Abstract
The mechanisms of intervertebral disc degeneration (IDD) involve numerous factors, including loss of the extracellular matrix (ECM) and vascular ingrowth. Melatonin has been reported to protect intervertebral discs (IVDs) from degeneration and to exert a potential anti-angiogenic effect. The aim of the present study was to investigate the anti-angiogenic and anabolic effects of melatonin in IVDs. Human nucleus pulposus (NP) and degenerative nucleus pulposus (DNP) cells were isolated and treated with melatonin. The results indicated that melatonin promoted ECM synthesis and NP cell proliferation. In addition, an NP/DNP and human umbilical vein endothelial cell (HUVEC) co-culture model was used to investigate the anti-angiogenesis effect of melatonin. Melatonin was indicated to suppress tube formation and migration of HUVECs in culture with NP cell-conditioned medium, as well as in an NP cell co-culture model. Fluorescence-labeled vascular endothelial growth factor (VEGF) was used to study the binding between VEGF and its receptor. The results of the present study indicated that melatonin exerts an angiogenic effect via inhibition of the binding of VEGF to its receptor in HUVECs. Taken together, these results suggest that melatonin is a potential agent to prevent IDD.
Keywords: melatonin, extracellular matrix, intervertebral disc degeneration, nucleus pulposus
Introduction
Lower back pain (LBP) is one of the most common complaints in adult patients, with total costs incurred by complications associated with LBP amounting to ~$100 billion/year in the US (1,2). Intervertebral disc degeneration (IDD) is closely linked to LBP and IDD-associated conditions are among the most common causes of disability among all workers aged 18-64 years in the US (3).
One of the characteristic features of IDD is the loss of intervertebral disc (IVD) extracellular matrix (ECM). The mechanisms of IDD are complex, involving numerous factors that may interact with each other (4). During the course of IDD, homeostasis of the ECM shifts toward a degenerative state (5). ECM degradation is caused by increases in the levels of destructive enzymes and a reduction in ECM synthesis. Certain drugs, including transforming growth factor-β1 (TGF-β1), which promote ECM synthesis and regeneration, may restore homeostasis within the IVD and reduce IDD (6-8).
Vascular ingrowth has been frequently observed in degenerated IVDs, while healthy IVDs are avascular (9,10). Intact aggrecan and notochordal cells are key inhibitors of neural and vascular ingrowth in healthy IVDs. However, with the degradation of ECM and the degeneration of nucleus pulposus (NP) cells, these barriers may also become dysfunctional (11,12). According to previous studies, degenerative NP cells secrete vascular endothelial growth factor (VEGF), which binds to VEGF receptor 2 (VEGFR-2) on the endothelial cell (EC) membrane surface (13).
Melatonin is an endogenous molecule released from the pineal gland that has a role in the regulation of the circadian rhythm and anti-oxidative stress and has an anti-inflammatory effect (14,15). Recent studies have reported that melatonin may protect IVDs from degeneration by improving cell survival and mitophagy induction (14,16). In addition, melatonin has been reported to exert a potential anti-angiogenic effect (17). However, whether melatonin is able to exert chondroprotective and anti-angiogenic effects in IVDs has yet to be established.
Current treatments for IDD are largely focused on symptom management, rather than treatment of the underlying causes of the disease (4,18). There is no satisfactory treatment available that is able to completely regenerate the degenerated disc and spinal fusion and disc arthroplasty may cause numerous problems, including fusion failure, pain and loss of function (19,20). In the present study, the potential anabolic and anti-angiogenic effects mediated by melatonin were examined in IVD cells. The study aimed to assess the biological function of melatonin in IDD to determine its suitability as a therapeutic agent.
Materials and methods
Cell culture
Human NP cells and degenerative human NP (DNP) cells were isolated from surgical specimens at Ningbo No. 6 Hospital (Ningbo, China). Normal NP cells were isolated from patients with lumbar fracture or neurilemmoma that underwent spinal fusion surgery and DNP cells were isolated from patients with IDD. Samples were collected between January 2018 and June 2019. Written informed consent was provided prior to sample collection. Institutional review board approval was given by the Ethics Committee of the Ningbo No. 6 Hospital (Ningbo, China; approval no. 2016031). A total of 15 patients (age, 30-75 years; mean age, 51 years; female, 10; male, 5) were included in the present study. All samples were used individually. NP and DNP cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin (HyClone; Cytiva) and 100 µg/ml streptomycin (HyClone; Cytiva). To achieve a better growth environment for the cells, high-glucose DMEM was used in the experiments. The concentration of glucose in the medium (4.5 g/l) was far below the harmful concentration reported in the literature (36 g/l) (21). Human umbilical vein ECs (HUVEC) were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Science (Shanghai, China) and cultured in DMEM/F12 media (Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. All cells were cultured in an incubator at 37˚C in a humidified atmosphere with 5% CO2 up to passages 2-3.
