Abstract
The chemistry, biochemistry, pharmacology and molecular biology of oxylipins (defined as a family of oxygenated natural products that are formed from unsaturated fatty acids by pathways involving at least one step of dioxygen-dependent oxidation) are complex and occasionally contradictory subjects that continue to develop at an extraordinarily rapid rate. The term includes docosanoids (e.g. protectins, resolvins and maresins, or specialized pro-resolving mediators), eicosanoids and octadecanoids and plant oxylipins, which are derived from either the omega-6 (n-6) or the omega-3 (n-3) families of polyunsaturated fatty acids. For example, the term eicosanoid is used to embrace those biologically active lipid mediators that are derived from C20 fatty acids, and include prostaglandins, thromboxanes, leukotrienes, hydroxyeicosatetraenoic acids and related oxygenated derivatives. The key enzymes for the production of prostanoids are prostaglandin endoperoxide H synthases (cyclo-oxygenases), while lipoxygenases and oxidases of the cytochrome P450 family produce numerous other metabolites. In plants, the lipoxygenase pathway from C18 polyunsaturated fatty acids yields a variety of important products, especially the jasmonates, which have some comparable structural features and functions. Related oxylipins are produced by non-enzymic means (isoprostanes), while fatty acid esters of hydroxy fatty acids (FAHFA) are now being considered together with the oxylipins from a functional perspective. In all kingdoms of life, oxylipins usually act as lipid mediators through specific receptors, have short half-lives and have functions in innumerable biological contexts.
Keywords: fatty acid metabolism, lipid mediators, polyunsaturated fatty acids
Introduction
Since the pioneering work of George and Mildred Burr (reviewed in [1]) some 90 years ago, it has been known that certain fatty acids are essential in human (and most other animal) diets. There are two types of essential fatty acids (EFAs), which belong to the n-3 (omega-3) and n-6 (omega-6) polyunsaturated fatty acid (PUFA) families (i.e. the last double bond in these acids is either three or six carbons from the methyl end of the chain). In the early experiments, fat-free diets were shown to give rise to a variety of physiological symptoms that could be alleviated by feeding linoleic (LA) and α-linolenic (ALA) acids. The reason why these fatty acids are essential in the diet is that humans and animals in general do not have the necessary desaturases (Δ12 and Δ15) to form them from oleic acid, i.e. to insert a new double bond after one that is pre-existing [2]. They are produced almost entirely in photosynthetic organisms that evolve oxygen—such as cyanobacteria, algae, mosses and higher plants. While LA and ALA can be regarded as the key EFAs of the n-6 and n-3 families, respectively, there may be a need for longer chain PUFAs, produced from these precursors by sequential elongation and desaturation reactions, for specific functions. Such acids (e.g. arachidonic (ARA), eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids) are termed ‘conditionally essential’ [3]. Moreover, there has been much recent interest in providing dietary 20 or 22C PUFAs and also ensuring that the ratio of dietary n-6/n-3 PUFAs is maintained at 3-4, which is much lower than in many current ‘Western’ diets [4].
Polyunsaturated fatty acids are important components of phospholipids in membranes to which they impart desirable physical properties. However, a major reason why we need EFA and why they produce so many diverse effects is because they are metabolised to give rise to lipid signalling molecules [5]. In the main, the latter are 20C (eicosanoids) or 22C (docosanoids) oxygenated derivatives, collectively termed ‘oxylipins’. Although linoleic acid is an important constituent of skin lipids [6], where it has vital functions, much of the dietary need for EFAs is to make longer-chain lipid signalling molecules. Nevertheless, there are a number of important signalling molecules in animals, which are formed from linoleic acid or α-linolenic acid per se (e.g. octadecanoids) [7].
In this article, we briefly describe the conversion of n-3 and n-6 PUFAs into various classes of lipid mediators. We also summarise the latter’s biological actions. More details of the metabolism and function of these lipid mediators will be found in subsequent chapters that cover eicosanoids, isoprostanes, specialised pro-resolving mediators (SPMs), endocannabinoids and jasmonates.
Conversion of essential fatty acids into eicosanoids
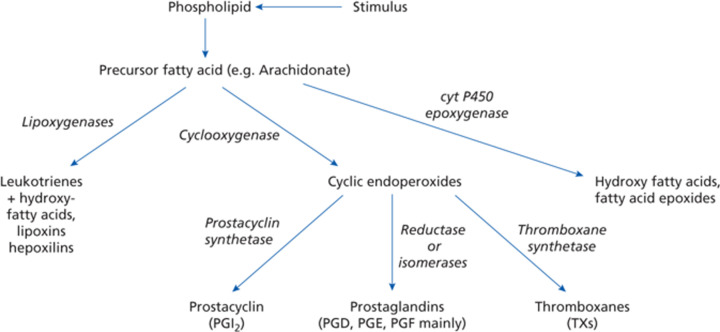
A simplified picture of the generation of classic eicosanoids (derived from the Greek for 20) is shown in Figure 1. Three different types of oxidation reactions utilise a 20-carbon unesterified fatty acid precursor, such as arachidonic acid (ARA, the main n-6 precursor) or eicosapentaenoic acid (EPA, the main n-3 precursor). These involve lipoxygenase, cyclooxygenase and cytochrome P450 oxidase or epoxygenase enzymes [8].
Figure 1. Generation of the classic eicosanoids (taken from [2] with permission).
Before reaction occurs, the PUFAs are usually released from membrane phospholipids mainly by phospholipase A2 (PLA2) action. A considerable number of PLA2 enzymes have been characterised and shown to occur in several unrelated protein families [9]. Overall, they can be broadly categorised into cytosolic calcium-dependent PLA2 (cPLA2), cytosolic calcium-independent PLA2 (iPLA2), and secreted PLA2 (sPLA2). The concentration of non-esterified PUFAs, such as arachidonic acid, in cells is normally far below the Km of enzymes such as cyclooxygenase (prostaglandin H2 synthase) so activation of hydrolytic enzymes, especially cPLA2, is a key regulatory reaction. For the 20C PUFAs, ARA and EPA, different lipid classes contain important relative concentrations. Thus, for ARA, two phosphoglyceride classes are important sources—phosphatidylcholine (the most abundant phosphoglyceride in most mammalian membranes) and phosphatidylinositol (and its phosphorylated derivatives) by virtue of the high concentration of ARA at the sn-2 position together with the specificity of cPLA2α [2]. Other potential sources of ARA or EPA are the plasmalogens but because these lipids are poor substrates for PLA2, they are usually hydrolysed by plasmalogenase (alkylglycerol monooxygenase) first [10,11].
Release of non-esterified PUFAs from membrane lipids can be enhanced by specific physiological stimulae (e.g. adrenaline, angiotensin II, certain antibody–antigen complexes) or non-specific pathological conditions. Of the many lipases that can be activated in this way, cPLA2α appears the most important as it has a marked specificity for phospholipids containing arachidonic acid in the sn-2 position. Hormonally induced mobilisation of Ca2+ leads to the movement of the enzyme from the cytosol to the endoplasmic reticulum and the nuclear envelope. Its activity is increased by phosphorylation. Two specific lipids—ceramide 1-phosphate and phosphatidylinositol 4,5-bisphosphate—bind to the enzyme and modify both its activity and its translocation within cells [12].
Secretory PLA2 (sPLA2) is also stimulated by Ca2+ but at the higher concentrations found outside the cell. The enzyme is rather non-specific towards different phosphoglycerides and towards the fatty acid present at the sn-2 position. There are suggestions that cPLA2 is involved in the rapid response in prostaglandin synthesis while sPLA2 is involved at later stages of prostaglandin stimulation after tissues have been activated further by cytokines, growth factors or inflammatory factors.
Cyclooxygenase activity
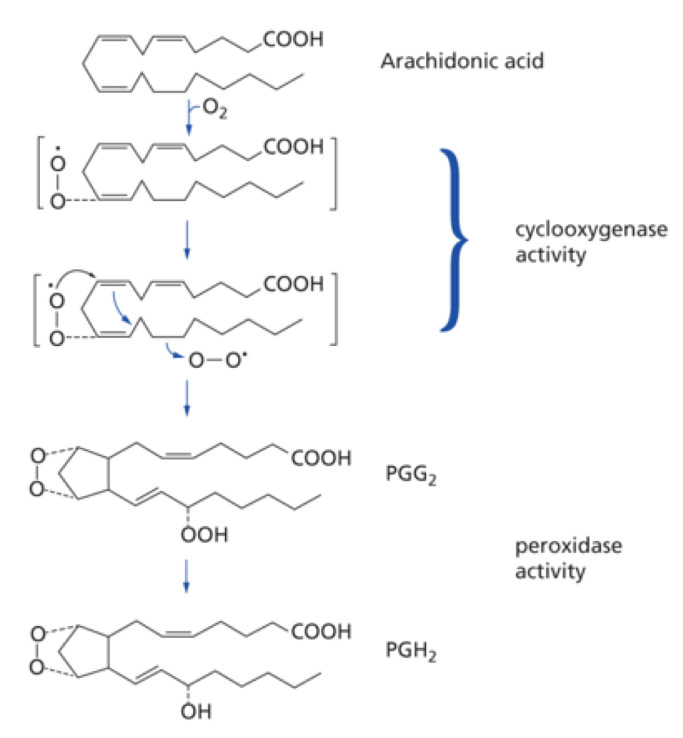
Once the precursor fatty acid (ARA or EPA usually) has been released from membrane lipids, it can be oxidised by cyclooxygenases (more correctly termed prostaglandin endoperoxide H synthases) [8,13]. Two reactions are catalysed by a single enzyme—a cyclooxygenase reaction where two molecules of oxygen are added to the substrate and a second peroxidation (Figure 2). In the case of ARA, prostaglandin PGG2 is the intermediate and PGH2 the first product.
Figure 2. Cyclooxygenase activity (taken from [2] with permission).
