Abstract
Background
New York City was among the epicenters during the COVID-19 pandemic. Oncologists must balance plausible risks of COVID-19 infection with the recognized consequences of delaying cancer treatment, keeping in mind the capacity of the health care system. We sought to investigate treatment patterns in gynecologic cancer care during the first two months of the COVID-19 pandemic at three affiliated New York City hospitals located in Brooklyn, Manhattan and Queens.
Methods
A prospective registry of patients with active or presumed gynecologic cancers receiving inpatient and/or outpatient care at three affiliated New York City hospitals was maintained between March 1 and April 30, 2020. Clinical and demographic data were abstracted from the electronic medical record with a focus on oncologic treatment. Multivariable logistic regression analysis was explored to evaluate the independent effect of hospital location, race, age, medical comorbidities, cancer status and COVID-19 status on treatment modifications.
Results
Among 302 patients with gynecologic cancer, 117 (38.7%) experienced a COVID-19-related treatment modification (delay, change or cancellation) during the first two months of the pandemic in New York. Sixty-four patients (67.4% of those scheduled for surgery) had a COVID-19-related modification in their surgical plan, 45 (21.5% of those scheduled for systemic treatment) a modification in systemic treatment and 12 (18.8% of those scheduled for radiation) a modification in radiation. Nineteen patients (6.3%) had positive COVID-19 testing. On univariate analysis, hospital location in Queens or Brooklyn, age ≤65 years, treatment for a new cancer diagnosis versus recurrence and COVID-19 positivity were associated with treatment modifications. On multivariable logistic regression analysis, hospital location in Queens and COVID-19 positive testing were independently associated with treatment modifications.
Conclusions
More than one third of patients with gynecologic cancer at three affiliated New York City hospitals experienced a treatment delay, change or cancellation during the first two months of the COVID-19 pandemic. Among the three New York City boroughs represented in this study, likelihood of gynecologic oncology treatment modifications correlated with the case burden of COVID-19.
1. Introduction
The COVID-19 pandemic has resulted in unprecedented challenges for the oncology community. The American Cancer Society estimates that nearly 5000 new cases of cancer will be diagnosed per day in the United States, however, the preferred approach to cancer care during the pandemic remains uncertain [1]. Early reports suggest that patients with cancer may be at increased risk for COVID-19 infection and may have more severe COVID-19 clinical events including admission to intensive care units, requirement for invasive ventilation and death [[2], [3], [4], [5], [6]]. A recent study at a New York City Hospital found a case fatality rate of 25% for patients with COVID-19 and solid tumors [5]. However, such studies are limited by small sample size, heterogeneous cancer types and several possible confounding variables [7]. Oncology providers face difficult decisions, 1) balancing plausible risks of COVID-19 infection for cancer patients with the recognized consequences of not treating cancer in an effective or timely manner, 2) mitigating the risks for significant care disruptions associated with social distancing behaviors, and 3) managing appropriate allocation of limited health care resources for both COVID-19 and cancer patients [5].
To flatten the growth curve of the COVID-19 pandemic, physicians have postponed or cancelled non-acute visits and procedures and transitioned millions of visits to telehealth. In New York City, elective procedures were prohibited beginning on March 22, 2020. Survivorship and surveillance visits for asymptomatic cancer patients are amenable to a telehealth platform. However, patients with active cancer requiring cancer-directed treatment require in-person physician evaluations and treatment [8]. Established frameworks to inform decisions about how to adapt cancer treatment during the COVID-19 pandemic based on patient and cancer-specific factors do not yet exist and providers are relying on evolving medical society guidelines [[9], [10], [11], [12], [13]]. For patients with active cancer, the physician and patient together must balance a delay in cancer treatment against the risk of potential COVID-19 exposure, keeping in mind the capacity of the local health care system to meet existing and projected needs [8]. This is especially challenging for gynecologic cancers because, although gynecologic oncologists have expertise in multi-modality cancer management, published literature regarding outcomes associated with surgical delays and extended neoadjuvant chemotherapy are retrospective, underpowered or single institution analyses with inconsistent results [[14], [15], [16]]. As New York City was among the early epicenters during the COVID-19 pandemic, we sought to investigate practice patterns in gynecologic cancer care during the first two months of the COVID-19 pandemic at affiliated New York City hospitals in three boroughs.