Cell proliferation assay
A commercial kit [Cell Counting Kit-8 (CCK-8); Dojindo Molecular Technologies, Inc.] was used to assess cell proliferation. NP cells were seeded in 96-well plates at a density of 5x103 cells per well. The concentration of melatonin was selected based on the result of preliminary experiments and values reported in the literature (16). In the present study, cells were incubated with melatonin (10 µM, 2.39 µg/ml) or TGF-β1 (10 ng/ml) for 1-6 days, followed by treatment with 10 µl CCK-8 solution and incubation at 37˚C for 2.5 h. The optical density (OD) of each well was measured at 450 nm with a microplate reader (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR (RT-qPCR)
NP cells were treated with melatonin (10 µM) or TGF-β1 (10 ng/ml) for 24 h. Following treatment, total RNA was extracted from NP cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), following the manufacturer's protocol. A total of 1 µg of total RNA was then incubated with the Prime Script™ Master Mix (Takara Bio, Inc.) to synthesize complementary DNA by RT. Gene expression was determined by qPCR using the SYBR Premix Ex Taq kit (Takara Bio, Inc.). The thermocycling conditions were as follows: Initialization at 95˚C for 3 min, followed by 40 cycles of 95˚C for 30 sec, 55˚C for 20 sec and 72˚C for 20 sec, with a final amplification at 95˚C for 15 sec. Gene expression was determined using the 2-ΔΔCq method (22). The primers were designed and selected using BLAST (https://blast.ncbi.nlm.nih.gov). Primer sequences are listed in Table I. GAPDH was used as an internal control.
Table I.
Primer sequences.
| Gene | Primer sequences (5'-3') |
|---|---|
| SOX9 | Forward AGCGAACGCACATCAAGAC |
| Reverse CTGTAGGCGATCTGTTGGGG | |
| ACAN | Forward ACTCTGGGTTTTCGTGACTCT |
| Reverse ACACTCAGCGAGTTGTCATGG | |
| COL2A1 | Forward TGGACGCCATGAAGGTTTTCT |
| Reverse TGGGAGCCAGATTGTCATCTC | |
| GAPDH | Forward TGTGGGCATCAATGGATTTGG |
| Reverse ACACCATGTATTCCGGGTCAAT |
SOX9, SRY-related HMG box-9; ACAN, aggrecan; COL2A1, collagen 2 type A1.
Immunohistochemistry
A total of 1x104/ml NP or DNP cells were seeded onto coverslips and treated with melatonin (10 µM) or TGF-β1 (10 ng/ml) for 3 or 7 days. Immunohistochemistry was then performed to determine the expression of collagen-II. Cells were fixed using 4% paraformaldehyde for 30 min at room temperature, prior to incubation with 0.2% Triton X-100 for 10 min at room temperature. After blocking with 2% bovine serum albumin (Sigma Aldrich; Merck KGaA) at room temperature for 1 h, cells were incubated with anti-collagen-II (cat. no. ab24118; 1:500; Abcam) antibody at 4˚C overnight. Cells were then treated with secondary antibody (cat. no. M00172; 1:2,000; Boster Biological Technology) at room temperature for 1 h, followed by color development with diaminobenzidine tetrahydrochloride (Dako; Agilent Technologies, Inc.). The images were observed under a light microscope (Olympus Corp.). The results of type II collagen staining were quantified by determining the integral OD (IOD) using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.)
Tube formation assay
NP cells were cultured in complete medium for 48 h. The medium was then collected and centrifuged at 200 x g at 4˚C for 5 min and the supernatant was collected as the conditioned medium. HUVECs were seeded at a density of 1x104/well in 96-well plates precoated with Matrigel®. Matrigel Matrix was coated at 12 mg/ml at 4˚C for 20 mins and then treated with melatonin (10 µM) or TGF-β1 (10 ng/ml) for 4 h at 37˚C. HUVECs were then incubated with NP conditioned medium in the presence or absence of 25 ng/ml VEGF for 6 h. The groups were as follows: HUVECs, HUVECs + melatonin (M), HUVECs + VEGF (V), HUVECs + V + M and HUVECs + V + TGF-β1 (T). The formation of tube-like structures was observed under a light microscope (Olympus Corp.) The images were observed under x40 magnification, with 5 fields in each sample randomly selected.
