. Absolute Neutrophil Counts and Neutrophil Toxicity Grade in Cycle 1.
A, Absolute neutrophil count (ANC) during cycle 1 of chemotherapy expressed on a logarithmic scale to better visualize the values at low ANC levels. Lower solid line is ANC grade 4 toxicity (0.5 cells × 10−6 /L), and upper solid line is ANC grade 3 toxicity (1.0 × 10−6 cells /L). B, Percentage of treated patients plotted against maximum neutrophil toxicity grade during treatment cycle 1.
Table. Days of Severe Neutropenia (DSN) in Cycle 1 for Patients Given Docetaxel and Either Pegfilgrastim or Plinabulin.
| DSN parameter | Docetaxel 75 mg/m2 plus pegfilgrastim 6 mg (n = 13)a | Docetaxel 75 mg/m2 plus plinabulin 20 mg/m2 (n = 14) | P value |
|---|---|---|---|
| Mean (SD), d | 0.15 (0.38) | 0.36 (0.93) | .76 |
| Median (range), d | 0 (0-1) | 0 (0-3) |
One patient randomized to the pegfilgrastim treatment arm was erroneously given plinabulin 20 mg/m2 throughout the study.
Pharmacokinetics
Plinabulin 5, 10, or 20 mg/m2 had no effect on ABMP (eFigures 1-6 in Supplement 2). Plinabulin had a negligible effect on Fridericia-corrected QT interval (eFigure 7 in Supplement 2). The plinabulin 20 mg/m2 dose was most effective in minimizing DSN and maximizing the ANC area above the curve, with the fewest adverse effects (eFigure 8 in Supplement 2). The plinabulin 40-mg fixed dose performed similarly as the plinabulin 20-mg/m2 dose (eFigure 9 in Supplement 2).
Safety
Adverse Events
Plinabulin was well tolerated at each dose level. Nonneutrophil hematologic toxic effects were similar among the 4 treatment arms (eTable 4 in Supplement 2). Other nonhematologic toxic effects with use of plinabulin 20 mg/m2 included alopecia (n = 4), diarrhea (n = 3), and bone pain (n = 1), none of which were grade 3 or 4 (eTable 4 in Supplement 2 and a full listing of all events in eTable 5 in Supplement 2). Three patients were withdrawn from the study with treatment-related adverse events. In the plinabulin 20 mg/m2 treatment arm, one patient was withdrawn following an event of weakness, dehydration, hypotension, neutropenia, septic shock, and vomiting, and one patient was withdrawn following a febrile neutropenic event. One patient in the plinabulin 5 mg/m2 treatment arm was withdrawn after having pneumonia.
Overall, 8 patients (2 in each treatment arm) had a total of 11 treatment-emergent serious adverse events (SAEs), though no single event was reported as serious in more than 1 patient and all are consistent with chemotherapy effect. The number of SAEs was slightly higher in the plinabulin 20 mg/m2 arm (5 SAEs: asthenia, dehydration, septic shock, and vomiting in 1 patient, and febrile neutropenia in another patient), compared with the pegfilgrastim (2 SAEs) and the plinabulin 5 mg/m2 (2 SAEs) and 10 mg/m2 (2 SAEs) treatment arms. One patient death occurred in each of the treatment arms in patients with refractory lung cancer. The investigator determined deaths were caused by respiratory failure onset in day 10 of cycle 1 with pegfilgrastim, septic shock onset in day 4 of cycle 1 with plinabulin 20 mg/m2, hemoptysis onset inday 9 of cycle 1 with plinabulin 10 mg/m2, and pneumonia onset in day 8 of cycle 2 with plinabulin 5 mg/m2. No deaths were considered by the investigators to be related to the study drug.
Blood Pressure
No detectable changes in every 15-minute BP measurements occurred among the 3 plinabulin treatment arms during day 1 of cycle 1. The median systolic and diastolic BP and heart rate (as the change from baseline) were similar for the plinabulin 20 mg/m2 and the pegfilgrastim 6 mg treatment arm (eFigures 1, 2, and 3 in Supplement 2). As pegfilgrastim was given on day 2, the BP taken on day 1 with pegfilgrastim therapy serves as a no-treatment or placebo control. Pretreatment BP on day 1 and day 8 and BP and heart rate at day 15 were also not different throughout all 4 cycles among the treatment arms (eFigures 4, 5, and 6 in Supplement 2).