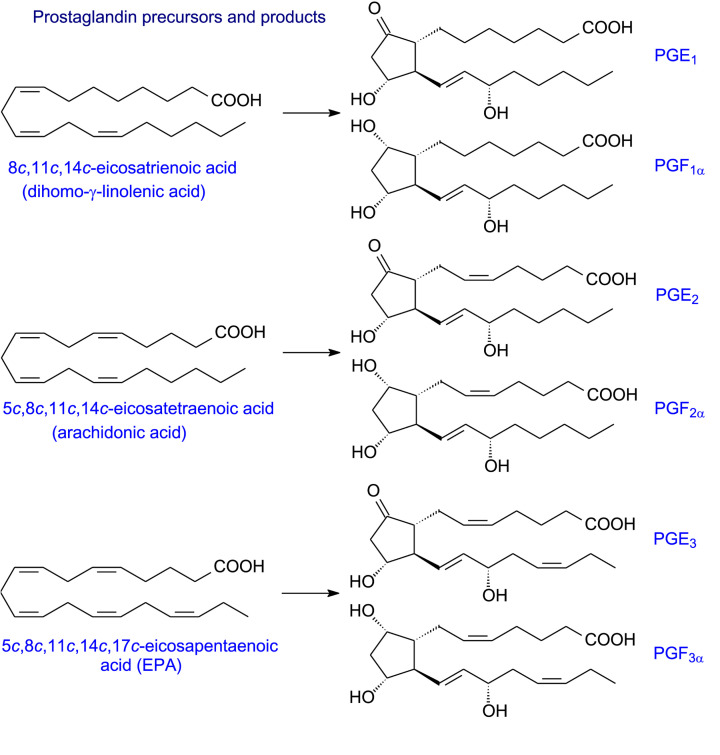
There are two major human cyclooxygenase isoforms, COX-1 and COX-2, which are haemoproteins and act as homodimers of 576 and 581 amino acids, respectively. COX-1 is constitutively expressed in many mammalian tissues. It is thought to be responsible for the formation of prostaglandins involved in the general regulation of physiological events. COX-2, on the other hand, is present at low levels until induced by inflammatory stimuli such as cytokines, endotoxins, tumour promoters and some lipids. Both isoforms have similar Vmax and Km values for ARA, undergo suicide inactivation and their reactions can be initiated by hydroperoxide. The COXs will use a broad range of 18 or 20C PUFA substrates although their substrate selectivities are somewhat different. For example, COX-2 needs lower concentrations of hydroperoxide for activation and has a wider substrate specificity (including those relevant to endocannabinoid metabolism) than COX-1 [14]. Moreover, COX-2 has a greater capacity to oxidise a number of PUFAs that are poor substrates for COX-1 including various n-3 PUFAs [14–16]. When acetylated by aspirin, COX-2 in contrast with COX-1 can catalyse a lipoxygenase-type reaction involved, for example, in the formation of various SPMs (see later). Furthermore, the products of COX reactions will also relate to the balance of substrates available. Some examples of prostaglandin precursors and their products are shown in Figure 3. The endoperoxide products, in turn, can form a host of different products (see Figure 4).
Figure 3. Prostaglandin precursors and products.
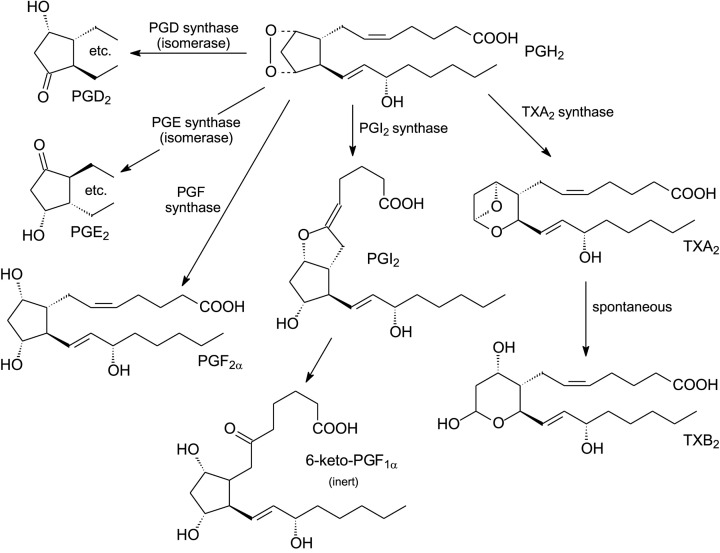
Figure 4. Conversion of cyclooxygenase endoperoxide products to different types of eicosanoids (adapted and re-drawn from [2]).
Cyclooxygenase endoperoxide products can be converted into various eicosanoids
Three general types of prostanoids can be synthesised from the endoperoxide produced by COX-1 or COX-2 (Figure 4). Prostaglandins PGD2, PDE2 and PGF2 are formed from PGH2, itself produced from ARA [17]. An alternative prostaglandin, PGI2 (also known as prostacyclin) [18,19] has a distinct function in promoting vasodilation and inhibiting platelet aggregation. Together with its action in inhibiting smooth muscle proliferation, PGI2 contributes to myocardial protection.
Instead of prostacyclin formation, PGH2 can be converted to thromboxane A2 via TxA2 synthase. TxA2 is extremely labile and rearranges spontaneously with a half-life of approximately 30 s to a stable but physiologically inert TxB2 (Figure 4). Because TxA2 causes platelet aggregation [20], there have been considerable efforts in searching for inhibitors of its synthesis. Since TxA2 and PGI2 have antagonistic effects on thrombosis and atherogenesis, it is obvious that their balance is essential for good cardiovascular health and maintenance of vascular homeostasis [21]. Thus, TxA2 is synthesised mainly in platelets and its production is enhanced during platelet activation to promote aggregation and vasoconstriction. On the other hand, prostacyclin (PGI2) is the main prostanoid produced by vascular endothelial cells. It will inhibit platelet aggregation and contributes substantially to cardiovascular protection (see [2,22]).
Prostanoids have receptors that mediate their actions
In the last two decades, several prostanoid receptors have been identified and partly characterised [23–25]. For prostaglandins, ten receptors have been characterised. In the case of PGE2, four receptors (EP1–4) have been identified and each has a different mechanism of action. Other receptors have been identified for PGD2, PGF2α, PGI2 and TxA2. The receptors have seven transmembrane segments and belong to the G protein-coupled receptor (GPCR) family which constitute the largest family of receptors in humans (approximately 800 coded in the human genome).
Apart from prostanoids [26], receptors have been identified for other lipid mediators [27]. For example, in the case of leukotrienes, LTB4 has BLT1 (high affinity) and BLT2 (low affinity and less specific) receptors while the cysteinyl leukotrienes have five (CysLT1, CysLT2, P2Y12, GPR17 and GPR99). Lipoxin [28] and SPMs [29] also have identified receptors.
By using knockout mice, precise functional roles for the individual receptors are being elucidated.
Prostanoids are produced in situ and are rapidly catabolised
The prostanoids can be regarded as ‘local hormones’. They are produced within tissues and have their main actions in that locality. In general, prostanoids have very short half-lives in vivo. For example, PGE2 and PGF2α are rapidly metabolised and do not survive a single pass through the circulation! The lung plays a major role in the catabolism with oxidation of the hydroxyl group at C15 being the usual target. This is followed by attack of the 13-double bond and then beta- and omega-oxidation. For the major active prostaglandins, their blood concentrations are less than 10−10M, emphasising their activity as local hormones (or autocoids). Moreover, unlike conventional hormones, they are produced in almost every cell in the body. The prostanoids are transported out of cells via carrier-mediated mechanisms and, once in the circulation, are deactivated rapidly [2,8].
Aspirin and non-steroidal anti-inflammatory drugs
Aspirin (acetylsalicylic acid) was originally utilised as a substitute for salicylate medicines which had been used for their health properties for 3,500 years [30,31]. Since 1897, when Bayer produced it, aspirin has been one of the World’s most commonly used medicines (approximately 40,000 tonnes or up to 120 billion pills per year!).
Of the various non-steroidal anti-inflammatory drugs (NSAIDs) (e.g. aspirin, ibuprofen, indomethacin, diclofenac), aspirin is the best known. NSAIDs will compete with PUFA substrates for the reaction site of COX enzymes but, in general, the therapeutic anti-inflammatory action of NSAIDs is caused by inhibition of COX-2. In contrast, the simultaneous inhibition of COX-1 causes most of the unwanted side-effects, such as gastric ulceration [32]. Once bound to the COX-1 active site, aspirin will cause irreversible inactivation through acetylation of serine 530. For COX-2, the acetylation reaction still allows oxygenation of ARA, in a similar manner to that of a lipoxygenase, but prostaglandin PGH2 is not formed (see section on resolvins).
Unlike aspirin, most of the other NSAIDs cause reversible inhibition of COX and compete with substrates such as ARA. Because NSAIDs inhibit both COX enzymes and it would be much more useful to only inhibit COX-2, considerable effort has been devoted to finding selective inhibitors [32,33]. Some success was achieved initially [34], but such compounds caused unwanted cardiovascular effects [35], which led to the clinical withdrawal of the initial products.
Lipoxygenase activity will produce hydroxy-eicosatetraenes, leukotrienes and other signalling molecules
Lipoxygenases can catalyse three different types of reactions (dioxygenation of lipids to give hydroperoxides; hydroperoxidation of the latter into keto lipids; formation of epoxy leukotrienes via leukotriene synthase reaction) due to their multifunctional nature. Several lipoxygenases (LOXs) occur in mammals and, in humans, six main family members have been listed—5-LOX, 12-LOX, 12/15-LOX (15-LOX type 1), 15-LOX type 2, 12R-LOX and epidermal LOX [36]. There are seven LOXs in mice. Orthologues of the same gene have different reaction selectivities in different species. This can often compromise extrapolation of data from animal experiments to human conditions. Each of the lipoxygenase proteins in animals has a single polypeptide chain of 75–80 kDa mass. The N-terminal ‘beta-barrel’ domain functions in substrate acquisition while a larger catalytic domain has a single non-heme iron atom which is bound to conserved histidine residues and to the carboxyl group of a conserved isoleucine at the C-terminus of the enzyme. The iron is active in the ferric state. For 5-LOX and 8-LOX activities the substrate ARA enters carboxyl group first while for 12-LOX or 15-LOX the methyl terminus enters the active site. While the PUFA substrate is held in a tight channel, smaller channels direct molecular oxygen towards the selected carbon to allow formation of specific hydroperoxy-eicosatetraenes (HPETEs), which are subsequently reduced to hydroxy-eicosatetraenes (HETEs). Each lipoxygenase acts with high regio- and stereo-specificity to produce HETE with distinctive biological functions in particular tissues.