EC migration
A total of 1x105 HUVECs were seeded on Transwell polycarbonate membrane inserts (8.0-µm pores; Corning, Inc.) and maintained in 100 µl high-glucose DMEM without serum. NP or DNP cells were cultured in complete medium with melatonin (10 µM) or TGF-β1 (10 ng/ml) in the lower chamber. The groups were as follows (upper chamber/lower chamber): HUVECs/NP, HUVECs/DNP, HUVECs/DNP + M, HUVECs/NP + V, HUVECs/NP + M + V, HUVECs/NP + V + T. All inserts were incubated for 24 h at 37˚C and 5% CO2 in an incubator. The inserts were washed with PBS and the cells on the top surface of the inserts were carefully removed using a cotton swab. The inserts were fixed with 4% paraformaldehyde for 15 min at room temperature, followed by staining with 0.1% crystal violet for 30 min at room temperature. Migrated cells were observed and counted under x40 magnification with 5 fields randomly selected in each sample using a light microscope (Olympus Corp.).
VEGF binding assay
HUVECs were seeded at a density of 1x104/well in 96-well plates for 24 h. Cells were pretreated with melatonin ((10 µM) or TGF-β1 (10 ng/ml) for 4 h, then 25 ng/ml VEGF labeled with Alexa Fluor 647® (Abace Biology) was added to the culture medium and the wells were incubated at room temperature for 4 h. The HUVECs were then washed with PBS 3 times and counterstained with DAPI (1:1,000 dilution) for 5 min at room temperature. VEGF bound to the remaining receptors and was observed under a confocal microscope (Olympus Corp.). The positive rate was calculated as the IOD/cell number using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.)
Statistical analysis
Values are expressed as the mean ± standard deviation from three independent repeats. Statistical analyses were performed with unpaired t-test or one-way analysis of variance (ANOVA) followed by Duncan's post-hoc test using SPSS 24.0 (IBM Corp.). For experiments with >3 groups, Tukey's post-hoc test was used following ANOVA. P<0.05 was considered to indicate a statistically significant difference.
Results
Melatonin promotes ECM-associated gene expression and NP cell proliferation
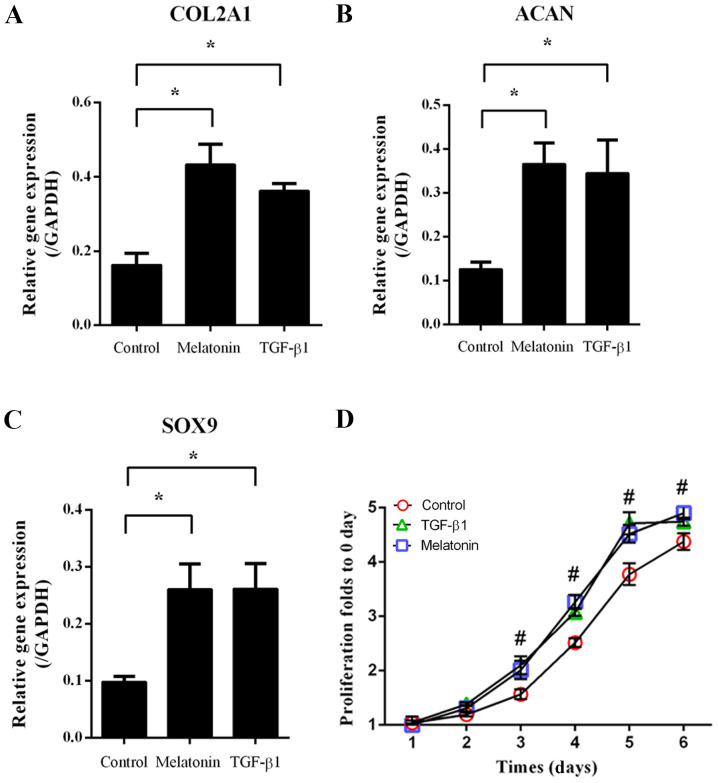
NP cells were treated with 10 µM melatonin or 10 ng/ml TGF-β1, followed by RT-qPCR to assess the expression of ECM-associated genes. As indicated in Fig. 1, melatonin and TGF-β1 significantly increased type II collagen AI (COL2A1), aggrecan (ACAN) and SRY-related HMG box-9 (SOX9) expression compared with that in the group subjected to the control treatment (P<0.05; Fig. 1A-C). In addition, the cell proliferation in the melatonin- and TGF-β1-treated groups was increased from day 3 onwards when compared with that in the control group (P<0.05; Fig. 1D). These results indicated that melatonin and TGF-β1 promoted NP cell proliferation and ECM-associated gene expression.
Figure 1.