Hospitalizations
Hospitalizations rates (all cause) among the 3 plinabulin arms and the pegfilgrastim arm were similar across all 4 treatment cycles (eTable 7 in Supplement 2).
Exploratory Efficacy End Points
Infections
The incidence of infections among the 3 plinabulin arms was similar throughout the study. The incidence of infections was similar for the plinabulin 20 mg/m2 and the pegfilgrastim 6 mg treatment arm (eTable 6 in Supplement 2).
Quality of Life, Bone Pain, and Thrombocytopenia
Fifty-five patients had evaluable quality of life information. Plinabulin 20 mg/m2 showed a significant improvement in global health status (P < .001) vs pegfilgrastim (Figure 3A). When compared with their baseline state, patients treated with plinabulin 20 mg/m2 significantly benefited in fatigue (P = .032; eFigure 10 in Supplement 2), pain (P = .027; eFigure 11 in Supplement 2), and insomnia (P = .05; eFigure 12 in Supplement 2), compared with the symptomatic deterioration in patients treated with pegfilgrastim.
Figure 3. Quality of Life, Bone Pain, and Thrombopenia.
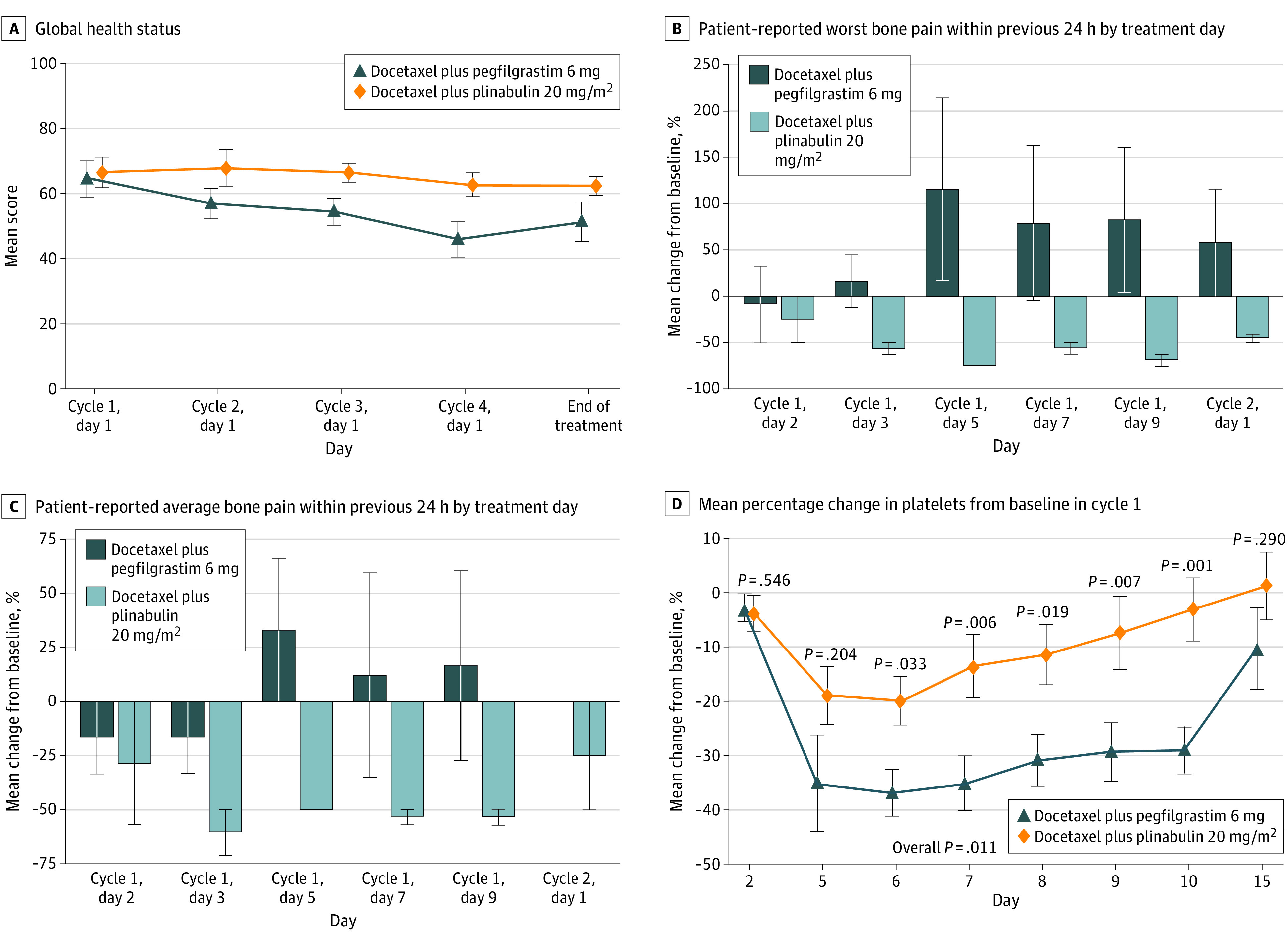
A, Patients’ measured global health status per treatment cycle. Higher score is better. B, Patient response to the measure of bone pain at its worst in the past 24 hours each day during cycle 1 expressed as a percentage change from baseline pain using the Brief Pain Inventory Short Form. Error bars indicate 95% CIs; a negative score indicates less pain. C, Patient response to the measure of bone pain on average in the past 24 hours each day during cycle 1 expressed as a percentage change from baseline pain using the Brief Pain Inventory Short Form. Error bars indicate 95% CIs; a negative score indicates less pain. D, Platelet counts by day during treatment cycle 1 expressed as percentage change from baseline. P values were based on the mixed-model repeated measures method with the terms of treatment, baseline value, postbaseline visit, and treatment by postbaseline visit interactions. The covariance is assumed to be compound symmetry