2. Methods
A prospective registry of all patients with active or presumed gynecologic cancer was maintained at three affiliated New York City Hospitals beginning March 1, 2020 through April 30, 2020. Institutional review board approval was obtained from each participating hospital. The hospitals included an 862-bed quaternary referral academic medical center in Manhattan (NewYork-Presbyterian Hospital - Weill Cornell Medical Center), a 651-bed tertiary care academic medical center in Brooklyn (NewYork-Presbyterian Brooklyn Methodist Hospital) and a 550-bed tertiary care academic medical center in Queens (NewYork-Presbyterian Queens). Patients were included if they were followed by the gynecologic oncology team (inpatient and/or outpatient) and had a diagnosis of an active cancer or presumed gynecologic cancer based on a suspicious clinical evaluation. Patients in remission were not included. Data for registry patients were manually abstracted from electronic health records with the use of a quality-controlled protocol and structured abstraction. De-identified data were maintained in a secure web platform supported by the participating institutions. Abstractors were clinicians and medical students trained to abstract standard data elements into a secure institutional data repository. Training was performed in several sessions, led by a single investigator (MF). Calibration was reinforced through daily meetings and ongoing collaboration through a remote shared communication platform. Abstracted data included patient demographics, clinical characteristics, comorbid conditions, COVID-19 symptoms and testing, oncologic history, planned oncology treatments and completed oncology treatments.
COVID-19-related modifications in cancer care were defined as cancellations of planned treatment, changes in planned treatment or delays of greater than 14 days due to the COVID-19 pandemic. This included patients with and without symptoms of COVID-19 and/or a diagnosis of COVID-19 as we aimed to capture the effect of the pandemic on all of gynecologic cancer care at participating sites. Data on COVID-19 testing was abstracted from the patients' electronic health records and cross-referenced with the institution's COVID-19 research data repository, which maintains all electronic COVID-19-related patient data. COVID-19 testing strategies were standardized across three hospitals, however, changes occurred over the course of data collection. In early March, COVID-19 testing was limited and only performed for symptomatic patients in the hospital at the discretion of emergency department and hospital physicians. As testing became more available, COVID-19 testing was offered at outpatient clinics for symptomatic patients and for all patients undergoing surgical procedures. The method of COVID-19 testing was standardized across the three hospitals with nasopharyngeal polymerase chain reaction (PCR) swab testing.
2.1. Statistical Analysis
The distribution of continuous variables was tested for normality via the Shapiro-Wilk normality test. Univariate tests were applied based on whether the variable of interest was distributed normally (i.e., t-test, analysis of variance) or not normally (i.e., Mann–Whitney U test, Kruskal-Wallis test). Associations between categorical variables were evaluated using the chi-square test or Fisher's exact tests, as appropriate for category size. Multivariable logistic regression analysis was explored to evaluate the independent effect of hospital location, race, age, medical comorbidities, cancer status (new diagnosis vs. recurrence), and COVID-19 status, on treatment modifications (i.e., binary outcome variable) during the study period. Ethnicity was not included in the multivariable model as it was collinear with race. Statistical significance was evaluated at the 0.05 alpha level, and two-sided 95% confidence intervals were calculated to assess the precision of all obtained estimates. Data were analyzed using SPSS Statistical software Version 20.0 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp) and R Version 3.6.1(R Foundation for Statistical Computing, Vienna, Austria, 2019).
3. Results
Three hundred and two patients with active or presumed gynecologic cancer were followed at three affiliated academic New York City Hospitals during the study period (Manhattan – 118, 39.1%, Queens – 75, 24.8%, Brooklyn – 109, 36.1%). The median patient age was 64.7 years (range 27.8–90.0). The distribution of patient race was as follows: White/Caucasian 117, 38.7%, Black/African American 88, 29.1%, Asian/Asian American 56, 18.5%, American Indian/Alaska Native 1, 0.3%, Native Hawaiian/Pacific Islander 1, 0.3%, other 10, 3.3% and unknown 29, 9.6%. Twenty-nine patients (9.6%) were Hispanic/Latino (Table 1 ).