Extracellular matrix-associated gene expression and proliferation in NP cells. NP cells were treated with melatonin (10 µM) or TGF-β1 (10 ng/ml) for 1-6 days. After 24 h, gene expression was measured by reverse transcription-quantitative PCR. (A-C) Gene expression of (A) COL2A1, (B) ACAN and (C) SOX9. (D) At days 1-6, cell proliferation was evaluated with a CCK-8. Values are expressed as the mean ± standard deviation. *P<0.05. #P<0.05, the melatonin group compared with the control group. ACAN, aggrecan; CCK-8, Cell Counting Kit-8; COL2A1, type II collagen A1; NP, nucleus pulposus; TGF-β1, transforming growth factor-β1.
Melatonin increases collagen-II expression in NP cells
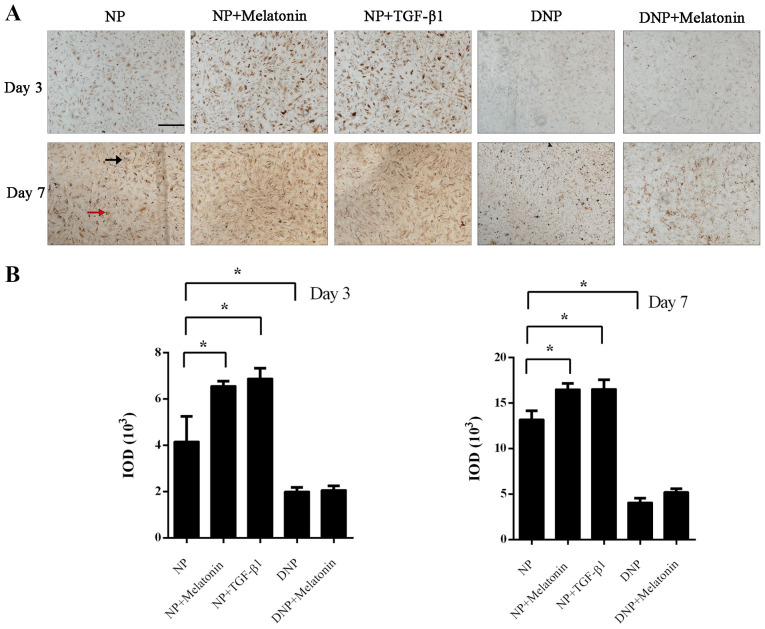
Immunohistochemistry was performed to assess collagen-II expression in NP cells after incubation with melatonin or TGF-β1. On day 3, positive staining was mainly present inside the NP cells, while at day 7, staining for collagen-II was observed both intracellularly (red arrow) and extracellularly (black arrow). The immunohistochemistry staining for collagen-II was markedly enhanced in the melatonin and TGF-β1 groups compared with that in the control group (P<0.05; Fig. 2A). Quantification of the IOD also indicated that the melatonin and TGF-β1 groups had increased collagen-II staining at day 3 and day 7 (P<0.05; Fig. 2B). There was less collagen-II staining in DNP cells and the rate of its increase was also markedly slower. Treatment of DNP cells with melatonin did not increase the collagen-II content. Although positive staining was increased on day 7 in the DNP + melatonin group, this was not statistically significant (P>0.05; Fig. 2B).
Figure 2.
Collagen-II protein expression in NP cells. NP cells were treated with melatonin (10 µM) or TGF-β1 (10 ng/ml) for 3 or 7 days. (A) At days 3 and 7, immunohistochemistry was performed to determine the collagen-II expression levels (scale bar, 20 µm). Black arrow, extracellular staining. Red arrow, intracellular staining. (B) The immunohistochemistry results were quantified as the IOD. Values are expressed as the mean ± standard deviation. *P<0.05. DNP, degenerative nucleus pulposus cells; IOD, integrated optical density; NP, nucleus pulposus cells; TGF-β1, transforming growth factor-β1.