During the first chemotherapy cycle, 41 patients had evaluable Brief Pain Inventory Short Form information. For the patient-reported outcome of worst bone pain in the past 24 hours, patients in the plinabulin 20 mg/m2 arm reported minimal to no bone pain. In contrast, patients in the pegfilgrastim 6 mg arm reported pain from day 3, which peaked on day 7 (90% change) before declining (Figure 3B). For the patient-reported outcome of bone pain on average, patients in the plinabulin 20 mg/m2 arm demonstrated an 8.3% increase in pain on day 5, which resolved to a slight improvement (negative change in pain). Patients in the pegfilgrastim 6 mg arm demonstrated an increase in pain from day 3, which peaked on day 7 (64.1%) and decreased thereafter (Figure 3C). Platelet counts (as a change from baseline) were significantly decreased with pegfilgrastim but not with plinabulin (Figure 3D). No patients treated with plinabulin had thrombocytopenia of any grade, but 35% of patients treated with pegfilgrastim had at least grade 1 thrombocytopenia.
Discussion
Recommended Plinabulin Dose for Phase 3 Study
Plinabulin 20 mg/m2, starting 30 minutes after completion of docetaxel chemotherapy, is the recommended phase 3 dose for testing in the supportive care setting. We base this conclusion on the study end point achieved at the plinabulin 20 mg/m2 dose within the prespecified noninferiority margin, the dose-response relationship of neutropenia seen with the 5, 10, and 20 mg/m2 doses, and the absence of hypertension and gastrointestinal toxic effects at the 20 mg/m2 dose (these toxic effects are seen at the 30 mg/m2 day 1 and day 8 dosing used in the anticancer program).24 Other adverse events and toxic effects were similar across the treatment arms and are largely attributable to the docetaxel chemotherapy (eTable 4 in Supplement 2).
The PK/PD analysis confirms the recommended phase 3 dose and also shows that a plinabulin 40-mg fixed-dose is equivalent to the plinabulin 20-mg/m2 dose. The equivalence was arrived at through the use of a semiphysiological population docetaxel-plinabulin combination treatment exposure–efficacy model. The model was developed to characterize plinabulin-induced prevention of docetaxel-induced neutropenia, with simulation of a virtual population of 2800 patients who were administered different fixed doses (20, 40, 60, or 80 mg) of plinabulin infused 30 minutes after a 1-hour infusion of docetaxel 75 mg/m2, and compared with the performance of the 20 mg/m2 body surface area–based dose of plinabulin (eFigure 10 in Supplement 2).