Table 1.
Patient demographics at three affiliated New York City hospitals.
| Total (302) |
Brooklyn (109, 36.1%) |
Manhattan (118, 39.1%) |
Queens (75, 24.8%) |
P Value |
|
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Age (median, range) | 64.67 (27.80–89.97) | 67.94 (30.97–84.85) | 66.55 (27.80–89.97) | 58.85 (34.72–89.89) | <0.001 |
| Race | <0.001 | ||||
| American Indian/Alaska Native | 1 (0.3%) | 1 (0.9%) | 0 (0%) | 0 (0%) | |
| Asian/Asian American | 56 (18.5%) | 5 (4.6%) | 15 (12.7%) | 36 (48.0%) | |
| Black/African American | 88 (29.1%) | 62 (56.9%) | 17 (14.4%) | 9 (12.0%) | |
| Native Hawaiian/Pacific Islander | 1 (0.3%) | 0 (0%) | 1 (0.8%) | 0 (0%) | |
| White/Caucasian | 117 (38.7%) | 38 (34.9%) | 67 (56.8%) | 13 (17.3%) | |
| Other | 10 (3.3%) | 1 (0.9%) | 8 (6.8%) | 0 (0%) | |
| Unknown | 29 (9.6%) | 2 (1.8%) | 10 (8.5%) | 17 (22.7%) | |
| Ethnicity | 0.004 | ||||
| Hispanic/Latino | 29 (9.6%) | 4 (3.7%) | 14 (11.9%) | 11 (14.7%) | |
| Not Hispanic/Latino | 235 (77.8%) | 100 (91.7%) | 93 (78.8%) | 42 (56.0%) | |
| Unknown | 38 (12.6%) | 5 (4.6%) | 11 (9.3%) | 22 (29.3%) | |
| Primary cancer | 0.077 | ||||
| Uterus | 122 (40.4%) | 57 (52.3%) | 40 (33.9%) | 25 (33.3%) | |
| Ovary | 123 (40.7%) | 33 (30.3%) | 58 (49.2%) | 32 (42.7%) | |
| Cervix | 37 (12.3%) | 12 (11.0%) | 12 (10.2%) | 13 (17.3%) | |
| Vulva | 14 (4.6%) | 5 (4.6%) | 4 (3.4%) | 5 (6.7%) | |
| Vagina | 3 (1.0%) | 1 (0.9%) | 2 (1.7%) | 0 (0%) | |
| Gastrointestinal primary | 3 (1.0%) | 1 (0.9%) | 2 (1.7%) | 0 (0%) | |
| New diagnosis of cancer | 199 (65.9%) | 66 (60.6%) | 68 (57.6%) | 65 (86.7%) | <0.001 |
| Medical comorbidities | 228 (75.5%) | 92 (84.4%) | 85 (72.0%) | 51 (68.0%) | 0.021 |
| COVID-19 Positive | 19 (6.3%) | 7 (6.4%) | 8 (6.8%) | 4 (5.3%) | 0.955 |
Among our cohort of patients, the primary cancer sites included ovary (123, 40.7%), uterus (122, 40.4%), cervix (37, 12.3%), vulva (14, 4.6%) and vagina (3, 1.0%). Three patients (1.0%) had surgery for a suspicious pelvic mass with final pathologic exam demonstrating a gastrointestinal primary malignancy. One hundred and ninety-nine patients (65.9%) were undergoing treatment for a newly diagnosed gynecologic malignancy and 103 (34.1%) were undergoing treatment for recurrent disease. Ninety-five patients (31.5%) were scheduled for surgery. Sixty-four patients (21.2%) were scheduled for radiation. Two hundred and nine patients (69.2%) were scheduled for systemic treatment. Among those scheduled for systemic treatment, 117 were scheduled for intravenous chemotherapy, 17 for hormonal therapy, 16 for PARP inhibitor treatment, 13 for combination intravenous chemotherapy and anti-angiogenic treatment, 12 for immunotherapy and 34 for other targeted therapies or combinations of intravenous chemotherapy, PARP inhibitor therapy, hormonal therapy, immunotherapy and oral chemotherapy. Among the cohort, 228 patients (75.5%) had a medical comorbidity in addition to their cancer diagnosis including hypertension (159, 52.6%), obesity (100, 33.1%), diabetes (65, 21.5%), pulmonary disease (38, 12.6%), coronary artery disease (15, 5%) and renal disease (6, 2%) (Table 1).