Melatonin suppresses tube formation and migration of HUVECs
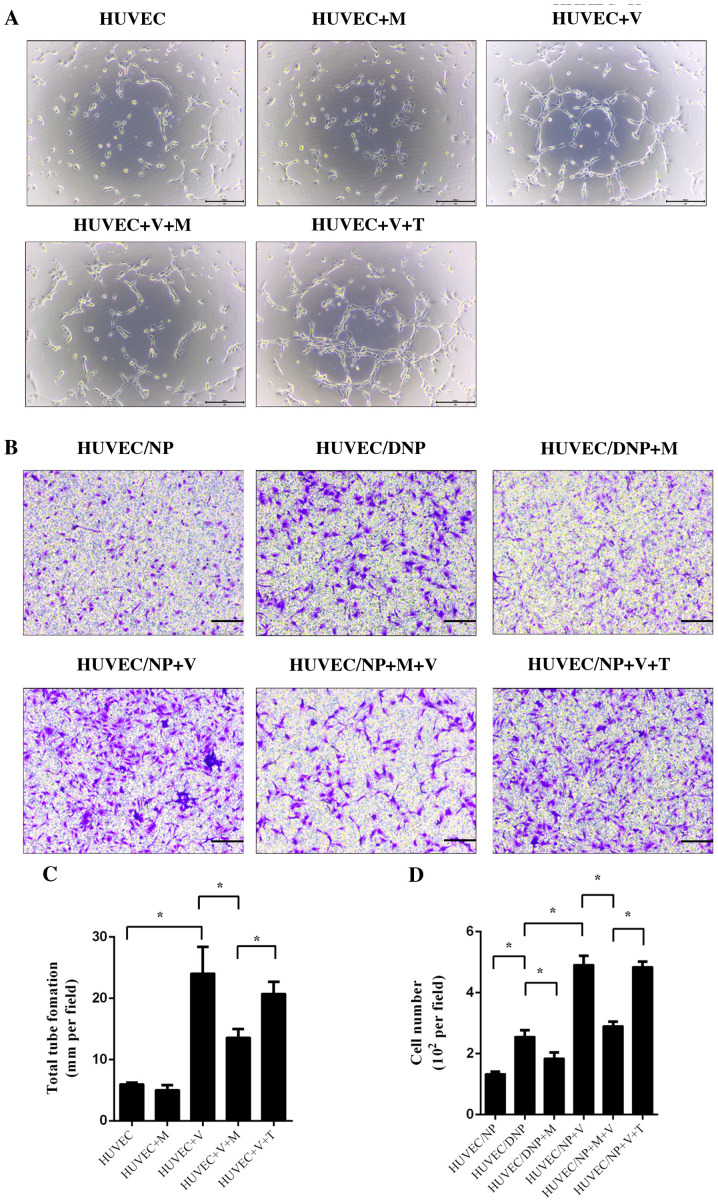
HUVECs were treated with conditioned medium with and without VEGF for 6 h. Tube formation was observed under a light microscope. After culture for 6 h, a small number of tube-like structures was observed. Treatment with melatonin alone did not influence tube formation of HUVECs (P>0.05; Fig. 3). Incubation with VEGF significantly promoted tube formation of HUVECs; however, this effect was inhibited by melatonin (P<0.05; Fig. 3A and C). TGF-β1 did not exert any significant influence on the tube formation of HUVECs after treatment with VEGF (P>0.05; Fig. 3A and C). An NP (DNP)/HUVEC co-culture model was set up to assess the migration of HUVECs. When compared with normal NP cells, DNP cells promoted increased HUVEC migration, which suggested that degeneration may be a cause of vascular ingrowth (P<0.05; Fig. 3B and D). The migration of HUVECs was also promoted by VEGF in NP/HUVEC models (P<0.05; Fig. 3B and C). Melatonin prevented the effect of DNP and VEGF-induced migration of HUVECs (P<0.05; Fig. 3B and C), but TGF-β1 did not (P>0.05; Fig. 3B and C).
Figure 3.
Tube formation and cell migration of HUVECs. HUVECs were pretreated with melatonin (10 µM) or TGF-β1 (10 ng/ml) for 4 h and then incubated with NP-conditioned medium and 25 ng/ml VEGF for 6 h. (A) Tube formation was observed under a light microscope. Scale bar, 50 µm. (B) HUVECs were co-cultured with NP or DNP and incubated with melatonin (10 µM) or TGF-β1 (10 ng/ml) for 24 h. After fixation and staining, HUVEC migration was observed under a light microscope. Scale bar, 50 µm). (C) Tube formation and (D) cell migration of HUVECs was quantified. Values are expressed as the mean ± standard deviation. *P<0.05. DNP, degenerative nucleus pulposus cells; HUVECs, human umbilical vein endothelial cells; M, melatonin; NP, nucleus pulposus cells; T/TGF-β1, transforming growth factor-β1; V/VEGF, vascular endothelial growth factor.