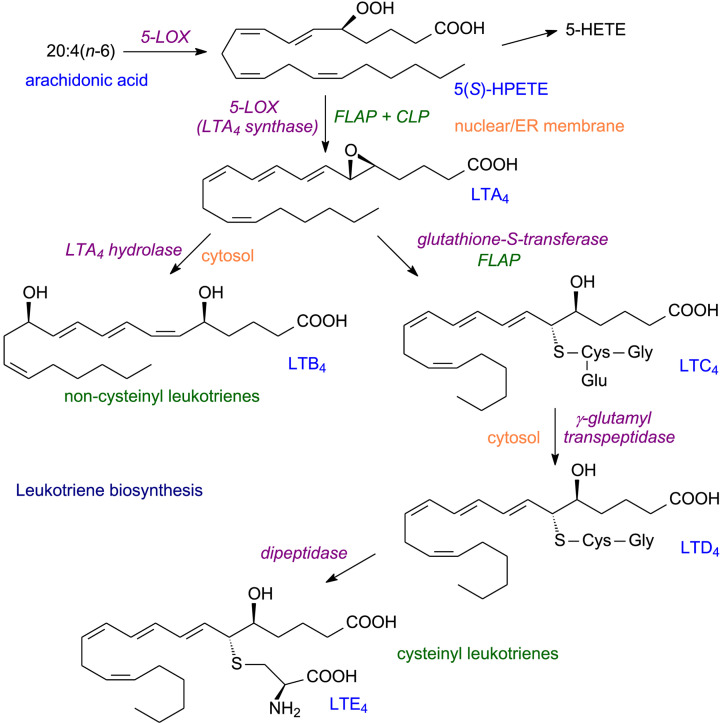
For formation of leukotrienes, 5-LOX is the key enzyme [37,38]. As for prostanoids, a non-esterified (free) fatty acid is the substrate. A two-step concerted reaction begins leukotriene formation (Figure 5) [8]. The first step is a dioxygenation of C5 to create, in the case of ARA, 5S-hydroperoxy-6t,8c,11c,14c-eicosatetraenoic acid (5-HPETE). For the second step, two accessory proteins are needed. These are the 5-lipoxygenase activating protein (FLAP) and coactosin-like protein (CLP). With the aid of these two proteins, 5-LOX converts 5-HPETE into 5, 6-epoxy-7t,9t,11c,14c-eicosatetraenoic acid (5-HETE) or leukotriene A4 (LTA4) (Figure 5).
Figure 5. Leukotriene biosynthesis.
The unstable LTA4 appears to have little biological function on its own but can be metabolised in two ways to yield physiologically important leukotrienes. First, it can be hydrolysed by LTA4 hydrolase, a zinc metalloprotein, to form the dihydroxy acid LTB4 [39]. Secondly, LTA4 is converted to a ‘cysteinyl leukotriene’ by the addition of gamma-glutamyl-cysteinyl glycine and with the assistance of FLAP, this yields LTC4 which can be reacted with a transpeptidase to remove glutamate and yield LTD4. Finally, a dipeptidase forms the final ‘cysteinyl leukotriene’ LTE4.
The leukotrienes have a variety of important biological effects [40]. LTB4 is a potent chemotactic agent and is one of the first signals that attract innate immune cells such as leukocytes to the site of insult. In contrast, LTB5 (which is formed from EPA) strongly inhibits the pro-inflammatory actions of LTB4. Leukotriene LTC4, together with other ‘cysteinyl leukotrienes’ (LTD4, LTE4) are the slow-acting substances of anaphylaxis, originally reported nearly 100 years ago. These lipid mediators exert a range of pro-inflammatory actions including constriction of airways and vascular smooth muscles. LTD4 and LTE4 are overexpressed in several types of cancer and are considered tumorigenic.
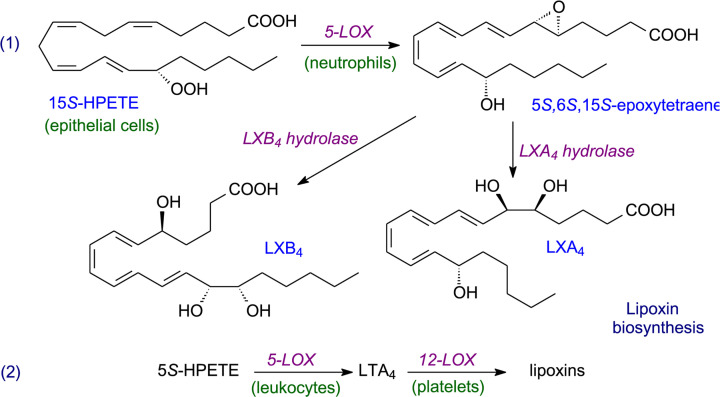
Instead of leukotrienes, ARA can form lipoxins, which are trihydroxy-eicosatetraenoic acids where the four double bonds are in conjugation (LXA4, LXB4) [41]. These are considered one of a group of specialised pro-resolving mediators (SPMs) which include resolvins, protectins and maresins (see later). Oxidation of ARA needs two different types of lipoxygenase in this case (see an example in Figure 6), and as few cell types have both of the necessary lipoxygenases, the synthesis of lipoxins needs trans-cellular pathways [42]. In such pathways, because a single cell lacks all of the enzymes necessary for a metabolic sequence, it has to combine with another cell type to complete a particular conversion. Of course, such cellular interactions need appropriate transport mechanism(s) associated with the pathway.
Figure 6. Examples of lipoxin biosynthesis.
Two other minor classes of lipoxygenase products are the eoxins and the hepoxilins. Eoxins are related to the cysteinyl-leukotrienes and are products of the 12/15-LOX in human eosinophils and mast cells. They are potent pro-inflammatory agents [43]. Hepoxilins are especially important in human epidermis [44,45] and are made in one of two pathways involving 12-LOX. Compounds analogous to those formed from ARA can be produced from EPA or DHA and, in skin, linoleate is an important substrate [46].
Cytochrome P450 oxidases, HETEs and epoxyeicosatetraenoic acids
In addition to cyclooxygenase or lipoxygenase activity, a third oxygenation of relevant PUFAs involves cytochrome P450 oxidases (Figure 1) [8,47,48]. These enzymes are membrane-bound hemoproteins that transfer a single oxygen to the substrate carbon i.e. they are monooxygenases which produce water as a second product. NADPH is also needed and the transfer of electrons requires a NADPH-cytochrome P450 reductase. Various cytochrome P450 oxidases can produce a mixture of hydroxy-eicosatetraenes (HETEs) (Figure 7). The balance of HETEs generated depends on the tissue, cell type and the catalytic efficiency of the individual cytochrome P450 oxidase isoforms [49]. In addition to their function in generating HETE isomers, the cytochrome P450 oxidases have other important roles in lipid metabolism [50].
Figure 7. Metabolism of arachidonic acid by the cytochrome P450 epoxygenase pathway (taken from [2] with permission).
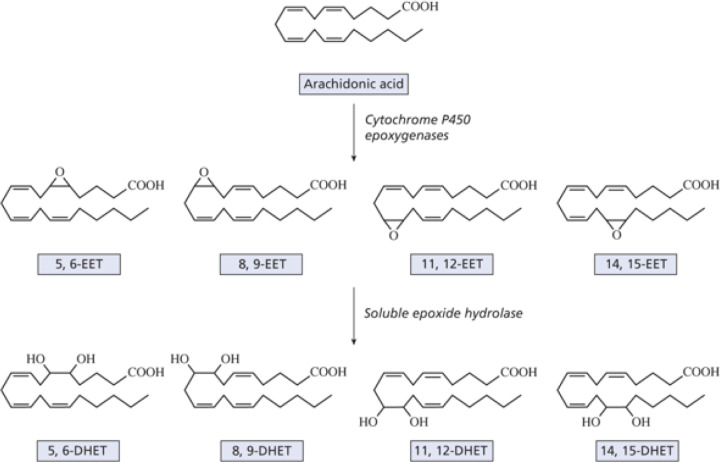
For ARA, three types of reaction can occur. First, a series of HETE products can be formed with cis-trans conjugated diols containing a hydroxyl group at one of six positions (5, 8, 9, 11, 12 or 15). Second, omega or omega-1 hydroxylases introduce a hydroxyl group at carbons 20 or 19, respectively, although minor activities with other oxidases can produce 16, 17 or 18 hydroxyl products. 20-HETE is pro-inflammatory and has largely detrimental functions, for example in increasing hypertension, in promoting systemic vasoconstriction and in tumour growth. It regulates vascular smooth muscle and endothelial cells by influencing their proliferation, migration, survival, and tube formation, acting via a specific G protein receptor (GPR75).
Third, cytochrome P450 oxidases can form a series of cis-epoxyeicosatrienoic acids (EETs) (14,15-, 11,12-, 8,9- and 5,6-EETs). Depending on the cytochrome 450 oxidase isoform, different EETs may predominate although most enzymes can produce all four isomers. The various EETs have major functions as autocrine and paracrine effectors in the cardiovascular and renal systems, which are believed to be largely beneficial. Because of the anti-hypertensive, fibrinolytic and anti-thrombotic properties of EETs, their presence in red blood cells has important implications for the control of circulation and the physical properties of the circulating blood. Both cis- and trans-EETs are synthesised and stored in erythrocytes, and they are produced and released in response to a low oxygen concentration as during exercise, for example.
EETs are rapidly metabolised in vivo to the corresponding dihydroxyeicosatrienoic acids (DHETs). This involves epoxide hydrolases, of which there are two isoforms, one membrane-bound and one soluble. The DHETs formed were once assumed to be inactivation products but now seem to have some biological activity of their own.