Patient demographics are displayed in Table 1. Forty-nine patients (16.2%) reported at least one symptom of COVID-19 including fever (21, 7%), cough (22, 7.3%), shortness of breath (20, 6.6%), gastrointestinal disturbance (15, 5%), anosmia or aguesia (3, 1%) and sore throat (2, 0.7%). Fifty-seven patients (18.9%) underwent testing for COVID-19 and 19 patients (6.3%) had a positive COVID-19 test. Additionally, one patient undergoing treatment for recurrent ovarian cancer had COVID-19 symptoms (fever, cough, and shortness of breath) and died in her nursing home facility, prior to confirmatory COVID-19 testing. There were no differences in rates of COVID-19 infection among gynecologic cancer patients at the three hospitals. The only patient variable associated with COVID-19 positive testing was the presence of at least one medical comorbidity. Among the 19 patients with COVID-19 positive testing, 18 (94.7%) had a medical comorbidity in addition to the cancer diagnoses versus 210 of the 283 patients (74.2%) without COVID-19 (P = 0.05). Table 2 provides a description of the 19 confirmed COVID-19 cases among our cohort of gynecologic oncology patients.
Table 2.
Patients with COVID-19 positive testing.
| Primary malignancy | Race | Ethnicity | Medical comorbidity | Treatment | COVID-19 symptoms | COVID-19 Course |
|---|---|---|---|---|---|---|
| Cervix | Black/African American | Not Hispanic/Latino | Hypertension | Radiation | Fever, shortness of breath, gastrointestinal symptoms | Mild symptoms |
| Vulva | Black/African American | Not Hispanic/Latino | Diabetes, hypertension, history of tobacco use | Radiation | Fever, cough, shortness of breath, chills | Hospitalized (> 1 month) |
| Uterus | White/Caucasian | Not Hispanic/Latino | Obesity | Intravenous chemotherapy and radiation | Asymptomatic | Asymptomatic |
| Uterus | Black/African American | Not Hispanic/Latino | Diabetes, hypertension, obesity | Surgery | Fever, myalgias | Mild symptoms |
| Uterus | White/Caucasian | Not Hispanic/Latino | Hypertension, obesity | Hormonal therapy | Fever, cough, shortness of breath, gastrointestinal symptoms, fatigue | Deceased due to COVID-19 |
| Ovary | Black/African American | Not Hispanic/Latino | Diabetes, hypertension, obesity | Antiangiogenic therapy | Fever, rhinorrhea | Mild symptoms |
| Uterus | Black/African American | Not Hispanic/Latino | Hypertension, pulmonary disease | Intravenous chemotherapy | Cough, shortness of breath, fatigue | Hospitalized (11 days) |
| Cervix | White/Caucasian | Hispanic/Latino | History of tobacco use | Hormonal therapy | Cough | Mild symptoms |
| Ovary | White/Caucasian | Not Hispanic/Latino | Obesity | PARP inhibitor | Cough, anosmia, sinus pressure, headache | Mild symptoms |
| Uterus | Black/African American | Not Hispanic/Latino | Hypertension, obesity | Intravenous chemotherapy | Cough, gastrointestinal symptoms, chills, myalgias, rhinorrhea | Mild symptoms |
| Ovary | White/Caucasian | Hispanic/Latino | Diabetes, hypertension, obesity | Intravenous chemotherapy | Fever, cough, shortness of breath, gastrointestinal symptoms, weakness | Deceased due to COVID-19 |
| Uterus | Black/African American | Not Hispanic/Latino | Diabetes, hypertension, obesity | Intravenous chemotherapy and immunotherapy | Fever, cough, shortness of breath | Hospitalized (9 days) |