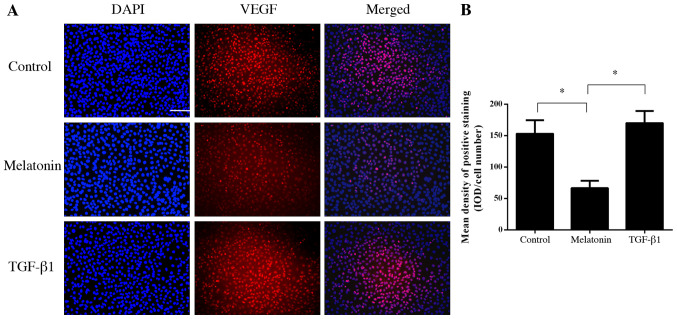
Melatonin inhibits the binding of VEGF to its receptor in HUVECs
To further investigate the mechanism of the anti-angiogenic effect of melatonin, fluorescence-labeled VEGF was used to bind to VEGFR on HUVECs. The results indicated that melatonin was able to inhibit the binding of VEGF to its receptor on HUVECs (P<0.05; Fig. 4). However, TGF-β1 did not influence the binding of VEGF to the VEGFR (P>0.05; Fig. 4).
Figure 4.
VEGF binding assay. HUVECs were pretreated with melatonin ((10 µM) or TGF-β1 (10 ng/ml) for 4 h then incubated with fluorescence-labeled VEGF with for 4 h. The VEGF binding rate was investigated by immunofluorescence. (A) Representative fluorescence microscopy images (scale bar, 100 µm). (B) Mean density of positive staining. Values are expressed as the mean ± standard deviation. *P<0.05. HUVECs, human umbilical vein endothelial cells; IOD, integrated optical density; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor.
Discussion
Melatonin is not only prevalent inside the human body but also in various plants and their products, including nuts, tomatoes, olive oil and wine (17,23,24). In addition to its function to synchronize the circadian rhythm (25) and its utility in the treatment of cardiovascular diseases and cancers (26), a beneficial application of melatonin for its anti-angiogenic effect has been previously reported (27,28). Melatonin has been suggested to inhibit angiogenesis indirectly by downregulating VEGF expression and secretion in various types of tumor cell (28,29). VEGF is an important growth factor in ECs. Overexpression of VEGF was identified in NP cells under inflammatory or degenerative conditions and was considered to be one of the major causes of vascular ingrowth in IVDs (30). Healthy IVDs are aneural and avascular, while neovascularization is considered to be an important pathological mechanism of symptomatic IVD degeneration (4). Vascular ingrowth has also been reported to be linked to nerve ingrowth and back pain (10,13). Vascular cells are able to produce nerve growth factor and brain-derived growth factor, the levels of which were indicated to be associated with increasing degeneration of the IVD (4,10). As the major angiogenic factor in IVD, VEGF binds to receptors on the surface of ECs (mainly VEGFR-2) to trigger proliferation and migration and promote the survival of ECs, ultimately resulting in vascular ingrowth in IVDs (31,32). Previous studies have also indicated that melatonin was able to inhibit HUVEC proliferation via modulating P53, Bax/Bcl-2 expression and ERK1/2/PI3K/AKT/protein kinase C/NF-Κb (33,34). Another study suggested that melatonin significantly inhibited VEGFR-2 phosphorylation (30%) and HUVEC migration (87%) at a high concentration of 1 mM (239 µg/ml) (17). Plants and their products contain melatonin at concentrations of up to 230 µg/g or ml (17,23,24). Calculation of the daily intake of melatonin and total plasma volume of adults indicated that the concentration of circulating melatonin would be 0.15-21 ng/ml, which is far below the therapeutic dose (17). The results of the present study demonstrated that melatonin exerted a potential anti-angiogenic effect at a relatively low concentration of 10 µM (2.39 µg/ml), which is comparatively easier to achieve.
Recently, the protective effect of melatonin in IDD was also reported. Ge et al (14) revealed that the melatonin was able to upregulate the expression of collagen-II and aggrecan via elevating the activity of the ERK signaling pathway in a rabbit model of IDD. Melatonin also ameliorated IDD via mitophagy induction and apoptosis inhibition (16). The present study provided similar results, in that melatonin promoted collagen-II and aggrecan expression and cell proliferation. Treatment with melatonin may repair degraded ECM and restore the normal morphology and biomechanics of IVDs. As a growth factor, TGF-β1 has been studied for decades and is thought to promote ECM synthesis in NP cells (8,35,36). In the present study, TGF-β1 was used as a positive control for promoting ECM synthesis and the results revealed that melatonin had a similarly promoting effect to TGF-β1 at current concentration.