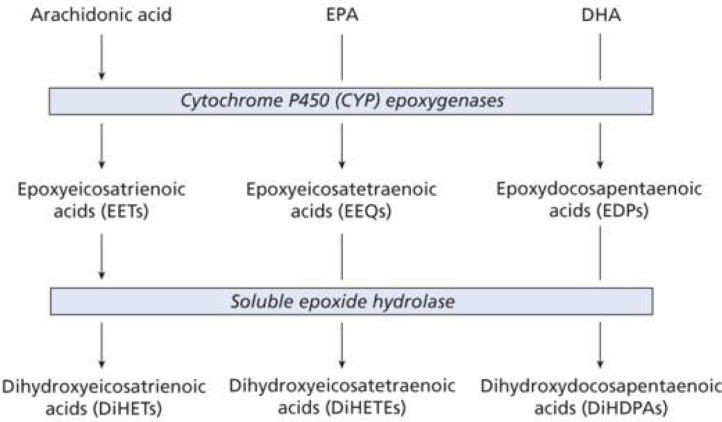
Historically, much of the original research with cytochrome P450 oxidases (as with cyclooxygenases and lipoxygenases) has focussed on ARA [48]. However, they have important activities with n-3 PUFAs such as EPA and DHA (Figure 8). In fact, the latter PUFAs are often the preferred substrates for some of the cytochrome P450 oxidases. Since their products often compete with metabolites from ARA, they can have beneficial effects, e.g. in alleviating pain. Such functions may account for some of the advantages of consuming significant dietary n-3 PUFAs. In addition, hydroxyoctadecadienoic acids (octadecanoids), which are formed by oxidation of linoleic acid, have a role in inflammation associated with important diseases, such as metabolic syndrome and cancer [7].
Figure 8. Action of CYP450 oxidases on EPA and DHA (taken from [2] with permission).
Specialised pro-resolving mediators
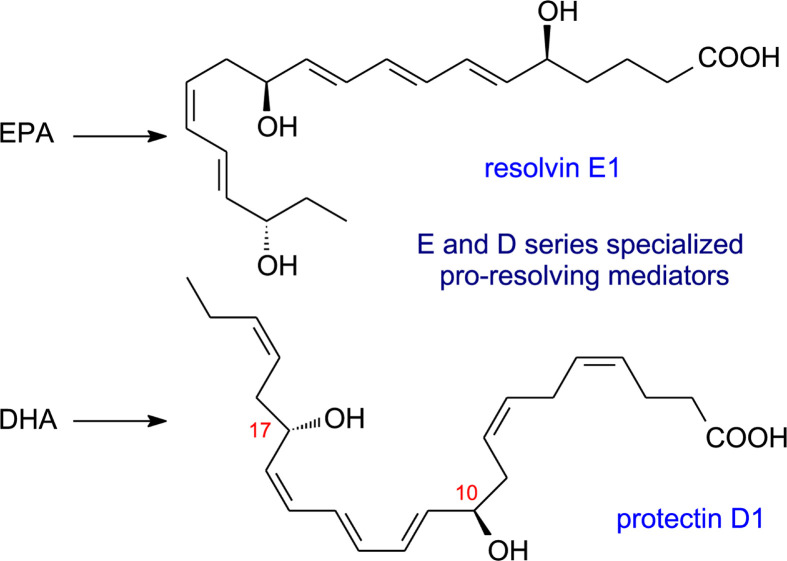
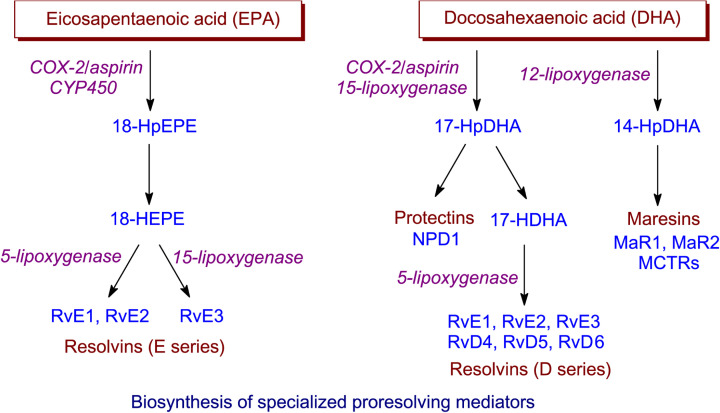
Specialised pro-resolving mediators (SPMs), so-called because of their intimate involvement in the resolution stage of inflammation, have been mentioned already when discussing lipoxins in connection with lipoxygenase action. Over the last 15 years there has been increasing interest in the discovery and, subsequently, elucidation of the biosynthesis and biological function of other SPMs—protectins, resolvins and maresins [51]. In contrast with lipoxins, which are formed from the n-6 PUFA ARA, these other three groups of lipid mediators have very long chain n-3 PUFAs as their precursors, i.e. EPA, n-3 docosapentaenoic acid (DPA) and DHA. Oxylipins derived from EPA are designated SPMs of the E series while those from DHA are SPMs of the D series (Figure 9). An overall picture of the formation of protectins, resolvins and maresins by dioxygen-dependent oxidation is shown in Figure 10, and it will be seen that the same three types of oxidising enzymes discussed previously are all involved but with a preponderance of lipoxygenase reactions.
Figure 9. Examples of specialised pro-resolving mediators (SPMs).
Figure 10. Biosynthesis of SPMs.
Protectins
When studies were first made of metabolite formation from DHA in brain tissue following aspirin treatment, new docosanoids were discovered. These were dihydroxylated E,E,Z-docosatrienes and were termed ‘neuroprotectins’ because of their protective action and their formation in nervous tissue such as brain [52] where DHA is a major fatty acid [53,54]. Since then, similar docosanoids have been found in many tissues so ‘protectins’ is the preferred nomenclature [55,56].
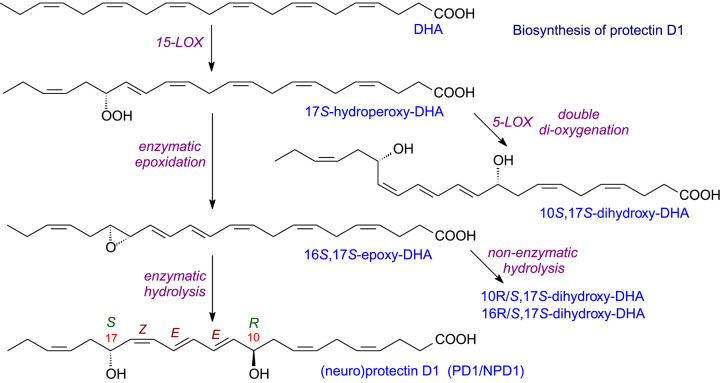
The biosynthetic pathway to protectin D1 (PD1, previously neuroprotection D1 or NPD1) is shown in Figure 11. Oxidation by 15-LOX yields a 17S-hydroperoxy-DHA that is converted into an epoxide and then hydrolysed. All the intermediates have very precise stereochemistry, which is also necessary for the biological activity of PD1. As illustrated in Figure 11, alternative reactions are possible via 5-LOX or non-enzymatic hydrolysis and these yield products which also have significant biological activity. Of interest is the reaction of 5-LOX which, when uncoupled from FLAP produces the SPM mediator (10S,17S-dihydroxy-DHA) rather than the inflammatory leukotriene B4. In addition to DHA, both 22:5(n-3) and 22:5(n-6) are good substrates for the 15-LOX. Their products as well as an omega-hydroxy analogue of PD1 (produced by CYP1) are all potent anti-inflammatory compounds.
Figure 11. Biosynthesis of protectin D1.
Aspirin-triggered protectins
Instead of the 17S intermediate produced by 15-LOX (Figure 11) in the production of PD1, aspirin treatment allows production of the 17R epimer (i.e. 10R,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid) from DHA. As mentioned before, aspirin is a classic inhibitor of COXs when it acetylates the active site. However, whereas it irreversibly inhibits COX-1, it only partially blocks the active site of COX-2, which retains lipoxygenase activity similar to that of 15-LOX but with the oxygen insertion in the R- rather than the S-configuration. With ARA as substrate the 15R-HETE is converted into lipoxins. The latter was the first type of lipid mediator known to start the resolution of inflammation. Thus, low-dose aspirin treatment will start resolution earlier than might be so otherwise (see chapter on ‘Specialised Pro-resolving Mediator Network’). Because most NSAIDs inhibit COXs reversibly, their use can delay complete resolution of inflammation.
Resolvins
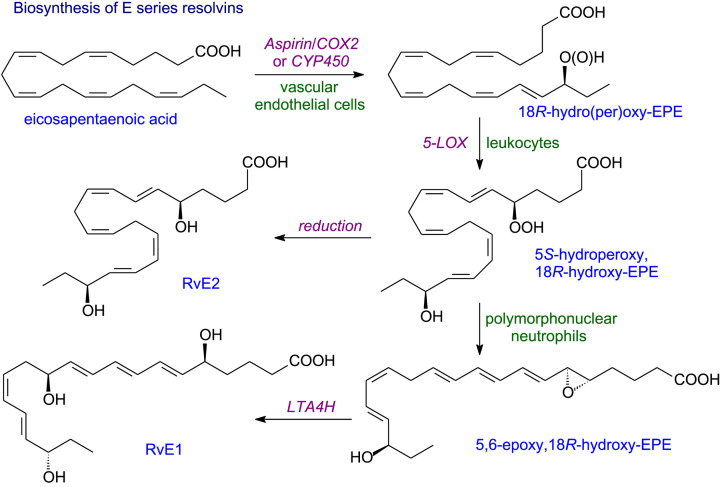
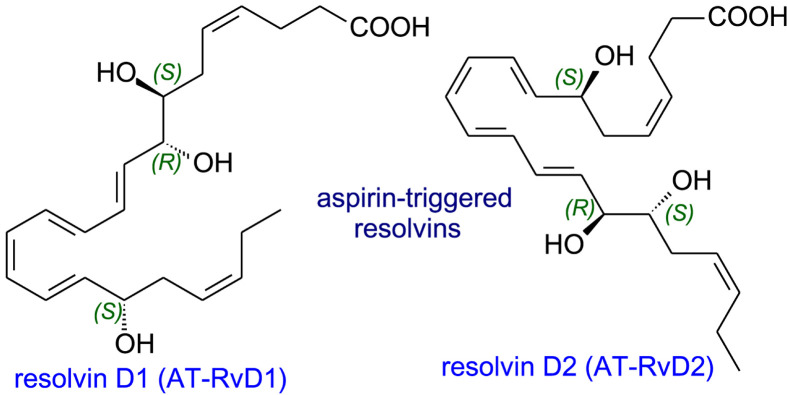
Resolvins (resolution phase interaction products) are produced from EPA, DPA and DHA and stop inflammation becoming chronic and, hence, prevent tissue damage and reduce the risk of a host of diseases arising [51,57]. COX-2 which has been acetylated by aspirin or a CYP 450 will introduce an 18R-hydroperoxy group into the EPA molecule (Figure 12). Reduction yields RvE2 while two steps are used for RvE1 formation. Since it is rare for all the enzymes needed to be found in a single cell, a trans-cellular sequence is needed as in the formation of lipoxins. Instead of EPA, DHA can be converted into 17R-resolvins by a similar aspirin-triggered COX-2 mechanism. The aspirin-triggered resolvins AT-RvD1 and AT-RvD2 (Figure 13) are formed when epoxidation takes place on 17R-hydroperoxy intermediate while a 5-LOX reaction with FLAP assistance forms AT-RvD3 or AT-RvD4.