| Ovary | White/Caucasian | Hispanic/Latino | None | Intravenous chemotherapy and antiangiogenic therapy | Shortness of breath, gastrointestinal symptoms, fatigue, myalgias, decreased oral intake | Mild symptoms |
| Ovary | White/Caucasian | Not Hispanic/Latino | History of tobacco use, obesity | Intravenous chemotherapy | Fever, cough, myalgias | Mild symptoms |
| Cervix | Black/African American | Not Hispanic/Latino | Hypertension, pulmonary disease, history of tobacco use | Surgery and radiation | Fever, gastrointestinal symptoms | Mild symptoms |
| Uterus | Black/African American | Not Hispanic/Latino | Hypertension | Surgery | Asymptomatic | Asymptomatic |
| Ovary | Unknown | Unknown | Hypertension | Intravenous chemotherapy | Asymptomatic | Asymptomatic |
| Ovary | Unknown | Unknown | Diabetes, pulmonary disease | Surgery | Fever, gastrointestinal symptoms, anosmia | Mild symptoms |
| Ovary | Asian/Asian American | Not Hispanic/Latino | Diabetes, hypertension | Antiangiogenic therapy | Cough, anosmia | Mild symptoms |
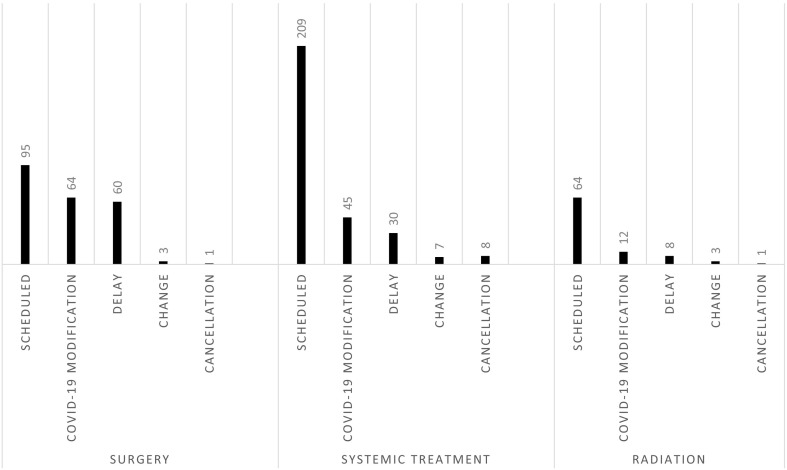
One hundred and seventeen patients (38.7%) experienced a modification in their oncology care due to the COVID-19 pandemic. Sixty-four patients (67.4% of those scheduled for surgery) had a COVID-19-related modification in their surgical plan. Forty-five patients (21.5% of those scheduled for systemic treatment) had a COVID-19-related modification in planned systemic treatment. Twelve patients (18.8% of those scheduled for radiation) had a COVID-19-related modification in planned radiation. This included one patient who had a modification in planned surgery and radiation and three patients with modifications in planned surgery and systemic treatment. For patients scheduled for surgery and radiation the most common treatment modification was delay followed by change in the treatment plan and cancellation was the least common modification. For patients scheduled for systemic treatment, treatment modifications in descending order were delay, cancellation and change (Fig. 1 ).
Fig. 1.
COVID-19-related modifications in surgery, systemic treatment and radiation.
We performed univariate analysis to determine if any patient variables were predictive of treatment modifications during the COVID-19 pandemic. Race, ethnicity, primary cancer site and presence of medical comorbidities were not predictive of treatment modifications. Patients treated at the hospital located in Queens were significantly more likely to have a treatment modification versus patients at Brooklyn and Manhattan and patients at Brooklyn were significantly more likely to have a modification versus patients at Manhattan (Queens – 46, 61.3%, Brooklyn – 42, 38.5%, Manhattan – 29, 24.6%, P < 0.001). Patients 65 years and older were less likely to have treatment delays than younger patients (48, 32.4% vs. 69, 44.8%, P = 0.04). Patients undergoing treatment for a new cancer diagnosis were more likely to undergo treatment modifications compared to patients being treated for recurrent disease (87, 43.7% vs. 30, 29.1%, respectively, P = 0.02). Patients testing positive for COVID-19 were also more likely to undergo treatment modifications than non-COVID-19 positive patients (15, 78.9% vs. 102, 36.0%, respectively, P < 0.001) (Table 3 ).