Exploratory End Points: Bone Pain, Patient-Reported Outcomes, and Thrombocytopenia
Use of pegfilgrastim was associated with more bone pain and more reduction in self-reported health outcomes than use of plinabulin. Bone pain is a noteworthy toxic effect associated with pegfilgrastim.25,26 In patients who had no bone pain at study entry, there was no bone pain after day 3 in the plinabulin 20 mg/m2 arm, while 35% of patients in the pegfilgrastim arm reported bone pain. Patients in all plinabulin arms also experienced less thrombocytopenia than patients receiving pegfilgrastim, with no patient experiencing any grade of thrombocytopenia. Patients treated with docetaxel and plinabulin 20 mg/m2 reported a lower reduction in patient-reported health outcomes compared with patients treated with docetaxel and pegfilgrastim.
Clinical Confirmation of the Mechanism of Action Difference Between Plinabulin and Pegfilgrastim Observed in Preclinical Models
With pegfilgrastim, the ANC nadir occurred in the first week of the cycle on day 6, and the ANC nadir was deep and the recovery duration narrow (Figure 2A). With plinabulin, the ANC nadir occurred in the second week on day 9, and the nadir was shallow and the recovery curve was broad. These differences are consistent with preclinical observations of a different mechanism of action for plinabulin compared with the G-CSF–derived therapies pegfilgrastim and filgrastim.11,14 Granulocyte colony-stimulating factor demarginates mature neutrophils and accelerates maturation and proliferation of neutrophil precursors. In contrast, plinabulin does not influence the time course of circulating neutrophil recovery after docetaxel chemotherapy. We postulate, based on the shape of the clinical neutrophil recovery curves and the in vitro data, that protection of hematopoetic stem cells from docetaxel-induced damage explains plinabulin’s neutrophil protective effects.
Limitations
The sampling duration of postchemotherapy ANC and complete blood count was limited for patient convenience and may have missed events later in the chemotherapy cycle. We are reassured by our observation that both plinabulin 20 mg/m2 and pegfilgrastim were equally effective against grade 4 neutropenia frequency, and all patients had ANC kinetics that trended toward recovery at the last ANC measurement on day 15 of cycle 1.
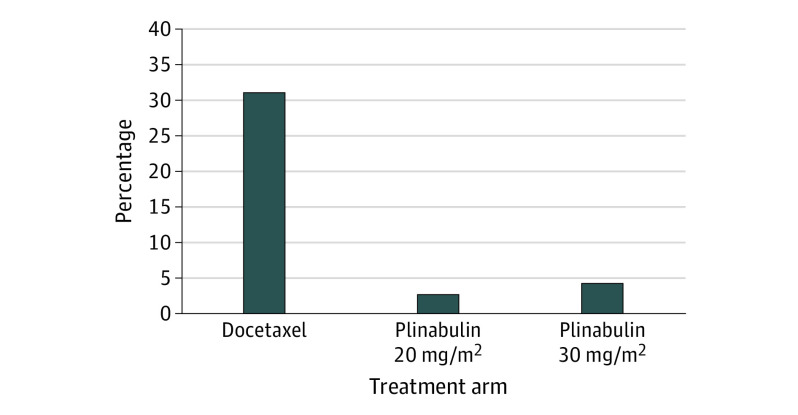
Although only 14 patients were treated in this trial with the recommended phase 3 dose of plinabulin 20 mg/m2, there is more clinical information with this dose. In a previously conducted phase 2 study of docetaxel for patients with NSCLC, 40 patients were treated with the plinabulin 20 mg/m2 dose.13 Day 8 grade 4 neutropenia developed in 2.6% of patients treated with plinabulin in contrast with 31% of patients treated with docetaxel alone (Figure 4). In regard to safety, 23% of the patients treated with docetaxel and plinabulin had transient hypertension of any grade, and 2 patients had grade 3 or 4 transient hypertension. With docetaxel and plinabulin 30 mg/m2, all toxic effects were more frequent. In addition, in a NSCLC therapeutic study with higher dose plinabulin (docetaxel and plinabulin 30 mg/m2 at day 1 and 8), transient hypertension occurred more often.24 Thus, the combined 64 patients from these studies provides reassurance that plinabulin 20 mg/m2 is a dose that balances efficacy with safety, and is the correct recommended phase 3 dose for the CIN indication.