Compared with healthy NP cells, treatment of DNP cells with melatonin did not produce any significant therapeutic effects in the present study. This may be attributed to two reasons: First, DNP cells were isolated from surgical specimens, which meant that the DNP cells were probably at an advanced stage of degeneration and were difficult to recover. Furthermore, DNP cells differed from healthy NP cells in numerous aspects, including gene expression, proliferative ability, ECM synthesis and morphology (37). Relatively long-term treatment may be required to gradually restore the biological function of DNP cells. The results of the present study also indicated that the collagen-II content in DNP cells was increased at day 7, although there was no statistical significance. Gene therapy may be effectively used to restore the function of DNP cells (38); however, the application of a widely used non-genetic agent may be safer and more acceptable for patients.
In conclusion, the results of the present study indicated that melatonin inhibited IVD degeneration by promoting ECM synthesis and suppressing angiogenesis in NP cells. However, the present study had certain limitations. To make the grouping clearer, DNP media was not used in the tube formation assays, which makes the results less persuasive. In addition, delivering agents precisely to the avascular IVD without iatrogenic damage while ensuring agents remain at an appropriate concentration in the long term is challenging. Therapeutic agents may also lose their activity because of the adverse microenvironment in degenerative IVD. Further research is required in order to address these problems.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CS, YL, LH, FZ and WW designed the study. CS, YL, YC, LH, FZ and WW performed the experiments. CS and YL analyzed data. CS and WW wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The current study was given institutional review board approval by the Ethics Committee of the Ningbo No. 6 Hospital (Ningbo, China; approval no. 2016031).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine (Phila Pa 1976) 1995;20:11–19. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88: (Suppl 2):S21–S24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331–341. doi: 10.1016/j.spinee.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Moon CS, Sul D, Lee J, Bae M, Hong Y, Lee M, Choi S, Derby R, Kim BJ, et al. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem. 2009;42:1504–1511. doi: 10.1016/j.clinbiochem.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Ellman MB, Yan D, An HS, Kc R, Li X, Chen D, Xiao G, Cs-Szabo G, Hoskin DW, et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J Cell Physiol. 2013;228:1884–1896. doi: 10.1002/jcp.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17 (Suppl 4):S441–S451. doi: 10.1007/s00586-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Cao C, Wu C, Yuan C, Gu Q, Shi Q, Zou J. TGF-βl suppresses inflammation in cell therapy for intervertebral disc degeneration. Sci Rep. 2015;5(13254) doi: 10.1038/srep13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 11.Johnson WE, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- 12.Kwon WK, Moon HJ, Kwon TH, Park YK, Kim JH. Influence of rabbit notochordal cells on symptomatic intervertebral disc degeneration: Anti-angiogenic capacity on human endothelial cell proliferation under hypoxia. Osteoarthritis Cartilage. 2017;25:1738–1746. doi: 10.1016/j.joca.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 13.He M, Pang J, Sun H, Zheng G, Lin Y, Ge W. Overexpression of TIMP3 inhibits discogenic pain by suppressing angiogenesis and the expression of substance P in nucleus pulposus. Mol Med Rep. 2020;21:1163–1171. doi: 10.3892/mmr.2020.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge J, Zhou Q, Niu J, Wang Y, Yan Q, Wu C, Qian J, Yang H, Zou J. Melatonin protects intervertebral disc from degeneration by improving cell survival and function via activation of the ERK1/2 signaling pathway. Oxid Med Cell Longev. 2019;2019(5120275) doi: 10.1155/2019/5120275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CC, Huang TY, Chen HY, Huang TC, Lin LC, Chang YJ, Hsia SM. Protective effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid Med Cell Longev. 2018;2018(9015765) doi: 10.1155/2018/9015765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Wu Y, Shi H, Wang J, Zheng Z, Chen J, Chen X, Zhang Z, Xu D, Wang X, Xiao J. Melatonin ameliorates intervertebral disc degeneration via the potential mechanisms of mitophagy induction and apoptosis inhibition. J Cell Mol Med. 2019;23:2136–2148. doi: 10.1111/jcmm.14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerezo AB, Hornedo-Ortega R, Álvarez-Fernández MA, Troncoso AM, García-Parrilla MC. Inhibition of VEGF-induced VEGFR-2 activation and HUVEC migration by melatonin and other bioactive indolic compounds. Nutrients. 2017;9(249) doi: 10.3390/nu9030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buser Z, Chung AS, Abedi A, Wang JC. The future of disc surgery and regeneration. Int Orthop. 2019;43:995–1002. doi: 10.1007/s00264-018-4254-7. [DOI] [PubMed] [Google Scholar]