Figure 12. Biosynthesis of E-series resolvins.
Figure 13. Aspirin-triggered resolvins.
Epimeric 18S-resolvins are also produced in vivo by related pathways with 15-LOX catalysing the first step (c.f. protectin formation) and these S-resolvins have their own distinct biological activities. Thus, in the absence of aspirin, 15-LOX generates 17S-hydroxyDHA which then goes on to form RvD1 and epimeric RvD2, both of which contain a 17S-hydroxyl group. A different intermediate from 17S-hydroxyDHA is transformed via an epoxide to RvD3 and RvD4. Both the products and intermediates from DHA have anti-inflammatory properties and two more resolvins, RvD5 and RvD6, have now been characterised.
In addition to EPA and DHA, DPA has been shown to be converted to three resolvins of which RvD1(n-3DPA) is the most abundant. Four further metabolites of DPA have a hydroxyl group at the 13-position and have been designated as 13-series resolvins (RvTs).
Tissue inactivation of resolvins has been studied best in the case of RvE1 and RVD1 where oxidation is key. For RvE1, at least four distinct oxidative pathways are involved.
Maresins
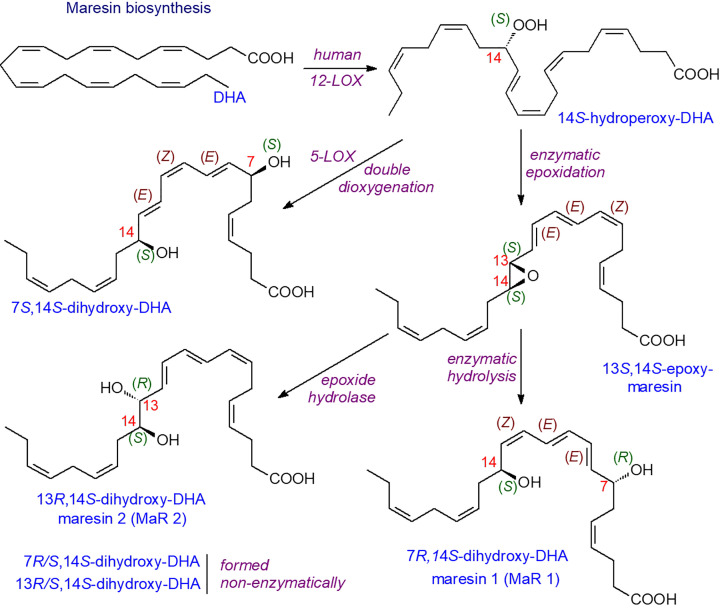
A single oxygenation by 12-LOX (in macrophages or platelets) to form a 14S-hydroperoxyDHA intermediate begins the formation of maresins (macrophage mediator in resolving inflammation) as illustrated in Figure 14 [58,59]. Some details of the pathway are still ill-defined but both MaR1 and MaR2 have potent anti-inflammatory and pro-resolving activities. Similar oxygenated compounds with anti-inflammatory properties are formed from 22:5(n-3) and 22:5(n-6) fatty acids.
Figure 14. Biosynthesis of maresins.
Some maresin-like di-oxygenated metabolites produced by sequential oxidation by 12-LOX and enzymes of the CYP450 family can occur in macrophages. For example, 14S, 21S-dihydroxy-docosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid and its epimers can be produced. This compound is induced by wounding and has been shown to promote wound healing. Similar 14, 22-dihydroxy metabolites are synthesised in leukocytes and platelets and also promote wound healing.
Sulphido-peptide conjugated mediators (analogous to cysteinyl-leukotrienes) have been detected for the SPMs in macrophages, initially for maresins and then for protectins and resolvins [51]. The various compounds have been detected in lymph nodes, serum and milk in humans and shown to regulate bacterial clearance and the repair and regeneration of damaged tissues.
Biological activities of SPMs
Given the diverse nature of SPMs, it is hardly surprising that they can give rise to a variety of biological effects. In general, their action is via specific G-protein coupled receptors which can lead to both rapid and long-term actions. Moreover, SPMs can antagonise pro-inflammatory receptors such as the leukotriene B4 receptor, BLT1.
As mentioned before, acute inflammation is a vital response to infection or tissue damage. However, the time scale needs to be kept as short as needed and chronic inflammation avoided. Indeed, occurrence of the latter will lead almost inevitably to tissue damage and loss of function. Under normal circumstances compounds involved in inflammation may initiate the resolution phase. For example, leukotriene LTB4 and prostaglandins, PGE2 and PGD2, will stimulate induction of 15-LOXs needed for later production of SPMs.
Early in inflammation there is a change in ARA metabolism from synthesis of leukotrienes to lipoxin formation. Local mobilisation of n-3 PUFAs, such as DHA, occurs followed by production of SPMs. As such mediators are formed, macrophages and mast cells remove excess neutrophils together with cell debris resulting from microorganisms and host defences. A noted ancillary effect is that low dose aspirin facilitates the resolution of inflammation by enhancing conversion of EPA and DHA to resolvins of the E and D series.
While the resolution process is on-going, a change in the phenotype of macrophages towards a pro-resolution state occurs. Initially, at the onset of inflammation, macrophages counter disease by removing invading pathogens by phagocytosis. However, these actions can cause trauma and tissue damage so SPMs, like lipoxins, have an important role in regulating and inhibiting these effects. Thus, RvE1 dramatically reduces dermal inflammation, peritonitis and interleukin production. A measure of its effectiveness is that it reduces inflammatory pain better than morphine! Furthermore, RvE2 has been shown to effectively reduce joint pain in arthritis. Similar beneficial effects of resolvins from DHA have been found (RvD2 ameliorating bacterial sepsis, RvD3 active in later stages of resolution, RvD4 helping clearance of apoptotic cells by skin fibroblasts).
The protectins have similar effects to resolvins but mainly in brain tissue [60–62]. They promote resolution of neuroinflammation and stimulate nerve regeneration. In animal models. PD1 has protective effects against stroke and Alzheimer’s disease. In non-neuronal tissues, PD1 promotes apoptosis of T cells, is beneficial towards asthma and may have potential in slowing viral replication. Protectins synthesised in white adipose tissue have anti-inflammatory effects on obesity and diabetes.
Maresin 1 is a powerful regulator of the resolution of inflammation, tissue regeneration and pain [62]. Notable effects are in lung, with vascular and metabolic diseases and bacterial infections. Maresins have been found especially important in tissue regeneration and wound healing in the latter stages of resolution. Indeed, they have been used successfully for specific surgical interventions.
Although SPMs are produced locally, they also reach the circulation and, therefore, may have effects in tissues other than where they are synthesised. Notably, SPMs have been found in bioactive concentrations in human milk and placenta. So, they may function in normal physiological development.
We have already highlighted the importance of SPMs in preventing chronic inflammatory diseases [63,64]. As a result of basic scientific knowledge, SPMs have been tested in a wide range of experimental models including peritonitis, colitis, arthritis, psoriasis, dry eye, cardiovascular disease, asthma and some cancers (see [65]). Although further clinical trials are necessary, it is hoped that the use of SPMs may be efficacious for many low-level inflammatory diseases. Particular attention is being paid to obesity [66], including insulin resistance, Type 2 diabetes, metabolic disease [67,68], non-alcoholic liver disease and cardiovascular complaints [69]. Overall, treatment of patients with a diet containing increased levels of very long chain n-3 PUFAs, together with low-dose aspirin may be a cost-effective method to ameliorate the clinical symptoms of many important disorders where inflammation is involved.
Other oxidised lipids with biological activities
In recent years, it has become apparent that various other oxidised lipids can be formed by both enzymatic and non-enzymatic means. For example, isoprostanes, neuroprostanes and phytoprostanes [70] are covered in a later chapter. Enzymatically oxidised phospholipids can be produced by reactions in which eicosanoids are attached in immune cells. Such lipids are represented by various chemically distinct families with important bioactivities. Lipoxygenase activities are usually involved in the formation of these oxidised phospholipids [71–73] which have important regulatory roles in both health and disease. For example, they have been implicated in ferroptosis, apoptosis, blood clotting, arthritis, diabetes and cardiovascular disease [74].
Production of VLCPUFAs from dietary essential fatty acids
The VLCPUFAs (>18C) which are almost the exclusive source of the lipid mediators discussed above are not produced in higher plants [75,76]. Instead, the vast majority are synthesised by marine microalgae at the base of the marine food web [77]. Nevertheless, even this supply is currently under threat due to climate change [4,78]. For humans, the precursor essential fatty acids (LA, ALA) can be converted to 20 and 22C PUFA but rather inefficiently [3,54]. Thus, a dietary supply of EPA and DHA is considered necessary (‘conditionally essential’) under certain circumstances [3] and, indeed, is considered desirable for all individuals, but especially for the newborn [79–82].