Table 3.
Univariate analysis evaluating association between clinical variables and cancer-directed treatment modifications.
| Treatment completed as planned (185) |
Treatment modified due to COVID-19 (117) |
P Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Age | 0.037 | ||
| Less than 65 years | 85 (55.2%) | 69 (44.8%) | |
| 65 years or more | 100 (67.6%) | 48 (32.4%) | |
| Hospital location | <0.001 | ||
| Brooklyn | 67 (61.5%) | 42 (38.5%) | |
| Manhattan | 89 (75.4%) | 29 (24.6%) | |
| Queens | 29 (38.7%) | 46 (61.3%) | |
| Race - Black/African American vs. other | 0.111 | ||
| Black/African American | 49 (55.7%) | 39 (44.3%) | |
| Non-Black/African American | 123 (66.5%) | 62 (33.5%) | |
| Unknown | 13 (44.8%) | 16 (55.2%) | |
| Race - Asian/Asian American vs. other | >0.99 | ||
| Asian/Asian American | 35 (62.5%) | 21 (37.5%) | |
| Non-Asian/Asian American | 137 (63.1%) | 80 (36.9%) | |
| Unknown | 13 (44.8%) | 16 (55.2%) | |
| Race - White/Caucasian vs. other | 0.192 | ||
| White/Caucasian | 80 (67.8%) | 38 (32.2%) | |
| Non-White/Caucasian | 92 (59.4%) | 63 (40.6%) | |
| Unknown | 13 (44.8%) | 16 (55.2%) | |
| Ethnicity | 0.106 | ||
| Hispanic or Latino | 14 (48.3%) | 15 (51.7%) | |
| Non-Hispanic or Latino | 154 (65.5%) | 81 (34.5%) | |
| Unknown | 17 (44.7%) | 21 (55.3%) | |
| Medical comorbidity | >0.99 | ||
| Yes | 140 (61.4%) | 88 (38.6%) | |
| No | 45 (60.8%) | 29 (39.2%) | |
| COVID-19 Status | 0.001 | ||
| Negative or not tested | 181 (64.0%) | 102 (36.0%) | |
| Test positive | 4 (21.1%) | 15 (78.9%) | |
| Cancer status | 0.019 | ||
| New diagnosis | 112 (56.3%) | 87 (43.7%) | |
| Recurrent disease | 73 (70.9%) | 30 (29.1%) | |
We performed a multivariable logistic regression analysis to evaluate the independent effect of age, hospital location, race, medical comorbidities, treatment for new vs. recurrent cancer, and COVID-19 status on COVID-19-related treatment modifications. On multivariable analysis, only hospital location and COVID-19 status remained significantly associated with treatment modifications (Table 4 ).
Table 4.
Multivariable logistic regression predicting cancer-directed treatment modification.
| Predictor | Adjusted OR | 95% CI: Low | 95% CI: High | P-Value |
|---|---|---|---|---|
| Age > 64 years | 0.81 | 0.45 | 1.45 | 0.472 |
| Hospital location: Queens vs. Manhattan (referent) | 4.31 | 1.97 | 9.8 | <0.001 |
| Hospital location: Brooklyn vs. Manhattan (referent) | 1.75 | 0.89 | 3.45 | 0.104 |
| Race: Black/African American vs. White/Caucasian (referent) | 1.35 | 0.7 | 2.62 | 0.377 |
| Race: Asian/Asian American/Other vs. White (referent) | 0.64 | 0.29 | 1.38 | 0.267 |
| Comorbidities: Yes vs. No (referent) | 0.88 | 0.46 | 1.69 | 0.692 |
| COVID-19 Status: Positive vs. Negative (referent) | 6.94 | 2.26 | 26.21 | 0.002 |
| Cancer status: New Diagnosis vs. Recurrence (referent) | 1.48 | 0.82 | 2.68 | 0.193 |
4. Discussion
The COVID-19 pandemic has forced the oncology community to rapidly adapt standard of care practices in order to minimize the risk of COVID-19 transmission to cancer patients and provide medical care within the capacity of strained health care systems. National guidelines have recommended postponing surgeries and considering postponing or cancelling nonsurgical cancer treatments based on shared-decision making with a review of the risk to benefit ratio [[9], [10], [11], [12], [13],17]. New York City had the first case of confirmed COVID-19 on March 1, 2020. On March 7th the governor of New York declared a state of emergency and on March 22 cancelled all non-critical elective surgeries. Not surprisingly, many women with gynecologic cancers throughout the country are experiencing significant delays in their cancer care [18]. In our study of hospitals in three New York City boroughs, 39% of patients experienced either a delay, cancellation or change in their planned cancer management due to the COVID-19 pandemic.