- 19.Freeman BJ, Davenport J. Total disc replacement in the lumbar spine: A systematic review of the literature. Eur Spine J. 2006;15: (Suppl 3):S439–S447. doi: 10.1007/s00586-006-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Eerenbeemt KD, Ostelo RW, van Royen BJ, Peul WC, van Tulder MW. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: A systematic review of the literature. Eur Spine J. 2010;19:1262–1280. doi: 10.1007/s00586-010-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Li P, Xu J, Wu X, Guo Z, Fan L, Song R, Wang J, Wei L, Teng H. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Biosci Rep. 2018;38(BSR20171454) doi: 10.1042/BSR20171454. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Murch SJ, Simmons CB, Saxena PK. Melatonin in feverfew and other medicinal plants. Lancet. 1997;350:1598–1599. doi: 10.1016/S0140-6736(05)64014-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Huo Y, Tan DX Liang Z, Zhang W, Zhang Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003;73:19–26. doi: 10.1016/s0024-3205(03)00252-2. [DOI] [PubMed] [Google Scholar]
- 25.Reiter RJ, Tan DX, Galano A. Melatonin: Exceeding expectations. Physiology (Bethesda) 2014;29:325–333. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 26.Reiter RJ, Tan DX, Korkmaz A. The circadian melatonin rhythm and its modulation: Possible impact on hypertension. J Hypertens (Suppl 27) 2009:S17–S20. doi: 10.1097/01.hjh.0000358832.41181.bf. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-García V, González A, Alonso-González C, Martínez-Campa C, Cos S. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc Res. 2013;87:25–33. doi: 10.1016/j.mvr.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Cui P, Yu M, Peng X, Dong L, Yang Z. Melatonin prevents human pancreatic carcinoma cell PANC-1-induced human umbilical vein endothelial cell proliferation and migration by inhibiting vascular endothelial growth factor expression. J Pineal Res. 2012;52:236–243. doi: 10.1111/j.1600-079X.2011.00933.x. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-García V, González A, Alonso-González C, Martínez-Campa C, Cos S. Regulation of vascular endothelial growth factor by melatonin in human breast cancer cells. J Pineal Res. 2013;54:373–380. doi: 10.1111/jpi.12007. [DOI] [PubMed] [Google Scholar]
- 30.Binch AL, Cole AA, Breakwell LM, Michael AL, Chiverton N, Cross AK, Le Maitre CL. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther. 2014;16(416) doi: 10.1186/s13075-014-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Ohba T, Haro H, Ando T, Wako M, Suenaga F, Aso Y, Koyama K, Hamada Y, Nakao A. TNF-alpha-induced NF-kappaB signaling reverses age-related declines in VEGF induction and angiogenic activity in intervertebral disc tissues. J Orthop Res. 2009;27:229–235. doi: 10.1002/jor.20727. [DOI] [PubMed] [Google Scholar]
- 33.Cui P, Yu M, Lou Z, Dai M, Han J, Xiu R, Yang Z. Intracellular signaling pathways involved in cell growth inhibition of human umbilical vein endothelial cells by melatonin. J Pineal Res. 2008;44:107–114. doi: 10.1111/j.1600-079X.2007.00496.x. [DOI] [PubMed] [Google Scholar]
- 34.Cui P, Luo Z, Zhang H, Su Y, Li A, Li H, Zhang J, Yang Z, Xiu R. Effect and mechanism of melatonin's action on the proliferation of human umbilical vein endothelial cells. J Pineal Res. 2006;41:358–362. doi: 10.1111/j.1600-079X.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Li Z, Chen F, Liu H, Wang H, Li X, Liu X, Wang J, Zheng Z. TGF-β1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral disc degeneration and inflammation-related pain in a rat model. Exp Mol Med. 2017;49(e379) doi: 10.1038/emm.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai F, Zhu L, Wang F, Shi R, Xie XH, Hong X, Wang XH, Wu XT. The paracrine effect of degenerated disc cells on healthy human nucleus pulposus cells is mediated by MAPK and NF-κB pathways and can be reduced by TGF-β1. DNA Cell Biol. 2017;36:143–158. doi: 10.1089/dna.2016.3230. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Li Y, Huang ZN, Wang ZY, Nan LP, Wang F, Zhou SF, Wang JC, Feng XM, Zhang L. The effect of intervertebral disc degenerative change on biological characteristics of nucleus pulposus mesenchymal stem cell: An in vitro study in rats. Connect Tissue Res. 2019;60:376–388. doi: 10.1080/03008207.2019.1570168. [DOI] [PubMed] [Google Scholar]
- 38.Luo XW, Liu K, Chen Z, Zhao M, Han XW, Bai YG, Feng G. Adenovirus-mediated GDF-5 promotes the extracellular matrix expression in degenerative nucleus pulposus cells. J Zhejiang Univ Sci B. 2016;17:30–42. doi: 10.1631/jzus.B1500182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.