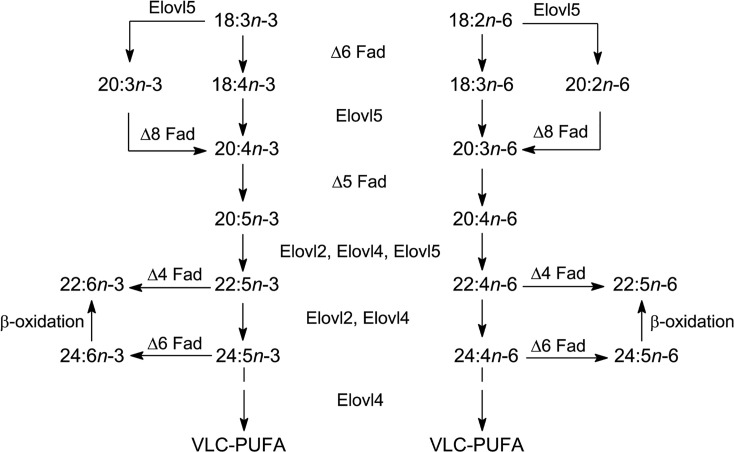
The essential fatty acids, LA and ALA are produced in photosynthetic organisms by specific desaturase enzymes (e.g. FAD3 and FAD7/8 for ALA). Then a series of desaturation and elongation reactions will convert LA to ARA or ALA to EPA and then DHA in both humans and fish (the usual source of VLCPUFA in our diets) (Figure 15). The most direct route to convert EPA to DHA is via an elongation to 22:5 and a Δ4-desaturase. However, Δ4-desaturase activity could not be detected in mammalian tissues and so the ‘Sprecher pathway’ involving a 24:6 intermediate followed by beta-oxidation was proposed (see [83]). A recent article has suggested a re-visiting of the situation [84] but, nevertheless, the Sprecher pathway seems to be key in humans and, with the exception of a few teleost species, in fish also [85]. Thus, because fish are the main dietary source of n-3 VLCPUFA for humans, it is important that they contain adequate levels of EPA and DHA, and oily fish sources should be from capture fisheries or from aquaculture with an adequate supply of n-3 VLCPUFA in the feed. Since aquaculture now supplies more than half the fish used [86] and some 75% of marine-sourced fish oils are currently used in aquaculture [87], it is easy to see that there is currently a serious problem that will only get worse as demand for EPA and DHA increases in the future [88].
Figure 15. Conversion of the essential fatty acids to 20- or 22-carbon PUFAs (re-drawn from [90] with permission).
For the conversion of LA and ALA into VLCPUFA, the same enzymes operate for both the n-3 and n-6 pathways (Figure 15). This means that the dietary ratio of n-6/n-3 PUFAs (mainly LA/ALA) in human diets is important. While many ‘Western diets’ have a ratio of over 10, for good health a ratio of 3-4 is recommended [4,54,77]. Furthermore, for some teleost fish the lack of a Δ5-desaturase means that conversion of ALA to EPA is not possible [89]. It should also be noted that expression of the necessary enzymes for VLCPUFA biosynthesis in fish is controlled by the diet [90].
In view of the problems faced in maintaining levels of EPA and DHA in fish being consumed by humans, alternative sources of these acids for aquaculture or, indeed, directly for human consumption have been sought. Thus, crops have been manipulated to allow the biosynthesis of EPA and/or DHA [88,91]. Two methods have been used. First, a polyketide synthase system was used to engineer oilseed rape to yield DHA [92]. This strategy mimicked the enzyme used by Schizochytrium to make the commercially successful oils used for infant formulations [93]. Second, various combinations of genes (mostly from microalgae) have been used to allow different higher plant systems to make EPA and/or DHA [94,95]. The success of these efforts in oilseed crops, especially oilseed rape (Canola) has been discussed [88]. Currently, there are a number of commercially available (or potentially available) new sources of EPA and DHA (see [88]).
As has been discussed before, adequate dietary n-3 VLCPUFAs has important implications for good health [96] and in the prevention of important complaints and illnesses [54,97–99]. These considerations will ensure that supply of the direct precursors (ARA, EPA, DHA) for lipid mediators will remain an important topic for years to come.
Plant oxylipins
In plants LA and, especially, ALA give rise to bioactive molecules which are very important for functions such as in stress responses and development [100]. The ‘lipoxygenase pathway ‘is initiated by lipoxygenase (9-LOX or 13-LOX) attack on a PUFA which, in leaves, is mainly ALA. LOX enzymes in plants are stable proteins, often present in high amounts, especially in leaves.
The fatty acid hydroperoxides that are produced by plant LOXs can, themselves, be metabolised to yield three types of derivatives. By a co-oxidation with peroxidase they can form a mixture of epoxy and hydroxy fatty acids that have roles in cutin formation. In contrast, the hydroperoxides can be cleaved by hydroperoxide lyase to give an aldehyde and an oxo-unsaturated fatty acid. The emission of C6 aldehydes and alcohols occurs rapidly in plants in response to wounding, and they contribute to protection against the invasion of fungi and insects, while taking part in abiotic stress responses by inducing the expression of stress-associated genes.The third reaction with hydroperoxides is by allene oxide synthase. This forms 12-oxophytodienoic acid which is the precursor for jasmonic acid and other jasmonates (with intriguing structural similarities to the cyclopentenone prostaglandins). These important effectors of growth, development and senescence are described in detail in a later chapter (‘Jasmonates’). Some recent updates on jasmonate functions can be found in a special edition of Plant and Cell Physiology [101–106].
Fatty acid esters of hydroxy fatty acids (FAHFA)
A range of unesterified fatty acids containing a hydroxyl group to which a further fatty acid is esterified have been found in the adipose tissue, serum, milk and many other tissues of mice and humans. These include several regio-isomers of the hydroxyl component as well as many different ester-linked fatty acids [107]. While little is yet known of the biosynthesis of these lipids, it has been established that they are produced endogenously with defined stereochemistry, i.e. the hydroxyl group has the R-configuration and peroxisomal enzymes are believed to be involved. Adipose tissue triacylglycerols act as a reservoir for these compounds, which have been classed as lipokines, i.e. lipid molecules derived from adipose tissue that act as hormonal regulators and coordinate an array of cellular processes.
Among those biological activities described to date, FAHFA have anti-diabetic and anti-inflammatory effects, even when administered orally and they protect against colitis by regulating gut innate and adaptive immune responses. They are believed to have an important role in maintaining normal blood sugar levels and insulin sensitivity, possibly by acting as selective agonists for the GPR40 and GPR120 receptors [108].
FAHFA have recently been reported in plants also [108] and have been comprehensively reviewed recently [109].
Summary
Oxylipins, formed from n-3 and n-6 polyunsaturated fatty acids, are important lipid mediators.
Three types of oxidase (cyclooxygenases, lipoxygenases, cytochrome P450 oxidases) are involved in their formation.
Oxylipins bind to receptors, are rapidly catabolised and usually act where they are produced—-as ‘local’ hormones.
Oxylipins have multiple functions in mammalian health and disease, as well as in other animals and plants.
Abbreviations
- ALA
α-linolenic
- ARA
arachidonic
- DHA
docosahexaenoic
- EFA
essential fatty acid
- EPA
eicosapentaenoic
- FAHFA
fatty acid esters of hydroxy fatty acids
- LA
linoleic
- PUFA
polyunsaturated fatty acid
- SPM
specialised pro-resolving mediator
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Open Access
Open access for this article was enabled by the participation of Cardiff University in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contribution
This is a joint publication, J.H and W.C equally contributed to the text and created the figures.
References
- 1.Spector A. and Kim H.-Y. (2015) Discovery of essential fatty acids. J. Lipid Res. 56, 11–21 10.1194/jlr.R055095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurr M.I., Harwood J.L., Frayn K.N., Murphy D.J. and Michell R.H. (2016) Lipids: biochemistry, biotechnology and health, 6th edn, Wiley/Blackwell, Oxford [Google Scholar]
- 3.Cunnane S.C. (2003) Problems with essential fatty acids: time for a new paradigm? Prog. Lipid Res. 42, 544–568 10.1016/S0163-7827(03)00038-9 [DOI] [PubMed] [Google Scholar]
- 4.Harwood J.L. (2019) Algae: critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 9, 708 10.3390/biom9110708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuelsson B. (2012) Role of basic science in the development of new medicines: examples from the eicosanoid field. J. Biol. Chem. 287, 10070–10080 10.1074/jbc.X112.351437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendal A.C., Kiezel-Tsugunova M., Brownbridge L.C., Harwood J.L. and Nicolaou A. (2017) Lipid functions in skin: differential effects of n-3 polyunsaturated fatty acids on cutaneous ceramides in a human skin organ culture model. Biochim. Biophys. Acta 1859, 1679–1689 10.1016/j.bbamem.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vangaveti V.N., Jansen H., Kennedy R.L. and Malabu U.H. (2016) Hydroxyoctadecadienoic acids: oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 785, 70–76 10.1016/j.ejphar.2015.03.096 [DOI] [PubMed] [Google Scholar]
- 8.Smith M.L. and Murphy R.C. (2016) The eicosanoids: cyclooxygenase, lipoxygenase and epoxygenase pathways. In Biochemistry of Lipids, Lipoproteins and Membranes 6th edn.(Ridgeway N.D. and McLeod R.S., eds), pp. 260–296, Elsevier, Amsterdam [Google Scholar]
- 9.Dennis E.A., Cao J., Hsu Y.H., Magrioti V. and Kokotos G. (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition and therapeutic interventions. Chem. Rev. 111, 6130–6185 10.1021/cr200085w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorman R.V., Toews A.D. and Horrocks L.A. (1977) Plasmalogenase activities in neuronal perikarya, astroglia and oligodendroglia isolated from bovine brain. J. Lipid Res. 18, 115–117 [PubMed] [Google Scholar]
- 11.Jenkins C.M., Yang K., Liu G., Moon S.H., Dilthey B.G. and Gross R.W. (2018) Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. J. Biol. Chem. 293, 8693–8709 10.1074/jbc.RA117.001629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leslie C.C. (2015) Cytosolic phospholipase A2; physiological function and role in disease. J. Lipid Res. 56, 1386–1402 10.1194/jlr.R057588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith W.L., Urade Y. and Jakobsson P.-J. (2011) Enzymes of the cyclooxygenase pathways of prostaglandin biosynthesis. Chem. Rev. 111, 5821–5865 10.1021/cr2002992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouzer C.A. and Marnett L.J. (2009) Cyclooxygenases: structural and functional insights. J. Lipid Res. 50, S29–S34 10.1194/jlr.R800042-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laneuville O., Breuer D.K., Xu N., Huang Z.H., Gage D.A., Watson J.T. et al. (1995) Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. J. Biol. Chem. 270, 19330–19336 10.1074/jbc.270.33.19330 [DOI] [PubMed] [Google Scholar]