Prior studies demonstrate health inequities across the five boroughs of New York City and suggest that the COVID-19 pandemic has disproportionately affected many minority and marginalized populations. [[19], [20], [21]] Early studies of COVID-19 demonstrate higher rates of COVID-19 hospitalizations and deaths in the boroughs with greater representation of racial/ethnic minorities. The boroughs, in order of decreasing COVID-19 hospitalizations and deaths, were the Bronx, Queens, Brooklyn, Staten Island and Manhattan [22,23]. We found significantly increased rates of oncologic care modifications among women treated at the location in Queens followed by Brooklyn compared to Manhattan. This finding is important given that these three hospitals are affiliated academic centers with shared standardized and evidence-based treatment algorithms for management of gynecologic cancers. The patient populations between hospital locations were also different, Brooklyn having the highest percentage of Black/African American patients, Manhattan the highest percentage of White/Caucasian patients, Queens the highest percentage of Asian/Asian American patients and Hispanic/Latino patients. Of note, we did not identify differences in treatment modifications by race or ethnicity. We did find that younger patients and patients with a new cancer diagnosis versus a recurrence were more likely to experience treatment modifications, however both patient characteristics were more common among the population treated at Queens and the differences disappeared when adjusting for hospital location.
Among the cohort of 302 patients, 19 (6.3%) had positive COVID-19 testing. This number likely represents an underestimate of the true COVID-19 prevalence as only 19% of patients underwent COVID-19 testing during the study period. All but one of the patients with COVID-19 had at least one medical comorbidity in addition to cancer. This is not surprising as a high burden of severe disease and death from COVID-19 has been consistently observed in patients with pre-existing medical comorbidities [24]. Two of the 19 patients (10.5%) died due to complications of COVID-19 infection and one patient living in a nursing home died with presumed COVID-19 prior to testing.
This study has important strengths and limitations. Strengths include the evaluation of patients with diversity in age, race/ethnicity and hospital location within New York City. Additionally, despite locations in three separate New York City boroughs, the hospitals have a close affiliation with standardization in evidence-based care. As a result, differences between hospitals are unlikely due to varying clinical approaches to disease management. Our study was unable to capture whether treatment delays resulted from hospital policies versus patient decision-making. Future studies are needed to better capture patient concerns and resulting treatment delays during the pandemic. While our study raises important questions about the risks of treating cancer during the pandemic and outcomes of patients with COVID-19 and a cancer diagnosis, similar to other early studies of COVID-19 and cancer, it is limited by small numbers of patients and potentially significant bias [[2], [3], [4], [5], [6]]. Finally, our study did not capture long-term effects of treatment modifications on cancer recurrence and overall survival. Large scale studies with long-term follow-up and high level, critically reviewed data are needed in order to prevent unduly influencing oncology practices and clinical guidelines during this crisis [7].
Our prospective review of gynecologic oncology patients treated at three affiliated New York City hospitals during the first two months of the COVID-19 pandemic demonstrates the tremendous burden of COVID-19 on the provision of cancer care, with more than a third of patients experiencing either a delay, cancellation or change in their planned disease management. Patients treated at hospitals in New York City boroughs with greater reported COVID-19 burden experienced significantly more delays. It is critical that we consider disease burden and hospital capacity for care of both cancer and COVID-19 as medical systems begin the process of re-entry into non-emergent clinical activity and prepare for future pandemics.
Declaration of Competing Interest
Kevin Holcomb serves as a consultant for Johnson and Johnson and receives research support from Fujirebio Diagnostics.
Peter Martin serves as a consultant for Beigene, Bayer, Celgene, Cellectar, Janssen, Karyopharm, Kite, Morphosys, Regeneron, Teneobio.