Figure 4. Neutrophil Toxicity in Previous Phase 2 Study of Docetaxel for Patients With Non–Small Cell Lung Cancer.
Neutrophil toxicity in a previous study conducted among patients with non–small cell lung cancer who were treated with docetaxel 75 mg/m2 on day 1. Incidence of grade 4 neutropenia (absolute neutrophil count <0.5 × 109 cells/L) on day 8 in the 40 patients treated with plinabulin 20 mg/m2 (2.6%) or the 55 patients treated with plinabulin 30 mg/m2 (4.3%) was compared with 31% in 50 patients treated with docetaxel without plinabulin. Both plinabulin doses reduced grade 4 neutropenia compared with patients treated with docetaxel alone.
Conclusions
Plinabulin 40 mg as a fixed day 1 dose will be tested in the phase 3 portion of this trial compared with pegfilgrastim alone, and the primary efficacy end point will be noninferior DSN. Secondary phase 3 end points will include bone pain reduction, thrombocytopenia reduction, and maintenance or improvement in quality of life. If successful, we hope to demonstrate that plinabulin has efficacy comparable with pegfilgrastim for CIN prevention but has more convenient dosing, superior safety and quality of life, has less associated bone pain, and less associated thrombocytopenia. A separate anticancer program is investigating plinabulin’s anticancer efficacy and should address concerns that plinabulin may have a detrimental effect on chemotherapy efficacy. Single-agent plinabulin, given the same day as chemotherapy, may fill the need for primary FN prophylaxis in patients receiving chemotherapy regimens with 10% to 20% FN risk, for which current guidelines do not recommend primary G-CSF prophylaxis.
Trial Protocol.
eTable 1 Demographics and Baseline Characteristics
eTable 2 Docetaxel Dose Intensity per treatment arm
eTable 3 Days of Grade 4 Neutropenia - Cycle 1
eTable 4 Number of Subjects with Most Common (≥ 20%) Treatment-Emergent Adverse Events (TEAE) -Safety Population
eTable 5 Number (%) of Patients with Treatment-Emergent Adverse Events Occurring in >1 Patient by System Organ Class and Preferred Term in any Treatment Arm
eTable 6 Number (%) of Patients with Treatment related adverse events – Infection
eTable 7 Hospitalization Rates – All Cause per Cycle
eFigure 1 Mean Change in Systolic Blood Pressure from Baseline -Cycle 1 Day 1
eFigure 2 Mean Change in Diastolic Blood Pressure from Baseline -Cycle 1 Day 1
eFigure 3 Mean Change in Heart Rate from Baseline: Cycle 1 Day
eFigure 4 Mean (+/- standard error) Systolic Blood Pressure (mmHg) by Time and Treatment (Safety Population)
eFigure 5 Mean (+/- standard error) Diastolic Blood Pressure (mmHg) by Time and Treatment (Safety Population)
eFigure 6 Mean (+/- standard error) Heart Rate (beats/minute) by Time and Treatment (Safety Population)
eFigure 7 Plinabulin had a negligible effect on QTcF (Fridericia-corrected QT interval)
eFigure 8 Predictive Performance Plot for the Docetaxel and Plinabulin Combination Model
eFigure 9 Relationship between plinabulin Area under the Curve (AUC) concentration and patient Weight or Body Surface Area (BSA) for a plinabulin fixed dose of 40 mg
eFigure 10 Fatigue
eFigure 11 Pain
eFigure 12 Insomnia
Data Sharing Statement.