- 16.Smith W.L. and Langenbach R. (2001) Why are there two cyclooxygenase isozymes? J.Clin. Invest. 107, 1491–1495 10.1172/JCI13271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo M.-J. and Oh D.-K. (2017) Prostaglandin synthases; molecular characterization and involvement in prostaglandin biosynthesis. Prog. Lipid Res. 66, 50–68 10.1016/j.plipres.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 18.Stritham J., Midgett C., Martin K.A. and Hwa J. (2011) Prostacyclin: an inflammatory paradox. Front. Pharmacol. 2, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pluchart H., Khouri C., Blaise S., Roustit M. and Cracowski J.-L. (2017) Targeting the prostacyclin pathway: beyond pulmonary arterial hypertension. Trends Pharm. Sci. 38, 512–523 10.1016/j.tips.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 20.Paul B.Z.S., Jin J. and Kunapuli S.P. (1999) Molecular mechanism of thromboxane A2-induced platelet aggregation. J. Biol. Chem. 274, 29108–29114 10.1074/jbc.274.41.29108 [DOI] [PubMed] [Google Scholar]
- 21.Smyth E.M. (2010) Thromboxane and the thromboxane receptor in cardiovascular disease. Clin. Lipidol. 5, 209–219 10.2217/clp.10.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell J.A. and Kirkby N.S. (2019) Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Brit. J. Pharm. 176, 1038–1050 10.1111/bph.14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata T. and Narumiya S. (2011) Prostanoid receptors. Chem. Rev. 111, 6209–6230 10.1021/cr200010h [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto Y., Inazumi T. and Tsuchiya S. (2015) Roles of prostaglandin receptors in female reproduction. J. Biochem. 157, 73–80 10.1093/jb/mvu081 [DOI] [PubMed] [Google Scholar]
- 25.Prostanoid receptors—G-protein-coupled receptors-UPHAR/BPS Guide to Pharmacology. www.guidetopharmacology.org [Google Scholar]
- 26.Matsuoka T. and Narumiya S. (2007) Prostaglandin receptor signalling in disease. Sci. World J. 7, 1329–47 10.1100/tsw.2007.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer J., Ripperger A., Frantz S., Ergun S., Schwedhelm E. and Benndorf R.A. (2014) Pathophysiology of isoprostanes in the cardiovascular system: implications of isoprostane-mediator thromboxane A2 receptor activation. Brit. J. Pharmacol. 171, 3115–3131 10.1111/bph.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye R.D., Boulay F., Wang J.M., Dahlgren C., Gerard C., Parmentier M. et al. (2009) Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 10.1124/pr.109.001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serhan C.N., Chiang N., Dalli J. and Levy B.D. (2015) Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 4, a016311 10.1101/cshperspect.a016311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravina E. (2011) The Evolution of Drug Discovery; from traditional medicine to modern drugs, John Wiley, ISBN 978-3-527-32669-3 [Google Scholar]
- 31.Montinari M.R., Minelli S. and De Caterin R. (2019) The first 3500 years of aspirin history from its roots - A concise summary. Vasc. Pharm. 113, 1–8 10.1016/j.vph.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 32.Limongelli V., Bonomi M., Marinelli L., Gervasio F.L., Cavalli A., Novellino E. et al. (2010) Molecular basis of cyclooxygenase enzumes (COXs) selective inhibition. Proc. Natl. Acad. Sci., U.S.A. 23, 5411–5416 10.1073/pnas.0913377107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurumbail R.G., Stevens A.M., Gierse J.K., McDonald J.J., Stegeman R.A., Pak J.Y. et al. (1996) Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 384, 644–648 10.1038/384644a0 [DOI] [PubMed] [Google Scholar]
- 34.Zarghi A. and Arfaei S. (2011) Selective COX-2 inhibitors: a review of their structure-activity relationships. Iranian J. Pharm. Res. 10, 655–683 [PMC free article] [PubMed] [Google Scholar]
- 35.Nissen S.E., Yeomans N.D., Soloman D.H. et al. (2016) Cardiovascular safety of celecoxib, naproxen or ibuprofen for arthritis. New Engl. J. Med. 375, 2519–2529 10.1056/NEJMoa1611593 [DOI] [PubMed] [Google Scholar]
- 36.Horn T., Adel S., Schumann R., Sur S., Kakularam K.R., Polamarasetty A. et al. (2015) Evolutionary aspects of lipoxygenases and genetic diversity of human leukotriene signaling. Prog. Lipid Res. 57, 13–39 10.1016/j.plipres.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rådmark O., Werz O., Steinhilber D. and Samuelsson B. (2015) 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta 1851, 331–339 10.1016/j.bbalip.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 38.Haeggström J.Z. (2018) Leukotriene biosynthetic enzymes as therapeutic targets. J. Clin. Invest. 128, 2680–2690 10.1172/JCI97945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan M., Tang X., Stsiapanava A. and Haeggström J.Z. (2017) Biosynthesis of leukotriene B4. Semin. Immunol. 33, 3–15 10.1016/j.smim.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 40.Jo-Watanabe A., Okuna T. and Yokomizo T. (2019) The role of leukotrienes as potential therapeutic targets in allergic disorders. Int. J. Mol. Sci. 20, 3580 10.3390/ijms20143580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandrasekharan J.A. and Sharma-Walia N. (2015) Lipoxins: nature's way to resolve inflammation. J. Inflamm. Res. 8, 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capra V., Rovati G.E., Mangano P., Buccellati C., Murphy R.C. and Sala A. (2015) Transcellular biosynthesis of eicosanoid lipid mediators. Biochim. Biophys. Acta 1851, 377–382 10.1016/j.bbalip.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 43.Feltenmark S., Gautam N., Brunnström A., Griffiths W., Backman L., Edenius C. et al. (2008) Eoxins are proinflammatory arachidonic metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc. Natl. Acad. Sci. U.S.A. 105, 680–685 10.1073/pnas.0710127105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pace-Asciak C.R. (2015) Pathophysiology of the hepoxilins. Biochim. Biophys. Acta 1851, 383–396 10.1016/j.bbalip.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 45.Muñoz-Garcia A., Thomas C.P., Keeney D.S., Zheng Y. and Brash A.R. (2014) The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochim. Biophys. Acta 1841, 401–408 10.1016/j.bbalip.2013.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krieg P. and Furstenberger G. (2014) Role of lipoxygenases in epidermis. Biochim. Biophys. Acta 1841, 390–400 10.1016/j.bbalip.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 47.Arnold C., Konkel A., Fischer R. and Schunck W.-H. (2010) Cytochrome P450-dependent metabolism of ω-6 and ω-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 62, 536–547 10.1016/S1734-1140(10)70311-X [DOI] [PubMed] [Google Scholar]
- 48.Capdevila J.H. and Falck J.R. (2018) The arachidonic acid monooxygenase: from biochemical curiosity to physiological/pathophysiological significance. J. Lipid Res. 59, 2047–2062 10.1194/jlr.R087882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeldin D.C. (2001) Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 276, 36059–62 10.1074/jbc.R100030200 [DOI] [PubMed] [Google Scholar]
- 50.Spector A.A. and Kim H.-Y. (2015) Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta 1851, 356–365 10.1016/j.bbalip.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalli J. (2017) The physiology and pharmacology of specialized pro-resolving mediators. Mol. Aspects Med. 58, 1–130, (special journal issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bazan N.G. (2018) Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol. Aspects Med. 64, 18–33 10.1016/j.mam.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Axelsen P.H. and Murphy R.C. (2010) Quantitative analysis of phospholipids containing arachidonate and docosahexaenoate chains in microdissected regions of mouse brain. J. Lipid Res. 51, 660–671 10.1194/jlr.D001750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall J.H. and Harwood J.L. (2017) Brain lipids in health and disease. In Food Lipids 4th edn(Akoh C.C., ed.), pp. 747–764, CRC Press, Boca Raton [Google Scholar]
- 55.Ariel A., Li P.-L., Wang W., Tang W.-X., Fredman G., Hong S. et al. (2005) The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apotoses via lipid raft clustering. J. Biol. Chem. 280, 43079–43086 10.1074/jbc.M509796200 [DOI] [PubMed] [Google Scholar]
- 56.Balas L. and Durand T. (2016) Dihydroxylated E,E,Z-docosatrienes. An overview of their synthesis and biological significance. Prog. Lipid Res. 61, 1–18 10.1016/j.plipres.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 57.Basil M.C. and Levy B.D. (2016) Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nature Rev. Immunol. 16, 51–67 10.1038/nri.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilroy D.W. and Bishop-Bailey D. (2019) Lipid mediators in immune regulation and resolution. Brit. J. Pharm. 176, 1009–1023 10.1111/bph.14587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang S., Wan M., Huang W., Stanton R.C. and Xu Y. (2018) Maresins: specialized proresolving lipid mediators and their potential role in inflammatory-related diseases. Mediators Inflamm. 2018, article 2380319 10.1155/2018/2380319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalli J. and Serhan C.N. (2019) Identification and structure elucidation of the pro-resolving mediators provides novel leads for resolution pharmacology. Brit. J. Pharm. 176, 1024–1037 10.1111/bph.14336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y., Calon F., Julien C., Winkler J.W., Petasis N.A., Lukiw W.J. et al. (2011) Docosahexaenoic acid-derived neuroprotection D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer's disease models. PLoS ONE 6, e15816 10.1371/journal.pone.0015816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serhan C.R., Dalli J., Colas R.A., Winkler J.W. and Chiang N. (2015) Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 1851, 397–413 10.1016/j.bbalip.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qu Q., Xuan W. and Fan G.-H. (2015) Roles of resolvins in the resolution of acute inflammation. Cell Biol. Int. 39, 3–22 10.1002/cbin.10345 [DOI] [PubMed] [Google Scholar]