Constantine Gorelick serves as a proctor for Intuitive Surgical.
None of the remaining authors have a conflict of interest to disclose.
Acknowledgements
Paul J. Christos and Charlene Thomas were partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
References
- 1.American Cancer Society . Atlanta; 2020. Cancer Facts & Figures 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 7.Robinson A.G., Gyawali B., Evans G. COVID-19 and cancer: do we really know what we think we know? Nat. Rev. Clin. Oncol. 2020;17(7):386–388. doi: 10.1038/s41571-020-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrag D., Hershman D.L., Basch E. Oncology practice during the COVID-19 pandemic. JAMA. 2020;323(20):2005–2006. doi: 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- 9.Oncology SoG . 2020. Society of Gynecologic Oncology: COVID-19 Resources. [Google Scholar]
- 10.Oncology ASoC . 2020. American Society of Clinical Oncology: COVID-19 Patient Care Information. [Google Scholar]
- 11.Ueda M., Martins R., Hendrie P.C., McDonnell T., Crews J.R., Wong T.L., et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J. Natl. Compr. Cancer Netw. 2020:1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez P.T., Chiva L., Eriksson A.G.Z., Frumovitz M., Fagotti A., Gonzalez Martin A., et al. COVID-19 global pandemic: options for management of gynecologic cancers. Int. J. Gynecol. Cancer. 2020;30(5):561–563. doi: 10.1136/ijgc-2020-001419. [DOI] [PubMed] [Google Scholar]
- 13.(AAGL) AAoGL. American Association of Gynecologic Laparoscopists (AAGL) 2020. COVID-19: Joint Statement on Minimally Invasive Gynecologic Surgery. [Google Scholar]
- 14.Shalowitz D.I., Epstein A.J., Buckingham L., Ko E.M., Giuntoli R.L. Survival implications of time to surgical treatment of endometrial cancers. Am. J. Obstet. Gynecol. 2017;216(3):268.e1–268.e18. doi: 10.1016/j.ajog.2016.11.1050. [DOI] [PubMed] [Google Scholar]
- 15.Vair B., Altman A.D., Nelson G. Time to surgery and the risk of cancer progression in patients with gynaecologic cancers of the lower genital tract. J. Obstet. Gynaecol. Can. 2015;37(4):338–344. doi: 10.1016/s1701-2163(15)30284-x. [DOI] [PubMed] [Google Scholar]
- 16.Fader A.N., Huh W.K., Kesterson J., Pothuri B., Wethington S., Wright J.D., et al. When to operate, hesitate and reintegrate: society of gynecologic oncology surgical considerations during the COVID-19 pandemic. Gynecol. Oncol. 2020;158(2):236–243. doi: 10.1016/j.ygyno.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tewari K.S., Java J.J., Eskander R.N., Monk B.J., Burger R.A. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG oncology/gynecologic Oncology group study. Ann. Oncol. 2016;27(1):114–121. doi: 10.1093/annonc/mdv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frey M., Blank S. Impact of the COVID-19 pandemic on quality of life for women with ovarian Cancer. Under Rev. Am. J. Obstetr. Gynecol. 2020;S0002-9378(20):30674–30678. [Google Scholar]
- 19.New York City Health Department of Health and Mental Hygiene . 2020. Summary of Vital Statistics 2017: The City of New York. [Google Scholar]
- 20.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farley J.H., Hines J., Lee N.K., Brooks S.E., Nair N., Brown C.L., et al. Promoting health equity in the era of COVID-19. Gynecol. Oncol. 2020;158(1):25–31. doi: 10.1016/j.ygyno.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadhera R.K., Wadhera P., Gaba P., Figueroa J.F., Joynt Maddox K.E., Yeh R.W., et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323(1):2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.New York City Health COVID-19 Data. 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page#download
- 24.Flaherty G.T., Hession P., Liew C.H., Lim B.C.W., Leong T.K., Lim V., et al. COVID-19 in adult patients with pre-existing chronic cardiac, respiratory and metabolic disease: a critical literature review with clinical recommendations. Trop. Dis. Travel Med. Vacc. 2020;6(16) doi: 10.1186/s40794-020-00118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]