References
- 1.Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328(18):1323-1332. doi: 10.1056/NEJM199305063281808 [DOI] [PubMed] [Google Scholar]
- 2.Crawford J, Ozer H, Stoller R, et al. . Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164-170. doi: 10.1056/NEJM199107183250305 [DOI] [PubMed] [Google Scholar]
- 3.Holmes FA, Jones SE, O’Shaughnessy J, et al. . Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13(6):903-909. doi: 10.1093/annonc/mdf130 [DOI] [PubMed] [Google Scholar]
- 4.Blackwell K, Semiglazov V, Krasnozhon D, et al. . Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26(9):1948-1953. doi: 10.1093/annonc/mdv281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartzberg LS, Lal LS, Balu S, et al. . Incidence of febrile neutropenia during chemotherapy among patients with nonmyeloid cancer receiving filgrastim vs a filgrastim biosimilar. Clinicoecon Outcomes Res. 2018;10:493-500. doi: 10.2147/CEOR.S168298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waller CF, Tiessen RG, Lawrence TE, et al. . A pharmacokinetics and pharmacodynamics equivalence trial of the proposed pegfilgrastim biosimilar, MYL-1401H, versus reference pegfilgrastim. J Cancer Res Clin Oncol. 2018;144(6):1087-1095. doi: 10.1007/s00432-018-2643-3 [DOI] [PubMed] [Google Scholar]
- 7.Glaspy JA, O’Connor PG, Tang H, Finck B. Randomized, single-blind, crossover study to assess the pharmacokinetic and pharmacodynamic bioequivalence of CHS-1701 to pegfilgrastim in healthy subjects. J Clin Oncol. 2017;35(15)(suppl):21693. doi: 10.1200/JCO.2017.35.15_suppl.e21693 [DOI] [Google Scholar]
- 8.Crawford J, Althaus B, Armitage J, et al. ; National Comprehensive Cancer Network . Myeloid growth factors clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3(4):540-555. doi: 10.6004/jnccn.2005.0030 [DOI] [PubMed] [Google Scholar]
- 9.Kubista E, Glaspy J, Holmes FA, Green MD, Hackett J, Neumann T; Pegfilgrastim Study Group . Bone pain associated with once-per-cycle pegfilgrastim is similar to daily filgrastim in patients with breast cancer. Clin Breast Cancer. 2003;3(6):391-398. doi: 10.3816/CBC.2003.n.003 [DOI] [PubMed] [Google Scholar]
- 10.Moore DC, Pellegrino AE. Pegfilgrastim-induced bone pain: a review on incidence, risk factors, and evidence-based management. Ann Pharmacother. 2017;51(9):797-803. doi: 10.1177/1060028017706373 [DOI] [PubMed] [Google Scholar]
- 11.La Sala G, Olieric N, Sharma A, et al. . Structure, thermodynamics, and kinetics of plinabulin binding to two tubulin isotypes. Chem. 2019;5(11):2969-2986. doi: 10.1016/j.chempr.2019.08.022 [DOI] [Google Scholar]
- 12.Mita MM, Spear MA, Yee LK, et al. . Phase 1 first-in-human trial of the vascular disrupting agent plinabulin(NPI-2358) in patients with solid tumors or lymphomas. Clin Cancer Res. 2010;16(23):5892-5899. doi: 10.1158/1078-0432.CCR-10-1096 [DOI] [PubMed] [Google Scholar]
- 13.Millward M, Mainwaring P, Mita A, et al. . Phase 1 study of the novel vascular disrupting agent plinabulin (NPI-2358) and docetaxel. Invest New Drugs. 2012;30(3):1065-1073. doi: 10.1007/s10637-011-9642-4 [DOI] [PubMed] [Google Scholar]
- 14.Tonra JR, Lloyd GK, Mohanlal R, Huang L. Plinabulin ameliorates neutropenia induced by multiple chemotherapies through a mechanism distinct from G-CSF therapies. Cancer Chemother Pharmacol. 2020;85(2):461-468. doi: 10.1007/s00280-019-03998-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford J, Becker PS, Armitage JO, et al. . Myeloid growth factors, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(12):1520-1541. doi: 10.6004/jnccn.2017.0175 [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138. [PubMed] [Google Scholar]