- 64.Serhan C.N. and Levy B.D. (2018) Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669 10.1172/JCI97943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moro K., Nagahishi M., Ramanathan R., Takabe K. and Wakai T. (2016) Resolvins and omega three polyunsaturated fatty acids: clinical implications in inflammatory diseases and cancer. World J. Clin. Cases 4, 155–164 10.12998/wjcc.v4.i7.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen T.V., Vik A. and Serhan C.N. (2019) The protectin family of pro-resolving mediators: potent immunoresolvents enabling innovative approaches to target obesity and diabetes. Front. Pharm. 9, 1582 10.3389/fphar.2018.01582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.López-Vicario C., Rius B., Alcarez-Quiles J., García-Alonso V., Lopategi A., Titos E. et al. (2016) Pro-resolving mediators produced from EPA and DHA: overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol. 785, 133–143 10.1016/j.ejphar.2015.03.092 [DOI] [PubMed] [Google Scholar]
- 68.Clària J., López-Vicario C., Rius B. and Titos E. (2017) Pro-resolving actions of SPM in adipose-tissue biology. Mol. Aspects Med. 58, 83–92 10.1016/j.mam.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 69.Kasikara C., Doran A.C., Cai A.S. and Tabas I. (2018) The role of non-resolving inflammation in atherosclerosis. J. Clin. Invest. 128, 2713–2723 10.1172/JCI97950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galano J.-M., Lee Y.Y., Oger C., Vigor C., Vercauteren J., Durand T. et al. (2017) Isoprostanes, neuroprostanes and phytoprostanes: an overview of 25 years of research in chemistry and biology. Prog. Lipid Res. 68, 83–108 10.1016/j.plipres.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 71.Clark S.R., Guy C.J., Scurr M.J., Taylor P.R., Kift-Morgan A.P., Hammond V.J. et al. (2011) Esterified eicosanoids are acutely generated by 5-lipoxygenase in primary human neutrophils and in human and murine infection. Blood 117, 2033–2043 10.1182/blood-2010-04-278887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Donnell V.B. and Murphy R.C. (2012) New families of oxidised phospholipids generated by immune cells. Identification and signalling actions. Blood 120, 1985–1992 10.1182/blood-2012-04-402826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banthiya S., Pekarova M., Kuhn H. and Haydeck D. (2015) Secreted lipoxygenase from Pseudomonas aeruginosa exhibits biomembrane oxygenase activity and induces hemolysis in human red blood cells. Arch. Biochem. Biophys. 584, 116–124 10.1016/j.abb.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 74.O'Donnell V.B., Aldrovandi M., Murphy R.C. and Krönke G. (2019) Enzymatically oxidized phospholipids assume center stage as essential regulators of innate immunity and cell death. Sci. Signal. 12, eaau2293, pii 10.1126/scisignal.aau2293 [DOI] [PubMed] [Google Scholar]
- 75.Harwood J.L. (1996) Recent advances in the biochemistry of plant fatty acids. Biochim. Biophys. Acta 1301, 7–56 10.1016/0005-2760(95)00242-1 [DOI] [PubMed] [Google Scholar]
- 76.Gunstone F.D., Dijkstra A. and Harwood J.L.(eds). (1996) The Lipid Handbook, 3rd ednTaylor and Francis, Boca Raton [Google Scholar]
- 77.Li-Beisson Y., Thelen J.J., Fedosejevs E. and Harwood J.L. (2019) The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 74, 31–68 10.1016/j.plipres.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 78.Colombo S.M., Rodgers T.F.M., Diamond M.L., Bazinet R.P. and Arts M.T. (2020) Projected declines in global DHA availability for human consumption as a result of global warming. Ambio 49, 865–880 10.1007/s13280-019-01234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aranceta J. and Pérez-Rodrigo C. (2012) Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids. Br. J. Nutr. 107, S8–S22 10.1017/S0007114512001444 [DOI] [PubMed] [Google Scholar]
- 80.Richter C.K., Skulas-Ray A.C. and Kris-Etherton P.M. (2016) Recommended intake of fish and fish oils worldwide. In Fish and Fish Oil in Health and Disease Prevention(Raatz S.K. and Bibus D.M., eds), pp. 27–48, Academic Press, Elsevier, New York [Google Scholar]
- 81.Global Recommendations for EPA and DHA intake (Rev. 19th Nov. 2014). www.goedomega3.com/index.php/files/download/304 [Google Scholar]
- 82.Koletzko B., Bregmann K., Brenna J.T., Calder P.C., Campoy C., Clandinin M.T. et al. (2020) Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Pediatrics and the Child Health Foundation Am. J. Clin. Nutr. 111, 10–16 [DOI] [PubMed] [Google Scholar]
- 83.Sprecher H. (2000) Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta 1486, 219–231 10.1016/S1388-1981(00)00077-9 [DOI] [PubMed] [Google Scholar]
- 84.Metherel A.H. and Bazinet R.P. (2019) Updates to the n-3 polyunsaturated fatty acid biosynthesis pathway: DHA synthesis rates, tetracosahexanoic acid and (minimal) retroconversion. Prog. Lipid Res. 76, 101008 10.1016/j.plipres.2019.101008 [DOI] [PubMed] [Google Scholar]
- 85.Castro L.F.C., Tocher D.R. and Monroig O. (2016) Long-chain polyunsaturated fatty acid biosynthesis in chordates: insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 62, 25–40 10.1016/j.plipres.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 86.Food and Agriculture Organisation (FAO) (2016) The State of the World Fisheries and Agriculture 2016. Contributing to Food Security and Nutrition for All Rome: FAO, 200 ISBN 978-92-5-109185-2 [Google Scholar]
- 87.IFFO (2017) The Marine Ingredients Organisation: Fishmeal and Fish Oil Statistical Yearbook 2016. www:ffo.net [Google Scholar]
- 88.Tocher D.R., Betancor M.B., Sprague M., Olsen R.E. and Napier J.A. (2019) mega-3 long-chain polyunsaturated fatty acids, EPA and DHA: bridging the gap between supply and demand. Nutrients 11, E89 10.3390/nu11010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tocher D.R. (2010) Fatty acid requirements in ontogeny of marine and freshwater fish. Aquacult. Res. 41, 717–732 10.1111/j.1365-2109.2008.02150.x [DOI] [Google Scholar]
- 90.Tocher D.R. (2015) Omega-3 long chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449, 94–107 10.1016/j.aquaculture.2015.01.010 [DOI] [Google Scholar]
- 91.Napier J.A., Olsen R.-E. and Tocher D.R. (2019) Update on GM canola crops as novel sources of omega-3 fish oils. Plant Biotech. J. 17, 703–705 10.1111/pbi.13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walsh T.A., Bevan S.A., Gachotte D.J., Larsen C.M., Moskal W.A., Merlo P.A. et al. (2016) Canola engineered with a microalgal polyketide synthase-like system produces oil enriched in docosahexaenoic acid. Nat. Biotechnol. 34, 881–887 10.1038/nbt.3585 [DOI] [PubMed] [Google Scholar]
- 93.Ratledge C. and Cohen Z.(eds). (2010) Single Cell Oils: microbial and algal oils, 2nd ednAOCS Press, Urbana, Ill., U.S.A. [Google Scholar]
- 94.Petrie J.R., Shretha P., Zhou X-R., Mansour M.P., Liu Q., Belide S. et al. (2012) Metabolic engineering plant seeds with fish-oil like levels of DHA. PLoS ONE 7, e49165 10.1371/journal.pone.0049165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiz-Lopez N., Haslem R.P., Usher S.L., Napier J.A. and Sayanova O. (2014) Successful high level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oil-seed crop. Plant J. 77, 198–208 10.1111/tpj.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calder P.C. (2018) Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc. Nutr. Soc. 77, 52–72 10.1017/S0029665117003950 [DOI] [PubMed] [Google Scholar]
- 97.Innis S.M. (2007) Dietary (n-3) fatty acids and brain development. J.Nutr. 137, 855–859 10.1093/jn/137.4.855 [DOI] [PubMed] [Google Scholar]
- 98.Campoy C., Escolano-Margaret V., Anjos T., Szajewska H. and Uauy R. (2012) Omega-3 fatty acids on child growth, visual acuity and neurodevelopment: a systematic review. Br. J. Nutr. 107, S85–S106 10.1017/S0007114512001493 [DOI] [PubMed] [Google Scholar]
- 99.Dalago-Lista J., Perez-Martinez P., Lopez-Miranda J. and Perez-Jimenez F. (2012) Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br. J. Nutr. 107, S201–S213 10.1017/S0007114512001596 [DOI] [PubMed] [Google Scholar]
- 100.Wasternack C. and Feussner I. (2018) The oxylipin pathways: biochemistry and function. Annu. Rev. Plant Biol. 69, 363–386 10.1146/annurev-arplant-042817-040440 [DOI] [PubMed] [Google Scholar]
- 101.Goosens A. and Farmer E.E. (2019) Regulatory oxylipins anno 2019: jasmonates galore in the plant oxylipin research community. Plant Cell Physiol. 60, 2609–2612 10.1093/pcp/pcz197 [DOI] [PubMed] [Google Scholar]
- 102.Savchenko T.V., Rolletschek H. and Dehesh K. (2019) Jasmonates-mediated rewiring of central metabolism regulates adaptive responses. Plant Cell Physiol. 60, 2613–2620 10.1093/pcp/pcz181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heitz T., Smirnova E., Marquis V. and Poirier L. (2019) Metabolic control within the jasmonate biochemical pathway. Plant Cell Physiol. 60, 2621–2628 10.1093/pcp/pcz172 [DOI] [PubMed] [Google Scholar]
- 104.Mielke S. and Gasperini D. (2019) Interplay between plant cell walls and jasmonate production. Plant Cell Physiol. 60, 2629–2637 10.1093/pcp/pcz119 [DOI] [PubMed] [Google Scholar]
- 105.Chen X., Wang D.-D., Fang X., Chen X.-Y. and Mao Y.-B. (2019) Plant specialized metabolism regulated by jasmonate signalling. Plant Cell Physiol. 60, 2638–2647 10.1093/pcp/pcz161 [DOI] [PubMed] [Google Scholar]
- 106.Acosta I.F. and Przybyl M. (2019) Jasmonate signalling during Arabidopsis stamen maturation. Plant Cell Physiol. 60, 2648–2659 10.1093/pcp/pcz201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Balas L., Feillet-Coudray C. and Durand T. (2018) Branched fatty acyl esters of hydroxyl fatty acids (FAHFAs) appealing beneficial endogenous fat against obesity and type-2 diabetes. Chem. Eur. J. 38, 9463–9476 10.1002/chem.201800853 [DOI] [PubMed] [Google Scholar]
- 108.Kolar M.J., Konduri S., Chang T., Wang H., McNerlin C., Ohlsson L. et al. (2019) Linoleic acid esters of hydroxyl linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 294, 10698–10707 10.1074/jbc.RA118.006956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brejchova K., Balas L., Paluchova V., rezinova M., Durand T. and Kuda O. (2020) Understanding FAHFAs: from structure to metabolic regulation. Prog. Lipid Res. in press [DOI] [PubMed] [Google Scholar]