- 17.Cleeland CS. The Brief Pain Inventory User Guide. MD Anderson Cancer Center; 2009. [Google Scholar]
- 18.Bedard G, Zeng L, Zhang L, et al. . Minimal important differences in the EORTC QLQ-C30 in patients with advanced cancer. Asia Pac J Clin Oncol. 2014;10(2):109-117. doi: 10.1111/ajco.12070 [DOI] [PubMed] [Google Scholar]
- 19.Phillips R, Gandhi M, Cheung YB, et al. . Summary scores captured changes in subjects’ QoL as measured by the multiple scales of the EORTC QLQ-C30. J Clin Epidemiol. 2015;68(8):895-902. doi: 10.1016/j.jclinepi.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 20.EORTC Quality of Life Group EORTC QLQ-C30 Reference Values and Bibliography. EORTC Quality of Life Unit; 2002. [Google Scholar]
- 21.Friberg LE, Karlsson MO. Mechanistic models for myelosuppression. Invest New Drugs. 2003;21(2):183-194. doi: 10.1023/A:1023573429626 [DOI] [PubMed] [Google Scholar]
- 22.Deming WE. Opportunities in mathematical statistics, with special reference to sampling and quality control. Science. 1943;97(2514):209-214. doi: 10.1126/science.97.2514.209 [DOI] [PubMed] [Google Scholar]
- 23.Alexopoulos K, Kouroussis C, Androulakis N, et al. . Docetaxel and granulocyte colony-stimulating factor in patients with advanced non-small-cell lung cancer previously treated with platinum-based chemotherapy: a multicenter phase II trial. Cancer Chemother Pharmacol. 1999;43(3):257-262. doi: 10.1007/s002800050892 [DOI] [PubMed] [Google Scholar]
- 24.Mohanlal R, Sun Y, Kloecker G, et al. . P2.01-23 DUBLIN-3, a phase (Ph) III trial comparing the plinabulin (P)/docetaxel(D) combination with docetaxel alone in stage IIIb/IV NSCLC. J Thorac Oncol. 2019;14(10):S647-S8. doi: 10.1016/j.jtho.2019.08.1367 [DOI] [Google Scholar]
- 25.Pawloski PA, Larsen M, Thoresen A, Giordana MD. Pegfilgrastim use and bone pain: a cohort study of community-based cancer patients. J Oncol Pharm Pract. 2016;22(3):423-429. doi: 10.1177/1078155215585188 [DOI] [PubMed] [Google Scholar]
- 26.Lambertini M, Del Mastro L, Bellodi A, Pronzato P. The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs). Crit Rev Oncol Hematol. 2014;89(1):112-128. doi: 10.1016/j.critrevonc.2013.08.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1 Demographics and Baseline Characteristics
eTable 2 Docetaxel Dose Intensity per treatment arm
eTable 3 Days of Grade 4 Neutropenia - Cycle 1
eTable 4 Number of Subjects with Most Common (≥ 20%) Treatment-Emergent Adverse Events (TEAE) -Safety Population
eTable 5 Number (%) of Patients with Treatment-Emergent Adverse Events Occurring in >1 Patient by System Organ Class and Preferred Term in any Treatment Arm
eTable 6 Number (%) of Patients with Treatment related adverse events – Infection
eTable 7 Hospitalization Rates – All Cause per Cycle
eFigure 1 Mean Change in Systolic Blood Pressure from Baseline -Cycle 1 Day 1
eFigure 2 Mean Change in Diastolic Blood Pressure from Baseline -Cycle 1 Day 1
eFigure 3 Mean Change in Heart Rate from Baseline: Cycle 1 Day
eFigure 4 Mean (+/- standard error) Systolic Blood Pressure (mmHg) by Time and Treatment (Safety Population)
eFigure 5 Mean (+/- standard error) Diastolic Blood Pressure (mmHg) by Time and Treatment (Safety Population)
eFigure 6 Mean (+/- standard error) Heart Rate (beats/minute) by Time and Treatment (Safety Population)
eFigure 7 Plinabulin had a negligible effect on QTcF (Fridericia-corrected QT interval)
eFigure 8 Predictive Performance Plot for the Docetaxel and Plinabulin Combination Model
eFigure 9 Relationship between plinabulin Area under the Curve (AUC) concentration and patient Weight or Body Surface Area (BSA) for a plinabulin fixed dose of 40 mg
eFigure 10 Fatigue
eFigure 11 Pain
eFigure 12 Insomnia
Data Sharing Statement.